Abstract
Objectives
Cardiovascular disease (CVD) prevention for patients with type 2 diabetes is accomplished through hypertension and dyslipidemia management. Although studies have established strategies for lowering low-density lipoprotein cholesterol (LDL-C) and blood pressure (BP), none have examined whether glycemia influences ability to achieve lipid and BP targets. This post-hoc analysis from the Stop Atherosclerosis in Native Diabetics Study (SANDS) examines the role of baseline glycemia in achieving standard and aggressive targets and outcomes after 36 months.
Methods
Diabetic individuals >age 40 with no cardiovascular events (N=499) were randomized to aggressive versus standard targets for LDL-C, non-high-density lipoprotein cholesterol (non-HDL-C), and systolic BP (SBP). Management algorithms were used for both groups. Carotid ultrasound and echocardiography were performed at baseline and after 36 months.
Results
No differences were observed in baseline hemoglobin A1c between treatment groups nor any significant change in A1c after 36 months in either group. Baseline A1c, however, was significantly and negatively related to achieving LDL-C (p=0.007), non-HDL-C (p=0.03), and SBP targets (p=0.007) and to changes in LDL-C (p=0.007), non-HDL-C (p=0.03), and SBP (p=0.001) in both groups. Baseline A1c failed to predict progression of carotid intima medial thickness (CIMT) (p=0.42) or left ventricular mass index (LVMI) (p=0.10), nor was it related to the effects of lipid and BP lowering on CIMT and LVMI over 36 months.
Conclusions
In diabetic adults with no CVD events, A1c was negatively associated with ability to achieve LDL-C, non-HDL-C, and SBP goals but was not independently related to treatment-associated changes in CIMT or LVMI over 36 months.
Keywords: LDL-C, A1c, cardiovascular disease, carotid arteries, diabetes
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of hospitalization and death for individuals with type 2 diabetes. Numerous trials have established that hypertension and dyslipidemia management is the cornerstone of CVD prevention in diabetes. The benefit of glycemia control in CVD in diabetic individuals is unclear. Epidemiologic studies have found a relation between glycemia concentration and CVD, but three large randomized clinical trials that tested the role of intensive glucose control in CVD prevention in diabetic individuals failed to show a mortality benefit or reduction in CVD incidence (Action to Control Cardiovascular Risk in Diabetes Study Group et al., 2008; Patel et al., 2007; Reaven et al., 2009; Duckworth et al., 2009; Skyler et al., 2009). This lack of benefit or possible detriment of intensive glucose management on CVD has created controversy regarding treatment recommendations. Furthermore, it is not known whether the degree of glycemia influences the ability to control CVD risk factors or the effects of risk factor reduction on subclinical atherosclerosis. For example, prior studies have documented an association between worse glycemia control and elevated triglycerides (Davidson et al., 2009; Smellie, 2006) and non-high density lipoprotein cholesterol (non-HDL-C). In addition, hyperglycemia and elevated hemoglobin A1c, the common index of long-term glycemia control, are correlated with increased arterial intima-medial thickness (Ho et al., 2009; Larsen et al., 2005) and arterial stiffness (Chen et al., 2009; Nestel, 2006), which could reduce the efficacy of antihypertensive drugs. Finally, hyperglycemia is associated with vascular smooth muscle dysfunction in animals (Popov & Constantinescu, 2008) and humans (Bjarnegard et al., 2009), providing another potential mechanism for resistance to antihypertensive therapy.
The Stop Atherosclerosis in Native Diabetics Study (SANDS) compared the effects of reducing systolic blood pressure (SBP), low-density lipoprotein cholesterol (LDL-C), and non-high-density lipoprotein cholesterol (non-HDL-C) to standard targets of 130 mmHg, 100 mg/dL, and 130 mg/dL versus aggressive targets of 115 mmHg, 70 mg/dL, and 100 mg/dL on preclinical atherosclerosis in diabetic adults from a population with high rates of diabetes and diabetes–associated CVD. In the group treated to lower targets, there was a reduction in carotid intima medial thickness (CIMT) and a greater reduction in left ventricular (LV) mass. Glycemia control, however, varied widely among the study participants. Therefore, we examined the relation between baseline glycemia and ability to achieve the BP, LDL-C, and non-HDL-C targets and whether glycemia control affected the ability of lipid and BP lowering to influence changes in carotid atherosclerosis and cardiac structure, as measured by CIMT and left ventricular mass index (LVMI). Such information could provide guidance in developing practice recommendations for patients with diabetes.
PATIENTS AND METHODS
Details of the SANDS design and methods have been published (Russell et al., 2006). All participants provided written informed consent. The study was approved by the institutional review boards of the National Institutes of Health and all participating institutions and American Indian communities.
