1 Introduction
An ENMC meeting was held in Naarden, the Netherlands (July 9–11th 2010) with 12 clinical and basic scientists and 3 industrial representatives from Europe (France, Belgium, Germany, Italy, Spain, Switzerland, the Netherlands and the UK) and the USA, to discuss their collective experience with the molecular underpinnings, diagnosis, and management of patients with deficiency of coenzyme Q10 (CoQ10).
CoQ10, also known as ubiquinone, is a unique electron carrier [1]. It is essential for aerobic organisms because of its role in mitochondrial energy production as a chaperone of electrons from complexes I and II (and also from the electron transfer flavoproteins, ETF) to complex III of the respiratory chain. In addition, this lipophilic molecule has numerous other vital functions in cells that include: serving as antioxidant in both lipoproteins and cell membranes; connecting energy production with important cellular pathways such as the cell cycle and DNA replication and repair through its role in pyrimidine biosynthesis; modulation of apoptosis through its regulation of the transition pore; and maintenance of body temperature via its action on uncoupling proteins.
The aims of this workshop were: to define how to clinically recognise and diagnose patients with CoQ10 deficiency; to optimize strategies to identify the underlying molecular genetic defects; to review basic CoQ10 biology; and to delineate therapeutic options for this group of disorders, which represent the most readily treatable subset of mitochondrial diseases.
2. Meeting outcomes
2.1 Biosynthesis of Coenzyme Q
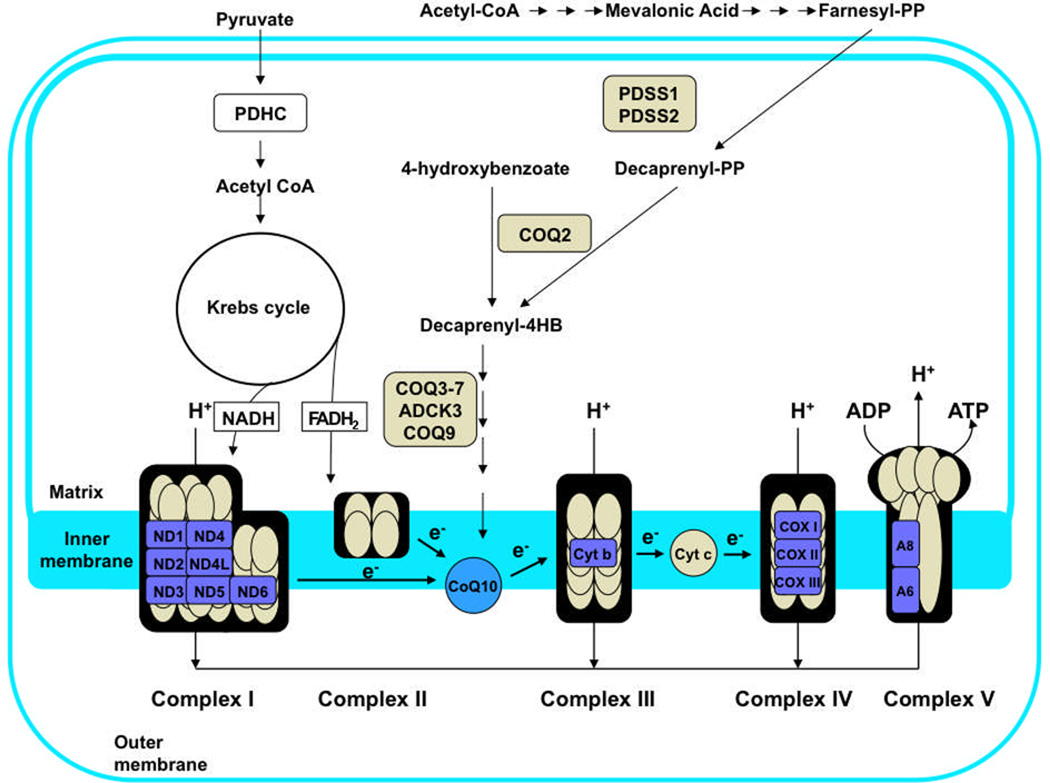
Primary CoQ10 deficiencies are caused by defects of the ubiquinone biosynthesis pathway, which is a complex and incompletely understood metabolic pathway [2]. In humans, the benzoquinone ring is synthesized from tyrosine or phenylalanine, while the polyprenyl side-chain is generated from acetyl-CoA through the mevalonate pathway and the enzymatic activity of decaprenyl diphosphate synthase. After condensation of 4-hydroxybenoate (4HB) with the polyprenyl tail, the ring undergoes decarboxylation, hydroxylation and methylation modifications to produce CoQ10. The biosynthesis of CoQ has been studied extensively in Escherichia coli and in several eukaryotic models including yeasts (Saccharomyces cerevisiae and Schizosaccharomyces pombe), Caenorhabditis elegans, plants, and animals [2–5]. An overview showing the CoQ branch of the isoprene biosynthetic pathway is shown in Figure 1. The polyprenyl tail of Q is produced by one of the non-sterol branch points emanating from a farnesyl-diphosphate precursor. The species specificity of the tail of CoQn (where n = the number of isoprene units) is determined by the Coq1 polypeptide in yeast, and by the Dps1/Dlp1 complex in S. pombe and the homologous Pdss1/Pdss2 complex in animal cells. In humans, the decaprenyl diphosphate synthase heterotetramer encoded by the PDSS1 and PDSS2 genes is responsible for producing the decaprenyl-diphosphate tail precursor of CoQ10.
Figure 1.
Biosynthesis of Coenzyme Q10 Deficiency
The aromatic ring precursor in coenzyme Q biosynthesis is 4HB. In contrast to bacteria which can produce 4HB via de novo synthesis [6], animals rely on a dietary source of essential amino acids, and utilize tyrosine and phenylalanine to generate the aromatic ring precursor of CoQ10. S. cerevisiae utilizes 4HB derived from tyrosine as the aromatic ring precursor of CoQ [4]. Recent independent and complementary studies by two groups show that para-aminobenzoic acid (pABA) also serves as an aromatic ring precursor of CoQ in S. cerevisiae [7, 8]. Pierrel et al. identified pABA as a Q biosynthetic precursor by studying the role of ferredoxin (Yah1) and ferredoxin reductase (Arh1) in Q biosynthesis [7]. They found that yeast mutants depleted in either Yah1 or Arh1 had defects in CoQ biosynthesis and accumulated a new prenylated intermediate with an amino substituent on the ring (3-hexapreny1-4-aminophenol). The discovery of this compound allowed the investigators to isolate pABA as a ring precursor in Q biosynthesis.
Marbois et al. [8] utilized a different approach to identify pABA as a new ring precursor in yeast Q biosynthesis. Using wild-type yeast cells, they identified a novel intermediate that was 1 Da less in mass than the previously characterized 3-hexapreny1-4-hydroxybenzoate intermediate. The mass difference and product ions suggested a ring with an amino substituent rather than a hydroxyl group. This notion was confirmed by demonstration that both 14C-labeled pABA and 13C6-pABA were shown to be incorporated into DMQ6 and CoQ6 by yeast. It is not yet clear how the nitrogen substituent is removed from the ring but the process likely includes a Schiff-base mediated deimination.
The use of pABA as a ring precursor in S. cerevisiae opens up questions regarding the possible use of pABA as a ring precursor in animal and human cells. In general, pABA is thought to compete with 4HB at the Coq2 step, and the product, prenyl-pABA is generally considered to be a dead-end product [1]. Several other aromatic ring inhibitors of Q biosynthesis, including 4-nitrobenzoic acid, also function as competitive inhibitors of Q biosynthesis in mammalian cells [9]. It may be important to test different concentrations of pABA and to re-evaluate with dose-response studies the role of pABA as a potential ring precursor in animal and human cells in culture.
