Abstract
HIV infection affects the central nervous system resulting in HIV associated neurocognitive disorder (HAND), which is characterized by depression, behavioral and motor dysfunctions. The HIV-1 viral envelope protein gp120 is known to induce the release of neurotoxic factors which lead to apoptotic cell death. Although the exact mechanisms involved in HIV-1 gp120-induced neurotoxicity are not completely understood, oxidative stress is suggested to play a vital role in the neuropathogenesis of HAND. Astrocytes represent major population of the non-neuronal cell type in the brain and play a critical role in the neuropathogenesis of HAND. Increased oxidative stress is known to induce nuclear factor erythroid derived 2-related factor 2 (Nrf2), a basic leucine zipper transcription factor which is known to regulate the antioxidant defensive mechanism. However, the role of Nrf2 in HAND has not been elucidated. We report that gp120 significantly upregulates Nrf2 in human astrocytes and is associated with stimulation of key antioxidant defensive enzymes Hemoxygenase (HO-1) and NAD(P)H dehydrogenase quinone1 (Nqo1). Pretreatment of the astrocytes with antioxidants or a specific calcium chelator BAPTA-AM, significantly blocked the upregulation of Nrf2, HO-1 and Nqo1. These results suggest a possible role of the intracellular calcium and oxidative stress in Nrf2 mediated antioxidant defense mechanism, which may have protective role in promoting cell survival.
Keywords: HIV-1 gp120, oxidative stress, Nrf2, Hemoxygenase, NAD(P)H dehydrogenase quinone1
Introduction
HIV associated neurocognitive disorder (HAND) is developed as a consequence of HIV infection in the central nervous system (CNS) occurring in about 50% of the HIV patients and is characterized by depression, behavioral and motor dysfunctions (Antinori et al., 2007; Ellis et al., 2007; McArthur and Brew, 2010). HIV infection in brain is associated with the release of several neurotoxic factors, generation of reactive oxygen species (ROS) (Ronaldson and Bendayan, 2008), stimulation of proinflammatory responses (Cysique et al., 2004; Gallo et al., 1989) eventually leading to cell death. The viral products implicated in the neurotoxicity of the HIV-1 infection include gp120, Tat, Nef, Vpr, Rev (Ellis et al., 2007; Nath and Geiger, 1998). HIV-1 gp120 is an envelope protein that mediates the entry of the virus into the host cell (Bazan et al., 1998; Gallo et al., 2003) and also induce the neurotoxicity through various pathways. The neurotoxicity of the HIV-1 gp120 is believed to be caused due to the infection of the astrocytes and microglia and stimulating the release of several soluble neurotoxic products (Ilyin and Plata-Salaman, 1997; Lipton, 1992; Ushijima et al., 1995).
Astrocytes represent a major non neuronal cell type in the brain and play important supportive role for the survival and functioning of the neurons (Anderson and Swanson, 2000; Chen et al., 2001; Mazzanti et al., 2001). Astrocytes are critical in homeostatic and regulatory functions that help in maintaining the neuronal integrity (Amiry-Moghaddam and Ottersen, 2003; Chen et al., 2001; Simard and Nedergaard, 2004). Productive infection of the astrocytes has been demonstrated to have significant impacts on the cell physiology. Astrocytes are natural host cells for HIV-1, specifically in advanced HAND and it has been shown that HIV infection in brain could affect astrocyte functioning by various pathways all of which contribute to the neuropathogenesis of HAND (Takahashi et al., 1996; Wang et al., 2008; Wang et al., 2004). Further, a higher rate of astrocyte apoptosis is reported in HIV patients with dementia (Shi et al., 1996).
Oxidative stress occurs due to an imbalance between the proto oxidant and anti-oxidant systems. Increased Oxidative stress results in cellular damage due to the oxidation of various essential molecules, activation of inflammatory response, receptor modulations, mitochondrial dysfunction (Betteridge, 2000; Pocernich et al., 2005; Valko et al., 2007; Yao et al., 2009). Increased evidences point to the role of oxidative stress in the neuropathogenesis of HAND (Blokhina et al., 2003; Nath, 2002; Pocernich et al., 2005; Yao et al., 2009). Earlier reports further suggest that chronic oxidative stress prevails in AIDS patients and the symptoms and consequences of this oxidative stress are reflected in the astrocytes and neurons of the HAND patients (Turchan et al., 2003).
Nuclear factor erythroid derived 2-related factor 2 (Nrf2), is a basic leucine zipper transcription factor that regulates the antioxidant defensive system and acts as a modifier of oxidative stress by binding to the antioxidant responsive element (ARE) located in the promoter regions of antioxidant genes (Ma et al., 2004; Motohashi and Yamamoto, 2004; Nguyen et al., 2005). Activation of Nrf2 is an adaptive or cytoprotective response and such activation has been shown to stimulate genes that code variety of detoxifying as well as antioxidant genes like Hemoxygenase-1 (HO-1) and NAD(P)H dehydrogenase quinone1 (Nqo1) in different conditions where oxidative stress is involved (Burdette et al., 2010; He et al., 2008). Although studies have demonstrated the role of Nrf2 in neurodegeneration (Shih et al., 2003), metal toxicity (He et al., 2008; Zhu et al., 2008), viral infections (Burdette et al., 2010; Qiang et al., 2004), its role in HAND is not completely understood. In this study we analyzed the effect of HIV-1 gp120 on Nrf2 mediated defensive mechanism which might have a cytoprotective role in human astrocytes.
Materials and Methods
Cell cultures and Treatments
Primary cultures of human astrocytes (HAs; catalog# 1800) were purchased from Sciencell Laboratories (Carlsbad, CA). Astrocytes were maintained in basal medium containing 10% fetal bovine serum, 100 units/ml penicillin, astrocyte growth supplement, and100 μg/ml streptomycin. Cells were maintained at 37°C in humidified incubator equilibrated with 5% CO2 and 95% air. Cultures consisted of >98% astrocytes based on immunohistochemical staining with glial fibrillary acidic protein. Primary cultures used in the current study were between passages 3-6.