Recruitment
Four hundred and ninety-nine men and women, ≥ age 40, who had type 2 diabetes and no history of a CVD event were enrolled between May 2003 and July 2004 at clinical centers in Oklahoma, Arizona, and South Dakota. The participants were randomized to one of two intervention groups: aggressive (AGG, n=252) or standard (STD, n=247), stratified by center and gender. All participants were American Indians as defined by Indian Health Service (IHS) criteria. Eligibility criteria included documented type 2 diabetes (1997 American Diabetes Association criteria), a successfully measured CIMT, LDL-C ≥ 100 mg/dL, and SBP > 130 mmHg within the previous 12 months. Major exclusion criteria included New York Heart Association class III or IV congestive heart failure; SBP > 180 mmHg; triglycerides ≥ 400 mg/dL; hepatic transaminase levels more than twice the upper limit of normal; and conditions that would cause hyperlipidemia, such as hyperthyroidism or nephrotic syndrome. Other traditional exclusion criteria included medical conditions that predicted survival of less than 3 years and concerns about potential adherence that might affect study completion. Those with asymptomatic CVD, such as carotid plaque and/or low ankle-brachial index, were not excluded.
Lipids and Blood Pressure
Study personnel performed BP and lipid management with equal frequency of contact for both groups. All other medical care, including diabetes management, was performed by the participants’ IHS providers, and participants were encouraged to attend regular visits for diabetes care.
The SBP was ≤115 mm Hg for the AGG and ≤130 mm Hg for the STD (Howard et al., 2008). The algorithm for hypertension management was based on the recommendations of the Sixth Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (1997). The step-1 drug was a renin-angiotensin system (RAS) blocker--either an angiotensin-converting enzyme (ACE) inhibitor, usually lisinopril, titrated to a dosage of 40mg/d, or an angiotensin receptor blocker, usually losartan, titrated to 100 mg/d. The latter choice was used as an alternative to the ACE inhibitor if there was an intolerance to ACE inhibitors. The step-2 drug was hydrochlorothiazide, in a dosage of 12.5 to 25 mg/d. It also could be used as a fixed-dose combination with losartan. A thiazide diuretic was chosen because of its efficacy in lowering BP with RAS inhibitors and in reducing CV morbidity and mortality in diabetic and nondiabetic patients. The step-3 drug was a beta-blocker or a calcium channel blocker. Atenolol could be titrated to 100 mg/d or amlodipine to 10 mg/d. The step-4 drug was the alternative to the step-3 medications. The step-5 drug was an alpha-blocker, doxazosin, which could be titrated to 8 mg daily. Step-6 drugs were direct-acting vasodilators, hydralazine up to 100 mg twice a day or minoxidil up to 10 mg twice a day. A loop diuretic could be substituted for hydrochlorothiazide as needed to achieve more volume reduction. Reserpine up to 0.3 mg also could be added if BP was not controlled with the previous medications. Those in the AGG averaged 2.3 medications and those in the STD 1.5 (Weir et al., 2009).
Goals for LDL-C were ≤70 mg/dL for the AGG and ≤100 mg/dL for the STD (Howard et al., 2008). The algorithm for achieving lipid goals was based on the recommendations of the National Cholesterol Education Program Adult Treatment Panel III (National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, 2002; Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, 2001). If lifestyle modification was unsuccessful, statin monotherapy was initiated. If the LDL-C goal was not reached at the maximal tolerable statin dosage, adjunctive therapy with ezetimibe or colesevelam was added. The non-HDL-C goal (≤100 mg/dL for the AGG and ≤ 130 mg/dL for the STD) (Howard et al., 2008) was then addressed using fenofibrate, omega-3 fatty acids, and/or niacin. Details of the management procedures have been published (Russell et al., 2006; Silverman et al., 2009).
Baseline and follow-up visits
All procedures followed standardized methods and were performed by trained, certified personnel (Howard et al., 2008). Based on the intention-to-treat principle, participants were followed from the date of entry until death, loss to follow up, or trial end. All participants were scheduled for initial follow-up visits at 1 month, and thereafter every 3 months until 36 months. At all follow-up visits, seated BP measures were obtained, and a lipid profile was measured using a Cholestech apparatus (Cholestech Corp., Hayward, CA) standardized against laboratory assay. Medications were adjusted to meet treatment goals, side effects were assessed, and information on health outcomes was obtained. Fasting glucose and hemoglobin A1c were measured by a central laboratory on fasting blood samples taken at baseline, 18, and 36 months.
