Abstract
Studies comparing young and older adults suggest a deficit in processing context information as a key mechanism underlying cognitive aging. However, the genetic architecture of context processing has not been examined. Consistent with previous results, we found evidence of functionally dissociable components of context processing accuracy in 1127 late middle-aged twins ages 51–60. One component emphasizes use of context cues to prepare responses (proactive cognitive control); the other emphasizes adjustment of responses after probes are presented (reactive control). Approximately one-quarter of the variance in each component was accounted for by genes. Multivariate twin analysis indicated that genetic factors underlying two important components of context processing were independent of one another, thus implicating more than one underlying mechanism. Slower reaction time (RT) on non-context processing trials was positively correlated with errors on the strongly proactive control component on which young adults outperform older adults, but RT was negatively correlated with errors on the strongly reactive control component on which older adults perform better. Although this RT measure was uncorrelated with chronological age in our age-homogeneous sample, slower RT was associated with performance patterns that were more like older adults. However, this did not generalize to other processing speed measures. Genetic correlations, which reflect shared genetic variance, paralleled the phenotypic correlations. There was also a positive genetic correlation between general cognitive ability and accuracy on the proactive control component, but there were still mostly distinct genetic influences underlying these measures. In contrast, the reactive control component was unrelated to general cognitive ability.
Keywords: twins, heritability, context processing, cognitive aging, processing speed
Some of the major accounts of key processes underlying cognitive change in middle and older adulthood focus on declines in overall processing speed (Salthouse, 1996), working memory capacity (Hultsch, Hertzog, Dixon, & Small, 1998; Salthouse, 1991; Wingfield, Stine, Lahar, & Aberdeen, 1988), episodic memory (Kausler, 1994), or efficiency of inhibitory control (Hasher & Zacks, 1988). A deficit in the ability to process context information (Braver & Barch, 2002; Braver et al., 2001) is another mechanism that has been proposed to account for age-related cognitive changes, and that may contribute to deficits in these other domains. Context representations consist of internally-represented, task-relevant information that are used to influence planning and behavior (Braver, Cohen, & Barch, 2002). Context representations serve both mnemonic and control functions in working memory (Braver, Satpute, Rush, Racine, & Barch, 2005). These representations become increasingly important as the required degree of cognitive control increases (e.g., when one must select between conflicting or strongly competing responses).
Phenotypic studies have shown functionally dissociable components of context processing that appear to be differentially affected by aging. There is also a strong theoretical model for this research paradigm which posits that the selective attention, working memory, and inhibitory processes of executive control functions can be accounted by a unitary underlying mechanism (Braver, Barch, & Cohen, 1999; Braver & Cohen, 2000; Cohen, Braver, & O’Reilly, 1996).
Examination of the components of context processing has been conducted almost exclusively at the phenotypic level. In the present study, we combined behavior genetic and cognitive neuroscience approaches in order to examine the genetic and environmental influences affecting the components of context processing and their relationship to general cognitive ability. It is important to keep in mind that underlying phenotypic and genetic factors are not necessarily the same (Friedman et al., 2008; Kremen et al., 2008). Elucidating the underlying genetic architecture of these processes would, thus, constitute an important step toward a fuller understanding of cognitive and brain aging. Elucidating these processes at the genetic level by means of the twin method could also serve as a guide for studies of specific genes that influence different components of context processing and cognitive aging.
Prefrontal Function, Dopamine, and Cognitive Aging
Both theoretical models and empirical findings strongly support the notion that reduced efficiency in context processing, changes in dopamine modulation, and changes in prefrontal cortex may account for many changes associated with cognitive aging (Bäckman, Lindenberger, Li, & Nyberg, 2010; Braver & Barch, 2002; Braver et al., 1999; Braver et al., 2001; Li, Lindenberger, & Backman, 2010; Li, Lindenberger, & Sikstrom, 2001; Suhara et al., 1991; Volkow et al., 2000; Volkow et al., 1998; West, 1996). Age-related structural differences tend to be greater in prefrontal cortex than in other parenchymal regions (Fjell et al., 2009; Jernigan et al., 2001; Raz & Rodrigue, 2006), and functional neuroimaging studies provide substantial evidence for age-associated differences in prefrontal function (Braver, Paxton, Locke, & Barch, 2009; Cabeza, 2002; Grady, Springer, Hongwanishkul, McIntosh, & Winocur, 2006) . Normal aging includes declines in dopamine function that affect prefrontal function, and the role of prefrontal dopamine modulation in cognitive control is a key feature of computational models of context processing (Bäckman et al., 2010; Braver & Barch, 2002; Braver et al., 1999; Braver et al., 2001; Li et al., 2010; Li et al., 2001; Suhara et al., 1991; Volkow et al., 2000; Volkow et al., 1998). Both computational modeling and empirical studies support the notion that dopamine receptor density and dopamine transmission (particularly for D1 and D2 receptors) are associated with the distinctiveness of neural (internal) representations as well as response speed (Bäckman et al., 2010; Braver & Barch, 2002; Braver et al., 1999; Li et al., 2010; Li et al., 2001). In turn, decreases in dopamine receptor density and/or efficiency of dopamine transmission in later life result in signal-to-noise reductions such that context representations are less robust and more susceptible to decay over time and to interfering effects of task-irrelevant inputs.
Reduced dopamine availability is also associated with reduced consistency of within-individual performance, a pattern that is consistent with idea that dopamine reductions result in less robust context representations which are indicators of greater neural noise (Bäckman et al., 2010; S. W. MacDonald, Cervenka, Farde, Nyberg, & Backman, 2009; Servan-Schreiber, Printz, & Cohen, 1990). These age-related changes in dopamine systems—particularly prefrontal dopamine—can result in declines in processing speed, working memory, updating, selective attention, and interference susceptibility. It is also well known that genetic factors play a substantial role in all of these processes (Bäckman et al., 2010; Bouchard & McGue, 2003; Kremen & Lyons, in press; Kremen, Prom-Wormley et al., 2010; Schmitt et al., 2007). Thus, genes that influence dopaminergic function may influence context processing and age-related changes in context processing as well.
Assessing Context Processing with the AX-CPT
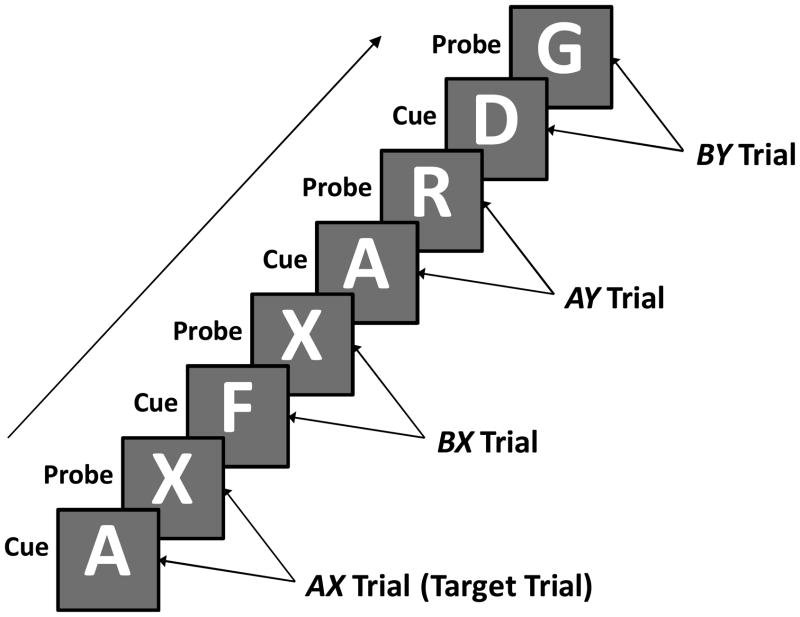
Many studies aimed at parsing the specific cognitive components of context processing have used a Continuous Performance Test (CPT), originally developed by Rosvold and colleagues (1956) and modified to examine components of context processing by Servan-Schreiber and colleagues (1996). In this modified version is referred to as the AX-CPT, letters are presented one at a time on a computer monitor in sequences of cue-probe pairs (see Figure 1). The goal is to make a target response to the X probe only when it immediately follows an A cue, and to make a non-target response to all other cues or probes. A high frequency of target (AX) trials introduces biases that interact differentially with context processing, thus allowing for a test of different context processing components. The tendency to make a target response to an X probe leads to a bias toward incorrect responses to X probes when they follow a non-A cue (referred to as BX trials, with B indicating any non-A cue). On trials in which the A cue is not followed by an X probe, attention to the cue’s predictive context will increase the bias toward a false alarm. Such trials are denoted as AY trials, with Y indicating any non-X probe.