HIV-1BAL gp120, recombinant was obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. The concentrations of gp120 used in the current study are within the range of earlier reports (Peng et al., 2008; Visalli et al., 2007; Yang et al., 2010). Further it has been reported that the levels of gp120 in the plasma of HIV patients has been 796ng/ml (Rychert et al., 2010). Heat inactivated gp120 served as a control. Further there was no difference between the heat inactivated controls vs. the normal control or among the controls at different time points.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Astrocytes were seeded (1×106cells/ml) in a six well plate and were treated with gp120. For time kinetics, astrocytes were treated with gp120 (50 ng/ml) for different time periods 24, 48 and 72h. Based on the time kinetics a dose study was performed using a range of gp120 concentrations (5-100ng/ml). At the end of the treatment periods, cells were harvested and RNA was isolated using RNAeasy mini kit. (Qiagen, GmbH, Germany). The cDNA was synthesized using high-capacity reverse transcriptase cDNA kit to perform qRT-PCR by Taqman gene expression assay primers (Applied Biosystems, CA, USA) for Nrf2 (Hs00975960_m1), NAD(P)H Quinone Oxido-Reductase (Hs00168547_m1), Hemoxygenase-1 (HS01110251_m1) and glutaldehyde-3-phosphate dehydrogenase (GAPDH) (Hs99999905_m1). GAPDH served as an internal control. Relative abundance of each mRNA species was assessed using brilliant Q-PCR master mix from Stratagene using MX 3000P instrument, which detects and plots the increase in fluorescence versus PCR cycle number to produce a continuous measure of PCR amplification. Relative mRNA species expression was quantitated and the mean fold change in expression of the target gene was calculated using the comparative CT method (Transcription accumulation Index, TAI=2−ΔΔ CT). All data were controlled for quantity of RNA input by performing measurements on an endogenous reference gene, GAPDH. In addition, results on RNA from treated samples were normalized to results obtained on RNA from the control.
Western Blotting
Primary astrocytes (1×106 cells/ml) were seeded in 10 cm dishes and grown to 80% confluence prior to treatments. At the end of the treatment periods, cells were washed twice with ice-cold PBS and harvested into lysis buffer containing protease inhibitors (Pierce Chemical, Rockford, IL). Protein content in each of the samples was determined using Bradford assay. Equal amounts of protein (20 μg) were loaded in each well and were run on an SDS-PAGE. The separated proteins were then electrophoretically transferred to polyvinylidene difluoride membranes at 100 V for 1 h. The membranes were blocked with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween-20 for 45 min at room temperature. The membranes were then incubated with anti-Nrf2 antibody (1:400) (Santa Cruz), anti-Nqo1 (1:1000), Anti-HO-1 (1:1000) or anti-β-actin antibody (1:5000) (Cell Signaling) at 4°C overnight, followed by washing and a second incubation in HRP-conjugated secondary antibodies for one hour (1:3000). Immunoreactive bands were visualized using a chemiluminescence system according to the manufacturer’s instructions (Pierce Chemical Co. Rockford, IL).
Analysis of Intracellular ROS
Intracellular ROS was monitored by employing 2′–7′ dichlorofluorescence diacetate (H2DCFDA) (Sigma, St. Louis, MO), which forms a fluorescent compound, dichlorofluorescein, upon oxidation with ROS. At the end of the treatment, cells were incubated with 10μM H2DCFDA for 30 minutes. Following the incubation, cells were washed twice with PBS. Cells were visualized and images were captured under inverted fluorescence microscope (Olympus) set at excitation and emission wave lengths of 485 and 535 nm respectively.
Statistical Analysis
All experiments were conducted in duplicate samples derived from three separate and independent experiments. The data were subjected to analysis of variance followed by Tukey’s test for comparison. Data were represented as mean SEM. p<0.05 was considered significant.
Results
HIV-1 gp120 increases ROS production in human astrocytes
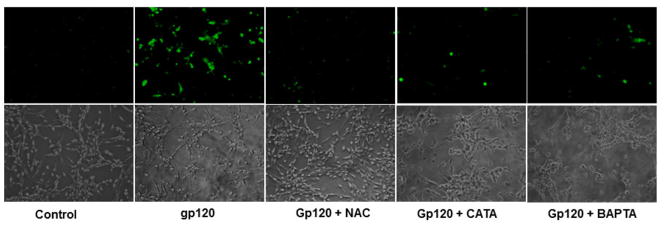
In order to test the role of oxidative stress in gp120 mediated toxicity, astrocytes were treated with gp120 for 48h following which the intracellular ROS was assessed using fluorescence probe. Our results demonstrate that the treatment of astrocytes with gp120 significantly increased the generation of intracellular ROS. Pretreatment of the cells with antioxidants NAC and Catalase suppressed the ROS production. Further, pretreatment with a calcium chelator BAPTA-AM also significantly inhibited the generation of ROS generation (Fig. 1).
Fig.1.
Astrocytes were treated with gp120 alone or gp120 plus either NAC, catalase or BAPTA-AM. Following the treatments, intracellular ROS was monitored by using a 2′–7′ dichlorofluorescence diacetate (H2DCFDA) (Sigma, St. Louis, MO), which forms a fluorescent compound, dichlorofluorescein, upon oxidation with ROS. Fluorescent images were captured under inverted fluorescence microscope (Olympus) set at excitation and emission wave lengths of 485 and 535 nm respectively. The cells treated with gp120 show higher fluorescence suggesting increased ROS production. Cells pretreated with antioxidants or BAPTA-AM showed significantly lesser ROS production as evidenced by reduced fluorescence.