2.2 Clinical recognition of coenzyme Q10 deficiency
Clinical recognition of CoQ10 deficiency is difficult because of extreme clinical heterogeneity, reflecting the heterogeneity of mitochondrial disease in general. However, some recognisable clinical phenotypes are emerging (Table 1). The first reported presentation of CoQ10 deficiency was of recurrent rhabdomyolysis associated with seizures and mental retardation [10]. Six cases from 4 families have been reported in total, with age of onset ranging from <2 to 15 years. Other clinical features associated with this phenotype include proximal muscle weakness, cerebellar symptoms, migraine, ptosis and lactic acidosis, and residual muscle CoQ10 levels are typically 4–16% of those observed in normal controls. In one patient with this encephalomyopathic phenotype plus ataxia, mutations were identified in the ADCK3 (CABC1) gene encoding a kinase that is thought to regulate CoQ10 biosynthesis [11,12] (see section 2.4).
Table 1.
Clinical phenotypes associated with coenzyme Q10 deficiency
| Clinical phenotype | Number of cases reported |
Associated genetic defects | References |
|---|---|---|---|
| Recurrent rhabdomyolysis with encephalopathy (seizures, mental retardation, or both) | 6 | Unknown in most cases; ADCK3 mutations in one family | [10–12] |
| Infantile-onset multisystem disorder (mainly encephalopathy plus nephropathy) | 13 | COQ2, PDSS1, PDSS2, COQ9, COQ6; other unknown gene(s) | [14–16, 30, 31] |
| Steroid-resistant nephrotic syndrome (+/− sensorineural hearing loss) | 10 | COQ2, COQ6 | [16, 18, 31, 32] |
| Ataxia | 31 | ADCK3, APTX; other unknown gene(s) | [12, 21, 34] |
| Leigh syndrome | 3 | PDSS2; other unknown gene(s) | [14, 24] |
| Myopathy | 9 | ETFDH; other unknown gene(s) | [26, 27] |
In 2000, Rötig et al. reported three siblings with a new phenotype of CoQ10 deficiency, namely a multisystem disorder of infancy with early-onset neurological disease initially manifesting as nystagmus and sensorineural hearing loss with subsequent ataxia, spasticity, dystonia, and cognitive dysfunction [13]. Severe steroid-resistant nephrotic syndrome due to glomerulosclerosis was fatal in one sibling and necessitated renal transplantation in the other two. Progressive visual failure in these children resulted from a combination of pigmentary retinopathy, optic atrophy, and cataracts. Seven additional cases of infantile-onset multisystemic disease and severe CoQ10 deficiency have been reported with prominent encephalopathy and nephropathy as the major clinical manifestations [14–18]. Most patients had steroid-resistant nephrotic syndrome with focal segmental glomerulosclerosis on renal biopsy. Other clinical features included Leigh syndrome, progressive ataxia, stroke-like episodes and hypertrophic cardiomyopathy. Most untreated cases of infantile-onset CoQ10 deficiency have been lethal in childhood; however, Mollet et al. described 14 and 22 year-old siblings with infantile onset deafness, macrocephaly, mental retardation, optic and peripheral neuropathies, obesity, cardiac valvulopathy, and livido reticularis [12]. Almost all patients reported with this phenotype have subsequently been shown to have mutations in genes encoding CoQ10 biosynthetic enzymes (see section 2.4).
The most frequent presentation of CoQ10 deficiency appears to be ataxia that typically begins in childhood and is often associated with seizures [19, 20]. At least 31 cases of this genetically heterogeneous condition have been reported and it is most frequently due to mutations in a kinase [12,19–21], ADCK3, that is thought to modulate CoQ biosynthesis. In addition, Quinzii and colleagues identified APTX mutations as a cause of secondary CoQ10 deficiency in three siblings with ataxia [22]. Le Ber and colleagues confirmed that APTX mutations caused secondary CoQ10 deficiency in muscle from five of six patients with oculomotor apraxia type 1 (A0A1) [23]. Less frequently observed phenotypes of CoQ10 deficiency include slowly progressive Leigh syndrome (initially reported in two adult sisters with encephalopathy and severe mental retardation associated with ataxia, deafness, growth retardation and lactic acidosis) [24] and isolated myopathy [25–27]. Patients with the myopathic form have presented with exercise intolerance between 6 and 33 years, associated with proximal muscle weakness, elevated CK (up to 20 times upper limit of normal) and lactic acidosis (up to 10 times normal levels after exercise) and no evidence of disease affecting other organ systems. There is typically a dramatic response to exogenous CoQ10. The genetic basis of CoQ10-deficient mild Leigh syndrome remains unknown, but most cases of myopathic CoQ10 deficiency appear to be secondary to multiple acyl-CoA dehydrogenase deficiency (MADD) [27](see section 2.4).
The considerable overlap of the CoQ10 deficient phenotypes with other mitochondrial disorders makes clinical recognition of CoQ10 deficiency extremely challenging. Nevertheless, it is important to be vigilant for clinical clues to diagnose patients so treatment can be initiated early in the disease. For example, it is important to suspect infantile-onset CoQ10 deficiency when evaluating young children with nephropathy, particularly steroid-resistant nephrotic syndrome, even in the absence of encephalopathy. Neuro-imaging may provide helpful diagnostic clues, since cerebellar atrophy is frequently seen in the CoQ10 deficiencies and is present in almost every subgroup of CoQ10 deficiency. However, this is a relatively nonspecific finding and cerebellar involvement is often seen in other mitochondrial respiratory chain disorders such as pontocerebellar hypoplasia type 6 (caused by mutations in the RARS2 gene which plays an essential role in mitochondrial translation), as well as in non-mitochondrial disorders such as the Joubert and Wolfram syndromes [28]. The triad of encephalopathy (seizures, mental retardation or both), myopathy with recurrent myoglobinuria, and ragged-red fibres is characteristic of the encephalomyopathic form of CoQ10 deficiency, whilst the combination of myopathy with markedly elevated CK and histological evidence of increased lipid have been observed in the myopathic form of CoQ10 deficiency. Although helpful, clinical features alone are insufficient to definitively diagnose CoQ10 deficiency or to distinguish between primary and secondary CoQ10 deficiencies. As a consequence, evaluation of patients with suspected CoQ10 deficiency relies on biochemical and molecular genetic investigation.
