Abstract
Parathyroid hormone receptors (PTHR) are promptly internalized upon stimulation by activating [PTH(1–84), PTH(1–34)] and non-activating [PTH(7–84), PTH(7–34)] ligands. Here, we characterized the mechanism regulating the sorting of internalized receptors between recycling and degradative pathways. PTHR recycles faster after challenge with PTH(1–34) than with PTH(7–34). PTHR recycling is complete by 2 hr after PTH(1–34) stimulation but incomplete at this time in cells treated with PTH(7–34). The slower and incomplete recycling induced by PTH(7–34) is due to proteasomal degradation. Both PTH(1–34) and PTH(7–34) induced PTHR polyubiquitination. Ubiquitination by PTH(1–34) was transient, whereas receptor ubiquitination following PTH(7–34) was sustained. PTH(1–34), but not PTH(7–34), induced expression of the PTHR-specific deubiquitinating enzyme USP2. Overexpression of USP2 prevented PTH(7–34)-induced PTHR degradation. We conclude that PTH(1–34) promotes coupled PTHR ubiquitination and deubiquitination, whereas PTH(7–34) activates only ubiquitination, thereby leading to PTHR downregulation. These findings may explain PTH resistance in diseases associated with elevated PTH(7–84) levels.
Keywords: PTH receptor, ubiquitination, deubiquitinase enzymes, recycling, downregulation, proteasome, G protein-coupled receptors, membrane trafficking
Introduction
The type 1 parathyroid hormone receptor (PTHR) is a G-protein coupled receptor (GPCR) that modulates bone turnover and extracellular mineral ion homeostasis. PTHR activation, desensitization, internalization and recycling proceed in a cyclical pathway, similar to other GPCRs.(1,2) Upon binding PTH, the receptor is rapidly phosphorylated, desensitized and internalized resulting in reduced cellular responses. The carboxy-terminus of the PTHR contains multiple phosphorylation sites and is a major regulatory domain controlling receptor interaction with β-arrestins and its endocytosis.(3–7) The PTHR undergoes rapid agonist-promoted endocytosis by a clathrin- and dynamin-dependent process.(4,8,9) The ligand is cleaved from the receptor and degraded. Normally the PTHR is recycled to the plasma membrane.(8)
PTHR activation occurs in a conspicuous cell- and ligand-dependent manner. However, naturally occurring amino-truncated PTH fragments can uncouple receptor activation from receptor inactivation and endocytosis.(10) PTH(7–84) and its analogue PTH(7–34), for instance, promote PTHR internalization in both kidney and bone cells lacking the cytoplasmic PDZ adaptor protein, NHERF1.(10–12) These amino-terminally truncated peptides lack intrinsic activity and are competitive inhibitors of the PTHR.(13–16) This raised the question as to the fate of the PTHR when it is internalized without undergoing antecedent or concurrent activation.
In certain disorders associated with secondary hyperparathyroidism and PTH resistance, amino-truncated PTH fragments accumulate to high levels as a consequence of preferential release and diminished peripheral metabolism.(17–21) Further, in this setting the PTHR is downregulated.(22–25)
Posttranslational receptor modification by ubiquitin(26) is a key molecular mechanism governing receptors degradation. Ubiquitination mediates the covalent conjugation of ubiquitin, a highly conserved polypeptide of 76 amino acids, to protein substrates.(27) This process is catalyzed by three enzymes acting in tandem: an E1, ubiquitin-activating enzyme, an E2, ubiquitin-carrying enzyme, and an E3, ubiquitin ligase.(28) Proteasome-dependent receptor degradation upon ligand activation has been demonstrated for various GPCRs.(29–32) Based on these considerations, we hypothesized that the sorting of internalized PTHR is a ligand dependent process with ubiquitin-mediated proteasomal degradation and deubiquitination the major responsible mechanisms determining down regulation or recycling. We tested this hypothesis in bone (ROS) and kidney (DCT and HK2) cell lines, major targets of PTH actions through PTH receptor in the organism. The results support this theory and suggest that selective downregulation of the PTHR by amino-truncated PTH fragments may contribute to PTH resistance.
Materials and Methods
Reagents
Monoclonal HA.11 antibody, affinity matrix mono HA.11(16B12) and monoclonal anti-Flag antibody were obtained from Covance (Berkeley, CA) and Sigma (St. Louis, MO), respectively. Polyclonal lysine48 (Lys48)-specific polyubiquitin antibody, polyclonal anti-ubiquitin antibody and polyclonal USP2 antibody were purchased from Cell Signaling Technology (Danvers, MA), Dako (Denmark) and Abgent (San Diego, CA), respectively. Human PTHR antiserum was obtained from Gramsch Laboratories (Schwabhausen, Germany) and characterized.(33) Zeocin, blasticidin and geneticin were purchased from Invitrogen (Carlsbad, CA); Horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody was from Pierce (Rockford, IL) and HRP-conjugated sheep anti-mouse antibody was from GE Healthcare (Piscataway, NJ). Protease inhibitor mixture Set I was from Calbiochem (San Diego, CA). Human PTH(1–34), PTH(7–34), PTH(1–84) and PTH(7–84) were obtained from Bachem (Torrance, CA). Polyclonal anti-EPS15 antibody, EPS15 shRNA and USP2 shRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Proteasome inhibitor (MG-132) was obtained from A.G. Scientific Inc (San Diego, CA). All other reagents were from Sigma (St. Louis, MO).
Cell culture
Renal proximal tubule cells (HK-2), mouse distal tubule kidney cells (DCT) and rat osteosarcoma (ROS) 17/2.8 were cultured in DMEM/F-12 50/50 medium supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin, 100 μg/ml streptomycin. CHO-N10 cells were cultured in Ham’s F-12 medium supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin, 100 μg/ml streptomycin, 0.4% zeocin and 10 μg/ml blasticidin.(34) 1.5% G418 was added to media used for CHO-N10 cells constitutively expressing the HA-PTHR. DCT cells stably expressing hPTHR-EGFP were generated by transiently transfecting hPTHR-EGFP(35) using FuGENE 6TM. After 48 hours, cells were trypsinized and plated in 150-mm dish containing culture media supplemented with 500 μg/ml G418 (Invitrogen, Carlsbad, CA) to select stable transfectants. Cells were maintained at 37°C in a humidified atmosphere of 5% CO2.
Plasmid constructs
PTHR–Hemagglutinin (HA)-tagged human PTHR in pcDNA3.1 were generated as described.(36) Flag-tagged PTHR was generated by converting the sequence DKEAPTGS (residues 94–101) in exon E2 to the Flag epitope, DYKDDDDK.(37) Cells were grown to 50–60% confluence and transfected, as indicated, with HA-PTHR, Flag-PTHR, USP2-HA (kindly provided by Dr Stefan Grimm, Imperial College, London UK), shEPS15 or shUSP2 using FuGENE 6 (Roche, Indianapolis, IN) according to the manufacturer’s protocol. Control conditions were transfected with empty vector DNA (pcDNA3.1) or scrambled shRNA, respectively. All experiments were performed 48 hours after transfection.
Immunoprecipitation and Immunoblot analysis
Cells were lysed with RIPA buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 7.4, and 150 mM NaCl) supplemented with protease inhibitor mixture I, 10 mM N-ethylmaleimide to inhibit deubiquitinase activity and incubated for 30 minutes on ice. Lysates were centrifuged for 20 minutes at 14,000 × g at 4°C.
Solubilized materials were incubated overnight at 4°C with anti-HA affinity matrix (Covance). Total lysates and immunoprecipitated protein, eluted by the addition of SDS sample buffer, were analyzed by SDS-polyacrylamide gels and transferred to Immobilon-P membranes (Millipore, Billerica, MA) using the semidry method (Bio-Rad, Hercules, CA). Nonspecific binding was blocked by incubating the membranes in 5% bovine serum albumin in Tris-buffered saline plus 0.1% tween-20 (TBST) (Lys48-linked polyubiquitin western blot) or 5% nonfat milk in TBST for 1 hour at room temperature followed by overnight incubation with the indicated antibodies (monoclonal anti-Flag and anti-HA antibodies, polyclonal anti-EPS15, anti-Lys48-linked polyubiquitin, human PTHR antibodies at 1:1000 and polyclonal anti-ubiquitin and anti-USP2 antibodies at 1:500) at 4°C. The membranes were then washed and incubated at room temperature for 1 hour in HRP-conjugated goat anti-rabbit IgG or sheep anti-mouse IgG diluted 1:2000. Protein bands were visualized with a luminol-based enhanced chemiluminescence substrate.