Outcome Measures
At the baseline, 18-, and 36-month visits, carotid and cardiac ultrasound studies were performed using standardized protocols (Silverman et al., 2009). These were performed by centrally trained ultrasonographers and interpreted by a single skilled physician reader blinded to patient assignment. For carotid ultrasound, B-mode imaging from multiple angles was performed to determine presence and location of plaque as well as arterial wall dimensions. End-diastolic B-mode images of the distal right and left common carotid artery were acquired in real-time, and a 1-cm segment of each far wall was measured using an automated system employing an edge detection algorithm with manual override capacity. One hundred separate dimensional measurements were obtained from the 1-cm segment and averaged to obtain mean CIMT and lumen diameter. Echocardiographic measures included assessment of left ventricular structure and function, location and severity of segmental wall motion abnormalities, and atrial function. The echocardiographic variable of primary interest in the current analysis was LV mass, calculated as described previously (Devereux, 2003).
Data Analysis
This post-hoc analysis examined the association between A1c and CIMT and LVMI at baseline and evaluated the potential effects of baseline A1c on achieving SBP, LDL-C, and non-HDL-C goals, as well as improvements in CIMT and LVMI for the STD and AGG at the study’s end. A1c measures were obtained in 491 of 499 SANDS participants at baseline and 426 at the study’s end. Change in A1c measures was available for 419 participants. No meaningful differences were observed between those with and without data for A1c change. Baseline variables were compared between glycemia control groups (A1c ≤ median or > median) using two-sided t-tests. The effect of baseline A1c on achieving LDL-C, non-HDL-C, and SBP treatment targets, and changes in these measures was examined using multiple linear regression models, adjusted for baseline SBP, LDL-C, non-HDL-C, diastolic blood pressure (DBP), age, gender, BMI, smoking status, and treatment group. Changes in LDL-C, non-HDL-C, and SBP were computed as the difference between the baseline measures and the average values over the last 12 months of the study. In addition, the proportion of participants reaching the LDL-C, non-HDL-C, and SBP targets was obtained by tertile of baseline A1c and compared between the AGG and STD (aggressive targets: SBP<117.5 mmHg, LDL-C <73.5 mg/dL, non-HDL-C<103.5 mg/dL; standard targets: SBP<136.5 mmHg, LDL-C <106.5 mg/dL, non-HDL-C<130 mg/dL). These values were defined as being within measurement error of the target goals.
Logistic regression analysis was used to predict the probability of reaching the SBP and lipid targets as a function of baseline A1c, adjusting for relevant covariates. In addition, the effect of A1c on carotid and cardiac measures at baseline, CIMT and LVMI, respectively, was analyzed using multiple linear regressions with robust standard errors adjusted for age, gender, BMI, LDL-C, non-HDL-C, HDL-C, SBP, DBP, smoking status, diabetes duration (in years), estimated glomerular filtration rate (eGFR), and albuminuria. All analyses were performed using Intercooled Stata 9.2 (StataCorp LP, College Station, TX), and the two-sided significance level required was 0.05.
RESULTS
Baseline characteristics are presented in Table I in groups stratified by glycemia control (above and below A1c of 7.5, the median value for all participants). In the overall sample, mean age was 56 years, two-thirds were women, mean BMI was 33, mean diabetes duration was 9.7 years, and mean A1c was 8.0. The randomization groups were similar at baseline in age, gender composition, renal function, smoking status, and aspirin use; average SBP was slightly higher in the STD group. At baseline 34% were taking aspirin, 73% were taking anti-hypertension medications: 64% taking ACE inhibitors, 10% diuretics, 9% beta blockers, and 18% Ca channel blockers. In addition, 36% were taking lipid lowering agents, 32% statins, and 5% fibrates. For diabetes treatment, 61% were taking oral agents, 6% insulin alone, and 17% a combination of insulin and oral agents. Those with higher A1c were younger, had higher eGFR and urine microalbumin, used significantly more insulin and oral hypoglycemic agents, and had a duration of diabetes that was longer by 3.5 years. As reported previously, A1c did not significantly change on average during the 36-month trial (change=−0.19 or 2.5%, p=0.09) but there was an increase of 0.17 (2.5%) in the A1c≤7.5 group and a decrease of 0.44 (−5%) in the A1c> 7.5 group (p<0.001 by baseline A1c strata).
Table I.