Figure 1.
Sample sequence AX-CPT trials. Letters appear one at a time. B refers to any non-A cue. Y refers to any non-X probe. The target button is the correct response only for probes on the target (AX) trials. The non-target button is the correct response for probes on all other trials and for all cues.
Both BX and AY trials involve combinations of response preparation, working memory, and inhibitory control that manifest differential patterns in cross-sectional studies of normal and pathological aging (Braver & Barch, 2002; Braver et al., 2001). If context processing is intact, BX trials will involve little response conflict, require little inhibitory control, and will not elicit high error rates. Once a non-A cue is presented, the examinee knows that the probe cannot be a target. If context maintenance and response preparation are solidly intact, AY trials will require enhanced inhibitory control and will be more likely to elicit false alarms or slow responses because the A-cue primes the examinee for a target response (incorrect on AY trials). If, however, the cue is not stably maintained, response conflict and the need for inhibitory control may be heightened by the X probe on BX trials, but these tendencies will be reduced on AY trials.
Therefore, if aging is associated with less efficient context processing, older adults should perform poorly on BX trials relative to their performance on AY trials in comparison with younger adults. Previous results support this prediction for both accuracy and reaction time (Braver et al., 2001; Braver et al., 2009; Braver et al., 2005; Paxton, Barch, Racine, & Braver, 2008; Paxton, Barch, Storandt, & Braver, 2006; Rush, Barch, & Braver, 2006), making it one of the rare cases in which older adults perform relatively faster than younger adults on a cognitive measure. Older adults with early stage Alzheimer’s disease have additional deficits in non-context processing, compared with age-matched healthy adults (Braver et al., 2005). These non-context processing errors are referred to as BY errors because they occur on trials with non-A cues followed by non-X probes, where there are no contextual biases.
Context Processing and General Cognitive Ability
Little is known about the relationship between general cognitive ability (sometimes referred to as Spearman’s g) and context processing. There is abundant evidence for substantial genetic influences on general cognitive ability (Bouchard & McGue, 2003; Lyons et al., 2009; Plomin & Spinath, 2002). Executive functions and working memory—which are important in AX-CPT performance—are also associated with general cognitive ability at both the phenotypic and genetic levels (Friedman et al., 2006; Luciano et al., 2001). It has even suggested that working memory may be essentially the same as general intellectual ability (Kyllonen, 1996). Yet, it is also well known that patients with frontal lobe damage may exhibit substantial executive function deficits but perform within normal limits on tests of general intellectual ability (Lezak, Howieson, & Loring, 2004). Friedman et al. (2006) found that only the executive function of updating—but not set-shifting or inhibition—was strongly associated with general cognitive ability. Their updating tasks involved working memory and context maintenance, which bear some similarity with the demands of the AX-CPT.
In a study that included the AX-CPT, MacDonald et al. (2005) identified two independent phenotypic factors: a context processing factor (with high loadings for AX and BX trials) and a preparatory factor (with high loadings for AY and AX trials). General cognitive ability was positively correlated with the context processing, but not the preparatory, factor. Examining genetic, in addition to phenotypic, associations with general cognitive ability would provide useful information about the different cognitive components underlying context processing and about which components may be leading indicators of age-related cognitive change.
Genetically-Informative Studies
We are aware of only two genetically-informative studies of context processing. One found no relationship between the catechol-O-methytransferance genotype and AX-CPT performance in 464 adults ages 30 to 54 (A. W. MacDonald, III, Carter, Flory, Ferrell, & Manuck, 2007). In the other, eight middle-aged and older male Apolipoprotein E ε4 homozygotes made more errors than other groups on AY trials (Reinvang, Winjevoll, Rootwelt, & Espeseth, 2009). If aging is associated with better AY performance because older adults have less efficient maintenance of context (and thus, a reduced tendency toward AY false alarms), then one might expect fewer AY errors in ε4 homozygotes because this group may be expected to manifest poorer cognitive aging. Thus, replication of this genetic association is warranted.
It is well known from twin studies that cognitive functions and regional brain structure are heritable, i.e., a significant proportion of variance in individual differences is accounted for by genetic influences (Bouchard & McGue, 2003; Kremen, Prom-Wormley et al., 2010; Peper, Brouwer, Boomsma, Kahn, & Hulshoff Pol, 2007; Schmitt et al., 2007). On the other hand, evidence that a particular cognitive domain is heritable does not mean that performance on all tests tapping that domain will be heritable (Kremen & Lyons, 2010). For example, some executive function and working memory measures have strong evidence of heritability (Ando, Ono, & Wright, 2001; Kremen, Jacobsen et al., 2007) whereas others do not (Chou, Kuo, Lin, & Chen, 2009; Kremen, Eisen, Tsuang, & Lyons, 2007). The lack of heritability may be due, in part, to the multi-determined nature of some tasks (e.g., Wisconsin Card Sorting Test), making it difficult to distinguish between overall performance indices and specific deficits (Kremen & Lyons, 2010). The design of the AX-CPT may circumvent this problem because the pattern of different error types and response tendencies is elucidated for each individual.
Twin studies are also important for elucidating genetic and environmental influences on cognitive and brain aging (Kremen & Lyons, 2010), and we are unaware of any twin studies of context processing. Although it seems intuitive that accumulated environmental exposures lead to a relative increase in the impact of environmental influences with age, twin studies have shown that the impact of genetic factors on variability in general cognitive ability or brain ventricular volume is greatest in older adults (Haworth et al., 2009; Kremen, Panizzon et al., 2010; Lyons et al., 2009). On the other hand, twin studies have indicated that the amount of cognitive change over time is often due almost entirely to environmental factors (Lyons et al., 2009; Reynolds, Finkel, Gatz, & Pedersen, 2002).
The Present Study
We utilized the AX-CPT to examine the genetic and environmental influences on accuracy for different components of context processing in a large twin sample of late middle-aged men. We estimated the heritability of a signal detection index (d′ context), error rates for AX, BX, and AY trials, and reaction times (RTs) for BY trials. We also conducted bivariate and multivariate twin analyses in order to examine the genetic architecture of the error measures, and the genetic and environmental relationship of accuracy (errors) to processing speed and general cognitive ability.
When genetic influences are observed in twin analyses, they provide direct evidence of underlying mechanisms because the direction of effect must go from gene to phenotype. Although there is evidence that the different AX-CPT trial types reflect functionally dissociable processes at the phenotypic level, the formal context processing model is based on the notion of a single underlying mechanism subserving these different processes (Braver et al., 1999; Braver & Cohen, 2000; Cohen et al., 1996). The factor analytic results of MacDonald et al. (2005) contradict the notion of a single mechanism at the phenotypic level, but it is possible to have one phenotypic factor and more than one genetic factor (Kremen et al., 2008) or the reverse (Friedman et al., 2008). Moreover, unlike that phenotypic analysis, the genetic analyses are directly informative about underlying mechanisms. In the present study, we used multivariate twin analyses to whether there was a single or common genetic factor underlying performance on AX, BX, and AY trials.
There is now a body of evidence suggesting that context processing is an important mechanism accounting for a number of age-related cognitive changes. Clarifying the genetics of different context processing components constitutes an important step toward elucidating the determinants of age-related cognitive changes. It can also be important for improving phenotype definition in association studies aimed at finding the specific genes that are associated with these different component processes.
