Abstract
Background
The burden of CMV-associated sensorineural hearing loss (SNHL) in populations with CMV seroprevalence approaching 100% is unknown. The purpose of this study was to assess the rate, associated factors and predictors of SNHL in CMV-infected infants identified by newborn screening in a highly seropositive maternal population.
Methods
Newborns with positive saliva CMV-DNA and confirmed by virus isolation in the first two weeks of life were enrolled in a prospective follow-up study to monitor hearing outcome.
Results
Of 12,195 infants screened, 121 (1%) were CMV-infected and 12 (10%) had symptomatic infection at birth. Hearing function could be assessed in 102/121 children who underwent at least one ABR testing at a median age of 12 months. SNHL was observed in 10/102 (9.8%; 95%CI: 5.1–16.7) children. Median age at the latest hearing evaluation was 47 months (12 to 84 months). Profound loss (>90dB) was found in 4/5 children with bilateral SNHL while all 5 children with unilateral loss had moderate to severe deficit. The presence of symptomatic infection at birth (OR 38.1; 95%CI: 1.6– 916.7) was independently associated with SNHL after adjusting for IUGR, gestational age, gravidity and maternal age. Among 10 infants with SNHL, six (60%) were born to mothers with non-primary CMV infection.
Conclusions
Even in populations with near universal immunity to CMV, congenital CMV infection is a significant cause of SNHL demonstrating the importance of CMV as a major cause of SNHL in children worldwide. As in other populations, SNHL is more frequently observed in symptomatic CMV infection.
Keywords: Cytomegalovirus, congenital infection, hearing loss, Brazilian children
INTRODUCTION
Congenital cytomegalovirus (CMV) infection has been reported to be an important cause of hearing loss in infants born in North America and northern Europe. Population based studies in Sweden1, Canada2 and USA3, 4 have reported that between 9.3% and 17% of infants with congenital CMV infection will have sensorineural hearing loss (SNHL). The rates of SNHL reported by these studies ranged between 22%–41% in children with clinically apparent or symptomatic infection and between 6–16% in those with subclinical or asymptomatic infection.
Recent studies have reported that hearing loss occurs at a similar frequency in children born to mothers who had primary CMV infection during pregnancy and offspring of women with non-primary infection with documented preconceptional seroimmunity5,6. These studies showed that 7–10% of infected infants from mothers with non-primary infection had SNHL whereas 11–15% of infants born to mothers with primary infection had SNHL. However, the prevalence and natural history of CMV-associated SNHL in maternal populations with near universal CMV seroimmunity have not been well defined. This feature of the natural history of congenital CMV infection is particularly relevant in regions of the world with transitional economies such as South America, Africa and southern Asia where near universal seroimmunity to CMV in maternal population has been reported. In a recent study, we have shown that that the birth prevalence of congenital CMV infection (1%) and the proportion of congenitally infected infants with symptomatic infection in our population with maternal seroprevalence rate of 96.7% to besimilar to that found in the populations with lower CMV seroprevalence rates7. Thus, more precise definition of the role of congenital CMV infection as a cause of hearing loss in offspring of women from highly seroimmune population is of considerable importance because understanding the rates of SNHL is relevant to the issue of vaccine prevention of maternal CMV infection. The objective of the current study was to assess the rate, associated factors and predictors of CMV-induced SNHL in a highly seropositive maternal population.
PATIENTS AND METHODS
Study population
One hundred twenty one infants with congenital CMV infection were identified from a prospective screening of 12,295 newborns in 2 public hospitals of Ribeirão Preto, State of São Paulo, Brazil, from March 2003 to May 2009. The first maternity hospital (MATER) provides care for low risk parturients. The second hospital, Clinical Hospital of Faculty of Medicine of Ribeirão Preto, University of São Paulo, serves as a referral center for high risk parturients but also provides care for low risk parturients. Infants with congenital CMV infection were identified by the detection of CMV DNA in saliva or urine specimens collected within the first 2 weeks of life and confirmed by virus isolation in tissue culture8. The study was approved by the Research Ethics Committee of the University Hospital (Processes 4782/2002, and 9145/2004), and written informed consent was obtained from all mothers.
