Abstract
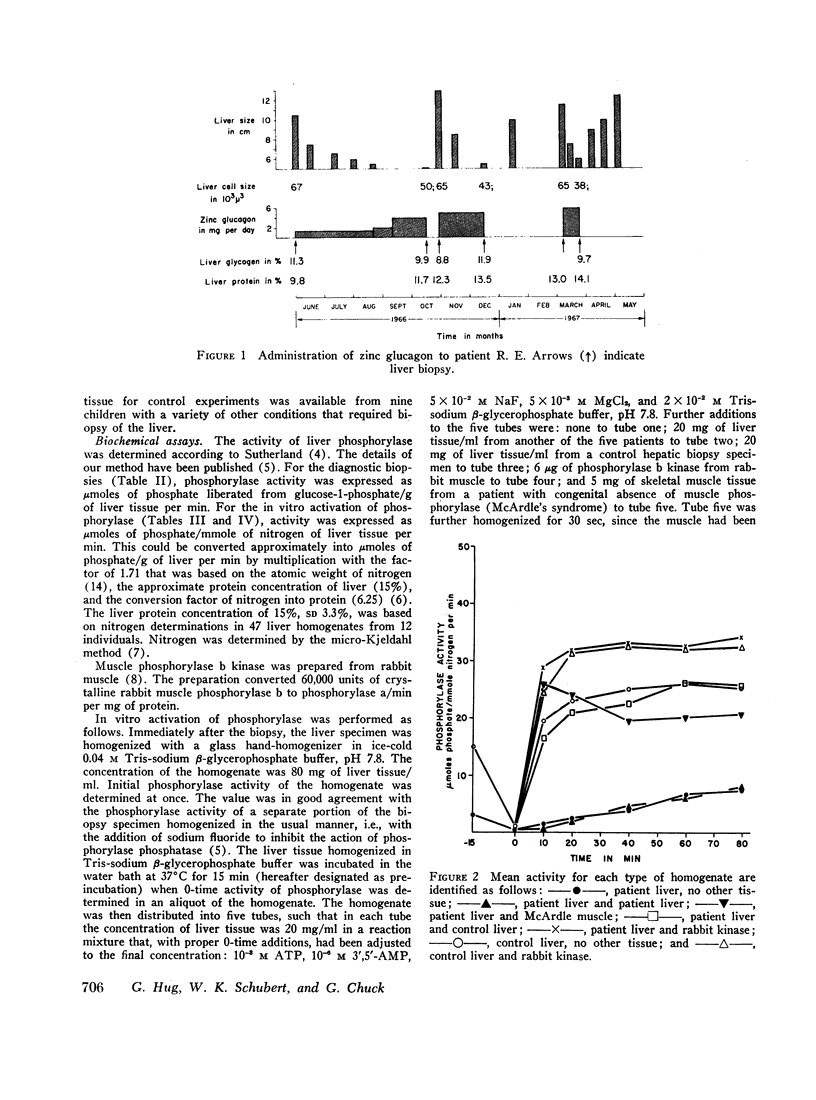
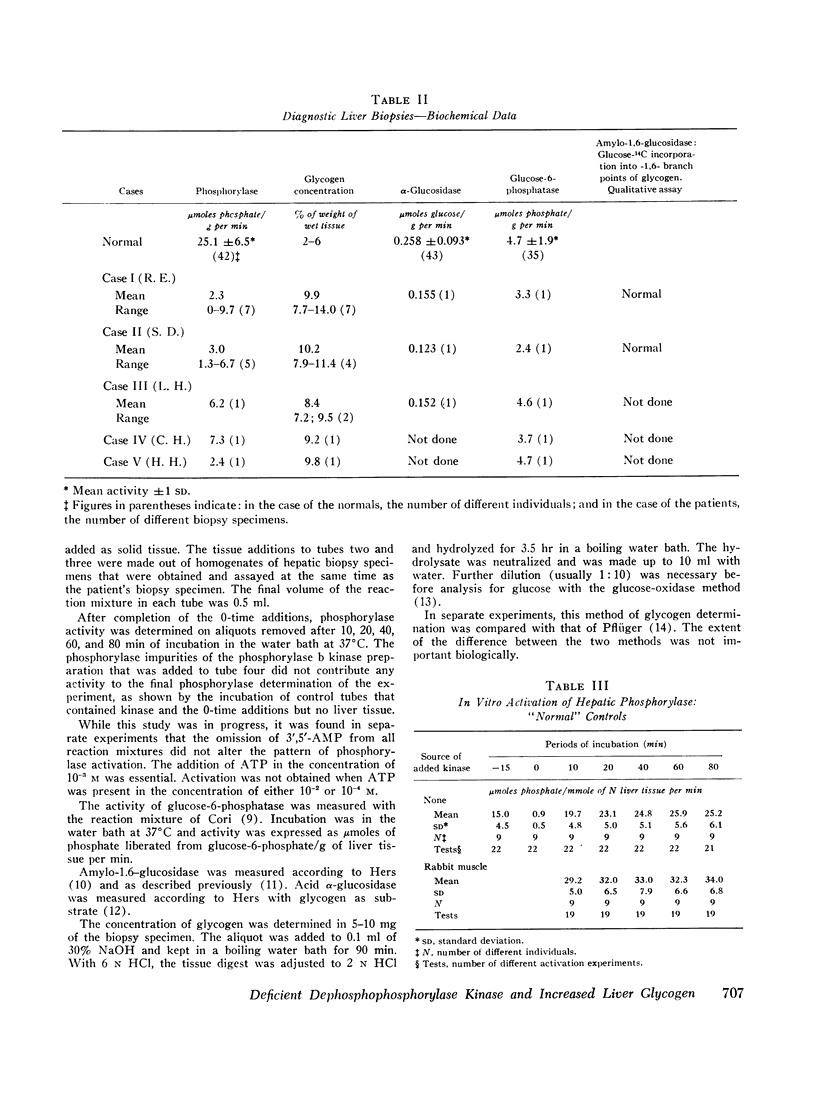
Low activity of phosphorylase and increased concentration of glycogen were found in liver tissue from five children with asymptomatic hepatomegaly. In vitro activation of liver phosphorylase in these patients occurred at the rate of 10% or less of normal. Elimination of the defect by the addition of kinase that activates phosphorylase demonstrated the integrity of the phosphorylase enzyme and the deficient activity of dephophophosphorylase kinase.
On the average, 60% of the phosphorylase enzyme of normal human liver was in the active form. Phosphorylase kinase of rabbit muscle activated phosphorylase