Method
Participants
The participants completed wave 1 of the longitudinal Vietnam Era Twin Study of Aging (VETSA). The goal of the VETSA was to establish a reasonably representative community-dwelling sample of middle-aged men at the baseline assessment. Thus, the only inclusion/exclusion criteria were that twins had to be between ages 51 and 59 at the time of recruitment, and both members of a pair had to agree to participate. The VETSA comprises 1237 male twins (614 pairs and 9 unpaired twins; 55% monozygotic [MZ] and 45% dizygotic [DZ] pairs) between the ages of 51 and 60 because four twins turned 60 by the time of their assessment (mean age=55.4, SD 2.5). The inclusion of unpaired twins—whose co-twin did not participate—allows for more precise estimates of the phenotypic correlations between the variables, despite their not being able to contribute to the genetic analyses. The mean level of formal education completed was 13.84 years (SD=2.11; range: 8–20). Most of the participants were married (79%), employed full-time 78%), and Caucasian (86%).
VETSA participants were randomly selected from a previous, large study of psychological health that included all available twins from the Vietnam Era Twin Registry (Tsuang, Bar, Harley, & Lyons, 2001). The Registry includes male-male MZ) and DZ twin pairs in which both twins served in the United States military at some time between 1965 and 1975. The majority of participants did not serve in combat or in Vietnam (Eisen, True, Goldberg, Henderson, & Robinette, 1987; Henderson et al., 1990). Demographic and health comparisons indicate that VETSA participants were largely representative of the Registry sample and of American men in their age range (Kremen et al., 2006; National Health and Nutrition Examination Survey (NHANES III), 1999–2004).
Zygosity was determined on the basis of 25 microsatellite markers. For a small number (97 [7.8%]) of participants whose DNA was not useable, zygosity was determined by a combination of DNA testing, questionnaire, and blood group methods (Eisen, Neuman, Goldberg, Rice, & True, 1989). For those with zygosity determined by genotype, the questionnaire-based method agreed with the DNA results in 95% of cases. Written informed consent was provided by all study participants.
Procedures and Measures
Participants live throughout the United States and were given the option of coming to the University of California, San Diego or Boston University for the same daylong series of assessments; 635 came to San Diego and 569 came to Boston, and 33 were tested in their hometowns. Tests and equipment were identical at each site. AX-CPT parameters were the same as in the baseline condition in the study of Braver et al. (2001). Letters were presented one at a time on a computer monitor. Participants who used their right hand to control the mouse were instructed to press the left mouse button on target trials and the right mouse button on non-target trials. These instructions were reversed for those who used their left hand to control the mouse. Target trials were defined as those in which an A cue was immediately followed by an X probe. The letters K and Y were not included because of their visual similarity to the letter X. Letters were presented in pseudorandom order with 70% of the trials being target (AX) trials. The 30% non-target trials comprised 10% BX trials consisting of an invalid (non-A) cue preceding the X target, 10% AY trials consisting of a valid (A) cue followed by a nontarget (non-X) probe, and 10% BY trials consisting of an invalid (non-A) cue followed by an invalid (non-X) probe.
Letters were presented centrally in red, 24-point upper case Helvetica font on a black background. Stimulus duration was 300 ms with a delay of 4,900 ms between presentation of the cue and probe, and an intertrial interval of 1,000 ms. Responses had to be within 1,300 ms of the stimulus to be counted. The test was presented via Presentation software, version 0.81 (Neurobehavioral Systems, Albany, CA) on Dell notebook computers with 15. 4 inch monitors. Log files generated by Presentation were transferred to an Access database.
The test consisted of six blocks of 30 trials each. Block 1 was considered a practice block, so that test scores were based on the 150 trials comprising blocks 2–5. Examiners used standardized written instructions to explain the task, including examples of the different trial types shown on paper and a sample letter shown on the computer monitor. After going through the standard instructions, examiners answered questions and reiterated portions of the instructions as needed to ensure that participants clearly understood the task. Brief breaks were provided between blocks. The AX-CPT was part of a larger neurocognitive test battery that has been reported on elsewhere (Franz et al., in press; Kremen et al., 2006).
A signal detection index (d′) has been computed in previous studies of the AX-CPT by using AX hits and BX false alarms rather than all false alarms. This measure is referred to as d′ context because on AX and BX trials, whether or not the probe is a target is determined by differences in context (Braver et al., 2001; Cohen, Barch, Carter, & Servan-Schreiber, 1999). The d′ context index was adapted from Corwin (1994): hit rate for AX trials – false alarm rate for BX trials. Correction factors were applied to avoid dividing by zero based on formulas provided by Corwin (1994): hit rate=(number of hits+.5)/number of target+.01); false alarm rate=(number of false alarms+.5)/(number of distracters+1).
We measured general cognitive ability with the Armed Forces Qualification Test (AFQT; Bayroff & Anderson, 1963), a 50-min paper-and-pencil test consisting of 100 multiple-choice items that was administered at age 20 on average and again during the VETSA at an average age of 55. As we have described elsewhere, the AFQT is highly correlated with measures of IQ or other indices of general intellectual ability and was highly stable (r=.74) over a period of 35 years (Lyons et al., 2009). AFQT scores are based on percentiles, but in statistical analyses we transformed the raw percentile scores to their normal deviates (Lyons et al., 2009). The mean AFQT percentile score for VETSA participants was 61.13 (interquartile range: 46–80.50) at age 20 and 64.07 (interquartile range: 50–81) during the VETSA assessment. These scores are comparable to a mean IQ score of approximately 104–105. The genetic correlation (defined in the Statistical Analysis section) between AFQT scores at age 20 and 55 was 1.0, indicating that the same genetic influences on AFQT performance were operating at both times.
Many age-related cognitive declines have been associated with age-related slowing of processing speed (Salthouse, 1996). As in previous AX-CPT studies, we included RT on BY trials—which are essentially unconfounded by demands for context processing—as a gauge of processing speed within this test. We also included three other external processing speed measures1. Simple reaction time (SRT) consisted of 10 left- and 10 right-hand trials in response to an asterisk appearing on the computer monitor. Choice reaction time (CRT) consisted of 21 trials in which the asterisk randomly appeared on either the left or right side of the screen and participants had to respond with the left or right hand, respectively. Trails 2 was the number sequencing condition of the Delis-Kaplan Executive Function System (Delis, Kaplan, & Kramer, 2001)Trail Making Test; it is similar to the traditional Trails A. Scores for the SRT and CRT were the mean RTs in milliseconds. The Trails 2 score was the time to completion in seconds.
Statistical Analysis
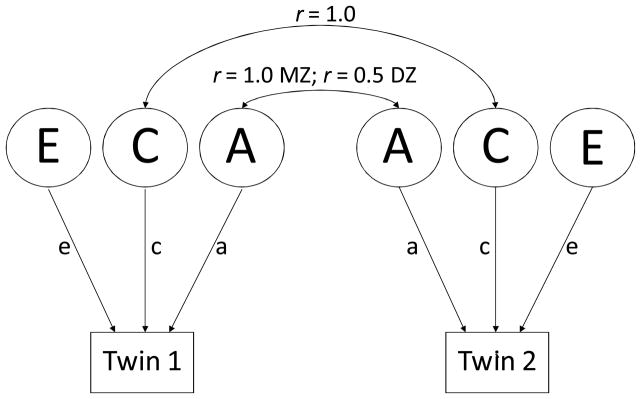
The twin method capitalizes on the fact that MZ twins shared 100% of their genes, whereas DZ twins share, on average, 50% of their genes. Both MZ and DZ twin pairs share some environmental experiences, but not others. These features can be used to construct models that are tested by means of the maximum-likelihood based structural equation modeling program Mx (Neale, Boker, Xie, & Maes, 2004). Given the proportions of shared genes, additive genetic factors are assumed to correlate 1.0 for MZ twins and 0.50 for DZ twins. In twin models, common environmental factors are assumed to correlate 1.0 for both types of twin pairs, and unique environmental factors are assumed to correlate 0.00 for both types of twins. These relationships are depicted in Figure 2 in what is referred to as the standard univariate ACE model in which variance of a phenotype is decomposed into additive genetic influences (A), common or shared environmental influences (C), and unique or nonshared environmental influences (E) (Eaves, Last, Young, & Martin, 1978; Neale & Cardon, 1992). When the values are standardized, squaring the coefficients denoted by the lowercase a, c, and e in Figure 2 provides the proportion of phenotypic variance accounted for by each component.