HIV-1 gp120 up regulates Nrf2 expression in human astrocytes
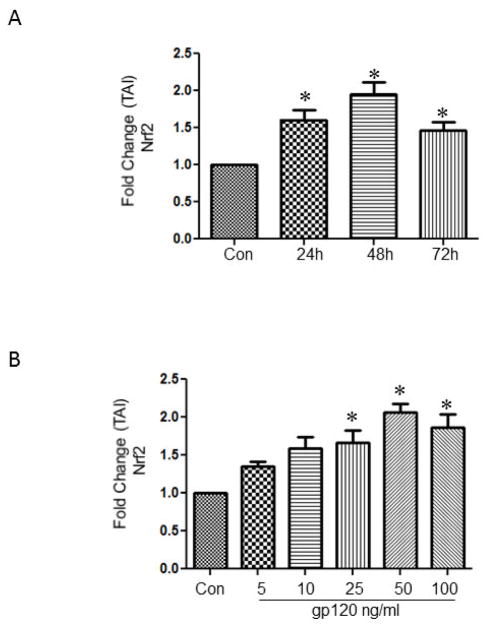
Activation of the Nrf2 is the initial event that has been shown to occur against stress related responses. In order to study if the treatment with gp120 leads to the increased expression of Nrf2, astrocytes were treated with 50 ng/ml of gp120 for different time intervals and analyzed for the relative gene abundance employing qRTPCR. The data presented in Fig 2A indicates a significant increase in the Nrf2 gene at 24h (TAI 1.67 ± 0.16), 48h (TAI 1.95 ± 0.15), and 72h (TAI 1.4 ± 0.11) respectively. Based on the time kinetics, 48h time point was selected to perform a dose response study. The results demonstrate that gp120, at 25ng/ml (TAI 1.67 ± 0.16), 50 ng/ml (TAI 2.07 ± 0.11) and 100 ng/ml (TAI 1.8 ± 0.19) significantly increased the expression of Nrf2 Fig 2B
Fig.2.
Astrocytes (1×106 cells/ml) treated with 50ng/ml gp120 for 24, 48 and 72h (A) and with various concentrations of gp120 (5-100ng/ml) (B). At the end of the treatments the cells were harvested, RNA was extracted, reverse transcribed, and the mRNA was measured by qRTPCR using the primers against Nrf2 and housekeeping gene GAPDH. The data expressed are Mean ± SEM of Transcription accumulation Index (TAI) values of three independent experiments. GAPDH served as the internal control. *P<0.05 compared to control.
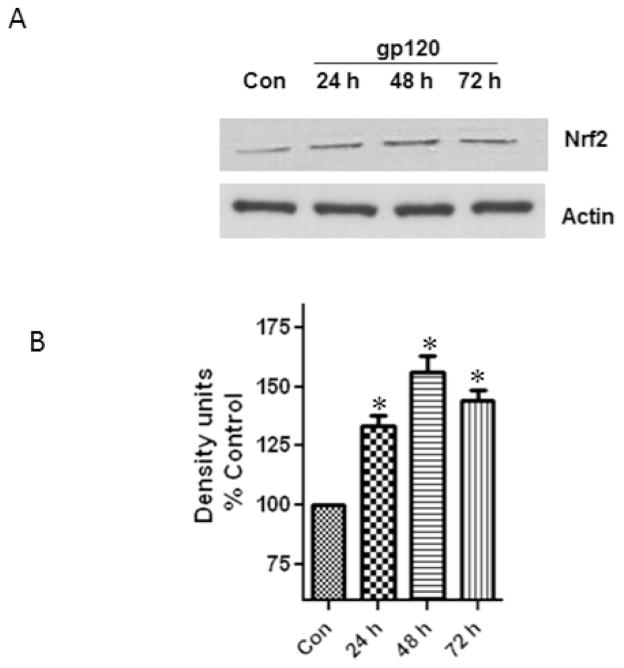
In order to examine if the treatment of astrocytes with gp120 altered the levels of the Nrf2 protein, we performed western blots from the total cell lysates obtained from the control and treated samples. Our results (Fig.3) show a significant increase of 28%, 52% and 39% at 24, 48 and 72h respectively. These results of the Nrf2 protein expression further correlated with the gene expression.
Fig.3.
Astrocytes (1×106 cells/ml) were treated with 50ng/ml gp120 for 24, 48 and 72 h after which the cells were harvested and the Nrf2 protein was analyzed. Untreated astrocytes served as controls. The figure shows a representative western blot indicating a significant upregulation of Nrf2 (A). Quantification of the band intensities of Nrf2 normalized against actin. Data were obtained from three independent experiments. Actin was used as a loading control.
*P<0.05 compared to control. Error bars represent mean ± SEM.
Induction of the Nrf2 is associated with the stimulation of the Antioxidant response
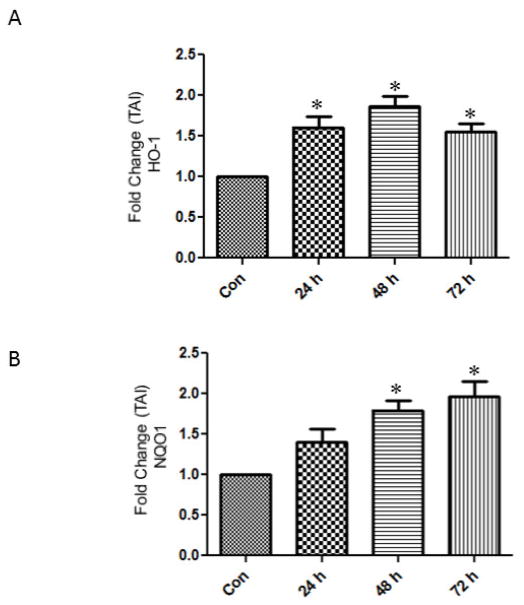
In order to examine whether the upregulation of Nrf2 is associated with the stimulation of of defensive genes Nqo1 and HO-1 astrocytes treated with gp120 were tested for Nqo1 and HO-1 gene and protein expressions. Our results show that the treatment of astrocytes with gp120 resulted in an increase of the HO-1 at 48h (TAI 1.8 ± 0.14) and 72 h (TAI 1.5 ± 0.11) respectively (Fig.4). Similarly the Nqo1 gene expression was significantly upregulated by 48h (TAI 1.7 ± 0.12) and 72 h (TAI 1.9 ± 0.2).