of normal human liver to a final value that was significantly higher than the one obtained in the absence of muscle phosphorylase kinase.
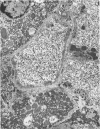
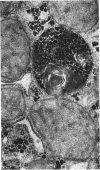
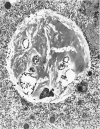
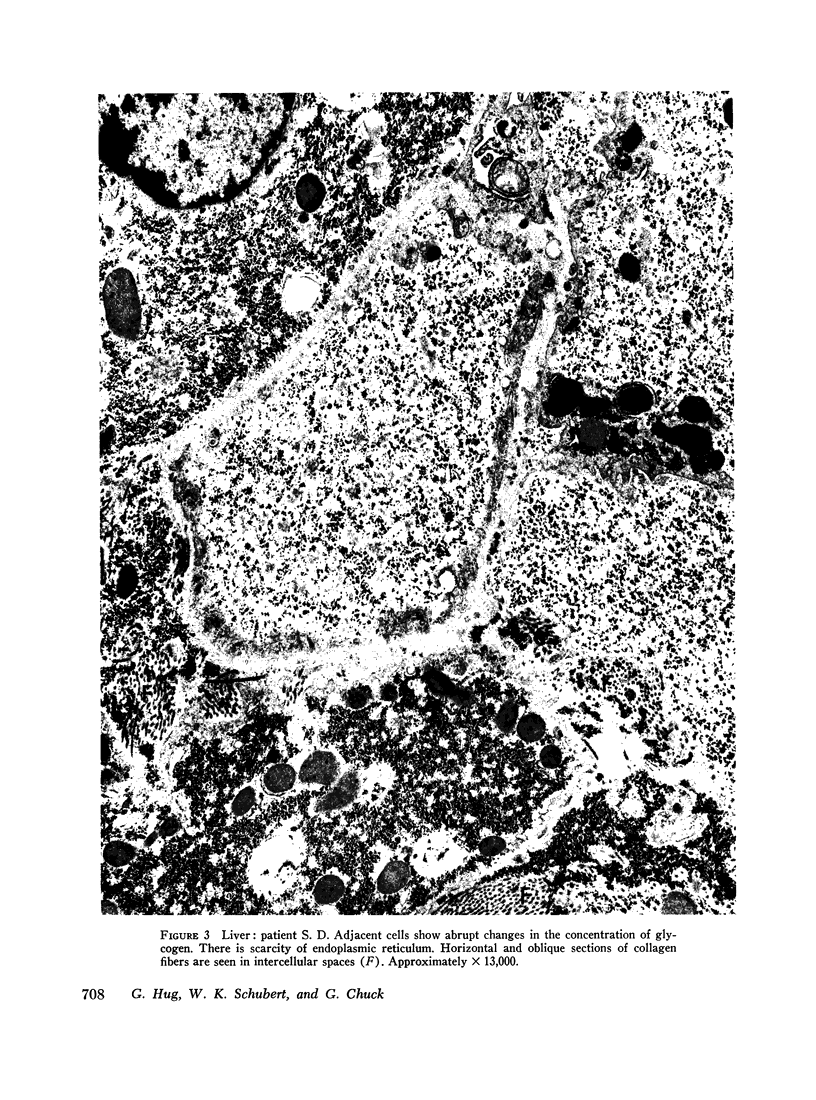
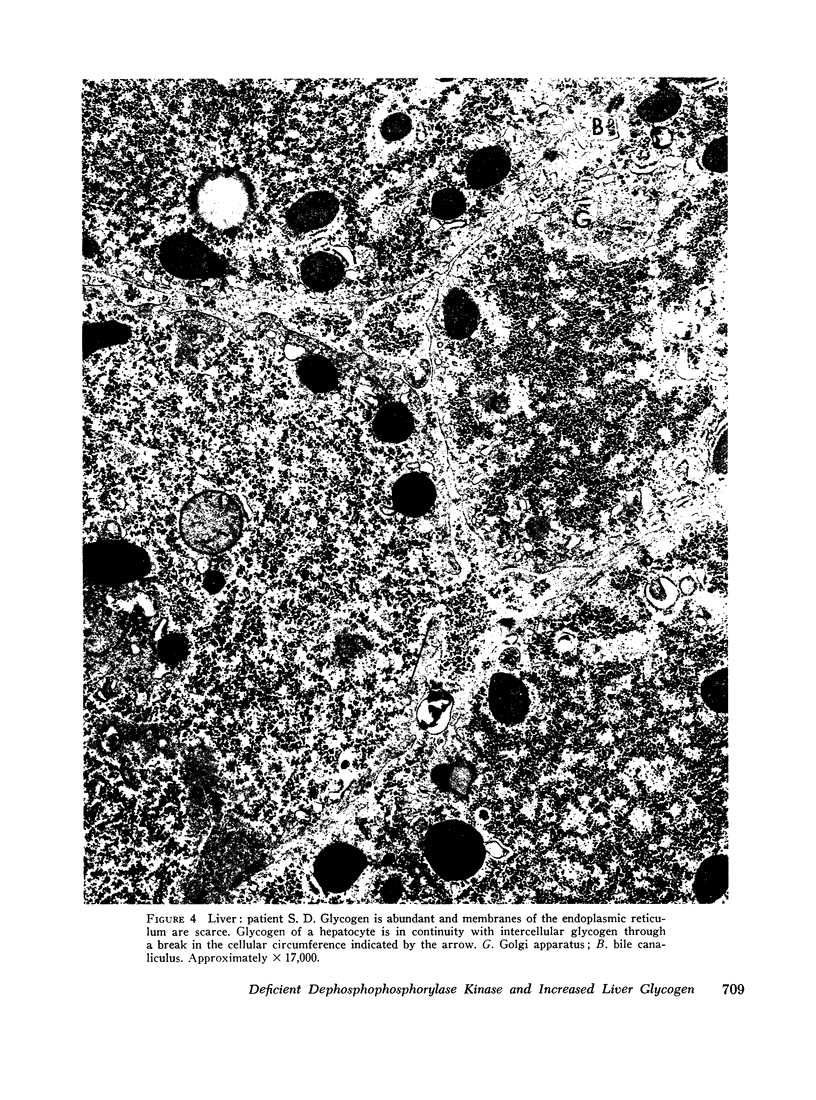
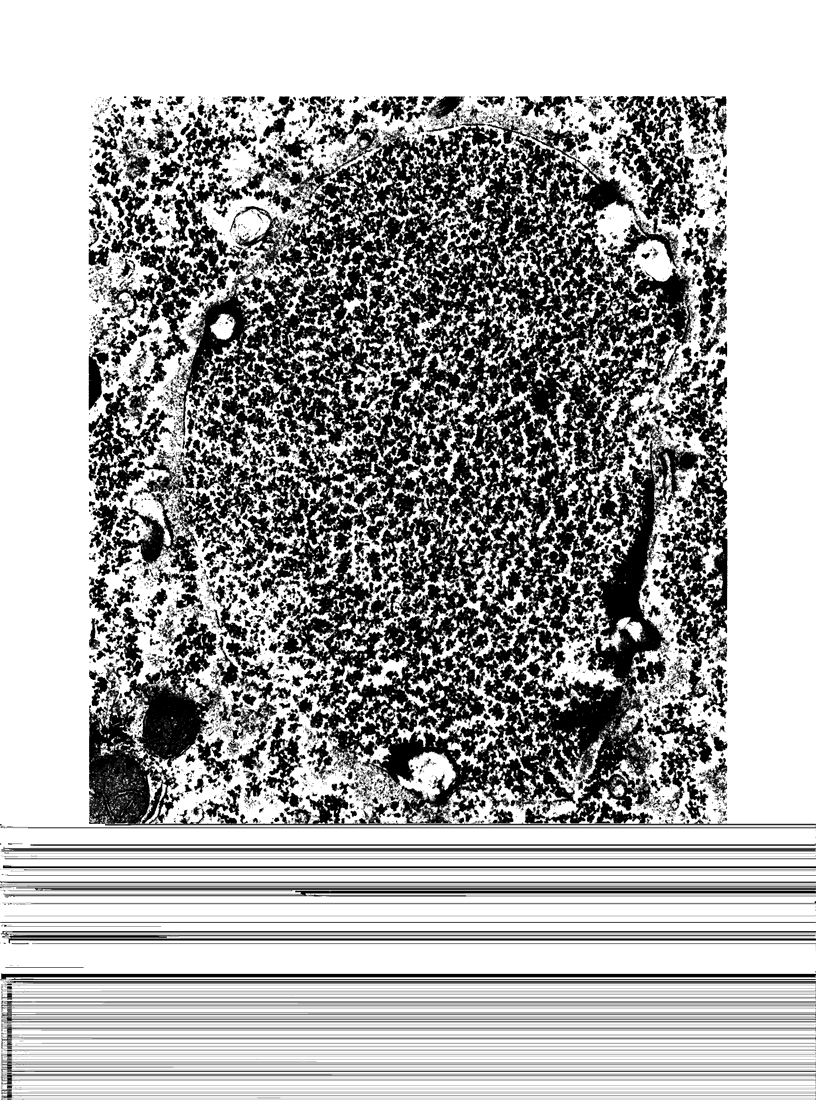
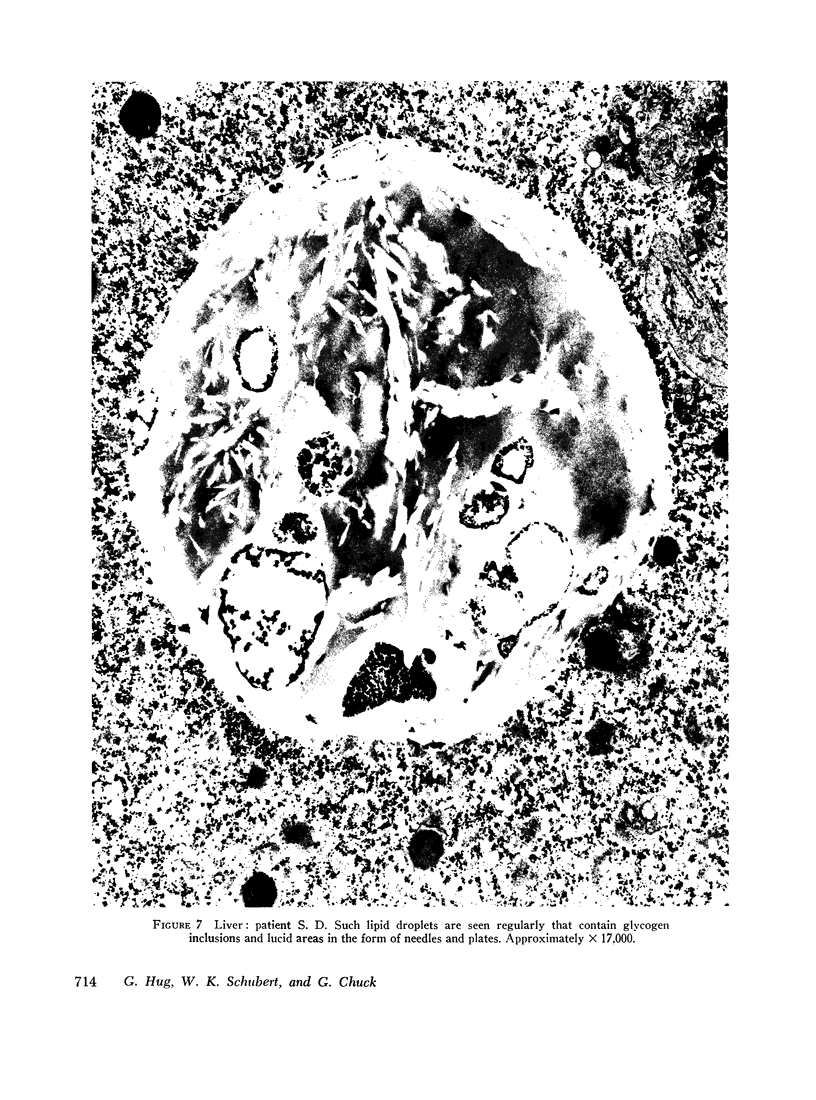
The ultrastructural examination of hepatic tissue from the five patients revealed increased amounts of glycogen. There was scarcity of endoplasmic reticulum. There was intercellular glycogen in continuity with the glycogen of the hepatocytes through breaks in their