Newborn evaluation and definition of congenital CMV disease
All infants identified as congenitally infected underwent a clinical evaluation, ophthalmological examination and computed tomography (CT) scan of brain. Congenitally infected neonates were classified as small for gestational age (≥5th percentile) or appropriate for gestational age (≥5th percentile) according to a standard reference curve9. Microcephaly was defined as previously described7. Infants were classified as symptomatic if they presented with at least one of the following findings suggestive of congenital infection including petechiae, cholestatic jaundice (conjugated bilirrubin level > 2mg/dl), hepatosplenomegaly, purpura, microcephaly, seizures, chorioretinitis or abnormal cranial CT7,10. Infants who were small for gestational age were not classified as having symptomatic infection if they did not exhibit any of the typical CMV-related findings10.
Audiologic Evaluation
The audiologic protocols consisted of an auditory brainstem evoked response (ABR) testing for all congenitally CMV infected infants within the first year of age and children younger than 3 years of age. During follow-up visits, pure tone conditioned play audiometry measurement was performed in children older than 3 years of age. The ABR register was performed using a standard protocol after infant's sedation with chloral hydrate when necessary11. All infants underwent otoscopic examination to detect middle-ear disorders before testing. The ABR threshold was defined as the lowest level at which the wave V could be detected and replicated. SNHL was suspected when the first ABR test showed air conduction thresholds above 30 dB in an infant with normal middle ear function. Confirmation of SNHL was made after at least two ABR evaluations performed on different occasions. A child was considered to have normal hearing when the first ABR threshold was ≤30 db and confirmed by subsequent ABR and/or pure tone audiometry measurements.
In March 2006, a newborn hearing screening program consisting of transient otoacoustic emission (OAE) testing (Acesscreen, Madsen, Denmark) of all infants born at the study hospital was instituted. The results of the OAE screening test was reported as pass or fail.
Definition of maternal CMV infection
Maternal CMV infection was considered primary when a CMV-specific immunoglobulin G (IgG) seroconversion occurred during pregnancy or when the first prenatal serum specimen contained CMV IgG antibodies of low avidity with a subsequent increase in the avidity index in the sample obtained at delivery. Women with CMV IgG antibodies before pregnancy and those with high avidity CMV IgG antibodies without CMV IgM within the first 25 weeks of gestation were classified as having non-primary CMV infection12.
Statistical analysis
Statistical analysis was performed by logistic regression models using the LOGISTIC procedure of SAS 9.0 statistical package, SAS Institute. Associations between newborn findings, maternal characteristics and hearing loss were analyzed initially by crude odds ratios (ORs) with 95% confidence intervals for each OR. To avoid confounding effects, multiple logistic regression models were performed simultaneously for each variable and adjusted ORs were calculated to determine the predictors independently associated with hearing loss.
RESULTS
Among 121 infants with congenital CMV infection, 12 (10%) had clinical findings at birth that were consistent with symptomatic congenital infection; 5 had multisystem disease (one died within the first week of life) and 4 showed at least one clinical finding. The remaining 3 infants had only cranial computerized tomography findings including abnormalities of neuronal migration, leading to polymicrogyria (1 infant), white matter gliosis and supratentorial ventriculomegaly (1 infant) and myelination delay in association with lissencephaly (1 infant). Five of 12 symptomatic infants received 6 weeks of intravenous ganciclovir therapy (6 mg/kg/per day in two doses) and four of them had multisystem disease. None of the infected infants had abnormal findings on ophthalmologic examination. Intrauterine growth restriction was observed in 35/121 (28.9%) infants. Among 12 symptomatic infants, 8 (66.7%) were small for gestational age.