Cell Fractionation
Cell fractionation was performed by differential centrifugation at 4°C.(36) ROS cells were transiently transfected with HA-PTHR, starved for 3 hours, and then incubated at 37°C for 5 minutes with PTH(1–34) or PTH(7–34). Cells were detached with a cell scraper, pelleted by centrifugation (1000 × g, 10 minutes), and lysed by sonication in phosphate-buffered saline (PBS) containing protease inhibitor cocktail Set I and 10 mM N-ethylmaleimide. The lysates were centrifuged at 1000 × g for 10 minutes to remove unbroken cells, including large cell debris and some nuclei. The supernatant was further centrifuged at 100,000 × g for 30 minutes. The resulting supernatant is the cytosolic fraction. The pellet that contains the plasma membranes and microsomes was solubilized in RIPA buffer supplemented with protease inhibitor cocktail Set I and 10 mM N-ethylmaleimide.
Receptor Recycling
Receptor recycling was measured as described(10,34) using HPCL-purified [125I][Nle8,18,Tyr34]-hPTH(1–34)NH2. PTH(1–34), PTH(7–34), or vehicle were added to fresh culture medium bathing confluent cells seeded on 24-well plates during 30 minutes. Cells were incubated with ~100,000 cpm of [125I][Nle8,18,Tyr34]-hPTH(1–34)NH2 on ice for 2.5 hours. Nonspecific binding was determined either by parallel incubation of nontransfected cells with PTHR or measured in parallel experiments carried out in the presence of 1 μM unlabeled PTH (1–34) and subtracted from total binding to calculate specific binding. After incubation, cells were washed twice with cold PBS and solubilized in 0.2N NaOH. Cell surface-bound [125I][Nle8,18,Tyr34]-hPTH (1–34) NH2 was counted by γ-spectrometry.
Receptor Surface Expression
PTHR surface expression was measured by ELISA in cells transiently transfected with HA-PTHR.(38) Confluent cells were treated with PTH(1–34), PTH(7–34), PTH(1–84) and PTH(7–84), as indicated, and fixed with 3.7% paraformaldehyde at room temperature. After 3 washes with PBS cells were blocked with 1% BSA for 45 minutes and incubated with polyclonal anti-HA antibody for 1 hour at room temperature. Cells were then washed with PBS, re-blocked with 1% BSA for 15 minutes and incubated with anti-IgG conjugated with alkaline phosphatase. After washing, alkaline phosphatase substrate was added for 30 minutes and 100 μl of the reaction mixture was transferred to a 96-well plate and absorbance was measured at 405 nm.
Statistics
Data are presented as the mean ± SE, where n indicates the number of independent experiments. Multiple comparisons were evaluated by analysis of variance with post-test repeated measures analyzed by the Bonferroni procedure (Prism; GraphPad). Differences greater than p ≤ 0.05 were assumed to be significant.
Results
Effect of ligand on PTHR recycling and abundance
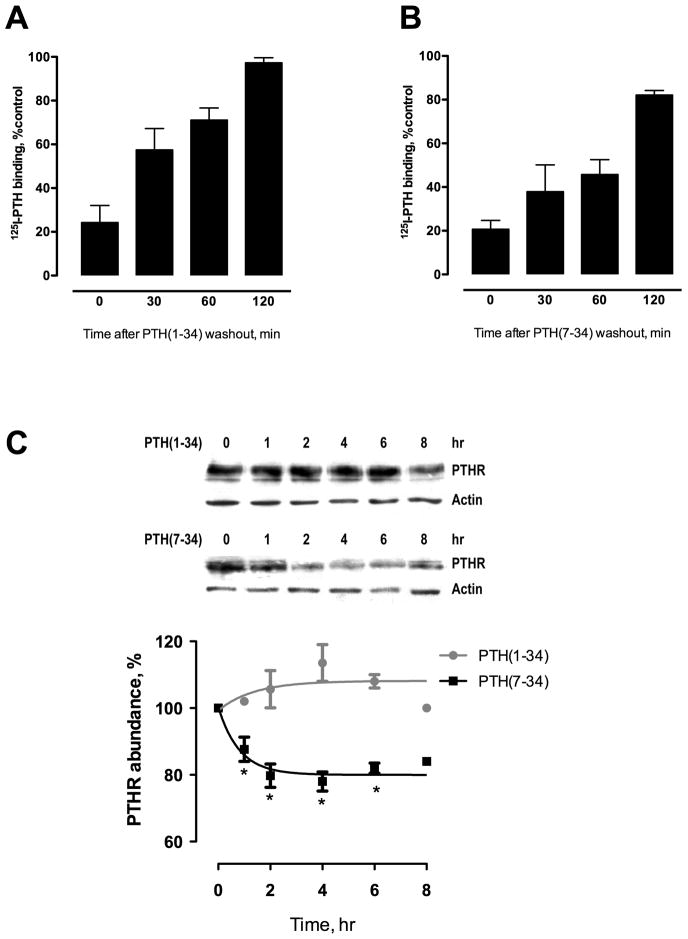
We first sought to determine if PTH(1–34) and PTH(7–34) exert different effects on PTHR recycling. Specific PTH binding is shown as a function of recycling time following a 30-minute exposure to saturating concentrations of the PTH(1–34) or PTH(7–34). After challenge with PTH(1–34) (Fig. 1A), more than 50% of receptor recycled by 30 minutes and fully recycled by 2 hours. In contrast, after challenge with PTH(7–34) (Fig. 1B), less than 50% of the receptor had recycled by 1 hour and complete recycling was not achieved by 2 hours (Fig. 1B). These results demonstrate that the PTHR recycles more quickly and completely after a brief exposure to the activating PTH peptide, PTH(1–34), than after PTH(7–34).
Fig. 1. PTHR recycling and abundance after stimulation by PTH(1–34) or PTH(7–34).
DCT cells were incubated with either (A) 100 nM PTH(1–34) or (B) 1 μM PTH(7–34) for 30 minutes at 37°C, rinsed, acid-washed to remove any residual bound ligand, and allowed to recycle for the times indicated. Receptor binding is shown as a function of recycling time and was measured by 125I-PTH(1–34) binding and expressed as the percent of specific binding relative to the total binding of radioligand in unstimulated cells. (C) HK-2 cells were treated with either 100 nM PTH(1–34) or 1 μM PTH(7–34) for 1–8 hours. Total lysates were extracted and immunoblotted as described in Materials and Methods. PTHR was detected using a specific primary human antibody (1:1000) and HRP-tagged antibody (1:1000). Average relative abundance of PTHR (shown as a percent of total receptor abundance in untreated HK-2 cells). Data are summarized as ± S.E. of 3 independent experiments. *p < 0.05 vs. 0 hr.
