Abstract
Despite the high prevalence of intestinal lactase deficiency in some racial groups and in patients with intestinal disease, the biochemical defect has not been characterized.
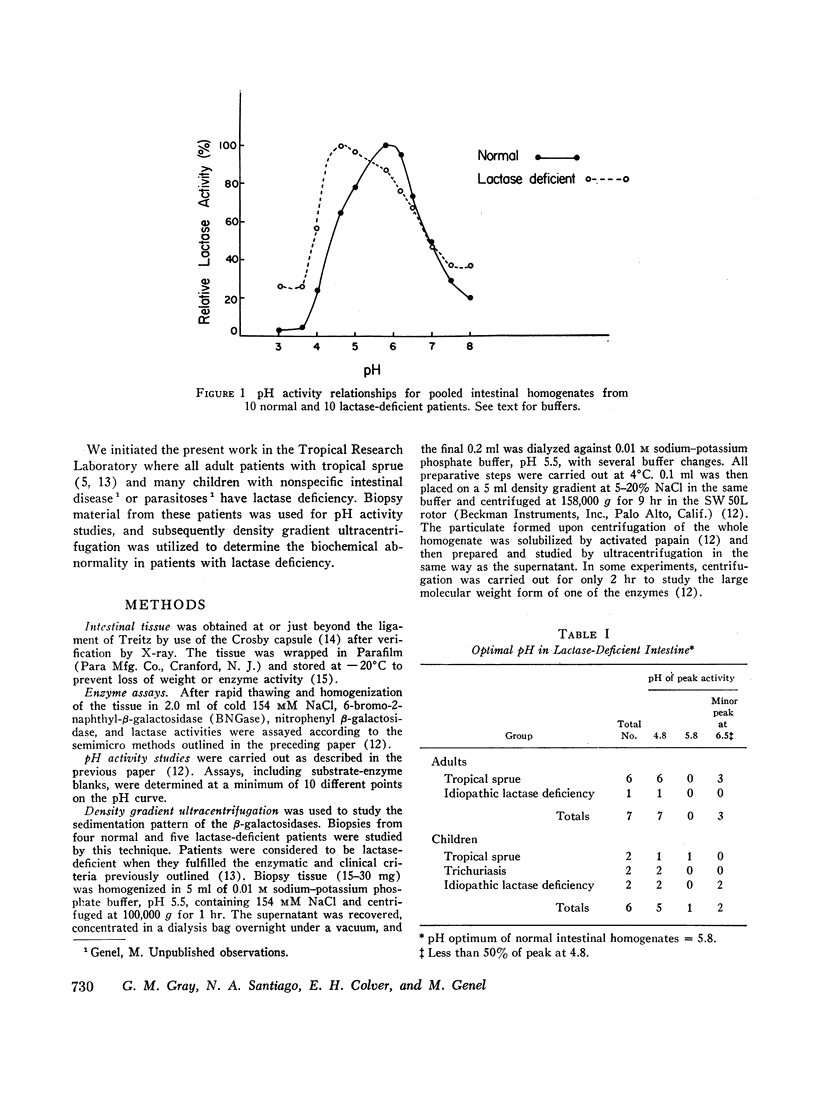
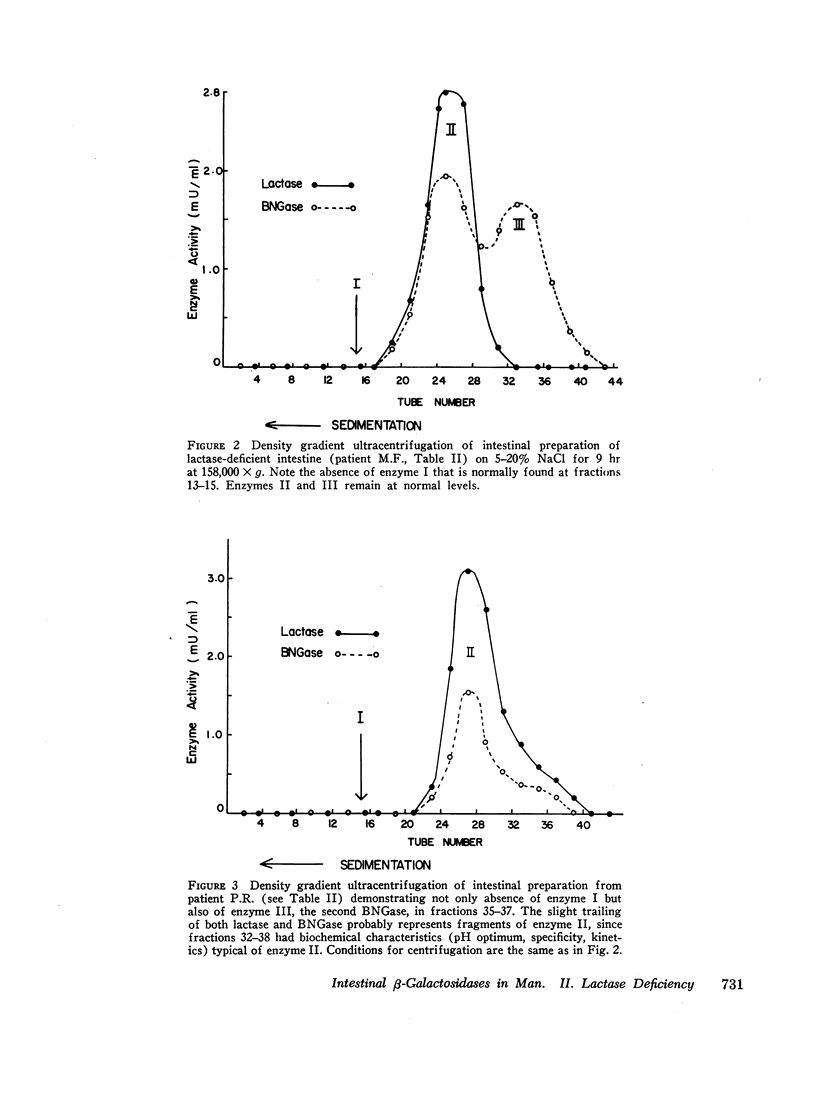
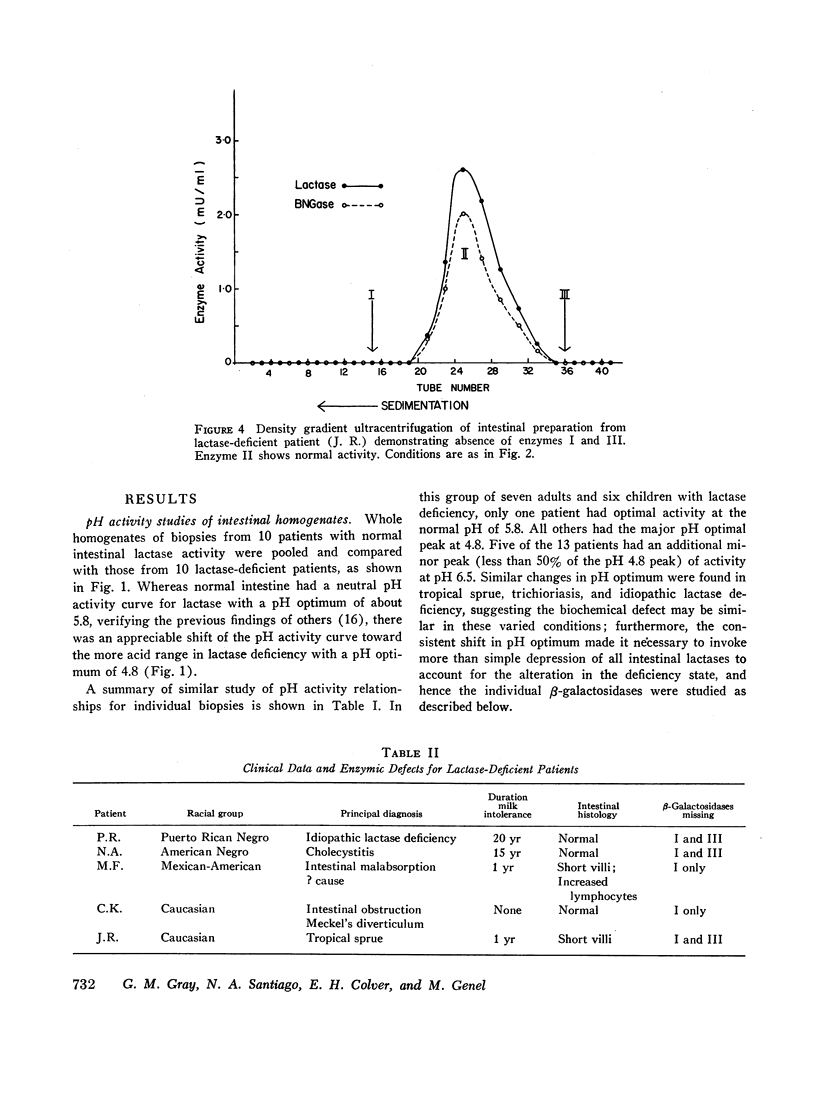
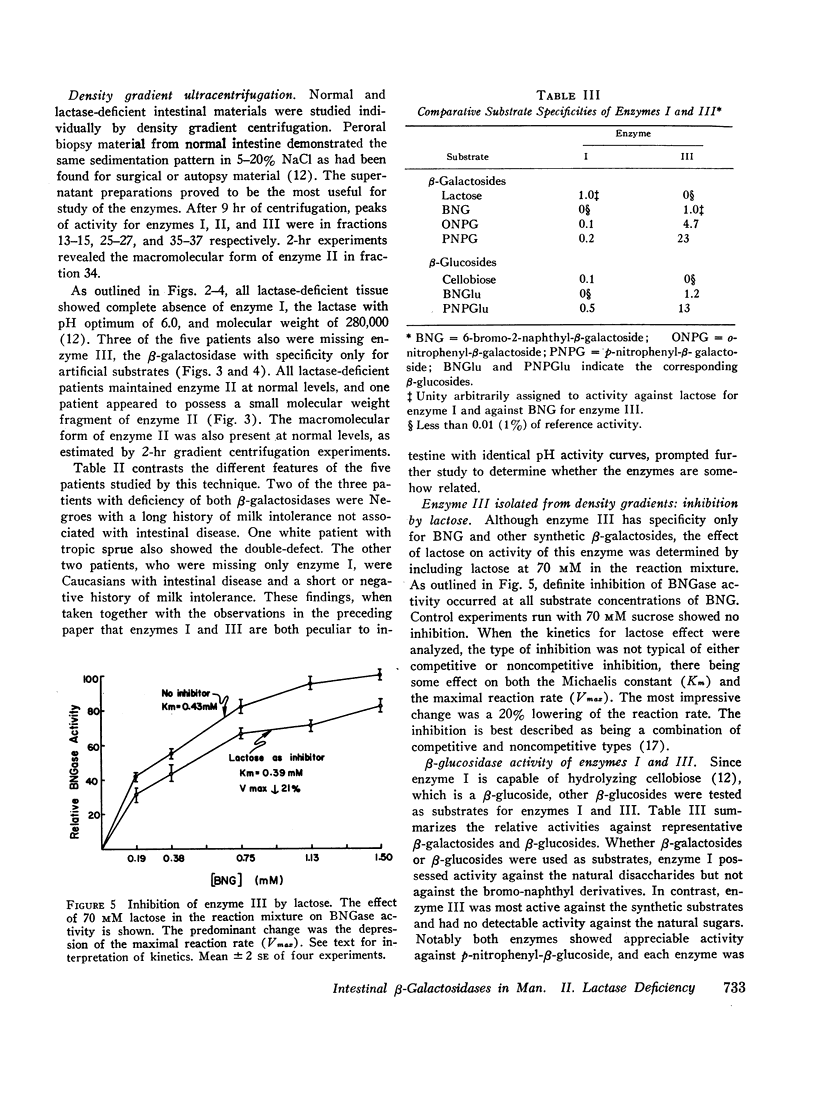
In the preceding paper normal intestine was found to have two lactases with distinctly different pH optima. Therefore, pH activity curves of homogenates from lactase-deficient intestine were studied, and the pH optimum was found to be shifted from the normal of 5.8 to 4.8. Density gradient ultracentrifugation of intestinal material from five lactase-deficient patients demonstrated absence of a lactase with pH optimum 6.0 and molecular weight 280,000. A second lactase with pH optimum 4.5 and molecular weights of 156,000 and 660,000 remained at normal levels accounting for the shift in the pH optimum in whole intestinal homogenates. In addition, three of the five patients had absence of a smaller β-galactosidase (molecular weight 80,000) that had specificity only for synthetic substrates. Although not a lactase, this enzyme had a pH optimum identical with the missing lactase, and its activity was inhibited by lactose in a partially competitive manner suggesting that it is capable of binding lactose. It is possible that this enzyme is a precursor or fragment of the missing lactase.