circumference. Lipid droplets with lucid areas in the form of needles and plates contained aggregates of glycogen. There were numerous lysosomes, some containing glycogen. Large vacuoles filled with glycogen and surrounded by a membrane were seen occasionally. The vacuoles might reflect the lysosomal pathway of glycogen degradation, since there was apparent fusion of such autophagic vacuoles with small vesicles resembling primary lysosomes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CORI G. T., CORI C. F. Glucose-6-phosphatase of the liver in glycogen storage disease. J Biol Chem. 1952 Dec;199(2):661–667. [PubMed] [Google Scholar]
- Cussen L. J. The structure of the normal human ureter in infancy and childhood. A quantitative study of the muscular and elastic tissue. Invest Urol. 1967 Sep;5(2):179–194. [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- HONG R., SCHUBERT W. K. Menghini needle biopsy of the liver. Am J Dis Child. 1960 Jul;100:42–46. doi: 10.1001/archpedi.1960.04020040044009. [DOI] [PubMed] [Google Scholar]
- HUG G., KRILL C. E., Jr, PERRIN E. V., GUEST G. M. Cori's disease (amylo-1,6-glucosidase deficiency). Report of a case in a Negro child. N Engl J Med. 1963 Jan 17;268:113–120. doi: 10.1056/NEJM196301172680301. [DOI] [PubMed] [Google Scholar]
- Hug G., Schubert W. K., Shwachman H. Imbalance of liver phosphorylase and accumulation of hepatic glycogen in a girl with progressive disease of the brain. J Pediatr. 1965 Nov;67(5):741–751. doi: 10.1016/s0022-3476(65)80362-6. [DOI] [PubMed] [Google Scholar]
- LAMY M., DUBOIS R., ROSSIER A., FREZAL J., LOEB H., BLANCHER G. [Glycogenosis caused by deficiency of hepatic phosphorylase]. Arch Fr Pediatr. 1960;17:14–37. [PubMed] [Google Scholar]
- SUTHERLAND E. W., WOSILAIT W. D. The relationship of epinephrine and glucagon to liver phosphorylase. I. Liver phosphorylase; preparation and properties. J Biol Chem. 1956 Jan;218(1):459–468. [PubMed] [Google Scholar]
- VASSELLA F. Die Glukagonbelastungsprobe beim gesunden Kind. Helv Paediatr Acta. 1957 Oct;12(4):331–360. [PubMed] [Google Scholar]