We next characterized the effect of chronic PTH exposure on abundance of endogenous PTHR in human kidney cells (HK-2). Exposure to PTH(7–34) for 1–8 hours promoted rapid PTHR degradation, with a 20–30% decrease of total receptor abundance within 1 hour (Fig. 1C). Treatment with PTH(1–34) for up to 8 hours had no significant effect on total PTHR expression (Fig. 1C). These findings suggest that PTH(7–34) preferentially reduces PTHR abundance. Taken together, these results raised the possibility that incomplete PTHR recycling induced by PTH(7–34) is due to a degradative process
Effect of PTH(7–34) in PTHR-proteasome depended degradation
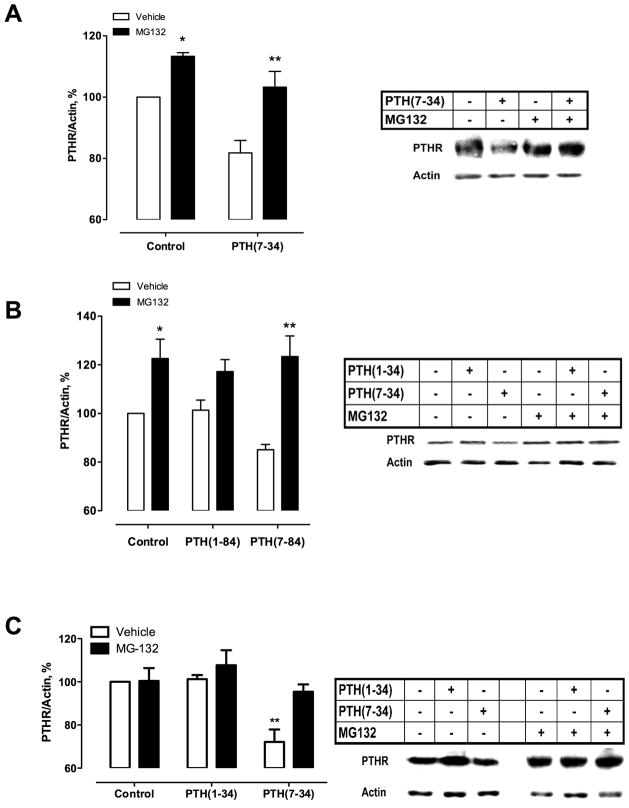
Proteasomes and lysosomes are two major cellular protein degradative pathways. Several GPCR such as μ- and δ-opioid receptors(39) and V2 vasopressin receptors(31) are degraded by proteasome. We first ascertained the participation of proteasomal degradation on the PTHR. We used MG132 (N-carbobenzyloxy-L-leucyl-L-leucyl-L-leucinal), a cell-permeable proteasomal inhibitor(40), to block the activity of the 26S proteasome and allow accumulation of protein otherwise degraded. Using CHO-N10-R3 cells, which stably express HA-PTHR, MG132 fully blocked PTHR degradation after 6-hour treatment with PTH(7–34) (Fig. 2A). Thus, PTHR degradation induced by PTH(7–34) is due to proteosomal degradation. Similar results were obtained with PTH(7–84) and measuring endogenous PTHR levels in more physiologically relevant HK-2 cells (Fig. 2B). ROS cells overexpressing HA-PTHR exhibited a comparable response to PTH(7–34) (Fig. 2C).
Fig. 2. Proteasome inhibition leads to PTHR accumulation.
CHO-N10-R3 (A), HK-2 (B) or ROS (C) cells were pre-treated with the proteasome inhibitor MG-132 at 25 μM for 1 hour before addition of either 100 nM PTH(1–84), 1 μM PTH(7–34) or 1 μM PTH(7–84) as indicated for an additional 6 hours at 37°C. Equal amounts of cell lysates were analyzed by immunoblot for HA-PTHR (A and C) or endogenous PTHR (B) and shown as the relative abundance as a percentage of total receptor abundance in untreated cells. Data illustrate 5 (A and B) or 4 (C) independent experiments. *p < 0.05; **p < 0.01 vs. corresponding vehicle condition.
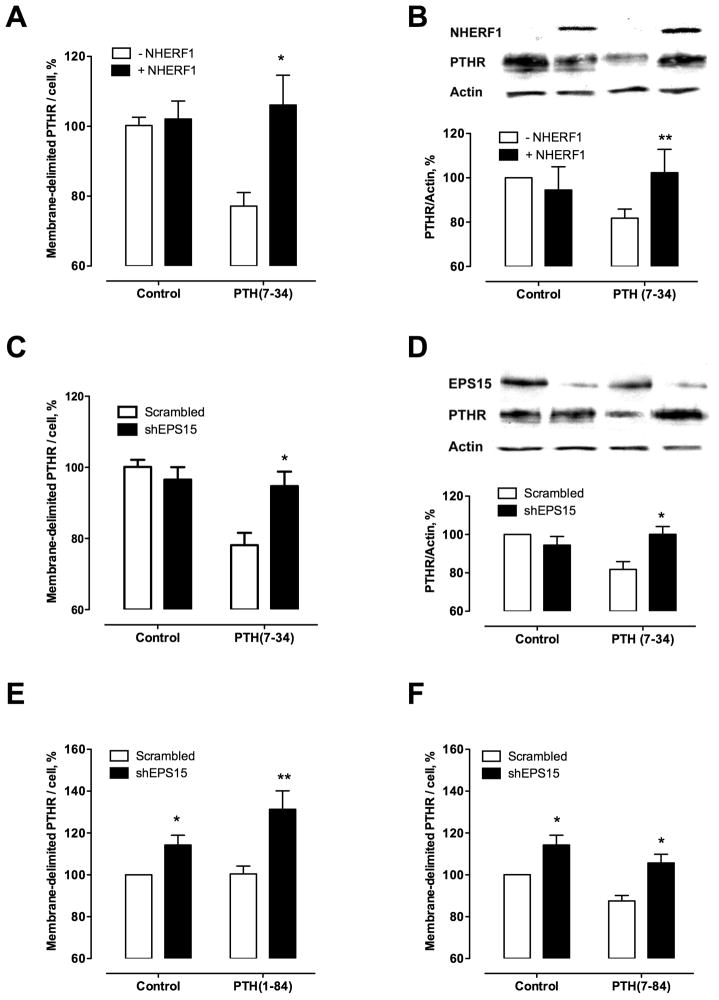
PTHR internalization is necessary for PTHR degradation induced by PTH(7–34) (Fig. 3). It is known that Na+/H+ exchanger regulatory factor-1 (NHERF1), implicated in protein targeting and in the assembly of protein complexes, inhibits PTHR internalization induced by PTH(7–34)(10). Here, we used a CHO cell model, where NHERF1 expression can be induced by tetracycline to permit a paired comparison of NHERF1 effect on PTHR degradation(11). Expression of NHERF1 itself did not affect membrane-delimited (Fig. 3A) or total (Fig. 3B) PTHR expression but blocked PTH(7–34)-induced receptor internalization and degradation (Fig. 3A-B). Eps15 is localized at clathrin-coated pits, where it interacts with the clathrin assembly AP-2 and the AP-2 binding protein, Epsin(41,42). The alteration of Eps15 and Epsin function inhibits receptor-mediated endocytosis of EGF and transferrin, demonstrating that both proteins are components of the endocytic machinery(41). We observed that shRNA-induced knockdown of Eps15 mRNA blocked the PTH(7–34)-stimulated decrease of total PTHR abundance in CHO-N10-R3 (Fig. 3C-D). PTH(1–84) did not induce PTHR degradation (Fig. 3E). PTHR degradation following stimulation with PTH(7–84) (Fig. 3F) in HK-2 cells was abolished by shRNA-Eps15. These findings support a key role of internalization on the PTHR degradative process triggered by PTH(7–34). Eps15 depletion in HK2 kidney cells increases membrane-delimited PTHR in control conditions. These results exhibit a differential effect of silencing Eps15 in control conditions in different cell models, suggesting a faster PTHR turnover in kidney cells compared to other cell lines such as CHON10R3 (Fig. 3C, E and F).
Fig. 3. PTHR downregulation requires antecedent internalization.
CHO-N10-R3 (A, C) and HK-2 cells (transiently transfected with HA-PTHR) (E, F) were incubated with 100 nM PTH(1–84), 1 μM PTH(7–34), or 1 μM PTH(7–84) as indicated for 3 hours. Receptor internalization was assayed in triplicate by ELISA as described in Materials and Methods. PTHR abundance was detected by western blot (B, D) in CHO-N10-R3 cells after a 3-hour exposure to 1 μM PTH(7–34). Data are the mean of 3 independent experiments performed. *p < 0.05; **p < 0.01 vs. corresponding –NHERF or scrambled conditions.