2.3 Laboratory investigation of Coenzyme Q10 deficiency
Biochemical screening tests
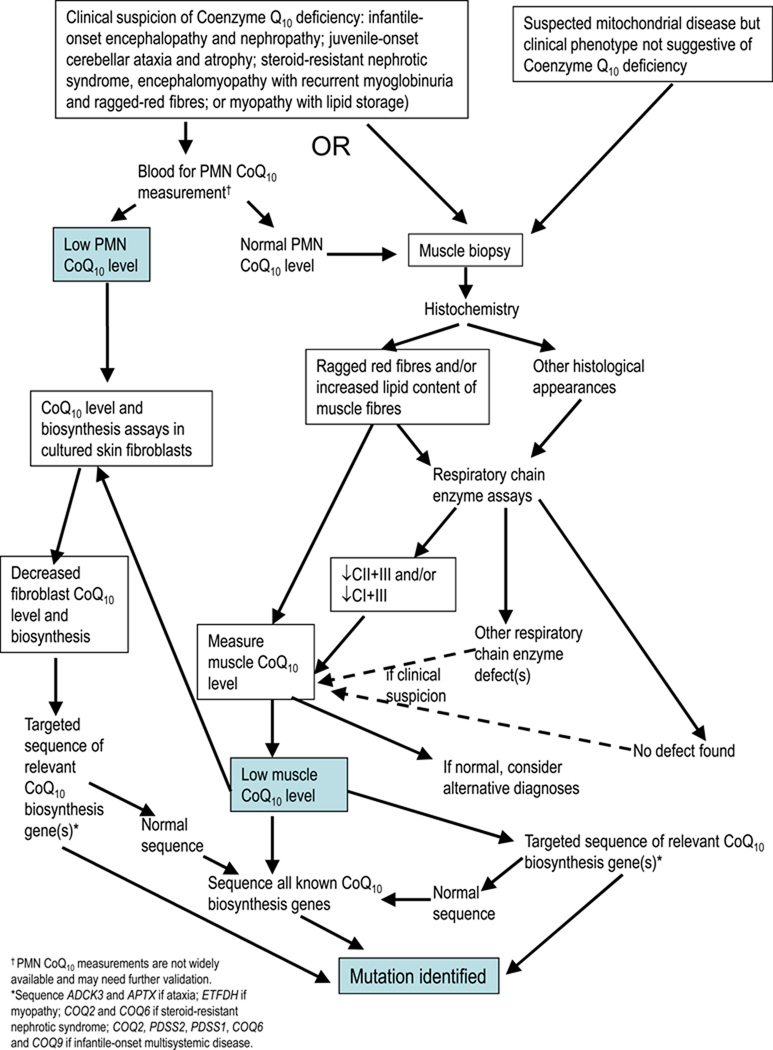
The best method for diagnosing CoQ10 deficiency biochemically was debated. Initial biochemical testing should include measurement of blood (plasma or serum) lactate, although a normal lactate level does not exclude CoQ10 deficiency. It was agreed that measurement of ubiquinone in a muscle biopsy remains the gold standard test for diagnosing CoQ10 deficiency. CoQ10 deficiency should be considered when muscle histology reveals ragged-red fibres and prominent lipid deposition, although these features are not a universal finding. It is important to assay NADH-cytochrome c reductase (a combined assay interrogating complexes I+III [CI+III]) or succinate cytochrome c reductase (complexes II+III [CII+III]) in frozen muscle homogenates, since these assays rely on endogenous CoQ10 levels and thus should be decreased in CoQ10 deficiency states even when individual respiratory chain activities are normal. However there are cases of mild CoQ10 deficiencies where activities of CI+III, CII+III, or both have been normal, and so the consensus recommendation is to measure the concentration of CoQ10 directly in skeletal muscle by high performance liquid chromatography (HPLC) in all cases where deficiency is suspected clinically (Figure 2).
Figure 2.
Investigation of Suspected Coenzyme Q10 Deficiency
Lack of commercially available non-physiological internal standards (IS) presents a major difficulty in the assessment of CoQ10 status in tissues. Initial studies used coenzyme Q9 as the IS of choice. However, CoQ9 is present in normal human tissues as a result of dietary contamination and synthesis by intestinal microorganisms. There is therefore a need for an alternative IS that is not influenced by exogenous or endogenous ubiquinones. For this reason, the non-physiological di-propoxy-CoQ10 IS has been synthesised, and this has enabled accurate determination of CoQ10 levels in muscle and other tissues [29].
Although skeletal muscle is generally accepted as the tissue of choice for the determination of CoQ10, muscle biopsy is an invasive procedure and it would be desirable to have a non-invasive screening test for CoQ10 deficiency. Initial screening investigations in blood focused on CoQ10 measurements in plasma. However, plasma CoQ10 appears to be highly dependent upon the concentration of lipoproteins, which act as carriers of CoQ10 in the circulation. Dietary intake also significantly influences plasma CoQ10 concentrations, contributing up to 25% of the total amount (9). Therefore, concentration of plasma CoQ10 may not accurately reflect the level in tissues. Assessment of blood mononuclear cells (MNC) has been suggested as an alternative tissue for CoQ10 evaluation. MNC represent a stable, easily isolated sample, which reflect changes in cellular CoQ10 status following supplementation [29]. Other tissues which may be considered are lymphoblastoid cell lines, primary fibroblasts or myoblasts. However, some patients with genetically confirmed CoQ10 biosynthetic defects had normal CoQ10 levels in fibroblasts [21], so it may be necessary to test more than one tissue to confirm the diagnosis.
Biosynthesis assays
Assays of CoQ10 biosynthesis in cultured skin fibroblasts can be used to confirm a defect in the CoQ10 biosynthetic pathway. These assays utilise radiolabelled substrates for the biosynthesis of CoQ10. Typically [3H]mevalonate and [14C]4HB are used in cell culture, whilst [3H]decaprenyl-PP is used in homogenized fibroblast extracts [14, 16]. The combined use of [14C]4HB and [3H]decaprenyl-PP may be useful to discriminate defects upstream or downstream of the reaction catalyzed by decaprenyl diphosphate synthase. For example, COQ2 mutant fibroblasts synthesised less radiolabelled CoQ10 with both the [14C]4HB and the [3H]decaprenyl-PP substrates, whereas radiolabelled CoQ10 production was reduced with [14C]4HB but normal with [3H]decaprenyl-PP in PDSS2 mutant fibroblasts [14, 16]. Multiple steps in the CoQ10 biosynthetic pathway cannot be distinguished using the available assays, but this may be possible in the future using tandem mass spectrometry methods to identify accumulation of abnormal metabolites. Preliminary work in this area was presented [Simon Eaton, personal communication].
2.4 Genetics of Coenzyme Q10 deficiency
Primary Coenzyme Q10 deficiency
Mutations in 6 genes encoding components of the CoQ10 biosynthetic pathway have been associated with human CoQ10 deficiency to date [12, 14–16, 21, 30, 31]. The first genetic defect was identified by homozygosity mapping; two siblings with nephropathy, one of whom also had an infantile onset encephalomyopathy, were demonstrated to have mutations in the COQ2 gene encoding the PHB-polyprenyl transferase [16]. COQ2 mutations have been described in six patients from 4 different families [15, 16, 32]. Phenotypes range from a fatal neonatal multisystem disorder to isolated nephrotic syndrome. Nephrotic syndrome was present in all reported patients and typically preceded the neurological manifestations. Preliminary data indicate that clinical variability depends at least in part on the residual activity of individual mutants, but environmental factors may also play an important role in determining the clinical phenotype of patients.
Rötig and co-workers used biochemical approaches to identify accumulating intermediates in cells with the infantile multisystem form of CoQ10 deficiency, followed by sequence analysis of candidate genes [13]. This led to demonstration of transprenyltransferase deficiency in one family, which was subsequently shown to be caused by a pathogenic mutation in the PDSS2 gene [13][Rötig, personal communication]. Transprenyltransferase, the enzyme that generates the decaprenyl side-chain of the CoQ10 molecule, is a heterotetramer composed of two different subunits encoded by the PDSS1 and PDSS2 genes in humans. In a second family, homozygosity mapping was used to identify a pathogenic mutation in the PDSS1 gene [15]. In an infant with fatal Leigh syndrome and nephrotic syndrome, compound heterozygous PDSS2 mutations were identified by candidate gene sequencing [14]. In toto, four families with PDSS1 or PDSS2 mutations have now been identified, all presenting with infantile-onset multisystemic disorders [15, 33][Rötig, personal communication]. Surprisingly, PDSS1-mutant patients have a very different clinical presentation from patients with PDSS2 mutations despite the fact that defects in either gene result in transprenyltransferase deficiency. The siblings with PDSS1 mutations had a complex clinical phenotype with deafness, macrocephaly, mental retardation, optic and peripheral neuropathies, obesity and cardiomyopathy without nephropathy and with prolonged survival. In contrast, patients with PDSS2 mutations have infantile-onset encephalopathy and steroid-resistant nephrotic syndrome, which if untreated can be fatal. Curiously, the nephro-encephalopathy phenotype of COQ2 deficiency overlaps with that of PDSS2 deficiency and the renal involvement in both disorders is recapitulated by PDSS2 mutant mice (see section 2.5). It is not known why patients with PDSS1 mutations do not develop nephropathy. Possible explanations include variations of CoQ10 levels in tissues, differences in reactive oxygen species overproduction, or variable expression levels of the two genes in different tissues.