Baseline Characteristics of SANDS Participants by A1c stratum
| Baseline Characteristics | ||
|---|---|---|
| HbA1c≤7.5 N=254 |
HbA1c≥7.5 N=237 |
|
| Mean (SD) | Mean (SD) | |
| Age (years) | 58 (9) | 54 (9)* |
| Female, N (%) | 164 (65) | 158 (67) |
| BMI, kg/m2 | 34(7) | 33(6) |
| Waist, cm | 111 (15) | 109(14) |
| % Smoker, N (%) | 53 (21) | 45 (19) |
| % Aspirin use (≥ 80 mg), N (%) |
186 (74) | 154 (65)* |
| Systolic BP, mmHg | 129 (16) | 132 (16) |
| Diastolic BP, mmHg | 74 (11) | 76 (10)* |
| CRP nmol/L *** | 24 (21, 28) | 29 (25, 34) |
| LDL cholesterol, mmol/L | 2.6 (0.7) | 2.8 (0.8)* |
| Non-HDL cholesterol, mmol/L | 3.5 (0.8) | 3.7 (0.9)* |
| HDL cholesterol, mmol/L | 1.2 (0.3) | 1.2 (0.3) |
| Triglycerides, mmol/L *** | 1.8 (1.7, 1.9) | 1.9 (1.8, 2.0)* |
| Hemoglobin A1c (%) | 6.6 (0.6) | 9.5 (1.5)** |
| eGFR (ml/min/1.73 m2) | 85 (21) | 95 (25)* |
| Urine albumin/creatinine ratio (mg/mmol) | 8.5 (44) | 18.7 (59) |
| Glucose, mmol/L | 6.6 (1.7) | 11 (4.3)* |
| Duration of diabetes | 7.3 (7.3) | 10.8 (7.1) |
| Diabetes Therapy | ||
| Lifestyle, N (%) | 47 (19) | 14 (6)* |
| Oral hypoglycemic agents, N (%) | 182 (72) | 196 (83)* |
| Insulin, N (%) | 35 (14) | 86 (37)* |
| Insulin plus oral, N (%) | 192 (76) | 226 (95)* |
| Endpoints | ||
| CIMT mean (mm) | 0.795 (0.17) | .815 (0.19) |
| LVMI(g/m2.7) | 41 (9) | 41 (9) |
Significant difference between HbA1c groups at baseline p<0.05
The goal was to show the means for each group, this difference must be significant by construction.
Geometric mean (95% confidence intervals) is presented. T-tests for baseline are conducted using log-transformed variables.
Abbreviations: BMI = body mass index; CRP = C-reactive protein; CIMT = carotid intima medial thickness; eGFR = estimated glomerular filtration rate; LVMI = left ventricular mass index.
During the final months of the trial, average SBP was lower in the AGG than in the STD in both A1c strata. The average decrease from baseline in SBP at the end of the trial was 11 mmHg in the AGG vs. 3 mmHg in the STD (p<0.001). LDL-C, non-HDL-C, and triglycerides substantially decreased in the AGG but changed minimally in the STD; the change was significantly different between groups (p<0.001 for LDL-C and non-HDL-C, p=0.06 for triglycerides ).
Baseline A1c was significantly related to changes in SBP, LDL-C, and non-HDL-C, in analyses adjusted for baseline SBP, LDL-C, non-HDL-C, DBP, BMI, age, smoking status, gender, and treatment group (Table II); one percentage point higher A1c at baseline was associated with the average decrease in SBP, LDL-C, non-HDL-C, and SBP being blunted by 0.91 mmHg, 1.64 mg/ dL (0.04 mmol/L), and 1.58 mg/ dL (0.04 mmol/L), respectively. Baseline A1c was significantly and directly related to baseline CIMT; in multiple linear regression models adjusted for confounders at baseline, CIMT increased by 0.013mm for a one percentage point increase in A1c. No evidence for a significant association between baseline A1c and LVMI was found in these models (p=0.37).
Table II.
Relationships between Baseline Variables and Changes in SBP, LDL-C, and Non-HDL-C at 36 months
| Change in SBP | Change in LDL-C | Change in Non-HDL-C | ||||
|---|---|---|---|---|---|---|
|
Independent Variables at baseline |
Coefficients (SE) |
p-value | Coefficients (SE) |
p-value | Coefficients (SE) |
p-value |
| SBP | −.72 (.039) | <.001 | −.01 (.076) | .86 | −.03 (.10) | .77 |
| LDL-C | .01 (.26) | .43 | −.94 (.037) | <.001 | ||
| non-HDL-C | −.86 (.05) | <.001 | ||||
| DBP | −.12 (.062) | .05 | .05 (.139) | .75 | .21 (.17) | .23 |
| BMI | −.13 (.064) | .05 | .07 (.165) | .68 | −.08 (.21) | .71 |
| Age | .11 (.07) | .11 | −.15 (.144) | .30 | −.26 (.18) | .14 |
| Smoker (yes=1) | .57 (1.15) | .62 | 1.28 (2.77) | .64 | 4.22 (3.5) | .23 |
| Gender (1=female) | −.47 (.87) | .59 | 2.58 (2.17) | .24 | 5.5 (2.6) | .04 |
| Treatment group (1=Aggressive) |
−11.67 (.88) | <.001 | −32.06 (2.03) | <.001 | −36.7 (2.5) | <.001 |
| Baseline A1c | .91 (.26) | <.001 | 1.64 (.60) | .007 | 1.58 (.73) | .03 |
| N | 459 | 453 | 453 | |||
| Model F statistic | 78.7 | <.001 | 106.2 | <.001 | 63.5 | <.001 |
| R2 | .65 | .68 | .58 | |||
Abbreviations: BMI= body mass index; DBP = diastolic blood pressure; LDL-C = low-density lipoprotein cholesterol; non-HDL-C = non-high-density lipoprotein cholesterol; SBP = systolic blood pressure.