Fig.4.
Effect of HIV-1gp120 on the gene expression of Hemoxygenase-1 (A) and NAD(P)H quinone oxidoreductase (Nqo1) (B). Human astrocytes (1×106 cells/ml) were treated with 50 ng/ml of gp120 and the mRNA was measured by qRTPCR using the primers against HO-1 and Nqo1 and housekeeping gene GAPDH. Untreated astrocytes served as controls. The data expressed are Mean ± SEM of Transcription accumulation Index (TAI) values of three independent experiments. GAPDH served as the internal control. *P<0.05 compared to control.
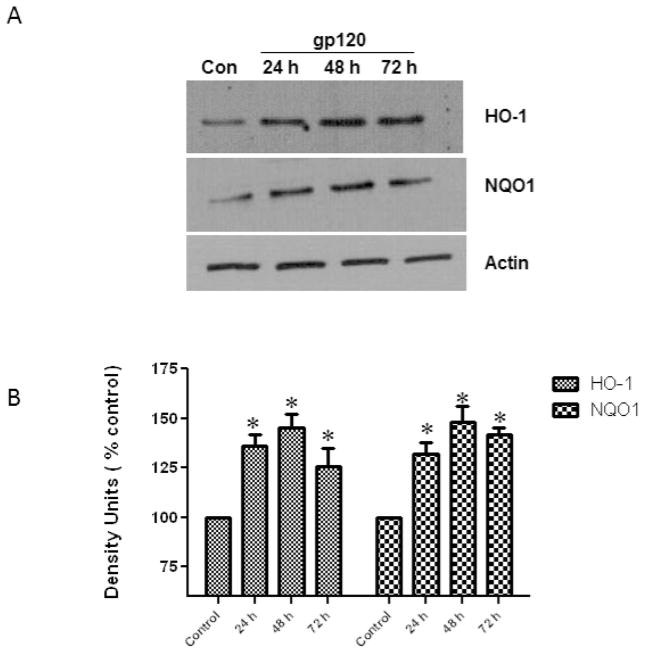
In order to examine HO-1 and Nqo1 protein expression, cell lysates, after treatment with gp120 were isolated, immunoblotted and analyzed to determine the expression levels at different time points. Our results (Fig. 5) demonstrate that the protein expression levels of HO-1and Nqo1 were significantly increased at 24, 48 and 72h.
Fig.5.
Effect of HIV-1gp120 on the protein expression of Hemoxygenase-1 and NAD(P)H quinone oxidoreductase (Nqo1). Astrocytes (1×106 cells/ml) were treated with gp120 (50 ng/ml) for 24, 48, and 72 h, after which the protein was extracted and analyzed by western blots using specific primary antibodies against HO-1 and Nqo1. Untreated astrocytes served as controls. The figure shows a representative blot demonstrating an increase in HO-1 and Nqo1 at all time points (A). Quantification of the protein band intensities normalized against actin (B). The data were collected from three independent experiments and represent the mean ± SEM. *P<0.05 compared to control.
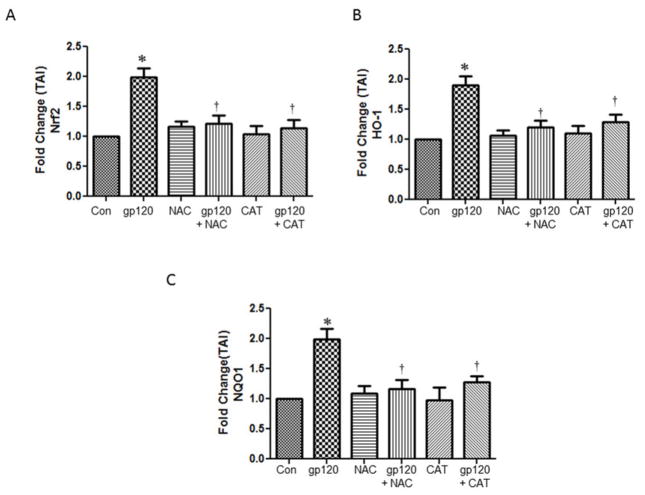
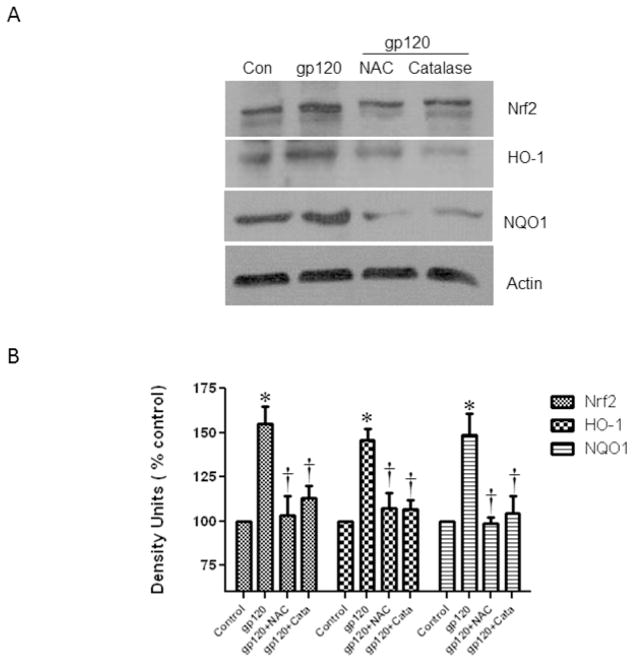
Pretreatment with antioxidants reduced the gp120-induced Nrf2, HO-1 and Nqo1
Previous studies have shown that gp120 induces ROS (Foga et al., 1997) (Pocernich et al., 2000). In order to evaluate if oxidative stress is playing a role in the Nrf2 stimulation by gp120 treatments, we pretreated the cells with known antioxidants and our results show that the N-Acetyl cysteine (NAC) and Catalase significantly attenuated the expression of gp120-induced Nrf2, HO-1 and Nqo1 mRNA expression (Fig. 6). In addition, our results also demonstrated that the pretreatment of astrocytes with antioxidants NAC and catalase inhibited the upregulation of the proteins Nrf2, HO-1 and Nqo1 proteins (Fig 7). Overall, our results suggest that suppression of oxidative stress by the antioxidant treatments attenuate the induction of Nrf2 and associated genes.