Mutations in ADCK3 were reported simultaneously by Lagier-Tourenne et al. and Mollet et al. who performed homozygosity mapping to identify the causative gene in families with autosomal recessive juvenile-onset cerebellar ataxia and atrophy [12, 21]. ADCK3 mutations have been reported in 12 patients (8 families) making this gene the most frequent cause of primary CoQ10 deficiency [12, 21, 34]. In addition to ataxia, some patients have had epilepsy, mild cognitive impairment, corticospinal tract signs, dystonia, and pes cavus. Fibroblasts from patients with ADCK3 mutations had normal or decreased levels of CoQ10, but muscle biopsies from four patients uniformly revealed CoQ10 deficiency. ADCK3 (CABC1) encodes a putative kinase, which appears to modulate biosynthesis of CoQ10. The homologous gene in S. pombe was initially postulated to encode a protein required for activity of bc1 complex, which led to the original gene name CABC1 [35–37]; however, subsequent studies from Catherine Clarke’s group demonstrated the role of this protein in CoQ10 biosynthesis [38, 39]. Studies of the yeast homologue, Coq8p, indicate that the protein is required to maintain stability of Coq3p, a ubiquinone biosynthetic protein [40].
Mutation of COQ9 has been reported in a single patient, who presented in the neonatal period with lactic acidosis and multisystem problems, including mild left ventricular hypertrophy, renal tubulopathy and a severe seizure and movement disorder associated with cerebral and cerebellar hypoplasia/atrophy [17]. Although there was only one affected child in this family, and the parents were not known to be related, a combined homozygosity mapping and candidate gene approach was successfully used to identify a homozygous nonsense mutation in COQ9 in this patient [30]. The precise function of COQ9 remains unknown, but the observations of an accumulated intermediate in HPLC analysis of CoQ species and reduced CoQ10 biosynthesis using [14C]4HB substrate in fibroblasts from the deficient patient, suggests that the COQ9 gene product is a bona fide CoQ10 biosynthetic enzyme.
This year, mutations in another gene, COQ6, have been reported in 11 patients from 5 different kindreds, with a phenotype similar to that observed in patients with mutations in COQ2 or PDSS2 [31]. All had nephrotic syndrome with onset in the first years of life, associated with sensorineural hearing loss in all 9 cases tested, and 3 also had CNS involvement with seizures or ataxia.
It has not been possible to identify the underlying genetic defect in more than 50 patients with CoQ10 deficiency despite sequencing all of the known biosynthetic genes [Quinzii, Navas, and Hirano, personal communication]. Possible explanations for this observation are that there may still be further biosynthetic enzymes, which have not yet been characterised in humans; the responsible mutations may be in regulatory sequences located distant to the genes of interest, or in as yet unknown regulatory genes; or that the CoQ10 deficiency in these patients may be a secondary phenomenon.
Secondary Coenzyme Q10 deficiencies
Mutations in the ETFDH gene (encoding the electron transfer flavoprotein dehydrogenase) were identified in patients who had been initially reported as having the isolated myopathy phenotype of CoQ10 deficiency [27]. These patients presented with fluctuating proximal and axial myopathy, and exercise intolerance. Episodic encephalopathy, hepatopathy and periodic vomiting, often triggered by metabolic stress, were also observed. A few patients experienced rhabdomyolysis and some died of coma or sudden unexplained death. Triggering factors included infections, metabolic stress, pregnancy, surgery, psychological stress and drugs. Biochemical investigations showed increased CK and lactate levels, with low carnitine and a characteristic acylcarnitine profile on tandem mass spectrometry suggestive of MADD [41]. Muscle biopsies exhibited mild to severe vacuolar changes, increased lipid content, ragged-red fibres, focal or diffuse SDH deficiency, COX-negative fibres, combined CI+III and CII+III deficiencies, and decreased level of CoQ10 in muscle (50% of normal). The mechanism of CoQ10 deficiency in MADD remains unknown and it does not appear to be a universal feature of this disorder, since some patients have been demonstrated to have normal CoQ10 levels [42,43].
Ataxia with oculomotor apraxia type I (AOA1) is one of a group of spinocerebellar ataxias with axonal neuropathy, but also has apraxia of eye movements [44]. Onset is typically in childhood with cerebellar symptoms followed by axonal sensorimotor neuropathy, often with cognitive impairment. The disorder is caused by recessive mutations in the APTX gene, which encodes a protein involved in single- and double-strand nuclear DNA break repair. In 2005 mutations in APTX were identified in one family with a cerebellar ataxic presentation of CoQ10 deficiency and clinical improvement with CoQ10 supplementation [22]. Low levels of CoQ10 were subsequently found in muscle and fibroblasts of some patients with typical AOA1 [23]. However in other AOA1 patients CoQ10 levels may be normal or even elevated. Clear genotype-phenotype correlations have yet to be established, and the mechanism of CoQ10 deficiency in this disorder remains unknown.
Secondary CoQ10 deficiency has also been reported in a number of patients with primary mtDNA mutations. For example, levels of CoQ10 in muscle from 25 patients with mitochondrial encephalopathies, mostly due to mtDNA mutations, were significantly lower than controls [45] while a multicenter study of 76 patients with heterogeneous mitochondrial diseases detected CoQ10 deficiency in 28 (37%) of which nine had pathogenic mtDNA mutations [46]. Five patients with MELAS had CoQ10 levels of ~40–60% of control levels [Navas, personal communication]. However the reasons for CoQ10 deficiency in these patients remain obscure, nor is the prevalence of secondary CoQ10 deficiency known.
2.5 Models of Coenzyme Q10 deficiency
Human cell culture models
Cell culture models have revealed insights into the pathogenesis of CoQ10 deficiency. Initial studies in cultured fibroblasts from two siblings with infantile-onset CoQ10 deficiency showed mild respiratory chain defects, but did not detect elevated superoxide anions, lipid peroxidation, or apoptosis-mediated cell death [47]. Lopez-Martin and colleagues showed that COQ2 mutant fibroblasts require uridine to sustain growth and proposed that deficiency of CoQ10 impaired de novo pyrimidine biosynthesis because of the dependence of dihydro-orotate dehydrogenase on ubiquinone [48]. In the same two cell lines plus two other cells lines from patients with genetically undefined CoQ10 deficiency, Rodriguez-Hernandez and colleagues noted increased autophagy of mitochondria [49].
Quinzii et al. initially observed that PDSS2 mutant fibroblasts have 12% residual CoQ10 and markedly reduced ATP synthesis, but do not show increased reactive oxygen species (ROS) or oxidative stress, while COQ2 mutant fibroblasts with 30% CoQ10 content have mild defects of ATP synthesis and significantly increased ROS production as well as oxidation of lipids and proteins and cell death [50]. They have extended the studies to other patient cell lines with variable degrees of CoQ10 deficiency due to different mutations in COQ2, ADCK3, and COQ9, and have observed similar correlations between levels of CoQ10 and cellular phenotype: 10–15% or >60% residual CoQ10 levels are not associated with significant ROS production whereas 30–50% residual CoQ10 content is associated with maximal ROS production and cell death [51]. Thus, in vitro, severe deficiency of CoQ10 causes bioenergetic defects, while moderate deficiency produces ROS, oxidative damage, and cell death.
Animal models
The first phenotype of CoQ deficiency described in C. elegans was the aging or clock phenotype [52]. Strains harbouring mutations in the clk-l/coq-7 gene showed a delayed aging phenotype including extension of lifespan. These animals had impaired CoQ production and accumulated demethoxy-CoQ (DMQ), an intermediate of CoQ biosynthesis indicating a primary CoQ deficiency [53]. Attempts to induce coq knockout (KO) strains mainly resulted in developmental arrest at early stages. The Coq-8 gene KO strain arrests late in life (Larval 3–4 [L3–L4] stages) and is therefore another useful model of primary CoQ deficiency [54]. Animal development was arrested at stages corresponding to higher expression of coq-8 in wild-type animals. Furthermore, sterility of these animals was caused by arrest at the 8th to 10th blastocyte division, corresponding to the stage of coq-8 expression to recycle new mitochondria. This animal model is useful not only to study development and lifespan phenotypes, but also genes involved in the regulation of the CoQ biosynthesis pathway.