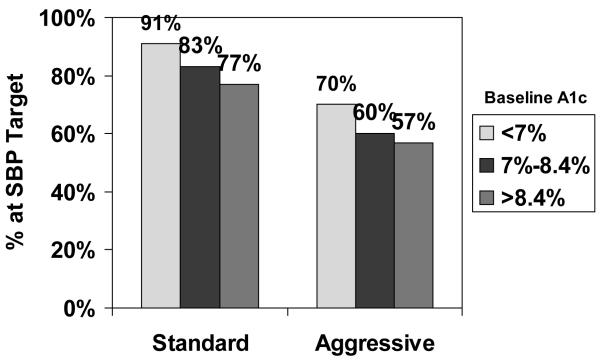
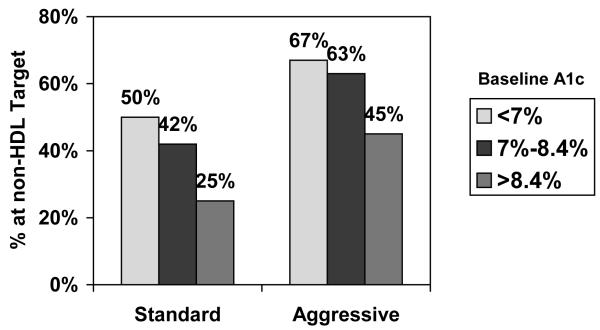
When goal achievement for SBP, LDL-C, and non-HDL-C was a dichotomous variable (yes/no) and baseline A1c was categorized into tertiles according to its distribution in the combined sample, the likelihood of reaching the three targets significantly declined in both randomization groups with increasing tertile of baseline A1c (Figures 1a, b, and c). No significant relations were observed between change in A1c and change in LDL-C, non-HDL-C, BP, or outcomes (data not shown).
Figure 1a. Percentage of SANDS participants who reached target SBP, by tertile of baseline A1c and treatment group.
Note: chi-square test for trend (standard group: p =.02; aggressive group: p = .09).
Figure 1b. Percentage of SANDS participants who reached target LDL-C, by tertile of baseline A1c and treatment group.
Note: chi-square test for trend (standard group: p = .002; aggressive group: p = .007).
Figure 1c. Percentage of SANDS participants who reached target non-HDL-C, by tertile of baseline A1c and treatment group.
Note: chi-square test for trend (standard group: p = .002; aggressive group: p = .004).
When SBP, LDL-C, and non-HDL-C goal achievement as binary variables were adjusted for baseline LDL-C, non-HDL-C, SBP, DBP, age, gender, BMI, smoking status, and treatment group, baseline A1c remained significantly and inversely associated with achieving targets for SBP, LDL-C, and non-HDL-C (p<0.001). These logistic regression models show that the probability of achieving the LDL-C target decreased from 0.72 to 0.55 in the AGG if A1c was 10% vs. 6% at baseline. Similarly, the probability of achieving the SBP target decreased from 0.73 to 0.51 for a similar difference in baseline A1c in the AGG.
Models controlled for baseline SBP, DBP, LDL-C, non-HDL-C, HDL-C, age, gender, BMI, smoking status, diabetes duration, and eGFR showed that baseline A1c was not a significant predictor of change in CIMT and LVMI (Table III), nor was there a significant interaction between A1c and either LDL-C or SBP in predicting change in CIMT or LVMI.
Table III.
Relation of Baseline A1C to Change in Outcome Measures
| Change in CIMT (n=434) | Change in LVMI (n=386) | |||
|---|---|---|---|---|
|
Variables at Baseline |
Coefficient | p-value | Odds Ratio | p-value |
| CIMT | −.23 | .004 | ||
| LVMI | −.37 | <.0001 | ||
| A1c | −.003 | .42 | .25 | .10 |
| Model R2 | .11 | .01 | .22 | <.0001 |
Abbreviations: CIMT = carotid intima medial thickness; LDL-C = low-density lipoprotein cholesterol; LVMI = left ventricular mass index.