PTH stimulates PTHR ubiquitination
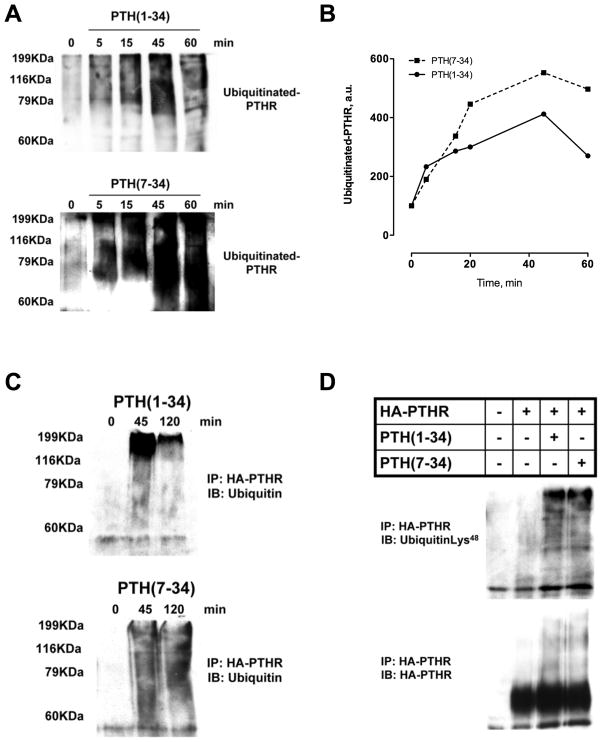
Agonist-promoted ubiquitination of GPCRs plays a key role in endocytic trafficking and receptor degradation. The observations described above led us to hypothesize that distinct patterns of ligand-induced ubiquitination and deubiquitination could explain the difference between the complete PTHR recycling induced by PTH(1–34) and PTHR downregulation elicited by PTH(7–34). To begin to test this theory, ROS cells were transiently transfected with HA-PTHR, myc-ubiquitin and treated with PTH(1–34) or PTH(7–34). As shown in Fig. 4, the PTHR was polyubiquitinated, shown as a smear on Western blots because of the variable number of conjugated ubiquitin moieties. Both PTH(1–34) and PTH(7–34) induced receptor ubiquitination within 5 minutes and reaching maximal within 45 minutes of agonist exposure (Fig. 4A and B). In response to PTH(1–34), PTHR ubiquitination was transient and began to diminish within 1 hour of ligand exposure, with just slight residual PTHR-ubiquitination 2 hours after treatment (Fig. 4C). In contrast, PTHR polyubiquitination induced by PTH(7–34) was sustained beyond 2 hours (Fig. 4C).
Fig. 4. PTHR ubiquitination.
ROS cells transfected with HA-PTHR and Myc-ubiquitin were pre-treated with MG-132 (25 μM; 1 hour) before incubation with either 100 nM PTH(1–34) or 1 μM PTH(7–34) for 5–120 minutes at 37°C. Cell lysates were immunoprecipitated using monoclonal anti-HA beads. Typical immunoblot of immunoprecipitates probed with polyclonal ubiquitin antibody (A, C) or Lys48 –specific ubiquitin antibody (D) are shown. Representative images of 3 independent experiments.
Ubiquitin chains can be assembled in several ways depending on the location of the array of internal lysines within ubiquitin itself used to form polyubiquitin chains.(43) Different types of ubiquitin modifications are associated with distinct cellular functions. Lys48-linked polyubiquitin chains regulate numerous nuclear, cytosolic and endoplasmic reticulum membrane proteins by targeting them for degradation by the 26S proteasome.(43) We analyzed polyubiquitinated PTHR forms to determine if PTH(1–34) and PTH(7–34) exerted ligand-selective ubiquitin modification. To answer this question, cells were pretreated with the proteasome inhibitor MG132, stimulated with PTH(1–34) or PTH(7–34) for 45 minutes, and immunoblotted with site-specific anti-Lys polyubiquitin antibodies. Lys48-linked ubiquitin chains are the dominant form for PTHR ubiquitination upon ligand stimulation (Fig. 4D). These data show that the PTHR is polyubiquitinated by both ligands. However, the differences in the PTHR polyubiquitination time-course, transient after PTH(1–34) stimulation or continuous after PTH(7–34) treatment, suggest that deubiquitination induced by PTH(1–34), but not PTH(7–34), removes the ubiquitin moieties from ubiquitinated PTHR.
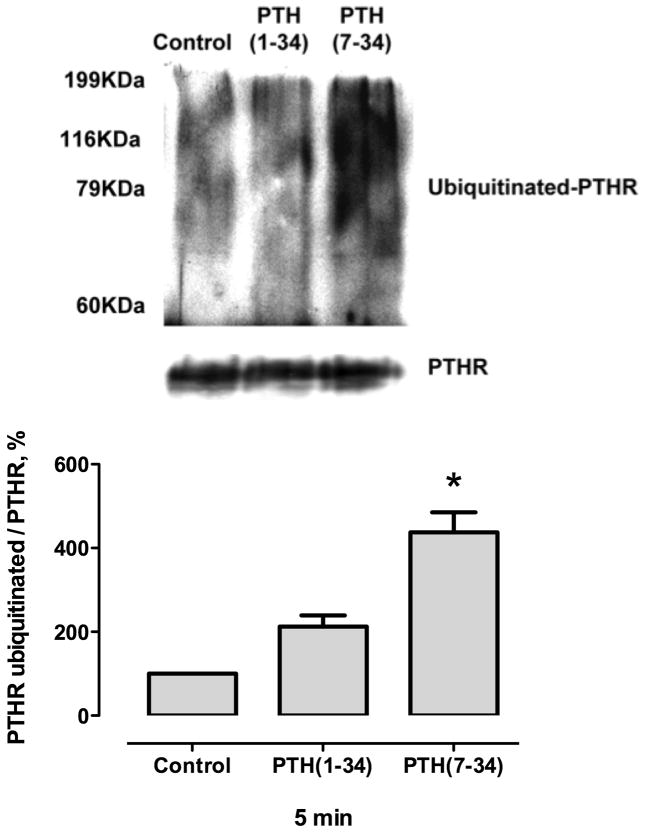
Cell fractionation experiments showed that the PTHR was ubiquitinated at the plasma membrane (Fig. 5). These data suggest that PTHR is ubiquitinated at the plasma membrane before its internalization.
Fig. 5. PTHR is ubiquitinated at the plasma membrane.
ROS cells were transfected with HA-PTHR. After 48 hours, the cells were treated with 100 nM PTH(1–34) or 1 μM PTH(7–34) for 5 minutes. Membrane proteins were isolated and immunoprecipitated using monoclonal anti-HA beads as described in Materials and Methods. Representative immunoblot of immunoprecipitate probed with polyclonal ubiquitin antibody. Values are mean ± SEM from 3 independent experiments.
USP2 regulates PTH receptor fate by deubiquitination
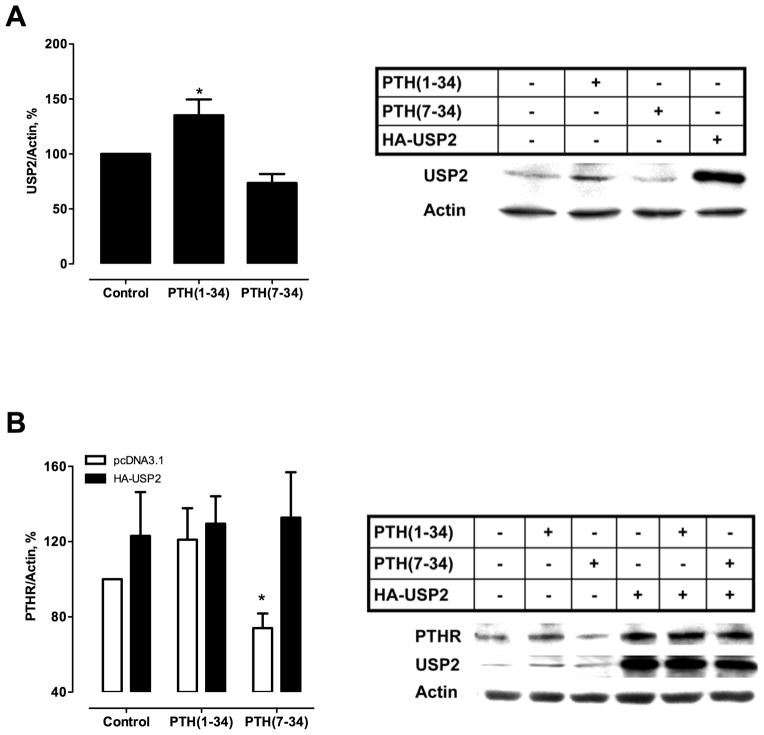
Ubiquitination is counteracted by deubiquitinating enzymes (DUBs) that deconjugate ubiquitin-modified proteins and rescue the substrate from proteasomal degradation.(44) We hypothesized that DUBs that hydrolyze ubiquitin-protein isopeptide bonds could rescue the PTHR from proteasomal degradation. To analyze the role of DUBs in the regulation of PTHR ubiquitination and stability, we focussed on ubiquitin-specific protease 2 (USP2) because its mRNA is rapidly upregulated in bone by PTH(1–38).(45) Here, USP2 protein levels significantly increased in ROS cells after PTH(1–34) stimulation but not with PTH(7–34) treatment (Fig. 6A). Furthermore, overexpression of USP2 in ROS cells rescued PTHR degradation induced by PTH(7–34) (Fig. 6B). These results suggest that USP2 protects the PTHR from PTH(7–34)-induced downregulation by virtue of direct deubiquitination of the receptor.