In the early 1970s, a spontaneous mutant mouse, termed kd, was identified to have autosomal recessive kidney disease [55]. Homozygous kd/kd mice appear healthy for at least the first 8 weeks of life, but histological examination of the kidneys at about 12 weeks reveals a mononuclear cell infiltrate and tubular dilation. Over time the entire kidney is involved and the mice die of renal failure [56]. The mutation responsible for the kd/kd phenotype was identified as a V117M amino acid substitution within PLMP, a prenyltransferase-like mitochondrial protein [57]. This polypeptide was subsequently identified as PDSS2, required for synthesis of the polyprenyl-diphosphate tail precursor in coenzyme Q synthesis [58, 59]. Although young homozygous Pdss2kd/kd mice have a normal content of CoQ9 and CoQ10 in kidney, they fail to produce the elevated levels of CoQ9 and CoQ10 present in kidneys of wild-type mice that normally occurs at about 40 days of age. The onset of proteinuria and kidney disease ensues several weeks after the normal increase in CoQ9 and CoQ10 fails to occur [59]. Conditional knockout mice showed that proteinuria and kidney disease could be recapitulated when Pdss2 was deleted in podocytes (Podocin/cre,Pdss2loxP/loxP) but not when targeted to renal tubular epithelium, monocytes or hepatocytes [58].
2.6 Treatment of Coenzyme Q10 deficiency
Clinical observations
In general, supplementation with oral CoQ10 (10–30 mg/kg/day in children and 1,200–3,000 mg/day in adults) seems effective in patients with COQ2 mutations, especially for the neurological and renal manifestations of this disorder. In contrast, poor responses to CoQ10 supplementation have been observed in patients with PDSS2 mutations and in the patient with a homozygous COQ9 mutation. The reasons for the disparate responses to CoQ10 are not known. A likely contributing factor is the poor bioavailability of CoQ10; less than 5% of oral CoQ10 reaches plasma in humans and rodent studies have demonstrated low uptake of CoQ by tissues with particularly little or no detectable uptake by brain except in aged rats [1, 60–62]. Thus, the blood-brain barrier appears to impair central nervous system uptake of CoQ10. Hence, tissue involvement may influence response to CoQ10 supplementation as illustrated by the ubiquinone-responsive CoQ10 deficient patients with COQ2 mutations who manifested nephrotic syndrome and in one case had stroke-like episodes suggesting involvement of vascular structures, which is likely to be amenable to CoQ10 supplementation [16,18, 32, 63]. In contrast brain tissue was clearly affected in the CoQ10-refractory patients with Leigh syndrome due to PDSS2 mutations and refractory seizures caused by COQ9 deficiency [14, 30].
Furthermore, because CoQ is highly lipophilic, exogenously administered CoQ will be integrated into plasma and other cellular membranes before reaching the inner mitochondrial membrane. This notion is supported by cell culture studies [33] (see In vitro treatment of cell models of coenzyme Q10 deficiency). Because of the poor bioavailability and delayed mitochondrial uptake of ubiquinone, early CoQ10 supplementation is crucial for the success of the therapy. Furthermore, it appears that CoQ10 can block the progression of the disease but cannot reverse established tissue damage. Therefore intense efforts should be directed towards a prompt diagnosis of these patients.
One study assessed the clinical outcome after CoQ10 therapy in patients presenting with ataxia, with or without associated CoQ10 deficiency [64]. This was an open-label prospective study in 14 patients classified into two groups according to CoQ10 values in muscle or fibroblasts (deficient or not). Mutation analysis of four genes involved in CoQ10 biosynthesis (COQ2, PDSS1, PDSS2 and ADCK3) revealed a missense point mutation in ADCK3 in a single patient, and the genetic basis of CoQ10 deficiency was unknown in the remaining cases. Patients were evaluated clinically (ICARS scale, MRI and video-tape registration) and biochemically at baseline and every 6 months during a period of 2 years after CoQ10 treatment (30 mg/Kg/day). Patients with CoQ10 deficiency showed a statistically significant reduction of ICARS scores (Wilcoxon test: p = 0.018) after 2 years of CoQ10 treatment as compared to baseline conditions. In patients without CoQ10 deficiency, no statistically significant differences were observed in total ICARS scores after therapy, although one patient from this group showed a remarkable clinical amelioration. Biochemical diagnosis of CoQ10 deficiency was a useful tool for the selection of patients who are good candidates for treatment, since all of them responded to therapy. However, the remarkable clinical response in one case without CoQ10 deficiency in fibroblasts or muscle (and the previous observation that some patients with ADCK3 mutations do not have CoQ10 deficiency) highlights the importance of therapeutic trials of CoQ10 in patients with ataxia, in order to identify those who are CoQ10-responsive.
Response to treatment is also variable in patients with secondary CoQ10 deficiency syndromes. In patients with myopathy due to ETFDH mutations, riboflavin supplementation was generally followed by clinical improvement and normalisation of CK and lactate levels. In a few patients, a positive effect was also shown after additional supplementation with CoQ10. A low fat and high carbohydrate diet should be recommended and patients should avoid fasting. Most patients are symptom-free on riboflavin alone, however about 15% of patients have a severe, early onset, multisystem disease with no improvement on therapy. In three siblings with cerebellar ataxia due to a homozygous stop codon mutation in APTX and secondary CoQ10 deficiency in muscle, high-dose CoQ10 supplementation (up to 3,000 mg daily) was associated with improved ambulation in all and resolution of seizures in the affected sister [22].
In vitro treatment of cell models of Coenzyme Q10 deficiency
Identification of factors that can influence the efficacy of CoQ10 supplementation may be useful to improve the treatment of CoQ10 deficiency. Results in fibroblasts with mutations in COQ9, COQ2 and PDSS2 revealed that the prolonged pharmacokinetics of CoQ10 to reach the mitochondrial respiratory chain critically affects restoration of energy status of human CoQ10 deficient cells [33]. This may explain the delayed clinical response of CoQ10 deficiency patients to oral supplementation with CoQ10. Additionally, it was demonstrated that short tail ubiquinone analogues cannot substitute for CoQ10 in the mitochondrial respiratory chain of human CoQ10 deficient fibroblasts, thus revealing the importance of the decaprenyl tail. Oxidative stress and cell death can be attenuated by the administration of lipophilic and hydrophilic antioxidants. Therefore, complementary administration of antioxidants with high bioavailability may be helpful in CoQ10 deficient patients.
Treatment of a Coenzyme Q10 deficient mouse model
Supplementation with CoQ10 (200 mg/kg/day, beginning at weaning) was shown to provide a dramatic, though partial, rescue of proteinuria and nephritis in the Pdss2kd/kd mice [59]. The supplementation did not result in elevated levels of CoQ10 in the kidney. It was speculated that the benefit of CoQ10 supplementation may be achieved through its action as an antioxidant or co-antioxidant in conjunction with vitamin E [3]. These results suggest that supplementation with CoQ10 may provide substantial benefit in treatment of human CoQ10 deficiencies with associated kidney disease, and in certain forms of human focal segmental glomerulosclerosis that mirror the renal disease in the Pdss2kd/kd mouse.
3. Future prospects
As a result of the workshop, an international consortium was established with the aim of optimising the diagnosis and treatment of patients with CoQ10 deficiency. The prevalence of CoQ10 deficiency remains unknown but, because of its novelty and relative obscurity, the consortium members suspect that this condition is underdiagnosed. The lack of a specific ‘CoQ10 phenotype’ hampers clinical recognition of this disorder, but it is hoped that the diagnostic algorithm presented here will help to increase awareness of and diagnostic rates for this condition. CoQ10 deficiency should especially be considered in patients with steroid-resistant nephrotic syndrome and those with an otherwise unexplained cerebellar ataxia. At present it is recommended that CoQ10 is measured routinely in all muscle samples from patients with suspected mitochondrial disease, and not just in those biopsies where reduced activities of complexes I+III or II+III are observed. The consortium will work to establish diagnostic criteria and standardise methods for laboratory diagnosis of CoQ10 deficiency, including systematic evaluation of methods for measuring CoQ10 in PMCs, lymphoblastoid cell lines and primary fibroblasts, as well as skeletal muscle. If sufficient laboratories are offering CoQ10 measurements, an External Quality Assessment (EQA) system will be set up.