Note: Models are adjusted for baseline BMI, age, gender, smoking status, change in LDL-C, and SBP.
DISCUSSION
The SANDS trial is unique in that rather than comparing two pharmacologic regimens, it compared two cohorts treated to aggressive vs. standard targets for SBP and lipid control, including non-HDL-C, in diabetic individuals. The degree of baseline glycemia was negatively correlated with the ability to achieve SBP, LDL-C, and non-HDL-C targets, and the likelihood of reaching these targets declined significantly in both groups with increasing tertile of baseline A1c. Although carotid atherosclerosis, indexed by CIMT, was significantly and directly related to baseline A1c, A1c did not influence the effects of lipid and SBP lowering on carotid atherosclerosis.
These findings are noteworthy for several reasons. First, they suggest that the improvements in subclinical measures of atherosclerosis and cardiac function observed in SANDS did not differ as a result of differences in glycemia control. They also suggest that the treatment strategies used to achieve the BP and lipid targets did not affect glycemia control, eliminating potential negative consequences of treatment regimens. Thiazide diuretics and beta blockers, which were steps 2 and 3 of the algorithm for BP control in SANDS (Weir et al., 2009) have known potential adverse effects on glycemia management, whereas ACE inhibitors may have beneficial effects. Had intensive lipid and BP treatment worsened glycemia control, that finding would have complicated clinical decision making because of the known benefits of glycemia control on microvascular complications.
Although a direct relationship was observed between baseline glycemia and CIMT, as seen in other studies (McNeely, 2009; Selvin et al., 2005), the degree of baseline glycemia did not significantly influence change in CIMT or LVMI over 36 months. This observation reinforces the need to focus on lipid and BP control in diabetes regardless of glycemia status, with the implication that improvements in CVD risk are possible despite level of glycemia.
Baseline A1c averaged approximately 8% and did not change significantly throughout the trial. Substantial variation occurred among individuals, but the change in A1c was not related to the change in endpoints. Both treatment groups were seen at similar intervals, but study personnel provided only lipid and BP management. Study communication to the participants and outside clinical personnel included the recommendation of an A1c of ≤7% for most patients in accordance with American Diabetes Association (ADA) recommendations (ADA, 2009) and the IHS. While only a small number of participants achieved the goal of ≤7%, the degree of glycemia control in the SANDS participants resembles that seen in other diabetic populations.
The reasons for a relationship between glycemia control and achievement of lipid and BP targets are not clear. The relationship was not influenced by adjustment for medication use and thus was unlikely due to specific hypoglycemic medications. Higher circulating glucose levels and their sequelae may impede the action of lipid and BP medications. The resultant glycation and sequential changes in basement membrane composition are known to lead to increased CIMT (Ho et al., 2009; Larsen et al., 2005) and vascular stiffness (Chen et al., 2009; Nestel, 2006), as well as smooth muscle dysfunction (Bjarnegard et al., 2009; Popov & Constantinescu, 2008). These effects may result in resistance to BP lowering agents. It is less likely that the glycation cascade would influence the action of the HMG CoA reductase inhibitors on LDL-C. However, significant increases in serum triglycerides occur with increasing glycemia (Davidson et al., 2009; Smellie, 2006), thereby increasing levels of non-HDL-C. In addition, changes in hepatic function occur with increasing glycemia, including accumulation of hepatic triglycerides; these changes may upregulate lipoprotein assembly and secretion, thus leading to higher LDL-C and non-HDL-C.
Alternatively, and more likely, the association of higher A1c with higher lipids and BP may reflect participant attributes that also affect achievement of lipid and BP targets. Although we did not have any objective measures of adherence, the similarity of the effects of baseline A1c on BP, LDL-C, and non-HDL-C suggest that glycemia control may predict adherence to BP- and lipid-lowering medications. Studies have established that level of adherence is related to achievement of the treatment goals, and that achieving high patient adherence to medications is a challenge for care providers. Although study staff expended great effort to promote adherence to the lipid and BP medications, and a large proportion of SANDS participants achieved and maintained BP and lipid goals, there remained less adherent participants who likely did not follow their prescribed regimen for glycemia control. This potential relation to adherence is important to providers, because in many cases those managing LDL-C and BP differ from those responsible for glycemia control. Studies that explore barriers to adherence are needed to improve attainment of lipid and BP targets as well as glycemia control.