Fig.6.
Effect of antioxidant treatment on gp120-induced gene expression. Human astrocytes (1×106 cells/ml) were pretreated with antioxidants, N-acetyl cysteine and catalase for 30 minutes prior to the treatment with 50 ng/ml of gp120 recombinant protein for 48 h. At the end of the treatment periods, relative mRNA abundance levels employing qRTPCR using the specific primers against Nrf2 (A), HO-1 (B) and Nqo1 (C) The data were collected from three independent experiments and represent the mean ± SEM. *P<0.05 compared to control; †compared to gp120, P<0.05.
Fig.7.
Effect of antioxidants (1×106 cells/ml) on gp120-induced protein expression. Human astrocytes were pretreated with antioxidants, N-acetyl cysteine and catalase for 30 minutes prior to the treatment with 50ng/ml of gp120 recombinant protein for 48 h. At the end of the treatment periods, cells were harvested and protein was extracted and immune-blotted against anti-Nrf2, anti-HO-1 and anti-Nqo1. The figure shows the representative blots of Nrf2 (A), HO-1(B) Nqo1 (C). Quantification of the protein band intensities normalized against actin (B). Data were obtained from three independent experiments and represented as mean ± SEM. *P<0.05 compared to control; †compared to gp120, P<0.05.
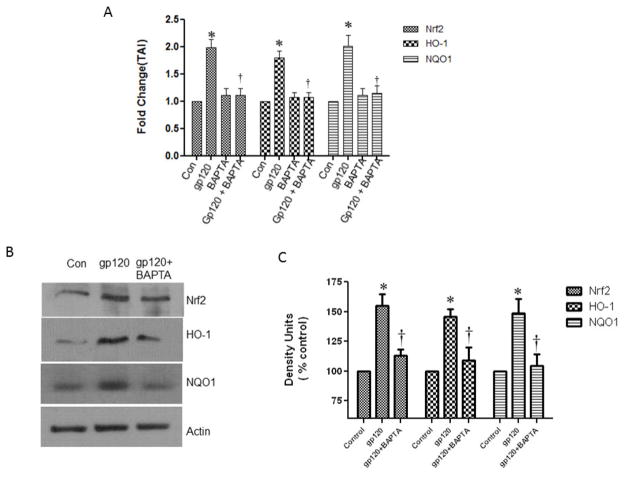
Pretreatment with BAPTA- AM reduced the gp120-induced Nrf2, HO-1 and Nqo1 expression
Earlier studies have demonstrated that gp120 induced oxidative stress is associated with the increased levels of intracellular calcium (Agrawal et al., 2010; Kaul and Lipton, 2006). Further, increased intracellular Ca2+ is also known to induce excess ROS and contribute the gp120-induced cellular oxidative damage. In order to evaluate the role of Ca2+ in gp120-induced Nrf2 activation, we have employed a calcium chelator, BAPTA-AM, at a nontoxic concentration (Kruman et al., 1998) which is very well known to suppress the intracellular Ca2+ levels. Pretreatment of the astrocytes with BAPTA-AM for 30 min prior to the gp120 treatment resulted in a significant decrease in Nrf2 gene and protein expression as well as in the inhibition of the HO-1 and Nqo1 gene and protein expression (Fig.8).
Fig.8.
Effect of the calcium chelator, BAPTA-AM on gp120-induced gene and protein expression: Cultured of human astrocytes (1×106 cells/ml) were pretreated with the calcium inhibitor BAPTA-AM for 30 minutes prior to the treatment with 50ng/ml of gp120 recombinant protein for 48 h. At the end of the treatment periods, cells were harvested, RNA and protein were extracted. RNA extracted from different groups of cells was reverse transcribed and analyzed for the mRNA abundance levels employing qRTPCR using the specific primers against Nrf2, HO-1 and Nqo1 (A). The protein samples were immune-blotted against anti-Nrf2, anti-HO-1 and anti-Nqo1. The figure shows the representative blots of Nrf2, HO-1 and Nqo1 expression (B). Quantification of the protein band intensities normalized against actin (C). Data were obtained from three independent experiments and represented as mean ± SEM. *P<0.05 compared to control; †compared to gp120, P<0.05.
Discussion
Increased production of free radicals has been shown to involve and initiate several important intracellular cascades. The gp120, one of the components of the viral envelope coating has been shown to be neurotoxic and cause apoptotic cell death (Bagetta et al., 1998; Yeung et al., 1998). Earlier studies have shown that gp120 infection causes an elevation in the production of the reactive oxygen species in vivo as well as in vitro (Foga et al., 1997; Pocernich et al., 2000). Our results also show a significant increase in the ROS levels in response to gp120 treatment (Fig. 1). The first line of defense against the oxidative stress is mediated by the upregulation of SOD and catalase. However when there is a continuous generation of ROS a second line of defensive action is deployed by the cell to elevate the antioxidant scavenging mechanisms. Activation of the cellular defensive pathways is the most important requirement when a cell responds to any insult or infection. One such member that stimulates the cellular protective mechanisms is Nrf2 (Motohashi and Yamamoto, 2004; Rushmore et al., 1991).