Genetic diagnosis of CoQ10 deficiency remains challenging because of the genetic heterogeneity of this disease group. A further difficulty is that primary and secondary CoQ10 deficiencies are often clinically indistinguishable. At present there is no clear genotype-phenotype correlation, but patients with an infantile presentation of encephalopathy and steroid-resistant nephrotic syndrome seem particularly likely to have primary CoQ10 deficiency. Targeted sequencing of the known human CoQ10 biosynthetic genes should be performed in this subgroup of patients, whilst patients with ataxic presentations should be screened for ADCK3 and/or APTX mutations. In other patients, radioisotope methods for CoQ10 biosynthesis assays may help to determine whether the observed CoQ10 deficiency is primary or secondary, and the likely level of the defect. In selected cases, a homozygosity mapping or whole exome sequencing approach may be fruitful. However the former requires consanguineous families and the latter may not detect defects outside the known likely candidate genes (i.e. genes already known to be involved in CoQ10 biosynthesis). In an attempt to reduce confusion regarding gene nomenclature of CoQ biosynthetic genes, we have successfully applied to HUGO and SGD to formally change the gene name from CABC1 to ADCK3. A database of known mutation and SNPs in CoQ biosynthetic genes is also planned.
Further evidence is needed regarding optimal treatment of CoQ10 deficiency, namely the most effective therapeutic agent (CoQ10, a synthetic analogue, or a combination of both), dosage regimens and route of administration. Furthermore, at present it is not at all clear which of the many functions of CoQ10 (electron transfer, antioxidant, pyrimidine biosynthesis, modulation of apoptosis or extramitochondrial roles) are most relevant to human disease in deficiency states. Understanding this will be critical to developing therapeutic advances for this group of potentially eminently treatable diseases.
4. Chairpersons
Shamima Rahman, UCL Institute of Child Health, London, UK
Michio Hirano, Columbia University, New York, USA
5. Workshop participants
-
Clinicians & Scientists
Catherine Clarke, UCLA, USA
Iain Hargreaves, National Hospital, London, UK
Michio Hirano, Columbia University, New York, USA
Rita Horvath, Institute of Genetic Medicine, Newcastle, UK
Luis C López, Universidad de Granada, Spain
Placido Navas, CABD, Seville, Spain
Maria del Mar O’Callaghan, Barcelona, Spain
Hélène Puccio, IGBMC, Strasbourg, France
Catarina Quinzii, Columbia University, New York, USA
Shamima Rahman, UCL Institute of Child Health, London, UK
Agnes Rötig, INSERM, Paris, France
Leonardo Salviati, University of Padova, Italy
Baziel van Engelen, Nijmegen, The Netherlands (ENMC)
- Industry representatives
- Nuri Gueven, Santhera, Switzerland
- Peter Lambrechts, Kaneka, Belgium
- Bill Shrader, Edison pharmaceuticals, USA
Acknowledgments
This Workshop was made possible thanks to the financial support of the European Neuromuscular Centre (ENMC) and ENMC main sponsors:
-
-
Deutsche Gesellschaft für Muskelkranke (Germany)
-
-
Muscular Dystrophy Campaign (UK)
-
-
Muskelsvindfonden (Denmark)
-
-
Prinses Beatrix Fonds (The Netherlands)
-
-
Schweizerische Stiftung für die Erforschung der Muskelkrankheiten (Switzerland)
-
-
Telethon Foundation (Italy)
-
-
Vereniging Spierziekten Nederland (The Netherlands)
and Associated members:
-
-
Association Française contre les Myopathies (France)
-
-
Muscular Dystrophy Association of Slovenia (Slovenia)
SR is supported by Great Ormond Street Hospital Children’s Charity. MH is supported by grants from the: NIH R01 HD057543 (cofunded by NICHD and the NIH Office of Dietary Supplement), R01 HD056103, RC1 NS070232, and P01 HD032062; the Muscular Dystrophy Association (MDA) USA, and by the Marriott Mitochondrial Disorder Clinical Research Fund (MMDCRF). CQ is funded by NIH K23 HD065871. CFC is supported by grants from the NIH R01 GM4592 and NSF 0919609.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Kawamukai M. Biosynthesis and bioproduction of coenzyme Q10 by yeasts and other organisms. Biotechnol Appl Biochem. 2009;53:217–226. doi: 10.1042/BA20090035. [DOI] [PubMed] [Google Scholar]
- 3.Bentinger M, Brismar K, Dallner G. The antioxidant role of coenzyme Q. Mitochondrion. 2007;(7 Suppl):S41–S50. doi: 10.1016/j.mito.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Olson RE, Rudney H. Biosynthesis of ubiquinone. Vitam Horm. 1983;40:1–43. doi: 10.1016/s0083-6729(08)60431-8. [DOI] [PubMed] [Google Scholar]
- 5.Tran UC, Clarke CF. Endogenous synthesis of coenzyme Q in eukaryotes. Mitochondrion. 2007;(7 Suppl):S62–S71. doi: 10.1016/j.mito.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siebert M, Severin K, Heide L. Formation of 4-hydroxybenzoate in Escherichia coli: characterization of the ubiC gene and its encoded enzyme chorismate pyruvate-lyase. Microbiology. 1994;140(Pt 4):897–904. doi: 10.1099/00221287-140-4-897. [DOI] [PubMed] [Google Scholar]
- 7.Pierrel F, Hamelin O, Douki T, Kieffer-Jaquinod S, Muhlenhoff U, Ozeir M, et al. Involvement of mitochondrial ferredoxin and para-aminobenzoic acid in yeast coenzyme Q biosynthesis. Chem Biol. 2010;17:449–459. doi: 10.1016/j.chembiol.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Marbois B, Xie LX, Choi S, Hirano K, Hyman K, Clarke CF. para-Aminobenzoic acid is a precursor in coenzyme Q6 biosynthesis in Saccharomyces cerevisiae. J Biol Chem. 2010;285:27827–27838. doi: 10.1074/jbc.M110.151894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forsman U, Sjoberg M, Turunen M, Sindelar PJ. 4-Nitrobenzoate inhibits coenzyme Q biosynthesis in mammalian cell cultures. Nat Chem Biol. 2010;6:515–517. doi: 10.1038/nchembio.372. [DOI] [PubMed] [Google Scholar]
- 10.Ogasahara S, Engel AG, Frens D, Mack D. Muscle coenzyme Q deficiency in familial mitochondrial encephalomyopathy. Proc Natl Acad Sci U S A. 1989;86:2379–2382. doi: 10.1073/pnas.86.7.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aure K, Benoist JF, Ogier de Baulny H, Romero NB, Rigal O, Lombes A. Progression despite replacement of a myopathic form of coenzyme Q10 defect. Neurology. 2004;63:727–729. doi: 10.1212/01.wnl.0000134607.76780.b2. [DOI] [PubMed] [Google Scholar]
- 12.Mollet J, Delahodde A, Serre V, Chretien D, Schlemmer D, Lombes A, et al. CABC1 gene mutations cause ubiquinone deficiency with cerebellar ataxia and seizures. Am J Hum Genet. 2008;82:623–630. doi: 10.1016/j.ajhg.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rötig A, Appelkvist EL, Geromel V, Chretien D, Kadhom N, Edery P, et al. Quinone-responsive multiple respiratory-chain dysfunction due to widespread coenzyme Q10 deficiency. Lancet. 2000;356:391–395. doi: 10.1016/S0140-6736(00)02531-9. [DOI] [PubMed] [Google Scholar]