Fig. 6. PTH(1–34) increases USP2 expression.
(A) ROS cells were transiently transfected with HA-PTHR and treated with 100 nM PTH(1–34) or 1 μM PTH(7–34) for 1 hour. USP2 expression was assayed by immunoblot. (B) ROS cells were transfected with HA-USP2 and Flag-PTHR. After 48 hours, cells were treated with agonist as indicated. PTHR was detected using an anti-Flag primary antibody (1:1000). Values are mean ± SEM from ≥ 3 independent experiments. *p<0.05 vs. PTH(7–34).
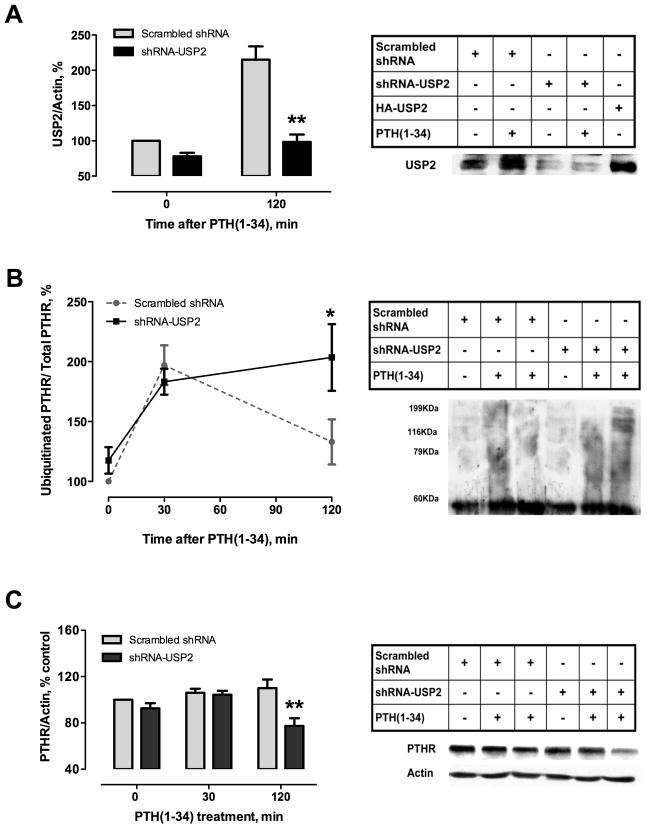
To address the mechanism of USP2 action, we examined the consequences of USP2 depletion on PTHR turnover in response to PTH(1–34). In ROS cells transfected with shRNA-USP2, PTH(1–34) treatment no longer augmented USP2 protein levels (Fig. 7A). Under these conditions, PTHR deubiquitination was abolished (Fig. 7B), indicating specific deubiquitinating activity of USP2 on the PTHR. As expected, blocking of USP2 deubiquitinase activity decreased PTHR abundance after PTH(1–34) stimulation (Fig. 7C), suggesting an increase of the degradative machinery activity. These results are consistent with the conclusion that USP2 dictates receptor fate after PTHR endocytosis induced by PTH(1–34).
Fig. 7. Silencing USP2 deubiquitinase promotes ubiquitinated PTHR accumulation and favors PTHR downregulation.
ROS cells were transfected with HA-PTHR. After 24 hours cells were transfected with shRNA-USP2 and incubated for 48 hours. Cells were then treated with 100 nM PTH(1–34) for 30 or 120 minutes. Total lysates and immunoprecipitated protein were analyzed by SDS-polyacrylamide gels and transferred to Immobilon-P membranes. Representative autoradiograms of USP2 (A) Ubiquitinated PTHR (B) and total PTHR (C) are shown. Values are mean ± SEM from ≥ 3 independent experiments. *p<0.05; **p < 0.01 vs. corresponding scrambled shRNA value.
Discussion
Ligand binding to several mammalian G protein-coupled receptors, such as the PTHR, results in conformational changes that not only initiate cellular signaling events but also lead to receptor phosphorylation and internalization. Internalized receptors are either recycled to the surface after dephosphorylation or are degraded.(1) The data reported here provide evidence that the cellular destination and fate of the internalized PTHR is dictated by the balance between polyubiquitination and deubiquitination.
Upon stimulation with either activating (PTH[1–34], PTH[1–84]) or non-activating (PTH[7–34], PTH[7–84]) peptides, the receptor is able to recycle to the membrane surface. The distinct kinetics of PTHR recycling suggests that resensitization is ligand-dependent. The coordinated events of desensitization, internalization, and resensitization protect against excessive receptor stimulation or periods of prolonged inactivity. In this manner, receptor activation and internalization are normally coupled. However, amino-truncated PTH peptides uncouple PTHR activation and inactivation.(10) Here, we show that PTHR recycling induced by PTH(7–34) is slower and incomplete compared with PTH(1–34). This phenomenon is opposite the actions of these peptides on PTHR internalization, where PTH(7–34)-induced receptor endocytosis was greater and more rapid than that elicited by PTH(1–34). Comparable agonist- and antagonist-induced endocytosis of serotonin, endothelin, and cholecystokinin receptors have been reported.(46–48) Similar to our results with the PTHR, serotonin 2a receptors are internalized upon stimulation by full agonists such as serotonin (5-HT) but also by partial agonists such dopamine. However, in contrast with the present results, both 5-HT and dopamine internalized receptors recycle to the cell surface with a similar timeframe.(49–51) Recycling of β2-adrenergic receptors (β2ARs) show similar kinetics regardless of ligand binding or agonist-induced activation.(52–54) Thus, the differential sorting between distinct recycling and degradative pathways after endocytosis is highly receptor-specific.(55) Notably, the PTHR can traffic to recycling or degradative pathways and this is controlled in a novel ligand-dependent fashion. Our findings suggest a regulatory role of PTH(7–34) on PTHR abundance that reduces the cellular response to activating ligands or excess agonist levels. Moreover, we show a significant decrease in total receptor abundance within 1-hour of exposure to PTH(7–34) that was sustained for up 8 hours. Complementary findings show a difference in PTHR half-life in the absence of ligand (12 hours) or decreasing receptor half-life in the presence of PTH(7–34) (7 hours).(56) Although it has been shown that 24–48 hour PTH(1–34) treatment downregulates total PTHR in opossum kidney cells,(57) we did not observe significant effects of PTH(1–34) on PTHR protein levels. Thus, the differences between activating and non-activating ligand-mediated internalization and recycling/degradation explains several aspects of the functional selectivity in receptor trafficking, activity and physiology.
Our observations support the idea that during its lifetime the PTHR undergoes degradation leading to reduced protein stability. In absence of ligand, blocking proteosomal degradation leads to increased PTHR expression of both endogenous or overexpressed PTHR. This suggests a constitutive degradative role for the 26S proteasome on basal PTHR turnover. However, PTH(7–34) preferentially augments this process.
Ligand stimulated, proteasome-mediated GPCR degradation is well established.(29–32) Classically, ubiquitination was identified as a pathway for degradation of short-lived cytosolic and nuclear proteins by the 26S proteasome. Considering the differences observed in receptor degradation induced by PTH(7–34) but not by PTH(1–34), we expected different ubiquitination patterns after PTH peptide challenge. However, both ligands induced polyubiquitination of PTHR, and specifically promoted Lys48-linked polyubiquitin chains that are targeted for degradative processing.(58) The rapid PTHR ubiquitination after ligand challenge (starting at 5 minutes and reaching maximal at 45 minutes) is similar to that reported for several GPCR, including the β2-AR (within 15 minutes)(32) and the human kappa-opioid receptor (30 minutes).(59)
The ubiquitin-proteasome system is essential for osteoblast proliferation under control and PTH-treated conditions. PTH enhances ubiquitination of protein substrates and stimulates proteasome activities by a cAMP-dependent mechanism.(60) The present results strongly support the conclusion the PTHR is itself one of the polyubiquitinated proteins. The ubiquitin ligase involved in this process is unknown. Recently, Smurf1 was identified as homologous to the E6-AP carboxyl terminus (HECT)-type ubiquitin ligase that was demonstrated to target different bone-related proteins such as Smad1, Smad5, Runx2, RhoA, bone morphogenetic protein (BMP) or transforming growth factor b (TGF-b) type I receptors for ubiquitination and degradation in osteoblasts.(61) Smurf1 promotes age-dependent bone mass increases in vivo.(62) Thus, Smurf1 could be a candidate linking ubiquitin moieties to the PTHR.