Figure 2.
Univariate twin model. A=latent additive genetic influences; C=latent common (shared) environmental influences; E=latent nonshared (unique) environmental influences. Lowercase a, c, and e refer to the parameter estimates (path coefficients); squaring these parameters yields the proportion of variance in the phenotype that is accounted for by the latent factors. Under the model, additive genetic influences correlate 1.0 for MZ twins and .50 for DZ twins; common environmental influences correlation 1.0 for all twins; and nonshared environmental influences (which include measurement error) are uncorrelated.
Note that the genetic and environmental variance components in these models are latent constructs; we do not know which or how many genes are involved, and we do not know what the specific environmental factors may be. Nevertheless, the proportions of variance accounted for can still be calculated. For example, an MZ twin correlation is derived from genes (100%) and environmental factors that are shared. Even without knowing what the environmental factors are, we can determine that if the MZ correlation for trait X is .70, 30% of the phenotypic variance (1 – .70) must be due to unique environmental experiences. In part because they are latent constructs, the definitions of these environmental variance components are sometimes misunderstood. They are statistical, rather than substantive, definitions (Carey, 2003). Common environment is defined as aspects of the environment that make twins similar. Unique environment is defined as aspects of the environment that make twins different; it also includes measurement error, which is assumed to be random, and therefore uncorrelated within twin pairs.
The univariate ACE model is easily extended to examine the genetic and environmental correlations between multiple variables. A correlation is simply the covariance between two variables divided by the square root of the product of the variance of each variable. Taking advantage of the ability of the twin design to decompose genetic and environmental variances, a genetic correlation is calculated by dividing the genetic covariance by the square root of the product of each variable’s genetic variance (Neale & Cardon, 1992). Essentially, it indicates the amount of genetic overlap between phenotypes. A unique environmental correlation is analogous to a genetic correlation except that it is based solely on the unique environmental covariance and the unique environmental variances for each variable.
In the present study, we tested multivariate twin models. The primary measures were error rates (misses and false alarms) for the different trial types. Error rates were not normally distributed and could not be normalized by data transformations. Due to fact that twin analyses assume that the variables of interest are normally distributed, we converted the error scores into ordinal measures with 6 levels (0–5) and calculated polychoric correlations to determine associations between the measures. BY RT was found to be normally distributed, but Mx does not currently allow for the simultaneous examination of ordinal and continuous data; therefore, we converted BY RT to a 10-level ordinal variable so that it could be analyzed alongside the error scores. SRT, CRT, Trails 2, and AFQT were also converted to ordinal variables.
In the first set of analyses, we examined the degree of genetic and environmental overlap between the AX, BX, and AY error scores. BY errors were not included because anyone with more than a very few BY errors is considered to have not understood or to have not been able to do the test. We initially fit a Cholesky decomposition model in order to estimate the heritability of each variable, as well as the genetic and environmental correlations between variables. The Cholesky decomposition is the simplest multivariate twin model in that it decomposes the phenotypic relationships into genetic and environmental components while imposing no formal structure on covariance. Relative to the Cholesky, we fit a common pathways model in order to determine whether common genetic and environmental factors underlie performance on the different trial types, and whether specific genetic and environmental for each trial type were present. The common pathway is a nested submodel of the Cholesky in which it is assumed that the covariation among the measures operates through a single latent phenotype2.
In the second set of analyses, we examined the genetic and environmental relationships between BY RT and BX and AY errors. In this analysis, we focused on the trial types that reflect the key processes of interest with respect to context processing while also taking processing speed into account. We only utilized a Cholesky model because our primary interest was in the correlations between BY RT and each of the two error types, and because we did not think it made sense to expect speed and accuracy phenotypes to be accounted for by a common factor. The third set of analyses tested bivariate models for AFQT and AX-CPT error scores.
For each set of analyses, model fits were compared against that of the full Cholesky using the likelihood-ratio chi-square test (LRC). The LRC is calculated by comparing the −2 log-likelihood (−2LL) of the full Cholesky to the −2LL of a nested submodel model, with degrees of freedom equal to the difference in the number of free parameters in most cases (Eaves et al., 1978; Neale & Cardon, 1992). A nonsignificant LRC indicates that there is not a significant reduction in fit for the reduced model, suggesting that the reduced model is more parsimonious because it has an adequate fit to the data with fewer parameters. We also used the Akaike Information Criterion to compare models (AIC; Akaike, 1987; Williams & Holahan, 1994). If two or more competing models have nonsignificant LRCs, the one with the lowest AIC is the most parsimonious because it achieves statistically equivalent goodness-of-fit with fewer parameters. Because a reduced model does not actually have a better fit to the data than its comparison model with more parameters, we refer to the model with the lowest AIC as the most parsimonious model instead of the more conventional label of best-fitting model.
Results
Signal Detection (d′ Context)
The heritability of d′ context was .40 (95% confidence interval [CI]=.31; .49), and the unique environmental variance was estimated at .60 (95% CI=.51; .69).
Genetic Architecture of AX, BX, and AY Errors
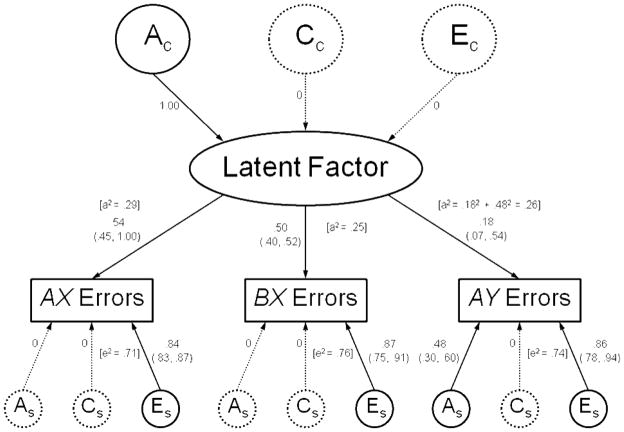
Table 1 presents the phenotypic correlations and the results from the AE Cholesky model (because dropping the C parameters had very little impact on the fit of the model). There were modest, but significant positive phenotypic correlations between AX and both BX and AY errors. The correlations were slightly stronger between AX and BX errors. BX and AY errors were not significantly correlated. In the full (ACE) trivariate model for AX, BX, and AY errors, the estimates for A were .20 for AX errors, .28 for BX errors, and .24 for AY errors. Common environmental influences (C) accounted for very small and nonsignificant proportions of variance (7% for AX errors, 0% for BX errors, and 2% for AY errors). Model-fitting results for the trivariate analysis of AX, BX, and AY errors are shown in Table 2. When compared with the full Cholesky, the most parsimonious model was a common pathways model with A-only covariance (Table 2, Model 5). This model, along with the parameter estimates derived from it, is depicted in Figure 3. As can be seen in Figure 3, it is referred to as an A-only covariance model because the covariance among the three error types can be explained entirely by common genetic influences.
Table 1.
Phenotypic, Genetic, and Unique Environmental Correlations for AX, BX, and AY Errors
| AX Errors | BX Errors | |
|---|---|---|
| Phenotypic Correlations
| ||
| BX Errors | .28 (.21, .34) | |
| AY Errors | .11 (.05, .18) | .06 (−.02, .13) |
|
| ||
| Genetic Correlations
| ||
| BX Errors | .95 (.60, 1.00) | |
| AY Errors | .40 (.09, .78) | .32 (−.11, .65) |
|
| ||
| Unique Environmental Correlations
| ||
| BX Errors | .03 (−.08, .15) | |
| AY Errors | .01 (−.11, .13) | −.04 (−.16, .12) |
Note. Genetic and unique environmental correlations are from the AE Cholesky model. Numbers in parentheses are 95% confidence intervals.
Table 2.