Nrf2 is a ubiquitously expressed transcription factor that is present in various tissues and cell types including astrocytes. It has been shown that the induction of Nrf2 regulates the basal and inducible expression of cytoprotective, detoxifying and antioxidant genes (Motohashi and Yamamoto, 2004). Earlier studies have demonstrated the role of Nrf2 in playing a protective role in several conditions such as cancer, viral infections, neurodegenerative disorders and chemical induced toxicities (Burdette et al., 2010; He et al., 2008; Martin-Montalvo et al., 2010; Qiang et al., 2004; Shih et al., 2003). A study by Zhang et al (Zhang et al., 2009) has shown the upregulation of Nrf2 by HIV-1 TAT in the HeLa-CD4-LTR-β-galactosidase indicator cells. However no studies have been focused on its role or the mechanisms of action of Nrf2 in human CNS cells. Overexpression of Nrf2 in HeLa cell line has been shown to provide additional protective effect against oxidative stress (Zhang et al., 2009). Interestingly, activation of the Nrf2 has also been shown to promote cell survival following endoplasmic reticular stress, which is well known to increase ROS levels as well as the intracellular calcium levels in hepatic cells infected with Hepatitis C virus (Burdette et al., 2010). Our results for the first time indicate that gp120 induces Nrf2 expression in astrocytes.
Reports from the existing literature reveal that the transcription factor Nrf2 is protective in in vitro and in vivo studies (Burdette et al., 2010; He et al., 2008; Shih et al., 2003) (Qiang et al., 2004). Induction of Nrf2 thereby upregulating defensive antioxidant mechanisms in neurons and glia in rat cortex through induction of the antioxidant response element (ARE)-driven genes has been reported (Shih et al., 2003). Further, supporting its protective role, it has been shown that the cells that lack the Nrf2 are highly susceptible to the cell damage due to inefficient antioxidant response. On the other hand those cells in which the Nrf2 was overexpressed showed higher resistance and were prevented from the brain damage against glutamate toxicity in astrocytes (Shih et al., 2003) as well as in against TAT in HeLa-CD4-LTR-β-galactosidase indicator cells against HIV long terminal activation (Zhang et al., 2009). In order to counter the oxidative stress mediated damage, organisms have developed a defensive mechanism by stimulating the antioxidant defensive mechanisms. Such mechanisms include the activation of Hemoxygenase and Nqo1. Earlier studies have shown that HO1 and Nqo1 both play an important role in mediating the anti-inflammatory and anti-oxidative effects in vitro and in vivo (Rushworth et al., 2008; Yeligar et al.). Since HAND is known to cause high levels of oxidative stress and inflammation, HO1 and Nqo1 may be activated to reduce the stress and inflammation. The role of HO-1 in monocytes of during HIV has been recently reported (Devadas et al., 2010; Hurttila et al., 2008). Moreover, over expression of HO-1 has been shown to promote anti-inflammatory response by suppressing proinflammatory cytokines and by increasing the expression of interleukin-10 (Hurttila et al., 2008; Seldon et al., 2007; Soares et al., 2004).
Activation of Nrf2 and the target genes may be elicited by an increase in ROS produced by gp120. N-acetyl cysteine and catalase are well known antioxidants that have the potential to inhibit ROS production. Pretreatment of the astrocytes with these antioxidants prior to the infection with gp120 inhibited the induction of Nrf2 and consequently blocked the upregulation of HO-1 and Nqo1, confirming that oxidative stress possibly results in the activation of these genes to protect itself from the gp120 induced cellular damage.
One of the initial events that occur in gp120 neurotoxicity is an elevation in the intracellular calcium levels. Calcium is also known to promote increased free radical generation and hence it is possible that the gp120 induced elevation of Ca2+ (Holden et al., 1999) may lead to the upregulation of Nrf2 associated antioxidant defense within the cell. Our results also suggest that the suppression of the intracellular Ca2+ by pretreating with BAPTA-AM, a calcium chelator results in the suppression of the Nrf2. Further our study shows that the inhibition of Ca2+ also blocked the upregulation of the genes, HO-1 and Nqo1 suggesting that the antioxidant enzyme activity regulation by Nrf2 against gp120 toxicity may be mediated by the Ca2+. Although the mechanisms associated with elevated calcium levels and oxidative stress is not clear, several studies have shown that increased Ca2+ can activate the calcium dependent enzyme activities, thereby producing higher amounts of peroxides and superoxides (Nakamura et al., 1989; Suzuki et al., 1985). Consistent with our results, inhibition of intracellular Ca2+ has been reported to reduce the stress and thus prevent the cellular damage in several in vitro as well as in vivo models (Burdette et al., 2010; Nakamura et al., 1989).
Taken together our results suggest that the astrocytes upregulate their antioxidant defense mechanisms in response to the gp120 induced stress by upregulation the Nrf2. Further, upregulation of Nrf2 or over expression of this gene might be of therapeutic significance against HIVgp120-induced neurotoxicity.
HIV-1gp120 upregulates second phase antioxidant defensive enzymes.
Antioxidants and calcium chelator blocked the upregulation of Nrf2, HO-1 and Nqo1.