This study has a number of strengths, including standardized treatment algorithms, systematic follow up, availability of data on risk factor management, as well as surrogate measures of disease. However, it was performed in a single population. This population has high rates of obesity and diabetes, and diabetes is associated with greatly increased rates of coronary heart disease and stroke (Howard et al., 1999; Zhang et al., 2008). Thus, it serves as a model for other populations in whom rates of obesity and diabetes are rapidly increasing. However, similar studies need to be performed in other groups. This was a post-hoc analysis; the study was not designed to directly examine effects of glycemia on lipids and BP. In addition, the modest number of participants, 3-year follow up, and small number of clinical CVD events precluded evaluation of potential interactions between glycemia and lipid and BP control relating to CVD events (Howard et al., 2008). Future studies are required to define the relations among the potential metabolic, pharmacologic, and patient-related factors and their effects on achieving lipid and BP targets and better glucose control, as well as to explore barriers to adherence.
In summary, while the intensity of the lipid and BP control was not correlated with glycemia control, baseline A1c was correlated with lesser reductions in SBP, LDL-C, and non-HDL-C and lower chances of achieving the targets for these measures. Although carotid atherosclerosis was significantly related to baseline A1c, degree of glycemia did not influence the effects of lipid and BP lowering on carotid atherosclerosis. This analysis shows that aggressive BP, LDL-C, and non-HDL-C targets can be pursued without worsening glucose control. Our findings help support the current ADA, American College of Cardiology, and American Heart Association recommendations that put pharmacological BP and lipid control as the principal non-lifestyle related CVD risk reduction strategies in patients with diabetes. Further investigation into the relation between glycemia and BP and lipid lowering may help explain the interaction between diabetes and CVD.
ACKNOWLEDGMENTS
We thank Rachel Schaperow, MedStar Health Research Institute, for editorial services.
This study was supported by grant U01-HL067031 from the National Heart, Lung and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association Standards of medical care in diabetes--2009. Diabetes Care. 2009;32(Suppl 1):S13–61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnegard N, Arnqvist HJ, Lindstrom T, Jonasson L, Jonsson A, Lanne T. Long-term hyperglycaemia impairs vascular smooth muscle function in women with type 1 diabetes mellitus. Diab Vasc Dis Res. 2009;6:25–31. doi: 10.3132/dvdr.2009.005. [DOI] [PubMed] [Google Scholar]
- Chen Y, Huang Y, Li M, Bi Y, Zhang Y, Gu W, Ning G. Association of arterial stiffness with HbA1c in 1000 type 2 diabetic patients with or without hypertension. Endocrine. 2009;36:262–7. doi: 10.1007/s12020-009-9221-z. [DOI] [PubMed] [Google Scholar]
- Davidson MB, Hu T, Hoar B, Stevenson C, Hoogwerf BJ. The relationship of glycemic control and triglycerides in patients with diabetes mellitus: a précis Database Study. Diabetes Obes Metab. 2009;11:118–22. doi: 10.1111/j.1463-1326.2008.00912.x. [DOI] [PubMed] [Google Scholar]
- Devereux RB, Roman MJ, Liu JE, Lee ET, Wang W, Fabsitz RR, Welty TK, Howard BV. An appraisal of echocardiography as an epidemiological tool: The Strong Heart Study. Annals of Epidemiology. 2003;13:238–244. doi: 10.1016/s1047-2797(02)00264-8. [DOI] [PubMed] [Google Scholar]
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD, VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the Third Report of the National Cholesterol Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Ho HC, Chen MF, Hwang JJ, Lee YT, Su TC. Intima-media thickness of lowerlimb arteries associated with fasting and post-challenge plasma glucose levels. J Atheroscler Thromb. 2009;7:748–755. doi: 10.5551/jat.836. [DOI] [PubMed] [Google Scholar]
- Howard BV, Roman MJ, Devereux RB, Fleg JL, Galloway JM, Henderson JA, Howard WJ, Lee ET, Mete M, Poolaw B, Ratner RE, Russell M, Silverman A, Stylianou M, Umans JG, Wang W, Weir MR, Weissman NJ, Wilson C, Yeh F, Zhu Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes: the SANDS randomized trial. JAMA. 2008;299:1678–1689. doi: 10.1001/jama.299.14.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard BV, Lee ET, Cowan LD, Devereux RB, Galloway JM, Go OT, Howard WJ, Rhoades ER, Robbins DC, Sievers ML, Welty TK. Rising tide of cardiovascular disease in American Indians. The Strong Heart Study. Circulation. 1999;99:2389–2395. doi: 10.1161/01.cir.99.18.2389. [DOI] [PubMed] [Google Scholar]
- Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure The sixth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC VI) 1997. NIH Publication No. 98-4080.