Model-Fitting Results for Trivariate Twin Analysis of AX, BX, and AY Errors
| Model | −2LL | df | LRC | Δdf | p | AIC |
|---|---|---|---|---|---|---|
| 1. ACE Cholesky | 10156.56 | 3348 | n/a | n/a | n/a | n/a |
| 2. AE Cholesky | 10156.89 | 3354 | 0.33 | 6 | .999 | −11.67 |
| 3. ACE Covariance Common Pathways | 10158.12 | 3351 | 1.56 | 3 | .668 | −4.44 |
| 4. AE Covariance Common Pathways | 10158.12 | 3355 | 1.56 | 7 | >.999 | −12.44 |
| 5. A Only Covariance Common Pathways | 10158.74 | 3358 | 2.18 | 10 | .995 | −17.82 |
| 6. Measurement Model | 10169.54 | 3359 | 12.98 | 11 | .295 | −9.02 |
−2LL = −2 Log-Likelihood; df = Degrees of Freedom; LRC = Likelihood Ratio Chi-square test, which is equal to the change in −2LL between the comparison model and the ACE Cholesky; Δdf = Change in df; AIC = Akaike’s Information Criterion, which is equal to [LRC – 2(Δdf)].
The fit of models 2–6 shown in the Table was determined relative to the ACE Cholesky.
An additional test of Model 6 relative to Model 5 (the most parsimonious model) indicated a significantly worse fit for Model 5 (LRC = 10.80; Δdf = 1; p = .001; AIC = 8.80).
Figure 3.
Common pathways model for AX, BX, and AY errors. Standardized parameter estimates with 95% confidence intervals are shown. To simplify the display, only one twin is represented in the diagram. A=Additive genetic influences; C=Common environmental influences; E=Unique environmental influences; a2=Proportion of variance accounted for by additive genetic influences (heritability); e2=Proportion of variance accounted for by unique environmental influences. Subscript C=Influences that are common to all three trial types. Subscript S=Influences that are specific to a particular trial type. Dotted lines represent variance components that were very small and could be dropped from the model without any significant reduction in fit.
*p<.05
In order to test for the presence of genetic influences specific to each variable, we also fit a measurement model to the data (Table 2, Model 6). The measurement model is the same as the common pathways model except that it assumes no specific genetic or common environmental effects. In other words, the specific A and C parameters are set to zero (Specific effects are denoted by the “s” subscripts in Figure 3). The E parameters can never be set to zero because the variables examined are never free of measurement error. The measurement model was more parsimonious than the full Cholesky, but it had a substantially worse fit to the data than the A-only covariance model (Model 5). In contrast to the measurement model, the A-only common pathways model does indicate specific genetic variance for AY errors. As can be seen in Figure 3, the heritabilities are of similar magnitude for each of the error types—ranging from .25 to .29. However, only a small amount of genetic variance in AY errors comes from the latent factor; most (72%) of the genetic influences on AY errors are accounted for by genetic factors that are specific to that trial type. Thus, there are common genetic factors that influence overall performance, but there are also other independent genetic factors that influence differences in reactive control, i.e., response selection following stimulus exposure rather than planning ahead.
Correlated Factors Model for BX and AY Errors Accounting for Processing Speed
With respect to phenotypic correlations, BY RT and BX errors were positively correlated (r=.30) whereas BY RT and AY errors were negatively correlated (r=−.32; see Table 3). The trivariate Cholesky model for BY RT and BX and AY errors generated the following standardized variance components for BY RT: A=.37 (95% CI=.21; .47); C=.00 (95% CI=.00; .0003); and E=.63 (95% CI=.53; .74). As already noted, the C estimates for BX and AY errors were near zero. Thus, an AE model was more parsimonious than the ACE model (−2LL=10301.28, df=3350, LRC=.25, Δdf=6, p>.999, AIC=−11.75). The genetic correlations followed a pattern similar to that of the phenotypic correlations: rg=.41 for BY RT and BX errors; and rg=−.38 for BY RT and AY errors. In this model, the unique environmental correlations also followed a pattern similar to that of the genetic correlations rg=.26 for BY RT and BX errors; and rg=−.30 for BY RT and AY errors.
Table 3.
Phenotypic Correlations, and Genetic and Unique Environmental Correlations from Most Parsimonious Model for BY Reaction Time (RT) and BX, and AY Errors
| BY RT | BX Errors | |
|---|---|---|
| Phenotypic Correlations
| ||
| BX Errors | .30 (.23, .36) | |
| AY Errors | −.32 (−.39, −.26) | .06 (−.02, .14) |
|
| ||
| Genetic Correlations
| ||
| BX Errors | .41 (.10, .66) | |
| AY Errors | −.38 (−.66, −.08) | .27 (−.15, .69) |
|
| ||
| Unique Environmental Correlations
| ||
| BX Errors | .26 (.12, .38) | |
| AY Errors | −.30 (−.42, −.24) | −.01 (−.16, .14) |
Note. Numbers in parentheses are 95% confidence intervals.
BX errors had a significant phenotypic correlation with Trails 2 (r=.17, p<.0001), but AY errors did not (r=−.02, p=.55). Although the direction of the correlations was again positive for BX and negative for AY errors, both had nonsignificant phenotypic correlations with SRT (r=.03, p=.30; r=.05, p=.09) and with CRT (r=−.05, p=.08; r=−.02, p=.52). Correlations of the other processing speed measures with BY RT were as follows: Trails 2 (r=.20; p<.0001); SRT (r=.29, p<.0001); CRT (r=.33; p<.0001). Given the general lack of significant phenotypic correlations between error scores and these additional processing speed measures, we did not perform multivariate genetic analyses with these processing speed measures.
Bivariate Genetic Analysis of AX-CPT Errors and General Cognitive Ability
With a genetic correlation between midlife and young adult general cognitive ability (AFQT scores) that did not differ from 1.0, there was virtually no difference in the results for AFQT scores at either time point. We did not examine genetic correlations for AFQT change scores because our previous work (Lyons et al., 2009) indicated that unique environmental factors primarily accounted for AFQT change; there were not significant genetic influences. With no significant genetic influences on the change phenotype, there could not be any genetic correlations between it and any other phenotype.
Age 20 and age 55 AFQT scores were not phenotypically correlated with AY errors (rs=.004 and .03, ps>.30). Table 4 shows the correlations of AFQT scores with AX and BX errors. Models were compared with a model that included ACE models for each variable and genetic, shared environmental, and unique environmental covariances. Here we summarize the results for the age 55 AFQT, but full model-fitting results for both age 20 and age 55 AFQT are available on request. In all cases, the most parsimonious models consisted of AE models for each of the individual variables and no shared environmental covariance. For age 55AFQT and AX errors, the most parsimonious model included the unique environmental covariance (−2LL= 9645.65, df=2343, LRC=.50, Δdf=3, p>.92, AIC=−5.50). For age 55 AFQT and BX errors, the most parsimonious model did not include the unique environmental correlation. As is readily apparent in Table 4, the results were essentially the same for AFQT scores in young adulthood and in late middle age. Thus, AX and BX errors (representing poor use of context to plan responses) both share some genetic influences with overall cognitive ability. AY errors (representing poor response selection when the ability to plan ahead is limited), which have some genetic influences that are distinct from AX and BX errors, have genetic influences that are distinct from overall cognitive ability as well.
Table 4.
Phenotypic Correlations, and Genetic and Unique Environmental Correlations from Most Parsimonious Models for General Cognitive Ability (AFQT) and AX and BX Error
| Age 20 AFQT | Age 55 AFQT | |
|---|---|---|
| Phenotypic Correlations
| ||
| AX Errors | −.13 (−.19, −.07) | −.19 (−.25, −.12) |
| BX Errors | −.15 (−.21, −.08) | −.22 (−.29, −.15) |
|
| ||
| Genetic Correlations
| ||
| AX Errors | −.29 (−.43; −.15) | −.30 (−.45, −.15) |
| BX Errors | −.33 (−.53; −.17) | −.49 (−.72; −.33) |
|
| ||
| Unique Environmental Correlations
| ||
| AX Errors | ---- | −.11 (−.22; −.001) |
| BX Errors | ---- | ---- |
Note. Numbers in parentheses are 95% confidence intervals. Separate bivariate analyses were performed for the age 20 and age 55 AFQT scores. There are empty cells for unique environmental correlations because those correlations were dropped from the models.