Oxidative stress and calcium play a role Nrf2 mediated events.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal L, et al. Dopaminergic neurotoxicity of HIV-1 gp120: reactive oxygen species as signaling intermediates. Brain Res. 2010;1306:116–30. doi: 10.1016/j.brainres.2009.09.113. [DOI] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci. 2003;4:991–1001. doi: 10.1038/nrn1252. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Antinori A, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagetta G, et al. HIV-1 gp120-induced apoptosis in the rat neocortex involves enhanced expression of cyclo-oxygenase type 2 (COX-2) Biochem Biophys Res Commun. 1998;244:819–24. doi: 10.1006/bbrc.1998.8321. [DOI] [PubMed] [Google Scholar]
- Bazan HA, et al. Patterns of CCR5, CXCR4, and CCR3 usage by envelope glycoproteins from human immunodeficiency virus type 1 primary isolates. J Virol. 1998;72:4485–91. doi: 10.1128/jvi.72.5.4485-4491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betteridge DJ. What is oxidative stress? Metabolism. 2000;49:3–8. doi: 10.1016/s0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- Blokhina O, et al. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003;91(Spec No):179–94. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette D, et al. Activation of transcription factor Nrf2 by hepatitis C virus induces the cell-survival pathway. J Gen Virol. 2010;91:681–90. doi: 10.1099/vir.0.014340-0. [DOI] [PubMed] [Google Scholar]
- Chen Y, et al. Astrocytes protect neurons from nitric oxide toxicity by a glutathione-dependent mechanism. J Neurochem. 2001;77:1601–10. doi: 10.1046/j.1471-4159.2001.00374.x. [DOI] [PubMed] [Google Scholar]
- Cysique LA, et al. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol. 2004;10:350–7. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- Devadas K, et al. Lipopolysaccharide suppresses HIV-1 replication in human monocytes by protein kinase C-dependent heme oxygenase-1 induction. J Leukoc Biol. 2010;87:915–24. doi: 10.1189/jlb.0307172. [DOI] [PubMed] [Google Scholar]
- Ellis R, et al. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Foga IO, et al. Antioxidants and dipyridamole inhibit HIV-1 gp120-induced free radical-based oxidative damage to human monocytoid cells. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:223–9. doi: 10.1097/00042560-199712010-00001. [DOI] [PubMed] [Google Scholar]
- Gallo P, et al. Human immunodeficiency virus type 1 (HIV-1) infection of the central nervous system: an evaluation of cytokines in cerebrospinal fluid. J Neuroimmunol. 1989;23:109–16. doi: 10.1016/0165-5728(89)90029-5. [DOI] [PubMed] [Google Scholar]
- Gallo SA, et al. The HIV Env-mediated fusion reaction. Biochim Biophys Acta. 2003;1614:36–50. doi: 10.1016/s0005-2736(03)00161-5. [DOI] [PubMed] [Google Scholar]
- He X, et al. Activation of Nrf2 in defense against cadmium-induced oxidative stress. Chem Res Toxicol. 2008;21:1375–83. doi: 10.1021/tx800019a. [DOI] [PubMed] [Google Scholar]
- Holden CP, et al. Role of Na+/H+ exchangers, excitatory amino acid receptors and voltage-operated Ca2+ channels in human immunodeficiency virus type 1 gp120-mediated increases in intracellular Ca2+ in human neurons and astrocytes. Neuroscience. 1999;91:1369–78. doi: 10.1016/s0306-4522(98)00714-3. [DOI] [PubMed] [Google Scholar]
- Hurttila H, et al. Oxidative stress-inducible lentiviral vectors for gene therapy. Gene Ther. 2008;15:1271–9. doi: 10.1038/gt.2008.75. [DOI] [PubMed] [Google Scholar]
- Ilyin SE, Plata-Salaman CR. HIV-1 gp120 modulates hypothalamic cytokine mRNAs in vivo: implications to cytokine feedback systems. Biochem Biophys Res Commun. 1997;231:514–8. doi: 10.1006/bbrc.1997.6131. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 2006;4:307–18. doi: 10.2174/157016206777709384. [DOI] [PubMed] [Google Scholar]
- Kruman, et al. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–88. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Models of neuronal injury in AIDS: another role for the NMDA receptor? Trends Neurosci. 1992;15:75–9. doi: 10.1016/0166-2236(92)90013-x. [DOI] [PubMed] [Google Scholar]
- Ma Q, et al. Induction of murine NAD(P)H:quinone oxidoreductase by 2,3,7,8-tetrachlorodibenzo-p-dioxin requires the CNC (cap 'n' collar) basic leucine zipper transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2): cross-interaction between AhR (aryl hydrocarbon receptor) and Nrf2 signal transduction. Biochem J. 2004;377:205–13. doi: 10.1042/BJ20031123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A, et al. NRF2, cancer and calorie restriction. Oncogene. 2010;30:505–20. doi: 10.1038/onc.2010.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti M, et al. Glutamate on demand: astrocytes as a ready source. Neuroscientist. 2001;7:396–405. doi: 10.1177/107385840100700509. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Brew BJ. HIV-associated neurocognitive disorders: is there a hidden epidemic? Aids. 2010;24:1367–70. doi: 10.1097/QAD.0b013e3283391d56. [DOI] [PubMed] [Google Scholar]
- Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–57. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, et al. Superoxide anion is the initial product in the hydrogen peroxide formation catalyzed by NADPH oxidase in porcine thyroid plasma membrane. J Biol Chem. 1989;264:4759–61. [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl 2):S193–8. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- Nath A, Geiger J. Neurobiological aspects of human immunodeficiency virus infection: neurotoxic mechanisms. Prog Neurobiol. 1998;54:19–33. doi: 10.1016/s0301-0082(97)00053-1. [DOI] [PubMed] [Google Scholar]
- Nguyen T, et al. Nrf2 controls constitutive and inducible expression of ARE-driven genes through a dynamic pathway involving nucleocytoplasmic shuttling by Keap1. J Biol Chem. 2005;280:32485–92. doi: 10.1074/jbc.M503074200. [DOI] [PubMed] [Google Scholar]