- 14.Lopez LC, Schuelke M, Quinzii CM, Kanki T, Rodenburg RJ, Naini A, et al. Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am J Hum Genet. 2006;79:1125–1129. doi: 10.1086/510023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mollet J, Giurgea I, Schlemmer D, Dallner G, Chretien D, Delahodde A, et al. Prenyldiphosphate synthase, subunit 1 (PDSS1) and OH-benzoate polyprenyltransferase (COQ2) mutations in ubiquinone deficiency and oxidative phosphorylation disorders. J Clin Invest. 2007;117:765–772. doi: 10.1172/JCI29089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quinzii C, Naini A, Salviati L, Trevisson E, Navas P, DiMauro S, et al. A Mutation in Para-Hydroxybenzoate-Polyprenyl Transferase (COQ2) Causes Primary Coenzyme Q10 Deficiency. Am J Hum Genet. 2006;78:345–349. doi: 10.1086/500092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahman S, Hargreaves I, Clayton P, Heales S. Neonatal presentation of coenzyme Q10 deficiency. J Pediatr. 2001;139:456–458. doi: 10.1067/mpd.2001.117575. [DOI] [PubMed] [Google Scholar]
- 18.Salviati L, Sacconi S, Murer L, Zaccello G, Franceschini L, Laverda AM, et al. Infantile encephalomyopathy and nephropathy with CoQ10 deficiency: a CoQ10-responsive condition. Neurology. 2005;65:606–608. doi: 10.1212/01.wnl.0000172859.55579.a7. [DOI] [PubMed] [Google Scholar]
- 19.Lamperti C, Naini A, Hirano M, De Vivo DC, Bertini E, Servidei S, et al. Cerebellar ataxia and coenzyme Q10 deficiency. Neurology. 2003;60:1206–1208. doi: 10.1212/01.wnl.0000055089.39373.fc. [DOI] [PubMed] [Google Scholar]
- 20.Musumeci O, Naini A, Slonim AE, Skavin N, Hadjigeorgiou GL, Krawiecki N, et al. Familial cerebellar ataxia with muscle coenzyme Q10 deficiency. Neurology. 2001;56:849–855. doi: 10.1212/wnl.56.7.849. [DOI] [PubMed] [Google Scholar]
- 21.Lagier-Tourenne C, Tazir M, Lopez LC, Quinzii CM, Assoum M, Drouot N, et al. ADCK3, an ancestral kinase, is mutated in a form of recessive ataxia associated with coenzyme Q10 deficiency. Am J Hum Genet. 2008;82:661–672. doi: 10.1016/j.ajhg.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinzii CM, Kattah AG, Naini A, Akman HO, Mootha VK, DiMauro S, et al. Coenzyme Q deficiency and cerebellar ataxia associated with an aprataxin mutation. Neurology. 2005;64:539–541. doi: 10.1212/01.WNL.0000150588.75281.58. [DOI] [PubMed] [Google Scholar]
- 23.Le Ber I, Dubourg O, Benoist JF, Jardel C, Mochel F, Koenig M, et al. Muscle coenzyme Q10 deficiencies in ataxia with oculomotor apraxia 1. Neurology. 2007;68:295–297. doi: 10.1212/01.wnl.0000252366.10731.43. [DOI] [PubMed] [Google Scholar]
- 24.Van Maldergem L, Trijbels F, DiMauro S, Sindelar PJ, Musumeci O, Janssen A, et al. Coenzyme Q-responsive Leigh's encephalopathy in two sisters. Ann Neurol. 2002;52:750–754. doi: 10.1002/ana.10371. [DOI] [PubMed] [Google Scholar]
- 25.Horvath R, Schneiderat P, Schoser BG, Gempel K, Neuen-Jacob E, Ploger H, et al. Coenzyme Q10 deficiency and isolated myopathy. Neurology. 2006;66:253–255. doi: 10.1212/01.wnl.0000194241.35115.7c. [DOI] [PubMed] [Google Scholar]
- 26.Lalani SR, Vladutiu GD, Plunkett K, Lotze TE, Adesina AM, Scaglia F. Isolated mitochondrial myopathy associated with muscle coenzyme Q10 deficiency. Arch Neurol. 2005;62:317–320. doi: 10.1001/archneur.62.2.317. [DOI] [PubMed] [Google Scholar]
- 27.Gempel K, Topaloglu H, Talim B, Schneiderat P, Schoser BG, Hans VH, et al. The myopathic form of coenzyme Q10 deficiency is caused by mutations in the electron-transferring-flavoprotein dehydrogenase (ETFDH) gene. Brain. 2007;130:2037–2044. doi: 10.1093/brain/awm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Namavar Y, Barth PG, Kasher PR, van Ruissen F, Brockmann K, Bernert G, et al. Clinical, neuroradiological and genetic findings in pontocerebellar hypoplasia. Brain. 2011;134:143–156. doi: 10.1093/brain/awq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duncan AJ, Heales SJ, Mills K, Eaton S, Land JM, Hargreaves IP. Determination of coenzyme Q10 status in blood mononuclear cells, skeletal muscle, and plasma by HPLC with di- propoxy-coenzyme Q10 as an internal standard. Clin Chem. 2005;51:2380–2382. doi: 10.1373/clinchem.2005.054643. [DOI] [PubMed] [Google Scholar]
- 30.Duncan AJ, Bitner-Glindzicz M, Meunier B, Costello H, Hargreaves IP, Lopez LC, et al. A nonsense mutation in COQ9 causes autosomal-recessive neonatal-onset primary coenzyme Q10 deficiency: a potentially treatable form of mitochondrial disease. Am J Hum Genet. 2009;84:558–566. doi: 10.1016/j.ajhg.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heeringa S, Chernin G, Chak iM, Zhou W, Sloan A, Ji Z, et al. COQ6 mutations cause nephrotic syndrome with sensorineural deafness in humans, concurrent with increased apoptosis. J Clin Invest. 2011;121:2013–2024. doi: 10.1172/JCI45693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diomedi-Camassei F, Di Giandomenico S, Santorelli FM, Caridi G, Piemonte F, Montini G, et al. COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J Am Soc Nephrol. 2007;18:2773–2780. doi: 10.1681/ASN.2006080833. [DOI] [PubMed] [Google Scholar]
- 33.Lopez LC, Quinzii C, Area E, Naini A, Rahman S, Schuelke M, et al. Treatment of CoQ10 deficient fibroblasts with ubiquinone, analogs, and vitamin C: time- and compound-dependent effects on bioenergetic and oxidative stress status. PLoS One. 2010;5:e11897. doi: 10.1371/journal.pone.0011897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerards M, van den Bosch B, Calis C, Schoonderwoerd K, van Engelen K, Tijssen M, et al. Nonsense mutations in CABC1/ADCK3 cause progressive cerebellar ataxia and atrophy. Mitochondrion. 2010;10:510–515. doi: 10.1016/j.mito.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Bousquet I, Dujardin G, Slonimski PP. ABC1, a novel yeast nuclear gene has a dual function in mitochondria: it suppresses a cytochrome b mRNA translation defect and is essential for the electron transfer in the be 1 complex. Embo J. 1991;10:2023–2031. doi: 10.1002/j.1460-2075.1991.tb07732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brasseur G, Tron G, Dujardin G, Slonimski PP, Brivet-Chevillotte P. The nuclear ABC1 gene is essential for the correct conformation and functioning of the cytochrome bc1 complex and the neighbouring complexes II and IV in the mitochondrial respiratory chain. Eur J Biochem. 1997;246:103–111. doi: 10.1111/j.1432-1033.1997.t01-1-00103.x. [DOI] [PubMed] [Google Scholar]
- 37.Iiizumi M, Arakawa H, Mori T, Ando A, Nakamura Y. Isolation of a novel gene, CABC1, encoding a mitochondrial protein that is highly homologous to Yyast activity of bc1 complex. Cancer Res. 2002;62:1246–1250. [PubMed] [Google Scholar]