Hearing outcome in children with congenital CMV infection
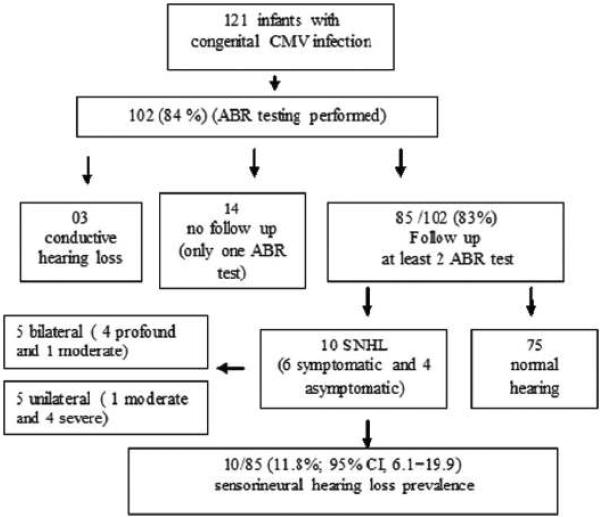
The overview of the study population and the results of hearing evaluations are shown in Figure 1. One hundred two of the 121 infants (84%) with congenital CMV infection underwent at least one ABR assessment and the median age of initial ABR testing was 12 months [range: 15 days to 51 months; 18/102 (17.6%) <6 months, and 32/102(30%) <12 months of age]. Three infants had conductive hearing loss at the time of this analysis, and fourteen infants (1 symptomatic) were lost to follow up after undergoing only one ABR test. All of these 14 infants had normal hearing. Of the 102 children who underwent at least one ABR testing, 10 (9.8%; 95%CI: 5.1–16.7) were demonstrated to have SNHL. Their median age at the latest hearing evaluation was 47 months (range: 12 to 84 months). Using more stringent criteria that included at least 2 ABR assessments and follow-up for at least 12 months, SNHL was confirmed in 10/85 (11.8%; 95% CI, 6.1–19.9) children; 6/11 with symptomatic (54.5%; CI95%: 25.9– 81.3%), and 4/75 (5.3%; CI95%: 1.7–12.4) with asymptomatic congenital CMV infection. Five children had bilateral hearing loss and among these, four (3 with multisystem disease and one asymptomatic in the neonatal period who had no abnormalities in the CT scan) had profound loss (>90 dB) and the remaining child with multisystem disease during neonatal period, had moderate SNHL (60 dB). All 5 children with unilateral involvement (2 symptomatic and 3 asymptomatic) had moderate to severe SNHL (60 to 90 dB).
Figure 1.
Overview of the study population
Sixty five of the 85 (76%) children whose hearing status could be ascertained completed follow-up for at least 36 months of age and underwent multiple hearing evaluations (median of 4 tests, range 2 to 6). Their median age at the latest hearing evaluation was 56 months (range: 36 to 84 months). None of the 65 children had progression in the hearing deficit.
The demographic characteristics and newborn findings were compared among study children with at least two ABR evaluations according to their hearing status (Table 1). No association was observed between hearing loss and intrauterine growth restriction, gestational age, gender, maternal age or gravidity. Although univariate analyses showed that children with abnormal findings at birth and those born to mothers younger than 20 years of age were more likely to develop hearing loss, only symptomatic congenital CMV infection remained independently associated with hearing loss. Additional risk factors for SNHL were observed in one infant who was born prematurely (32 weeks) and received intravenous aminoglycosides for more than 5 weeks after birth.
Table 1.