- Peng F, et al. Platelet-derived growth factor protects neurons against gp120-mediated toxicity. J Neurovirol. 2008;14:62–72. doi: 10.1080/13550280701809084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocernich CB, et al. In-vivo glutathione elevation protects against hydroxyl free radical-induced protein oxidation in rat brain. Neurochem Int. 2000;36:185–91. doi: 10.1016/s0197-0186(99)00126-6. [DOI] [PubMed] [Google Scholar]
- Pocernich CB, et al. Proteomic analysis of oxidatively modified proteins induced by the mitochondrial toxin 3-nitropropionic acid in human astrocytes expressing the HIV protein tat. Brain Res Mol Brain Res. 2005;133:299–306. doi: 10.1016/j.molbrainres.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Qiang W, et al. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol. 2004;78:11926–38. doi: 10.1128/JVI.78.21.11926-11938.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, Bendayan R. HIV-1 viral envelope glycoprotein gp120 produces oxidative stress and regulates the functional expression of multidrug resistance protein-1 (Mrp1) in glial cells. J Neurochem. 2008;106:1298–313. doi: 10.1111/j.1471-4159.2008.05479.x. [DOI] [PubMed] [Google Scholar]
- Rushmore TH, et al. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266:11632–9. [PubMed] [Google Scholar]
- Rushworth SA, et al. Lipopolysaccharide-induced expression of NAD(P)H:quinone oxidoreductase 1 and heme oxygenase-1 protects against excessive inflammatory responses in human monocytes. J Immunol. 2008;181:6730–7. doi: 10.4049/jimmunol.181.10.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychert J, et al. Detection of HIV gp120 in plasma during early HIV infection is associated with increased proinflammatory and immunoregulatory cytokines. AIDS Res Hum Retroviruses. 2010;26:1139–45. doi: 10.1089/aid.2009.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldon MP, et al. Heme oxygenase-1 inhibits the expression of adhesion molecules associated with endothelial cell activation via inhibition of NF-kappaB RelA phosphorylation at serine 276. J Immunol. 2007;179:7840–51. doi: 10.4049/jimmunol.179.11.7840. [DOI] [PubMed] [Google Scholar]
- Shi B, et al. Apoptosis induced by HIV-1 infection of the central nervous system. J Clin Invest. 1996;98:1979–90. doi: 10.1172/JCI119002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, et al. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M, Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience. 2004;129:877–96. doi: 10.1016/j.neuroscience.2004.09.053. [DOI] [PubMed] [Google Scholar]
- Soares MP, et al. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172:3553–63. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- Suzuki H, et al. Enhancement by Ca2+ or Mg2+ of catalytic activity of the superoxide-producing NADPH oxidase in membrane fractions of human neutrophils and monocytes. J Biol Chem. 1985;260:3635–9. [PubMed] [Google Scholar]
- Takahashi K, et al. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann Neurol. 1996;39:705–11. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- Turchan J, et al. Oxidative stress in HIV demented patients and protection ex vivo with novel antioxidants. Neurology. 2003;60:307–14. doi: 10.1212/01.wnl.0000042048.85204.3d. [DOI] [PubMed] [Google Scholar]
- Ushijima H, et al. Exposure to gp120 of HIV-1 induces an increased release of arachidonic acid in rat primary neuronal cell culture followed by NMDA receptor-mediated neurotoxicity. Eur J Neurosci. 1995;7:1353–9. doi: 10.1111/j.1460-9568.1995.tb01126.x. [DOI] [PubMed] [Google Scholar]
- Valko M, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Visalli V, et al. N-acetylcysteine prevents HIV gp 120-related damage of human cultured astrocytes: correlation with glutamine synthase dysfunction. BMC Neurosci. 2007;8:106. doi: 10.1186/1471-2202-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, et al. Proteomic modeling for HIV-1 infected microglia-astrocyte crosstalk. PLoS One. 2008;3:e2507. doi: 10.1371/journal.pone.0002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, et al. Effects of human immunodeficiency virus type 1 on astrocyte gene expression and function: potential role in neuropathogenesis. J Neurovirol. 2004;10(Suppl 1):25–32. doi: 10.1080/753312749. [DOI] [PubMed] [Google Scholar]
- Yang Y, et al. Cocaine potentiates astrocyte toxicity mediated by human immunodeficiency virus (HIV-1) protein gp120. PLoS One. 2010;5:e13427. doi: 10.1371/journal.pone.0013427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, et al. Cocaine and human immunodeficiency virus type 1 gp120 mediate neurotoxicity through overlapping signaling pathways. J Neurovirol. 2009;15:164–75. doi: 10.1080/13550280902755375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeligar SM, et al. Ethanol-induced HO-1 and NQO1 are differentially regulated by HIF-1alpha and Nrf2 to attenuate inflammatory cytokine expression. J Biol Chem. 2010;285:35359–73. doi: 10.1074/jbc.M110.138636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung MC, et al. Inhibition of HIV-1 gp120-induced apoptosis in neuroblastoma SK-N-SH cells by an antisense oligodeoxynucleotide against p53. Aids. 1998;12:349–54. doi: 10.1097/00002030-199804000-00002. [DOI] [PubMed] [Google Scholar]
- Zhang HS, et al. Nrf2 is involved in inhibiting Tat-induced HIV-1 long terminal repeat transactivation. Free Radic Biol Med. 2009;47:261–8. doi: 10.1016/j.freeradbiomed.2009.04.028. [DOI] [PubMed] [Google Scholar]
- Zhu H, et al. Antioxidants and phase 2 enzymes in macrophages: regulation by Nrf2 signaling and protection against oxidative and electrophilic stress. Exp Biol Med (Maywood) 2008;233:463–74. doi: 10.3181/0711-RM-304. [DOI] [PubMed] [Google Scholar]