The residual lactase activity provided by the lactase with low pH optimum represents 20-70% of the activity of the missing enzyme, and yet these patients are not able to digest dietary lactose. Thus it appears that the residual enzyme plays no significant role in the hydrolysis of ingested lactose.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AURICCHIO S., RUBINO A., PRADER A., REY J., JOS J., FREZAL J., DAVIDSON M. INTESTINAL GLYCOSIDASE ACTIVITIES IN CONGENITAL MALABSORPTION OF DISACCHARIDES. J Pediatr. 1965 Mar;66:555–564. doi: 10.1016/s0022-3476(65)80120-2. [DOI] [PubMed] [Google Scholar]
- AURICCHIO S., RUBINO A., TOSI R., SEMENZA G., LANDOLT M., KISTLER H., PRADER A. DISACCHARIDASE ACTIVITIES IN HUMAN INTESTINAL MUCOSA. Enzymol Biol Clin (Basel) 1963;74:193–208. doi: 10.1159/000458059. [DOI] [PubMed] [Google Scholar]
- Bayless T. M., Rosensweig N. S. A racial difference in incidence of lactase deficiency. A survey of milk intolerance and lactase deficiency in healthy adult males. JAMA. 1966 Sep 19;197(12):968–972. [PubMed] [Google Scholar]
- CROSBY W. H., KUGLER H. W. Intraluminal biopsy of the small intestine; the intestinal biopsy capsule. Am J Dig Dis. 1957 May;2(5):236–241. doi: 10.1007/BF02231100. [DOI] [PubMed] [Google Scholar]
- Cady A. B., Rhodes J. B., Littman A., Crane R. K. Significance of lactase deficit in ulcerative colitis. J Lab Clin Med. 1967 Aug;70(2):279–286. [PubMed] [Google Scholar]
- Chung M. H., McGill D. B. Lactase deficiency in Orientals. Gastroenterology. 1968 Feb;54(2):225–226. [PubMed] [Google Scholar]
- Cook G. C., Dahlqvist A. Jejunal hetero-beta-galactosidase activities in Ugandans with lactase deficiency. Gastroenterology. 1968 Sep;55(3):328–332. [PubMed] [Google Scholar]
- Cook G. C., Kajubi S. K. Tribal incidence of lactase deficiency in Uganda. Lancet. 1966 Apr 2;1(7440):725–729. doi: 10.1016/s0140-6736(66)90888-9. [DOI] [PubMed] [Google Scholar]
- Elliott R. B., Maxwell G. M. Predominance of lactase of small molecular size in duodenal and jejunal mucosa of Australian aboriginal children. Aust J Exp Biol Med Sci. 1966 Dec;44(6):709–713. doi: 10.1038/icb.1966.69. [DOI] [PubMed] [Google Scholar]
- Gray G. M., Santiago N. A. Intestinal beta-galactosidases. I. Separation and characterization of three enzymes in normal human intestine. J Clin Invest. 1969 Apr;48(4):716–728. doi: 10.1172/JCI106029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. M., Walter W. M., Jr, Colver E. H. Persistent deficiency of intestinal lactase in apparently cured tropical sprue. Gastroenterology. 1968 Apr;54(4):552–558. [PubMed] [Google Scholar]
- Hsia D. Y., Makler M., Semnza G., Prader A. Beta-galactosidase activity in human intestinal lactases. Biochim Biophys Acta. 1966 Feb 14;113(2):390–393. doi: 10.1016/s0926-6593(66)80077-2. [DOI] [PubMed] [Google Scholar]
- Huang S. S., Bayless T. M. Milk and lactose intolerance in healthy Orientals. Science. 1968 Apr 5;160(3823):83–84. doi: 10.1126/science.160.3823.83-a. [DOI] [PubMed] [Google Scholar]
- PLOTKIN G. R., ISSELBACHER K. J. SECONDARY DISACCHARIDASE DEFICIENCY IN ADULT CELIAC DISEASE (NONTROPICAL SPRUE) AND OTHER MALABSORPTION STATES. N Engl J Med. 1964 Nov 12;271:1033–1037. doi: 10.1056/NEJM196411122712003. [DOI] [PubMed] [Google Scholar]
- Zoppi G., Hadorn B., Gitzelmann R., Kistler H., Prader A. Intestinal beta-galactosidase activities in malabsorption syndromes. Gastroenterology. 1966 Apr;50(4):557–561. [PubMed] [Google Scholar]