Characteristics of 85 infants with congenital CMV infection according to sensorineural hearing status.
| Hearing loss(n=10) | Normal hearing(n=75) | Crude OR(95% CI) | Adjusted OR(95% CI) | |
|---|---|---|---|---|
| Type of congenital CMV infection | ||||
| Asymptomatic (n=75) | 4 (5.3 %) | 71 (94.7%) | 1.0 | 1.0 |
| Symptomatic (n=10) | 6 (60.0%) | 4 (40.0%) | 26.6 (5.3– 134.1) | 38.1 (1.6– 916.7) |
| Intrauterine growth restriction | ||||
| Adequate for gestational age (n=63) | 4 (6.4%) | 59 (93.6%) | 1.0 | 1.0 |
| Small for gestational age (n=22) | 6 (27.3%) | 16 (72.7%) | 5.5 (1.4–22.0) | 7.3 (0.7–72.9) |
| Gestational age (weeks) | ||||
| Term ≥ 37 (n=60) | 8 (13.3%) | 52 (86.7%) | 1.0 | 1.0 |
| Preterm < 37 (n=25) | 2 (8.0%) | 23 (92.0%) | 1.7 (0.3– 9.0) | 7.2 (0.5– 106.2) |
| Gender | ||||
| Male (n=49) | 3 (6.1%) | 46 (93.9%) | 1.0 | 1.0 |
| Female (n=36) | 7 (19.4%) | 29 (80.1%) | 3.7 (0.9– 15.5) | 12.4 (0.9– 163.9) |
| Maternal age (years) | ||||
| ≥ 20 (n=55) | 3 (5.5%) | 52 (94.5%) | 1.0 | 1.0 |
| < 20 (n=30) | 7 (23.3%) | 23 (76.7%) | 5.3 (1.3– 22.2) | 1.1 (0.1– 22.8) |
| Gravidity | ||||
| Multiparous (n=37) | 2 (4.3%) | 45 (95.7%) | 1.0 | 1.0 |
| Primiparous (n=44) | 8 (21.1%) | 30 (78.9%) | 6.0 (1.2– 30.2) | 9.5 (0.5– 182.7) |
Newborn Hearing Screening Findings
Seven of 10 infants with confirmed hearing loss had been tested by OAE within the first month of life and six of these infants failed OAE. In all six children, ABR confirmed loss in the same ear in which OAE testing resulted in failure, suggesting that hearing loss was present at birth. In one child who had normal hearing at 2 months of age, ABR testing at 10 months of age revealed unilateral profound SNHL (109dB). Among 75 infants with normal hearing, 30 had been screened by OAE in the neonatal period. Two of them had failed OAE in one ear, however, subsequent testing by OAE and at least two ABR assessments revealed normal hearing. Delayed onset SNHL could not be excluded in 3 children in whom hearing loss was detected at the time of their first ABR evaluations at older ages (21, 28, and 40 months) and did not undergo OAE testing during the neonatal period.
Maternal immune status and CMV-associated hearing loss
The data on association between the type of maternal CMV infection and hearing loss are shown in Table 2. Forty three (50%) of the mothers of 85 children adequately evaluated by at least two ABR tests had serum samples collected during their first prenatal visit and at delivery. Seven infants of these 43 mothers had hearing loss and six of them were born to mothers with non-primary maternal CMV infection as determined by the presence of CMV-IgG antibodies before pregnancy in one mother and by the presence of CMV-IgG antibodies of high avidity in serum samples obtained between 6 to 25 weeks of gestation in 5. The mother of the remaining infant with SNHL had primary infection as indicated by low avidity CMV IgG antibodies in the serum sample obtained at 9 weeks of gestation.
Table 2.
CMV- related hearing loss according to type of maternal infection
| Hearing status | Maternal CMV infection (n=85) | ||
|---|---|---|---|
| Primary n= 3 | Non primary n= 40 | Indeterminate or samples not available n= 42 | |
| Moderate to severe unilateral HL | 0 | 4 | 1 |
| Moderate to profound bilateral HL | 1 | 2 | 2 |
| Normal | 2 | 34 | 39 |
DISCUSSION
This prospective follow-up study of children with congenital CMV infection identified by newborn screening of Brazilian infants demonstrated that congenital CMV infection is an important cause of SNHL even in this population with near universal maternal CMV seroimmunity. The frequency of hearing loss detected in our study (9.8%) is similar to that reported for populations with lower CMV seroprevalence in which the majority of congenital CMV infections were presumed to be a consequence of primary maternal CMV infections2, 6 and for populations with higher CMV seroprevalence in the developed world3, 13, 14. Thus, the findings of our study confirm that although the prevalence of congenital CMV infection may vary with underlying CMV seroprevalence rates and demographic factors, it constitutes an important cause of SNHL worldwide.