Ubiquitination can be a reversible process, where deubiquitinating enzymes (DUBs) remove the ubiquitin moieties from ubiquitinated substrates. Our results indicate that PTH(1–34) elicits a transient PTHR ubiquitination signal, whereas PTH(7–34) treatment triggers a continuous signal for PTHR ubiquitination. The present data support the view that USP2 recognizes specifically ubiquitinated PTHRs. This leads to deubiquitination of the receptor, thereby preventing proteasomal degradation and facilitating receptor recycling. PTH(1–34) increase USP2 levels, favoring the balance towards rapid deubiquitination and recycling of the receptor. In contrast, PTH(7–34) does not increase USP2. The slow deubiquitination leads to accumulation of ubiquitin tags on the PTHR and subsequent receptor degradation by the proteasomal pathway. Hence, our data imply that USP2 effects depend on protein amount.
DUBs can display specificity at multiple levels to distinguish between the many ubiquitin-like molecules, isopeptides (using an ε-amino group) and linear peptides (using an α-amino group), and between different types of ubiquitin linkage and chain structure.(63) Ligand-induced deubiquitinases have been shown for only a few GPCRs. The Adenosine A2 receptor is deubiquitinated by USP4;(64) the ubiquitination status and trafficking of the EGFR growth factor receptor is regulated by the USP8-STAM complex;(65) the β2-AR undergoes increased agonist-stimulated ubiquitination, lysosomal trafficking, and degradation after knockdown of USPs 20 and 33.(66) DUBs are remarkably specific. Overexpression of USP4 that promotes cell surface targeting of adenosine A2 receptors(64) does not affect β2-AR ubiquitination.(66) Thus, receptor deubiquitination involves specific USP isoforms. Many DUBs have been described to remove ubiquitin moieties from histones, pro-oncogenes, tumor suppressor proteins like p53, E3 ligases, Smad4 and a large number of signaling protein as NF-κB signaling cascade. Consequently, the physiological roles of DUBs are as pervasive as the ubiquitin proteasome system itself.(63)
Berthouze and co-workers propose that fast recycling from early endosomal compartments mainly involves dephosphorylation of non-ubiquitinated receptors, whereas slow recycling of ubiquitinated receptors from deeper subcellular compartments is regulated by DUB and phosphatase activities.(66) The PTHR is a member of class B, characterized by slow recycling. Previous studies reported that upon PTH(1–34) stimulation, the PTHR localizes in early endosomes but not in late endosomes(67) and after 30–40 minutes the receptor traffics to the Golgi apparatus.(68,69) These observations support the idea that slow PTHR recycling may arise not only because some receptors are in deeper subcellular compartments but also a consequence of the localization of DUBs to the specific substrate.
The physiological relevance of the proposed mechanism could explain some aspects of PTH resistance in chronic renal failure. End-stage renal failure is accompanied by PTH resistance and secondary hyperparathyroidism.(70) Furthermore, high levels of PTH(7–84) are associates with PTHR downregulation.(22–24) It has been proposed that the PTH resistance of end-stage renal failure results from competitive inhibition of intact PTH with these amino-terminally truncated PTH fragments.(15) PTH receptor downregulation, however, would be inconsistent with the view that PTH(7–84) acts exclusively as an antagonist. Rather, we propose that the selective induction of deubiquitinase activity induced by PTH(1–34) but not by PTH(7–34) modulates receptor levels and PTH resistance in bone and kidney.
In summary, this study reveals the importance of the balance between the ubiquitination and the deubiquitination process in the regulation of PTHR abundance and fate. Further, these are the first results to show that PTHR recycling and can be coupled to ligand-selective deubiquitination. USP2 is critical for PTHR recycling after PTH(1–34) stimulation, thus, underscoring the role for regulated deubiquitination in safeguarding physiological responsiveness. Consistent with this view, we previously reported that a decrease in PTHR abundance at the cell membrane affects both cAMP and ERK signaling pathways, thereby reducing their signaling (38). We now suggest that proteasome-dependent degradation of the receptor triggered by the uncoupling of ubiquitination and deubiquitination induced by the PTH(7–34) may have similar inhibitory effects on PTHR signaling due to reduced numbers of functional PTH receptors at the cell membrane. Considering possible pathophysiological roles of PTH(7–34), new agents that activate renal DUBs might rescue receptor recycling, and with it PTHR resensitization, and provide a novel treatment for chronic kidney disease.
Acknowledgments
Funding: National Institutes of Health, DK54171 (PAF) and DK071158 (AB)
This work was supported by National Institutes of Health grants R01 DK54171 (PAF) and DK071158 (AB)
Footnotes
Conflict of interests: No authors have conflicts of interest
Contributor Information
Verónica Alonso, Email: vea7@pitt.edu.
Clara E. Magyar, Email: cmagyar@mednet.ucla.edu.
Bin Wang, Email: biw5@pitt.edu.
Alessandro Bisello, Email: alb138@pitt.edu.
Peter A. Friedman, Email: paf10@pitt.edu.
References
- 1.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: The role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 2.Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic. 2007;8:462–470. doi: 10.1111/j.1600-0854.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 3.Malecz N, Bambino T, Bencsik M, Nissenson RA. Identification of phosphorylation sites in the G protein-coupled receptor for parathyroid hormone. Receptor phosphorylation is not required for agonist-induced internalization. Mol Endocrinol. 1998;12:1846–1856. doi: 10.1210/mend.12.12.0203. [DOI] [PubMed] [Google Scholar]
- 4.Chauvin S, Bencsik M, Bambino T, Nissenson RA. PTH receptor recycling: role of receptor dephosphorylation and β-arrestin. Mol Endocrinol. 2002;16:2720–2732. doi: 10.1210/me.2002-0049. [DOI] [PubMed] [Google Scholar]
- 5.Tawfeek HA, Qian F, Abou-Samra AB. Phosphorylation of the receptor for PTH and PTHrP is required for internalization and regulates receptor signaling. Mol Endocrinol. 2002;16:1–13. doi: 10.1210/mend.16.1.0760. [DOI] [PubMed] [Google Scholar]
- 6.Vilardaga JP, Krasel C, Chauvin S, Bambino T, Lohse MJ, Nissenson RA. Internalization determinants of the parathyroid hormone receptor differentially regulate β-arrestin/receptor association. J Biol Chem. 2002;277:8121–8129. doi: 10.1074/jbc.M110433200. [DOI] [PubMed] [Google Scholar]
- 7.Miedlich SU, Abou-Samra AB. Eliminating phosphorylation sites of the parathyroid hormone receptor type 1 differentially affects stimulation of phospholipase C and receptor internalization. Am J Physiol Endocrinol Metab. 2008;295:E665–E671. doi: 10.1152/ajpendo.00036.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrari SL, Behar V, Chorev M, Rosenblatt M, Bisello A. Endocytosis of ligand-human parathyroid hormone receptor 1 complexes is PKC-dependent and involves β-arrestin2. Real-time monitoring by fluorescence microscopy. J Biol Chem. 1999;274:29968–29975. doi: 10.1074/jbc.274.42.29968. [DOI] [PubMed] [Google Scholar]
- 9.Conway BR, Minor LK, Xu JZ, D’Andrea MR, Ghosh RN, Demarest KT. Quantitative analysis of agonist-dependent parathyroid hormone receptor trafficking in whole cells using a functional green fluorescent protein conjugate. J Cell Physiol. 2001;189:341–355. doi: 10.1002/jcp.10028. [DOI] [PubMed] [Google Scholar]