Discussion
To our knowledge, this is the first study of both genetic and environmental influences on AX-CPT performance. There was moderate heritability for d′ context, but further examination of specific component processes served to more fully elucidate the genetic influences underlying AX-CPT performance. Genetic influences accounted for about one-quarter of the variance in AX, BX, and AY errors. The remaining variance was primarily accounted for by unique environmental influences. Common environmental influences could be dropped from the models without a significant reduction in fit. The strongest phenotypic correlation was between AX and BX errors. Similar to previous studies (e.g., Braver et al., 2001), BX and AY errors were not significantly phenotypically correlated. There was a far smaller degree of genetic overlap between AX and AY errors, and there was no overlap in the environmental influences on any of the error types. Given that the genetic correlation between AX and BX errors was not significantly different from 1.0 and the unique environmental correlation was near zero, we can conclude that the phenotypic correlation was almost entirely due to fact that the same genetic influences were operating on each trial type. Taken together, the results indicate that there are far more common genetic influences underlying the common processes shared by AX and BX trials than there are in the common processes shared across AX and AY trials. The common process in AX and BX trials is that using context cues to prepare responses maximizes performance. On AY trials, the context cue works against optimal performance and response choice can only be made after the probe is presented.
These conclusions were supported by the trivariate model-fitting results for these three trial types. The A only covariance model supported by that analysis indicates that the shared variance among the three trial types can be accounted for solely by common genetic influences that operate through a common latent factor. All of the genetic influences on AX and BX errors are from the genes that underlie the latent factor. In contrast, most of the genetic influences on AY errors come from genes that are specific to that trial type and independent of the genes underlying the latent genetic factor. Thus, it is largely different genetic factors that influence performance on BX and AY trials. This relative independence indicates that these phenotypic functionally dissociable processes are genetically dissociable as well. MacDonald et al. (2005) found that BX and AY error scores loaded on independent phenotypic factors. The present results provide a causal mechanism in that there are also some independent genetic influences underlying BX and AY performance.
Overall, our results suggest that a single underlying mechanism—as proposed in the formal context processing models—is insufficient to account for the performance patterns. The absence of a phenotypic correlation between BX and AY performance casts doubt on the notion of a single underlying mechanism, but the presence of independent genetic factors indicated by the lack of a genetic correlation between the two essentially confirms the presence of at least two underlying mechanisms. Different genetic influences contributing to different components of context processing may make it easier to account for varied within-individual performance or differential changes with age. This finding appears to be consistent with the dual mechanisms of control model which postulates that “it should be possible to modulate reactive control without affecting proactive control” (p. 7355) (Braver et al., 2009). Reactive control refers to reliance on probe information to determine responses, whereas proactive control refers to reliance on cue information which allows for planning ahead (Braver et al., 2009; Braver et al., 2005). Strategy differences might make it necessary to postulate an additional underlying mechanism for modulating shifts between different trial types, i.e., for adaptively switching between emphasis on proactive or reactive cognitive control modes. Modulating speed of response could be one way to do that. Alternatively, it may be that AY performance primarily reflects a response style that is independent of both BX performance and overall cognitive ability. It might be analogous to response bias (β) in signal detection paradigms, i.e., how liberal or conservative people are in their threshold for calling something a target.
In virtually all previous cross-sectional, non-genetically informative studies (Braver et al., 2001; Braver et al., 2009; Braver et al., 2005; Paxton et al., 2008; Paxton et al., 2006; Rush et al., 2006), chronological age and slower BY RTs were both associated with increased BX errors and fewer AY errors. With the chronologically age-homogeneous sample that is part of the VETSA study design, chronological age was not associated with BX or AY errors. Yet, the same pattern of correlations between BY RT and either BX or AY errors was present in our sample. Thus, VETSA participants who tend to have more BX and fewer AY errors (and slower BY RT) appear to be functioning in a way that is similar to chronologically older individuals; that is, they appear to be relying more heavily on reactive control relative to proactive control strategies.
On the other hand, this same pattern of correlations with BX and AY errors was not present for any of our other external processing speed measures. Taken together, these results suggest that BY RT is an indicator of task-specific, rather than general, processing speed. Thus, a subset of our relatively young VETSA participants may be functioning like older adults on the AX-CPT, but not necessarily on other tests. With these participants being only in their 50s at the time of this assessment, it may be that those with AX-CPT performance that is more similar to that of older adults are at increased risk for earlier or greater cognitive declines in VETSA follow-up assessments. As an index of cognitive variation this is independent of chronological age in this sample, it might be tentatively suggested this pattern of performance could constitute a cognitive analog of BioAge (Baltes & Lindenberger, 1997; Wahlin, MacDonald, deFrias, Nilsson, & Dixon, 2006).
Given substantial prior evidence indicating that executive function is associated with general cognitive ability, it was also of interest to examine the relationship of context processing components to overall cognitive ability. These associations can be somewhat puzzling because the ability of patients with frontal lobe damage and impaired executive function to manifest little or no impairment on IQ tests suggests a dissociation between executive function and general cognitive ability. This paradoxical set of findings may be partially explained by findings such as those of Friedman et al. (2006) who showed that updating, but not shifting or inhibition, were strongly associated with general cognitive ability. Our results are partially consistent with that pattern. AX and BX errors had negative genetic correlations with general cognitive ability, the stronger relationship being with BX errors. This indicates that there are shared genetic influences between context processing and general cognitive ability; however, the correlations were far from −1.00, indicating that there are substantially different genetic influences as well. In contrast, AY errors were independent of general cognitive ability.
This pattern is consistent with the phenotypic factor analysis of MacDonald et al. (2005) in which the factor with strong AY loadings was independent of the factor containing strong BX loadings, and only the latter factor was significantly correlated with general cognitive ability. MacDonald et al. suggested a parallel between AY trials and go/no-go tasks, a class of tasks that require inhibitory reactive control and tend to be impaired when there is greater impulsivity. Given evidence that response inhibition is poorer in older than in young adults (Zacks, Hasher, & Li, 2000; Zacks, Radvansky, & Hasher, 1996), this view makes it difficult to account for older adults having better AY performance than young adults. The logic of the context processing model has been that AY performance is better because older adults have poorer context maintenance, not because they have better inhibitory control. If older adults—or those functioning more like older adults—do not maintain the context well, the prepotent response tendency (expectation of an X following an A) will be weaker and the downstream effect would be that less inhibitory control is needed. Therefore, AY trials may have a strong go/no-go component, but only for individuals with good context maintenance.
Consistent with this notion, Rush et al. (2006) found that AY errors were significantly correlated with go/no-go errors in young adults (mean age=20 years) but not in older adults (mean age=75 years). AY errors were also significantly positively correlated with BX errors in the study of Rush et al. (2006), but only in the older adults. Again, this pattern of correlations is consistent with the idea that AY performance may be more strongly influenced by maintenance of context in older adults, but more by response inhibition in younger adults. This idea may also account for an apparent paradox; a more conservative approach, which is often seen in older adults, would be consistent with fewer AY false alarms, but not faster RTs. However, if AY trials effectively lose their go/no-go element when individuals reach a certain age, they would not experience the response conflict and subsequent slowing of younger adults. Like the young adults, BX and AY errors were uncorrelated in middle-aged adults (mean age=55 years in the present study; mean age=44 years in the MacDonald et al. (2005) study). We might, therefore, expect to see this pattern change as VETSA participants get older.