Although the type of maternal CMV infection could only be determined in 7/10 infants with CMV-related hearing loss, six of these children were born to mothers with non-primary maternal infection and only one was born to a mother with primary infection. The findings of our study confirms previous evidence from populations in the U.S. and Northern Europe that the frequency of hearing loss is similar in congenitally infected infants irrespective of the maternal CMV serologic status prior to pregnancy5, 6. Further, our findings demonstrate the occurrence of bilateral and severe to profound SNHL in congenitally infected children born to women with non-primary maternal CMV infection.
Although the exact prevalence of SNHL at birth in the study population has not been delineated, available data suggests that the prevalence of hearing loss at birth in Brazilian infants (0.96 per 1000 living newborns)15 is similar to that observed in the U.S. (1.6 per 1000)16 and Europe (0.78 per 1000)17. Considering the 1% birth prevalence rate of congenital CMV infection in our population, the hearing impairment due to congenital CMV infection would affect at least 6 per 10,000 live births or 1800 of the 3 million Brazilian infants born annually. Even though most (4/5, 80%) of the infants with bilateral severe hearing loss had been identified due to the presence of multisystem CMV-related signs at birth, approximately half of the children (4/10) with SNHL had no detectable clinical abnormalities at birth and therefore, would not have been identified during the neonatal period if they were not screened for CMV.
A limitation of our study is that the hearing evaluation of CMV-infected children were not performed at similar ages and at least two ABR assessments were only performed in 85/121 study children with congenital CMV infection. Of the seven study children with confirmed CMV-associated SNHL who also underwent OAE screening during the neonatal period, only one child with normal hearing during initial testing developed SNHL at a follow-up evaluation at 12 months. However, since newborn hearing screening was not in place during the first 3 years of the study, it is not possible to determine the exact time of onset of hearing impairment or document late-onset and/or progressive SNHL in some of the CMV-infected children with SNHL. In addition, 76% of the study children were followed for at least 36 months. Therefore, we believe that our data provides reliable estimates of CMV-associated SNHL in a highly seropositive population and suggests that current strategies to prevent morbidity associated with congenital CMV infection including the development of prophylactic vaccines to prevent primary maternal infections during pregnancy may have limited efficacy in these populations.
Similar to the findings from studies conducted in populations with different CMV seroprevalence rates in the U.S. and Europe, the results of our study indicated that symptomatic infants were significantly more likely to develop SNHL than those with asymptomatic infection1–3. Thus, the presence of CMV-related symptoms at birth is a strong predictor of hearing loss, even in populations with high maternal CMV seroprevalence rate. Our findings demonstrate that congenital CMV infection is an important cause of hearing loss, including bilateral and severe to profound deficit, even in countries with transitional economies in which maternal seroimmunity is nearly universal.
ACKNOWLEDGEMENTS
FUNDING This work was supported by grants from the National Institutes of Health (NIAID AI 49537; Fogarty International Center, R03 TW006480 to WJB), (NIDCD DC04162 to SBB) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil, Process number 02/04166-6.
We are grateful to Patricia Frizzo de Carvalho e Oliveira and Lauro Juliano Marin for technical assistance with laboratory assays.