- 10.Sneddon WB, Syme CA, Bisello A, Magyar CE, Weinman EJ, Rochdi MD, Parent JL, Abou-Samra AB, Friedman PA. Activation-independent parathyroid hormone receptor internalization is regulated by NHERF1 (EBP50) J Biol Chem. 2003;278:43787–43796. doi: 10.1074/jbc.M306019200. [DOI] [PubMed] [Google Scholar]
- 11.Wheeler DG, Sneddon WB, Wang B, Friedman PA, Romero G. NHERF-1 and the cytoskeleton regulate the traffic and membrane dynamics of G protein-coupled receptors. J Biol Chem. 2007;282:25076–25087. doi: 10.1074/jbc.M701544200. [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Yang Y, Abou-Samra AB, Friedman PA. NHERF1 regulates parathyroid hormone receptor desensitization; interference with β-arrestin binding. Mol Pharmacol. 2009;75:1189–1197. doi: 10.1124/mol.108.054486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura T, Sakamoto H, Filburn CR. Parathyroid hormone 1–34, but not 3–34 or 7–34, transiently translocates PKC in cultured renal (OK) cells. Biochem Biophys Res Commun. 1989;159:1352–1358. doi: 10.1016/0006-291x(89)92259-6. [DOI] [PubMed] [Google Scholar]
- 14.Fujimori A, Cheng S-L, Avioli LV, Civitelli R. Structure-function relationship of parathyroid hormone: activation of phospholipase-C, protein kinase-A and -C in osteosarcoma cells. Endocrinology. 1992;130:29–36. doi: 10.1210/endo.130.1.1727705. [DOI] [PubMed] [Google Scholar]
- 15.Slatopolsky E, Finch J, Clay P, Martin D, Sicard G, Singer G, Gao P, Cantor T, Dusso A. A novel mechanism for skeletal resistance in uremia. Kidney Int. 2000;58:753–761. doi: 10.1046/j.1523-1755.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- 16.Gentili C, Boland R, de Boland AR. PTH stimulates PLCβ and PLCγ isoenzymes in rat enterocytes: influence of ageing. Cell Signal. 2001;13:131–138. doi: 10.1016/s0898-6568(00)00145-5. [DOI] [PubMed] [Google Scholar]
- 17.Lepage R, Roy L, Brossard JH, Rousseau L, Dorais C, Lazure C, D’Amour P. A non-(I-84) circulating parathyroid hormone (PTH) fragment interferes significantly with intact PTH commercial assay measurements in uremic samples. Clin Chem. 1998;44:805–809. [PubMed] [Google Scholar]
- 18.John MR, Goodman WG, Gao P, Cantor TL, Salusky IB, Jüppner H. A novel immunoradiometric assay detects full-length human PTH but not amino-terminally truncated fragments: Implications for PTH measurements in renal failure. J Clin Endocrinol Metab. 1999;84:4287–4290. doi: 10.1210/jcem.84.11.6236. [DOI] [PubMed] [Google Scholar]
- 19.Coen G, Bonucci E, Ballanti P, Balducci A, Calabria S, Nicolai GA, Fischer MS, Lifrieri F, Manni M, Morosetti M, Moscaritolo E, Sardella D. PTH 1–84 and PTH “7–84” in the noninvasive diagnosis of renal bone disease. Am J Kidney Dis. 2002;40:348–354. doi: 10.1053/ajkd.2002.34519. [DOI] [PubMed] [Google Scholar]
- 20.Monier-Faugere MC, Geng Z, Mawad H, Friedler RM, Gao P, Cantor TL, Malluche HH. Improved assessment of bone turnover by the PTH-(1–84)/large C-PTH fragments ratio in ESRD patients. Kidney Int. 2001;60:1460–1468. doi: 10.1046/j.1523-1755.2001.00949.x. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita H, Gao P, Cantor T, Futata T, Murakami T, Uchino S, Watanabe S, Kawamoto H, Fukagawa M, Noguchi S. Large carboxy-terminal parathyroid hormone (PTH) fragment with a relatively longer half-life than 1–84 PTH is secreted directly from the parathyroid gland in humans. Eur J Endocrinol. 2003;149:301–306. doi: 10.1530/eje.0.1490301. [DOI] [PubMed] [Google Scholar]
- 22.Ureña P, Kubrusly M, Mannstadt M, Hruby M, Trinh Trang Tan M-M, Silve C, Lacour B, Abou-Samra AB, Segre GV, Drüeke T. The renal PTH/PTHrP receptor is down-regulated in rats with chronic renal failure. Kidney Int. 1994;45:605–611. doi: 10.1038/ki.1994.79. [DOI] [PubMed] [Google Scholar]
- 23.Tian J, Smogorzewski M, Kedes L, Massry SG. PTH-PTHrP receptor mRNA is downregulated in chronic renal failure. Am J Nephrol. 1994;14:41–46. doi: 10.1159/000168684. [DOI] [PubMed] [Google Scholar]
- 24.Edwards RM, Contino LC, Gellai M, Brooks DP. Parathyroid hormone-1 receptor down-regulation in kidneys from rats with chronic renal failure. Pharmacology. 2001;62:243–247. doi: 10.1159/000056102. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Zhang J, Lin S. Down-regulation of PTH/PTHrP receptor in the kidney of patients with renal impairment. Chin Med J (Engl) 1998;111:24–27. [PubMed] [Google Scholar]
- 26.Shenoy SK. Seven-transmembrane receptors and ubiquitination. Circ Res. 2007;100:1142–1154. doi: 10.1161/01.RES.0000261939.88744.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wojcikiewicz RJ. Regulated ubiquitination of proteins in GPCR-initiated signaling pathways. Trends Pharmacol Sci. 2004;25:35–41. doi: 10.1016/j.tips.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Hurley JH, Stenmark H. Molecular mechanisms of ubiquitin-dependent membrane traffic. Annu Rev Biophys. 2010 doi: 10.1146/annurev-biophys-042910-155404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cook LB, Zhu C-C, Hinkle PM. Thyrotropin-releasing hormone receptor processing: role of ubiquitination and proteasomal degradation. Mol Endocrinol. 2003;17:1777–1791. doi: 10.1210/me.2003-0073. [DOI] [PubMed] [Google Scholar]
- 30.Cohen BD, Bariteau JT, Magenis LM, Dias JA. Regulation of follitropin receptor cell surface residency by the ubiquitin-proteasome Pathway. Endocrinology. 2003;144:4393–4402. doi: 10.1210/en.2002-0063. [DOI] [PubMed] [Google Scholar]
- 31.Martin NP, Lefkowitz RJ, Shenoy SK. Regulation of V2 vasopressin receptor degradation by agonist-promoted ubiquitination. J Biol Chem. 2003;278:45954–45959. doi: 10.1074/jbc.M308285200. [DOI] [PubMed] [Google Scholar]
- 32.Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated β2-adrenergic receptor and β-Arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 33.Lupp A, Klenk C, Rocken C, Evert M, Mawrin C, Schulz S. Immunohistochemical identification of the PTHR1 parathyroid hormone receptor in normal and neoplastic human tissues. Eur J Endocrinol. 2010;162:979–986. doi: 10.1530/EJE-09-0821. [DOI] [PubMed] [Google Scholar]
- 34.Wang B, Bisello A, Yang Y, Romero GG, Friedman PA. NHERF1 regulates parathyroid hormone receptor membrane retention without affecting recycling. J Biol Chem. 2007;282:36214–36222. doi: 10.1074/jbc.M707263200. [DOI] [PubMed] [Google Scholar]
- 35.Zhang P, Jobert AS, Couvineau A, Silve C. A homozygous inactivating mutation in the parathyroid hormone/parathyroid hormone-related peptide receptor causing Blomstrand chondrodysplasia. J Clin Endocrinol Metab. 1998;83:3365–3368. doi: 10.1210/jcem.83.9.5245. [DOI] [PubMed] [Google Scholar]
- 36.Wang B, Yang Y, Friedman PA. Na/H Exchange regulator factor 1, a novel Akt-associating protein, regulates extracellular signal-related signaling through a B-Raf-mediated pathway. Mol Biol Cell. 2008;19:1637–1645. doi: 10.1091/mbc.E07-11-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang B, Yang Y. Generation of human PTH1R construct with Flag epitope located internally: comparison of two-fragment assembly by using PCR overlap extension or ligase. J Biomol Tech. 2009;20:195–200. [PMC free article] [PubMed] [Google Scholar]
- 38.Alonso V, Ardura JA, Wang B, Sneddon WB, Friedman PA. A naturally occurring isoform inhibits parathyroid hormone receptor trafficking and signaling. J Bone Miner Res. 2011;26:143–155. doi: 10.1002/jbmr.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaturvedi K, Bandari P, Chinen N, Howells RD. Proteasome involvement in agonist-induced down-regulation of mu and delta opioid receptors. J Biol Chem. 2001;276:12345–12355. doi: 10.1074/jbc.M008054200. [DOI] [PubMed] [Google Scholar]