- 38.Do TQ, Hsu AY, Jonassen T, Lee PT, Clarke CF. A defect in coenzyme Q biosynthesis is responsible for the respiratory deficiency in Saccharomyces cerevisiae abc1 mutants. J Biol Chem. 2001;276:18161–18168. doi: 10.1074/jbc.M100952200. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh EJ, Dinoso JB, Clarke CF. A tRNA(TRP) gene mediates the suppression of cbs2-223 previously attributed to ABC1/COQ8. Biochem Biophys Res Commun. 2004;317:648–553. doi: 10.1016/j.bbrc.2004.03.096. [DOI] [PubMed] [Google Scholar]
- 40.Tauche A, Krause-Bucholz U, Rodel G. Ubiquinone biosynthesis in Saccharomyces cerevisiae: the molecular organization of O-methylase Coq3p depends on Abc1p/Coq8p. FEMS Yeast Res. 2008;8:1263–1275. doi: 10.1111/j.1567-1364.2008.00436.x. [DOI] [PubMed] [Google Scholar]
- 41.Olsen RK, Olpin SE, Andresen BS, Miedzybrodzka ZH, Pourfarzam M, Merinero B, et al. ETFDH mutations as a major cause of riboflavin-responsive multiple acyl-CoA dehydrogenation deficiency. Brain. 2007;130:2045–2054. doi: 10.1093/brain/awm135. [DOI] [PubMed] [Google Scholar]
- 42.Liang WC, Ohkuma A, Hayashi YK, Lopez LC, Hirano M, Nonaka I, et al. ETFDH mutations, CoQ10 levels, and respiratory chain activities in patients with riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency. Neuromuscul Disord. 2009;19:212–216. doi: 10.1016/j.nmd.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohkuma A, Noguchi S, Sugie H, Malicdan MC, Fukuda T, Shimazu K, et al. Clinical and genetic analysis of lipid storage myopathies. Muscle Nerve. 2009;39:333–342. doi: 10.1002/mus.21167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Ber I, Moreira MC, Rivaud-Pechoux S, Chamayou C, Ochsner F, Kuntzer T, et al. Cerebellar ataxia with oculomotor apraxia type 1: clinical and genetic studies. Brain. 2003;126:2761–2772. doi: 10.1093/brain/awg283. [DOI] [PubMed] [Google Scholar]
- 45.Matsuoka T, Maeda H, Goto Y, Nonaka I. Muscle coenzyme Q10 in mitochondrial encephalomyopathies. Neuromuscul Disord. 1991;1:443–447. doi: 10.1016/0960-8966(91)90007-f. [DOI] [PubMed] [Google Scholar]
- 46.Sacconi S, Trevisson E, Salviati L, Ayme S, Rigal O, Redondo AG, et al. Coenzyme Q10 is frequently reduced in muscle of patients with mitochondrial myopathy. Neuromuscul Disord. 2010;20:44–48. doi: 10.1016/j.nmd.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 47.Geromel V, Kadhom N, Ceballos-Picot I, Chretien D, Munnich A, Rötig A, et al. Human cultured skin fibroblasts survive profound inherited ubiquinone depletion. Free Radic Res. 2001;35:11–21. doi: 10.1080/10715760100300551. [DOI] [PubMed] [Google Scholar]
- 48.Lopez-Martin JM, Salviati L, Trevisson E, Montini G, DiMauro S, Quinzii C, et al. Missense mutation of the COQ2 gene causes defects of bioenergetics and de novo pyrimidine synthesis. Hum Mol Genet. 2007;16:1091–1097. doi: 10.1093/hmg/ddm058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguéz-Hernandez A, Cordero MD, Salviati L, Artuch R, Pineda M, Briones P, et al. Coenzyme Q deficiency triggers mitochondrial degradation by mitophagy. Autophagy. 2009;5:19–32. doi: 10.4161/auto.5.1.7174. [DOI] [PubMed] [Google Scholar]
- 50.Quinzii CM, Lopez LC, Von-Moltke J, Naini A, Krishna S, Schuelke M, et al. Respiratory chain dysfunction and oxidative stress correlate with severity of primary CoQ10 deficiency. FASEB J. 2008;22:1874–1885. doi: 10.1096/fj.07-100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quinzii CM, Lopez LC, Gilkerson RW, Dorado B, Coku J, Naini AB, et al. Reactive oxygen species, oxidative stress, and cell death correlate with level of CoQ10 deficiency. FASEB J. 2010;24:3733–3743. doi: 10.1096/fj.09-152728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ewbank JJ, Barnes TM, Lakowski B, Lussier M, Bussey H, Hekimi S. Structural and functional conservation of the Caenorhabditis elegans timing gene clk-1. Science. 1997;275:980–983. doi: 10.1126/science.275.5302.980. [DOI] [PubMed] [Google Scholar]
- 53.Padilla S, Jonassen T, Jimenez-Hidalgo MA, Fernandez-Ayala DJ, Lopez-Lluch G, Marbois B, et al. Demethoxy-Q, an intermediate of coenzyme Q biosynthesis, fails to support respiration in Saccharomyces cerevisiae and lacks antioxidant activity. J Biol Chem. 2004;279:25995–26004. doi: 10.1074/jbc.M400001200. [DOI] [PubMed] [Google Scholar]
- 54.Asencio C, Navas P, Cabello J, Schnabel R, Cypser JR, Johnson TE, et al. Coenzyme Q supports distinct developmental processes in Caenorhabditis elegans. Mech Ageing Dev. 2009;130:145–153. doi: 10.1016/j.mad.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 55.Lyon MF, Hulse EV. An inherited kidney disease of mice resembling human nephronophthisis. J Med Genet. 1971;8:41–48. doi: 10.1136/jmg.8.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sibalic V, Fan X, Wuthrich RP. Characterisation of cellular infiltration and adhesion molecule expression in CBA/CaH-kdkd mice with tubulointerstitial renal disease. Histochem Cell Biol. 1997;108:235–242. doi: 10.1007/s004180050163. [DOI] [PubMed] [Google Scholar]
- 57.Peng M, Jarett L, Meade R, Madaio MP, Hancock WW, George AL, Jr, et al. Mutant prenyltransferase-like mitochondrial protein (PLMP) and mitochondrial abnormalities in kd/kd mice. Kidney Int. 2004;66:20–28. doi: 10.1111/j.1523-1755.2004.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peng M, Falk MJ, Haase VH, King R, Polyak E, Selak M, et al. Primary coenzyme Q deficiency in Pdss2 mutant mice causes isolated renal disease. PLoS Genet. 2008;4:el000061. doi: 10.1371/journal.pgen.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saiki R, Lunceford AL, Shi Y, Marbois B, King R, Pachuski J, et al. Coenzyme Q10 supplementation rescues renal disease in Pdss2kd/kd mice with mutations in prenyl diphosphate synthase subunit 2. Am J Physiol Renal Physiol. 2008;295:F1535–F1544. doi: 10.1152/ajprenal.90445.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bentinger M, Dallner G, Chojnacki T, Swiezewska E. Distribution and breakdown of labeled coenzyme Q10 in rat. Free Radic Biol Med. 2003;34:563–575. doi: 10.1016/s0891-5849(02)01357-6. [DOI] [PubMed] [Google Scholar]
- 61.Bhagavan HN, Chopra RK. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res. 2006;40:445–453. doi: 10.1080/10715760600617843. [DOI] [PubMed] [Google Scholar]
- 62.Ferrante KL, Shefner J, Zhang H, Betensky R, O'Brien M, Yu H, et al. Tolerance of high-dose (3,000 mg/day) coenzyme Q10 in ALS. Neurology. 2005;65:1834–1836. doi: 10.1212/01.wnl.0000187070.35365.d7. [DOI] [PubMed] [Google Scholar]
- 63.Montini G, Malaventura C, Salviati L. Early coenzyme Q10 supplementation in primary coenzyme Q10 deficiency. N Engl J Med. 2008;358:2849–2850. doi: 10.1056/NEJMc0800582. [DOI] [PubMed] [Google Scholar]
- 64.Pineda M, Montero R, Aracil A, O'Callaghan MM, Mas A, Espinos C, et al. Coenzyme Q(10)-responsive ataxia: 2-year-treatment follow-up. Mov Disord. 2010;25:1262–1268. doi: 10.1002/mds.23129. [DOI] [PubMed] [Google Scholar]