Footnotes
Potential Conflicts Of Interest: No author has any potential conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ahlfors K, Ivarsson SA, Harris S, et al. Congenital cytomegalovirus infection and disease in Sweden and the relative importance of primary and secondary maternal infections. Preliminary findings from a prospective study. Scand J Infect Dis. 1984;16(2):129–137. doi: 10.3109/00365548409087131. [DOI] [PubMed] [Google Scholar]
- 2.Saigal S, Lunyk O, Larke RP, Chernesky MA. The outcome in children with congenital cytomegalovirus infection. A longitudinal follow-up study. Am J Dis Child. 1982;136(10):896–901. doi: 10.1001/archpedi.1982.03970460026006. [DOI] [PubMed] [Google Scholar]
- 3.Dahle AJ, Fowler KB, Wright JD, Boppana SB, Britt WJ, Pass RF. Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J Am Acad Audiol. 2000;11(5):283–290. [PubMed] [Google Scholar]
- 4.Fowler KB, Boppana SB. Congenital cytomegalovirus (CMV) infection and hearing deficit. J Clin Virol. 2006;35(2):226–231. doi: 10.1016/j.jcv.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Ross SA, Fowler KB, Ashrith G, et al. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J Pediatr. 2006;148(3):332–336. doi: 10.1016/j.jpeds.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Foulon I, Naessens A, Foulon W, Casteels A, Gordts F. A 10-year prospective study of sensorineural hearing loss in children with congenital cytomegalovirus infection. J Pediatr. 2008;153(1):84–88. doi: 10.1016/j.jpeds.2007.12.049. [DOI] [PubMed] [Google Scholar]
- 7.Mussi-Pinhata MM, Yamamoto AY, Moura Brito RM, et al. Birth prevalence and natural history of congenital cytomegalovirus infection in a highly seroimmune population. Clin Infect Dis. 2009;49(4):522–528. doi: 10.1086/600882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto AY, Mussi-Pinhata MM, Marin LJ, Brito RM, Oliveira PF, Coelho TB. Is saliva as reliable as urine for detection of cytomegalovirus DNA for neonatal screening of congenital CMV infection? J Clin Virol. 2006;36(3):228–230. doi: 10.1016/j.jcv.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 10.Boppana SB, Pass RF, Britt WJ, Stagno S, Alford CA. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11(2):93–99. doi: 10.1097/00006454-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Stapells DR, Kurtzberg D. Evoked potential assessment of auditory system integrity in infants. Clin Perinatol. 1991;18(3):497–518. [PubMed] [Google Scholar]
- 12.Lazzarotto T, Spezzacatena P, Pradelli P, Abate DA, Varani S, Landini MP. Avidity of immunoglobulin G directed against human cytomegalovirus during primary and secondary infections in immunocompetent and immunocompromised subjects. Clin Diagn Lab Immunol. 1997;4(4):469–473. doi: 10.1128/cdli.4.4.469-473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fowler KB, McCollister FP, Dahle AJ, Boppana S, Britt WJ, Pass RF. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr. 1997;130(4):624–630. doi: 10.1016/s0022-3476(97)70248-8. [DOI] [PubMed] [Google Scholar]
- 14.Williamson WD, Demmler GJ, Percy AK, Catlin FI. Progressive hearing loss in infants with asymptomatic congenital cytomegalovirus infection. Pediatrics. 992;90(6):862–866. [PubMed] [Google Scholar]
- 15.Bevilacqua MC, Alvarenga KF, Costa OA, Moret ALM. The universal newborn hearing screening in Brazil: from identification to intervention. Int J Pediatr Otorhinolaryngol. 2010;74:510–15. doi: 10.1016/j.ijporl.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Korver AM, Konings S, Dekker FW, et al. Decibel Collaborative study Group Newborn hearing screening vs later hearing screening and developmental outcomes in children with permanent chidhood hearing impairment. JAMA. 2010;304(15):1701–8. doi: 10.1001/jama.2010.1501. [DOI] [PubMed] [Google Scholar]
- 17.Mehl AL, Thomson V. The Colorado newborn hearing screening project, 1992–1999: on the threshold of effective population-based universal newborn hearing screening. Pediatrics. 2002;109(1):e7. doi: 10.1542/peds.109.1.e7. [DOI] [PubMed] [Google Scholar]