- 40.Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 41.Salcini AE, Chen H, Iannolo G, De Camilli P, Di Fiore PP. Epidermal growth factor pathway substrate 15, Eps15. Int J Biochem. 1999;31:805–809. doi: 10.1016/s1357-2725(99)00042-4. [DOI] [PubMed] [Google Scholar]
- 42.de Melker AA, van der Horst G, Borst J. Ubiquitin ligase activity of c-Cbl guides the epidermal growth factor receptor into clathrin-coated pits by two distinct modes of Eps15 recruitment. J Biol Chem. 2004;279:55465–55473. doi: 10.1074/jbc.M409765200. [DOI] [PubMed] [Google Scholar]
- 43.Kuhlbrodt K, Mouysset J, Hoppe T. Orchestra for assembly and fate of polyubiquitin chains. Essays Biochem. 2005;41:1–14. doi: 10.1042/EB0410001. [DOI] [PubMed] [Google Scholar]
- 44.Reyes-Turcu FE, Ventii KH, Wilkinson KD. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem. 2009;78:363–397. doi: 10.1146/annurev.biochem.78.082307.091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miles RR, Sluka JP, Halladay DL, Santerre RF, Hale LV, Bloem L, Patanjali SR, Galvin RJ, Ma L, Hock JM, Onyia JE. Parathyroid hormone (hPTH 1–38) stimulates the expression of UBP41, an ubiquitin-specific protease, in bone. J Cell Biochem. 2002;85:229–242. doi: 10.1002/jcb.10129. [DOI] [PubMed] [Google Scholar]
- 46.Willins DL, Berry SA, Alsayegh L, Backstrom JR, Sanders-Bush E, Friedman L, Roth BL. Clozapine and other 5-hydroxytryptamine-2A receptor antagonists alter the subcellular distribution of 5-hydroxytryptamine-2A receptors in vitro and in vivo. Neuroscience. 1999;91:599–606. doi: 10.1016/s0306-4522(98)00653-8. [DOI] [PubMed] [Google Scholar]
- 47.Bhowmick N, Narayan P, Puett D. The endothelin subtype A receptor undergoes agonist- and antagonist-mediated internalization in the absence of signaling. Endocrinology. 1998;139:3185–3192. doi: 10.1210/endo.139.7.6105. [DOI] [PubMed] [Google Scholar]
- 48.Roettger BF, Ghanekar D, Rao R, Toledo C, Yingling J, Pinon D, Miller LJ. Antagonist-stimulated internalization of the G protein-coupled cholecystokinin receptor. Mol Pharmacol. 1997;51:357–362. [PubMed] [Google Scholar]
- 49.Bhattacharyya S, Puri S, Miledi R, Panicker MM. Internalization and recycling of 5-HT2A receptors activated by serotonin and protein kinase C-mediated mechanisms. Proc Natl Acad Sci USA. 2002;99:14470–14475. doi: 10.1073/pnas.212517999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhattacharyya S, Raote I, Bhattacharya A, Miledi R, Panicker MM. Activation, internalization, and recycling of the serotonin 2A receptor by dopamine. Proc Natl Acad Sci USA. 2006;103:15248–15253. doi: 10.1073/pnas.0606578103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raote I, Bhattacharya A, Panicker MM. Serotonin 2A (5-HT2A) Receptor Function: Ligand-Dependent Mechanisms and Pathways. In: Chattopadhyay A, editor. Serotonin Receptors in Neurobiology. 2011/01/05. Boca Raton: 2007. pp. 105–132. [PubMed] [Google Scholar]
- 52.Kurz JB, Perkins JP. Isoproterenol-initiated β-adrenergic receptor diacytosis in cultured cells. Mol Pharmacol. 1992;41:375–381. [PubMed] [Google Scholar]
- 53.von Zastrow M, Kobilka BK. Ligand-regulated internalization and recycling of human beta 2-adrenergic receptors between the plasma membrane and endosomes containing transferrin receptors. J Biol Chem. 1992;267:3530–3538. [PubMed] [Google Scholar]
- 54.Moore RH, Sadovnikoff N, Hoffenberg S, Liu S, Woodford P, Angelides K, Trial JA, Carsrud ND, Dickey BF, Knoll BJ. Ligand-stimulated β2-adrenergic receptor internalization via the constitutive endocytic pathway into rab5-containing endosomes. J Cell Sci. 1995;108 (Pt 9):2983–2991. doi: 10.1242/jcs.108.9.2983. [DOI] [PubMed] [Google Scholar]
- 55.Tsao PI, von Zastrow M. Type-specific sorting of G protein-coupled receptors after endocytosis. J Biol Chem. 2000;275:11130–11140. doi: 10.1074/jbc.275.15.11130. [DOI] [PubMed] [Google Scholar]
- 56.Klenk C, Schulz S, Calebiro D, Lohse MJ. Agonist-regulated cleavage of the extracellular domain of parathyroid hormone receptor type 1. J Biol Chem. 2010;285:8665–86674. doi: 10.1074/jbc.M109.058685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abou-Samra AB, Goldsmith PK, Xie LY, Jüppner H, Spiegel AM, Segre GV. Down-regulation of parathyroid (PTH)/PTH-related peptide receptor immunoreactivity and PTH binding in opossum kidney cells by PTH and dexamethasone. Endocrinology. 1994;135:2588–2594. doi: 10.1210/endo.135.6.7988447. [DOI] [PubMed] [Google Scholar]
- 58.Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- 59.Li JG, Haines DS, Liu-Chen LY. Agonist-promoted Lys63-linked polyubiquitination of the human kappa-opioid receptor is involved in receptor down-regulation. Mol Pharmacol. 2008;73:1319–1330. doi: 10.1124/mol.107.042846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murray EJ, Bentley GV, Grisanti MS, Murray SS. The ubiquitin-proteasome system and cellular proliferation and regulation in osteoblastic cells. Exp Cell Res. 1998;242:460–469. doi: 10.1006/excr.1998.4090. [DOI] [PubMed] [Google Scholar]
- 61.Zhao L, Huang J, Guo R, Wang Y, Chen D, Xing L. Smurf1 inhibits mesenchymal stem cell proliferation and differentiation into osteoblasts through JunB degradation. J Bone Miner Res. 2010;25:1246–1256. doi: 10.1002/jbmr.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamashita M, Ying SX, Zhang GM, Li C, Cheng SY, Deng CX, Zhang YE. Ubiquitin ligase Smurf1 controls osteoblast activity and bone homeostasis by targeting MEKK2 for degradation. Cell. 2005;121:101–113. doi: 10.1016/j.cell.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Komander D, Clague MJ, Urbe S. Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol. 2009;10:550–563. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 64.Milojevic T, Reiterer V, Stefan E, Korkhov VM, Dorostkar MM, Ducza E, Ogris E, Boehm S, Freissmuth M, Nanoff C. The ubiquitin-specific protease Usp4 regulates the cell surface level of the A2A receptor. Mol Pharmacol. 2006;69:1083–1094. doi: 10.1124/mol.105.015818. [DOI] [PubMed] [Google Scholar]
- 65.Berlin I, Schwartz H, Nash PD. Regulation of epidermal growth factor receptor ubiquitination and trafficking by the USP8. STAM complex. J Biol Chem. 2010;285:34909–34921. doi: 10.1074/jbc.M109.016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berthouze M, Venkataramanan V, Li Y, Shenoy SK. The deubiquitinases USP33 and USP20 coordinate beta2 adrenergic receptor recycling and resensitization. EMBO J. 2009;28:1684–1696. doi: 10.1038/emboj.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, Vilardaga JP. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 2009;5:734–742. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garrido JL, Wheeler D, Vega LL, Friedman PA, Romero G. Role of phospholipase D in parathyroid hormone receptor type 1 signaling and trafficking. Mol Endocrinol. 2009;23:2048–2059. doi: 10.1210/me.2008-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feinstein TN, Wehbi VL, Ardura JA, Wheeler DS, Ferrandon S, Gardella TJ, Vilardaga JP. Retromer terminates the generation of cAMP by internalized PTH receptors. Nat Chem Biol. 2011;7:278–284. doi: 10.1038/nchembio.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Llach F. Secondary hyperparathyroidism in renal failure: The trade-off hypothesis revisited. Am J Kidney Dis. 1995;25:663–679. doi: 10.1016/0272-6386(95)90541-3. [DOI] [PubMed] [Google Scholar]