- Larsen JR, Brekke M, Bergengen L, Sandvik L, Arnesen H, Hanssen KF, Dahl-Jorgensen K. Mean HbA1c over 18 years predicts carotid intima media thickness in women with type 1 diabetes. Diabetologia. 2005;48:776–779. doi: 10.1007/s00125-005-1700-z. [DOI] [PubMed] [Google Scholar]
- McNeely MJ, McClelland RL, Bild DE, Jacobs DR, Jr, Tracy RP, Cushman M, Goff DC, Jr, Astor BC, Shea S, Siscovick DS. The association between hemoglobin A1c and subclinical cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. Diabetes Care. 2009;32:1727–1733. doi: 10.2337/dc09-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- Nestel P. Relationship between arterial stiffness and glucose metabolism in women with metabolic syndrome. Clin Exp Pharmacol Physiol. 2006;33:883–886. doi: 10.1111/j.1440-1681.2006.04459.x. [DOI] [PubMed] [Google Scholar]
- Patel A, ADVANCE Collaborative Group. MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M, Glasziou P, Grobbee DE, Hamet P, Heller S, Liu LS, Mancia G, Mogensen CE, Pan CY, Rodgers A, Williams B. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- Popov D, Constantinescu E. Arterial smooth muscle cells dysfunction in hyperglycaemia and hyperglycaemia associated with hyperlipidemia: from causes to effects. Arch Physiol Biochem. 2008;114:150–160. doi: 10.1080/13813450802033990. [DOI] [PubMed] [Google Scholar]
- Reaven PD, Moritz TE, Schwenke DC, Anderson RJ, Criqui M, Detrano R, Emanuele N, Kayshap M, Marks J, Mudaliar S, Rao R Harsha, Shah JH, Goldman S, Reda DJ, McCarren M, Abraira C, Duckworth W, Veterans Affairs Diabetes Trial Intensive glucose lowering therapy reduces cardiovascular disease events in VADT participants with lower calcified coronary atherosclerosis. Diabetes. 2009 Aug 3;58:2642–2648. doi: 10.2337/db09-0618. [Epub ahead of print] PMID: 19651816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M, Fleg JL, Galloway J, Henderson JA, Howard J, Lee ET, Poolaw B, Ratner RE, Roman MJ, Silverman A, Stylianou M, Weir MR, Wilson C, Yeh F, Zhu J, Howard BV. Examination of lower targets for low-density lipoprotein cholesterol and blood pressure in diabetes--the Stop Atherosclerosis in Native Diabetics Study (SANDS) Am Heart J. 2006;152:867–875. doi: 10.1016/j.ahj.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Selvin E, Coresh J, Golden SH, Boland LL, Brancati FL, Steffes MW. Atherosclerosis risk in communities study. Glycemic control, atherosclerosis, and risk factors for cardiovascular disease in individuals with diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2005;28:1965–1973. doi: 10.2337/diacare.28.8.1965. [DOI] [PubMed] [Google Scholar]
- Silverman A, Huang C-CJ, Russell M, Mete M, Roman MJ, Stylianou M, Lee ET, Yeh F, Fleg J, Wilson C, Henderson JA, Weir MR, Ratner RE, Howard BV. Stop Atherosclerosis in Native Diabetics Study (SANDS): Baseline Characteristics of the Randomized Cohort. Journal of Health Disparities Research and Practice. 2009;3:29–42. [Google Scholar]
- Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, Howard BV, Kirkman MS, Kosiborod M, Reaven P, Sherwin RS, American Diabetes Association. American College of Cardiology Foundation. American Heart Association Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. J Am Coll Cardiol. 2009;53:298–304. doi: 10.1016/j.jacc.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Smellie WS. Hypertriglyceridemia in diabetes. BMJ. 2006;333:1257–1260. doi: 10.1136/bmj.39043.398738.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir MR, Yeh F, Silverman A, Devereux RB, Galloway JM, Henderson JA, Howard WJ, Russell M, Wilson C, Ratner R, Sorkin J, Umans JG, Fleg JL, Stylianou M, Lee E, Howard BV. Safety and feasibility of achieving lower systolic blood pressure goals in persons with type 2 diabetes: The SANDS trial. Journal of Clinical Hypertension. 2009;11:1–9. doi: 10.1111/j.1751-7176.2009.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Galloway JM, Welty TK, Wiebers DO, Whisnant JP, Devereux RB, Kizer JR, Howard BV, Cowan LD, Yeh J, Howard WJ, Wang W, Best L, Lee ET. Incidence and risk factors for stroke in American Indians: the Strong Heart Study. Circulation. 2008;118:1577–1584. doi: 10.1161/CIRCULATIONAHA.108.772285. [DOI] [PMC free article] [PubMed] [Google Scholar]