It is important to note that previous studies of AX-CPT performance and aging have been cross-sectional, with the difference in patterns of performance between young and old adults suggesting an age-associated shift from more proactive to more reactive control (Braver et al., 2009; Braver et al., 2005; Rush et al., 2006). That is, younger adults perform better on BX trials, reflecting an emphasis on planning ahead for responses based on context cues. Older adults perform better on AY trials, reflecting an emphasis on making response decisions after appearance of a probe. Braver et al. (2009), however, also suggested that variability in these two cognitive strategies may be present even within a small sample of young adults. These findings raise some key questions that we may begin answer in the ongoing longitudinal assessment of the large VETSA sample: whether some individuals experience an age-related shift toward greater reliance on reactive control, and some do not; and whether particular patterns of AX-CPT performance in midlife indicate increased risk for mild cognitive impairment or dementia.
In the present study, we examined AX-CPT performance to determine the genetic and environmental influences on components of context processing in late middle age. Approximately 40% of the variance in signal detection (d′ context) and one-quarter of the variance in individual error scores were accounted for by genetic influences. Multivariate analyses demonstrated common genetic influences across the different component processes, but additional independent genetic influences on AY performance. We concluded that there are genetic influences on reactive cognitive control (based on AY scores) that are independent of the genetic influences on proactive cognitive control (based primarily on BX scores). Unlike proactive control, reactive control was also independent of general cognitive ability. Within a narrow age range, slower CPT RTs were associated with error patterns similar to older adults, but these associations did not generalize to external RT measures. Given our results indicating more than one genetic mechanism underlying context processing performance, further investigation is warranted to determine how these mechanisms may differentially affect age-related changes in context processing components.
Acknowledgments
This work was supported by National Institute on Aging Grants R01 AG018386, AG022381, and AG022982 (to William S. Kremen), and R01 AG018384 (to Michael J. Lyons). The U.S. Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance in the conduct of this study, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University; VA San Diego Healthcare System. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible.
Footnotes
The inclusion of additional external processing speed measures was suggested by the editor and an anonymous reviewer.
We also considered testing an independent pathways model, but with only three phenotypes, we could not empirically test an independent pathways model against the full (Cholesky) model (McArdle & Goldsmith, 1990).
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/pag
Contributor Information
William S. Kremen, Department of Psychiatry, Center for Behavioral Genomics, University of California, San Diego, and VA San Diego Healthcare System
Matthew S. Panizzon, Department of Psychiatry, University of California, San Diego
Hong Xian, Department of Internal Medicine, Washington University, and St. Louis VA Medical Center.
Deanna M. Barch, Departments of Psychology, Psychiatry, and Radiology, Washington University
Carol E. Franz, Department of Psychiatry, Center for Behavioral Genomics, University of California, San Diego
Michael D. Grant, Department of Psychology, Boston University
Rosemary Toomey, Department of Psychology, Boston University.
Michael J. Lyons, Department of Psychology, Boston University
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Ando J, Ono Y, Wright MJ. Genetic structure of spatial and verbal working memory. Behavior Genetics. 2001;31:615–624. doi: 10.1023/a:1013353613591. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Lindenberger U, Li SC, Nyberg L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: Recent data and future avenues. Neuroscience and Biobehavioral Reviews. 2010;34:670–657. doi: 10.1016/j.neubiorev.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive fucntions across the adult life span. Psychology and Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Bayroff AG, Anderson AA. Development of Armed Forces Qualification Tests 7 and 8 (Technical Research Report 1122) Alexandria, VA: U.S. Army Research Institute; 1963. [Google Scholar]
- Bouchard TJ, Jr, McGue M. Genetic and environmental influences on human psychological differences. Journal of Neurobiology. 2003;54:4–45. doi: 10.1002/neu.10160. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neuroscience and Biobehavioral Reviews. 2002;26:809–817. doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Cohen JD Tech. Rep. PDP.CNS.99.1. Mechanisms of cognitive control: Active memory, inhibition, and the prefrontal cortex. Pittsburgh, PA: Carnegie Mellon University; 1999. [Google Scholar]
- Braver TS, Barch DM, Keys BA, Carter CS, Cohen JD, Kaye JA, et al. Context processing in older adults: Evidence for a theory relating cognitive control to neurobiology in healthy aging. Journal of Experimental Psychology: General. 2001;130:746–763. [PubMed] [Google Scholar]
- Braver TS, Cohen JD. On the control of control: The role of dopamine in regulating prefrontal function and working memory. In: Monsell S, Driver J, editors. Attention and performance XVIII: Control of cognitive processes. Cambridge, MA: MIT Press; 2000. pp. 713–737. [Google Scholar]
- Braver TS, Cohen JD, Barch DM. The role of prefrontal cortex in normal and disordered cognitive control: A cognitive neuroscience perspective. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford, England: Oxford University Press; 2002. pp. 428–448. [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proceedings of the National Academy of Sciences U S A. 2009;106:7351–7356. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Satpute AB, Rush BK, Racine CA, Barch DM. Context processing and context maintenance in healthy aging and early stage dementia of the Alzheimer’s type. Psychology and Aging. 2005;20:33–46. doi: 10.1037/0882-7974.20.1.33. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Carey G. Human Genetics for the Social Sciences. Thousand Oaks, CA: Sage Publications; 2003. [Google Scholar]
- Chou LN, Kuo PH, Lin CC, Chen WJ. Genetic and environmental influences on the Wisconsin Card Sorting Test performance in healthy adolescents: A twin/sibling study. Behavior Genetics. 2009 doi: 10.1007/s10519-009-9299-3. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processig deficits in schizophrenia: Converging evidence from three theoretically motivated cognitive tasks. Journal of Abnormal Psychology. 1999;108:120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, O’Reilly RC. A computational approach to prefrontal cortex, cognitive control and schizophrenia: Recent developments and current challenges. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 1996;351:1515–1527. doi: 10.1098/rstb.1996.0138. [DOI] [PubMed] [Google Scholar]
- Corwin J. On measuring discrimination and response bias: Unequal numbers of targets and distractors and two classes of distractors. Neuropsychology. 1994;8:110–117. [Google Scholar]
- Deary IJ, Johnson W, Starr JM. Are processing speed tasks biomarkers of cognitive aging? Psychology and Aging. 2010;25:219–228. doi: 10.1037/a0017750. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Eaves LJ, Last KA, Young PA, Martin NG. Model-fitting approaches to the analysis of human behavior. Heredity. 1978;41:249–320. doi: 10.1038/hdy.1978.101. [DOI] [PubMed] [Google Scholar]
- Eisen SA, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam Era Twin Registry: An approach using questionnaires. Clinical Genetics. 1989;35:423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Eisen SA, True WR, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: Method of construction. Acta Geneticae Medicae et Gemellologiae. 1987;36:61–66. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, et al. High consistency of regional cortical thinning in aging across multiple samples. Cerebral Cortex. 2009 doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz CE, Lyons MJ, O’Brien RC, Panizzon MS, Kim K, Bhat R, et al. Depression and cognitive ability in midlife adults: Accounting for pre-onset cognitive ability. American Journal of Geriatric Psychiatry in press. [Google Scholar]
- Friedman NP, Miyake A, Corley RP, Young SE, Defries JC, Hewitt JK. Not all executive functions are related to intelligence. Psychological Science. 2006;17:172–179. doi: 10.1111/j.1467-9280.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, Defries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. Journal of Cognitive Neuroscience. 2006;18:227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and new view. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. New York: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Haworth CM, Wright MJ, Luciano M, Martin NG, de Geus EJ, van Beijsterveldt CE, et al. The heritability of general cognitive ability increases linearly from childhood to young adulthood. Molecular Psychiatry. 2009 doi: 10.1038/mp.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson WG, Eisen SE, Goldberg J, True WR, Barnes JE, Vitek M. The Vietnam Era Twin Registry: A resource for medical research. Public Health Reports. 1990;105:368–373. [PMC free article] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Dixon RA, Small BJ. Memory change in the aged. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, et al. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Kausler DM. Learning and memory in normal aging. San Diego, CA: Academic Press; 1994. [Google Scholar]
- Kremen WS, Eisen SA, Tsuang MT, Lyons MJ. Is the Wisconsin Card Sorting Test a useful neurocognitive endophenotype? American Journal Medical Genetics (Neuropsychiatric Genetics) 2007;144B:403–406. doi: 10.1002/ajmg.b.30527. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Jacobsen KC, Xian H, Eisen SA, Eaves LJ, Tsuang MT, et al. Genetics of verbal working memory processes: A twin study of middle-aged men. Neuropsychology. 2007;21:569–580. doi: 10.1037/0894-4105.21.5.569. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Jacobson KC, Panizzon MS, Xian H, Eaves LJ, Eisen SA, et al. Factor structure of planning and problem-solving: A behavioral genetic analysis of the Tower of London task in middle-aged twins. Behavior Genetics. 2008 doi: 10.1007/s10519-008-9242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Lyons MJ. Behavior genetics of aging. In: Schaie KW, Willis SL, editors. Handbook of the psychology of aging. 7. San Diego, CA: Elsevier; 2010. pp. 93–107. [Google Scholar]
- Kremen WS, Lyons MJ. Behavior genetics of aging. In: Schaie KW, Willis SL, editors. Handbook of the psychology of aging. 7. San Diego, CA: Elsevier; in press. [Google Scholar]
- Kremen WS, Panizzon MS, Neale MC, Fennema-Notestine C, Prom-Wormley E, Eyler LT, et al. Heritability of brain ventricle size: Converging evidence from inconsistent results. Neurobiology of Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Prom-Wormley E, Panizzon MS, Eyler LT, Fischl B, Neale MC, et al. Genetic and environmental influences on the size of specific brain regions in midlife: The VETSA MRI study. Neuroimage. 2010;49:1213–1223. doi: 10.1016/j.neuroimage.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Thompson-Brenner H, Leung YJ, Grant MD, Franz CE, Eisen SA, et al. Genes, environment, and time: The Vietnam Era Twin Study of Aging (VETSA) Twin Research and Human Genetics. 2006;9:1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- Kyllonen PC. Is working memory capacity Spearman’s g? In: Dennis I, Tapsfield P, editors. Human abilities: Their nature and measurement. Mahwah, NJ: Erlbaum; 1996. pp. 49–75. [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, editors. Neuropsychological Assessment. 4. Oxford: Oxford University Press; 2004. [Google Scholar]
- Li SC, Lindenberger U, Backman L. Dopaminergic modulation of cognition across the life span. Neuroscience and Biobehavioral Reviews. 2010;34:625–630. doi: 10.1016/j.neubiorev.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Sikstrom S. Aging cognition: From neuromodulation to representation. Trends in Cognitive Sciences. 2001;5:479–486. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- Luciano M, Wright MJ, Smith GA, Geffen GM, Geffen LB, Martin NG. Gentic covariance among measures of information processing speed, working memory, and IQ. Behavior Genetics. 2001;31:581–592. doi: 10.1023/a:1013397428612. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, York TP, Franz CE, Grant MD, Eaves LJ, Jacobson KC, et al. Genes determine stability and environment determines change in cognitive ability during 35 years of adulthood. Psychological Science. 2009;11:1146–1152. doi: 10.1111/j.1467-9280.2009.02425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, III, Carter CS, Flory JD, Ferrell RE, Manuck SB. COMT val158Met and executive control: A test of the benefit of specific deficits to translational research. Journal of Abnormal Psychology. 2007;116:306–312. doi: 10.1037/0021-843X.116.2.306. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Goghari VM, Hicks BM, Flory JD, Carter CS, Manuck SB. A convergent-divergent approach to context processing, general intellectual functioning, and the genetic liability to schizophrenia. Neuropsychology. 2005;19:814–821. doi: 10.1037/0894-4105.19.6.814. [DOI] [PubMed] [Google Scholar]
- MacDonald SW, Cervenka S, Farde L, Nyberg L, Backman L. Extrastriatal dopamine D2 receptor binding modulates intraindividual variability in episodic recognition and executive functioning. Neuropsychologia. 2009;47:2299–2304. doi: 10.1016/j.neuropsychologia.2009.01.016. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Goldsmith HH. Alternative common factor models for multivariate biometric analyses. Behavior Genetics. 1990;20:569–608. doi: 10.1007/BF01065873. [DOI] [PubMed] [Google Scholar]
- National Health and Nutrition Examination Survey (NHANES III) Trends in health and aging. 1999–2004. p. 2007. Retrieved April 20, 2007. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6. Richmond, VA: Department of Psychiatry, Medical College of Virginia; 2004. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- Paxton JL, Barch DM, Racine CA, Braver TS. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cerebral Cortex. 2008;18:1010–1028. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton JL, Barch DM, Storandt M, Braver TS. Effects of environmental support and strategy training on older adults’ use of context. Psychology and Aging. 2006;21:499–509. doi: 10.1037/0882-7974.21.3.499. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Hulshoff Pol HE. Genetic influences on human brain structure: A review of brain imaging studies in twins. Human Brain Mapping. 2007;28:464–473. doi: 10.1002/hbm.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, Spinath FM. Genetics and general cognitive ability (g) Trends in Cognitive Sciences. 2002;6:169–176. doi: 10.1016/s1364-6613(00)01853-2. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral Reviews. 2006;30 doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinvang I, Winjevoll IL, Rootwelt H, Espeseth T. Working memory deficits in healthy APOE epsilon 4 carriers. Neuropsychologia. 2009;48:566–573. doi: 10.1016/j.neuropsychologia.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Reynolds CA, Finkel D, Gatz M, Pedersen NL. Sources of influence on rate of cognitive change over time in Swedish twins: An application of latent growth models. Experimental Aging Research. 2002;28:407–433. doi: 10.1080/03610730290103104. [DOI] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Sarason I, Bransome ED, Beck LH. A continuous performance test of brain damage. Journal of Consulting Psychology. 1956;20:343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Rush BK, Barch DM, Braver TS. Accounting for cognitive aging: Context processing, inhibition or processing speed? Aging, Neuropsycholoy, and Cognition. 2006;13:588–610. doi: 10.1080/13825580600680703. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Mediation of adult age differences in cognition by reductions in working memory and speed of processing. Psychological Science. 1991;2:179–183. [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Eyler LT, Giedd JN, Kremen WS, Kendler KS, Neale MC. Review of twin and family studies on neuroanatomic phenotypes and typical neurodevelopment. Twin Research and Human Genetics. 2007;10:683–694. doi: 10.1375/twin.10.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic deficits in the processing of context: A test of a theoretical model. Archives of General Psychiatry. 1996;53:1105–1112. doi: 10.1001/archpsyc.1996.01830120037008. [DOI] [PubMed] [Google Scholar]
- Servan-Schreiber D, Printz H, Cohen JD. A network model of catecholamine effects: gain, signal-to-noise ratio, and behavior. Science. 1990;249:892–895. doi: 10.1126/science.2392679. [DOI] [PubMed] [Google Scholar]
- Suhara T, Fukuda H, Inoue O, Itoh T, Suzuki K, Yamasaki T, et al. Age-related changes in human D1 dopamine receptors measured by positron emission tomography. Psychopharmacology. 1991;103:41–45. doi: 10.1007/BF02244071. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Bar JL, Harley RM, Lyons MJ. The Harvard Twin Study of Substance Abuse: What we have learned. Harvard Review of Psychiatry. 2001;9:267–279. [PubMed] [Google Scholar]
- Volkow ND, Logan J, Fowler JS, Wang GJ, Gur RC, Wong C, et al. Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. American Journal of Psychiatry. 2000;157:75–80. doi: 10.1176/ajp.157.1.75. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Ding YS, Gur RC, Gatley J, et al. Parallel loss of presynaptic and postsynaptic dopamine markers in normal aging. Annals of Neurology. 1998;44:143–147. doi: 10.1002/ana.410440125. [DOI] [PubMed] [Google Scholar]
- Wahlin Å, MacDonald SW, deFrias CM, Nilsson LG, Dixon RA. How do health and biological age influence chronological age and sex differences in cognitive aging: moderating, mediating, or both? Psychology and Aging. 2006;21:318–332. doi: 10.1037/0882-7974.21.2.318. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120(2):272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Williams LJ, Holahan PJ. Parsimony-based fit indices for multiple-indicator models: Do they work? Structural Equation Modeling. 1994;1:161–189. [Google Scholar]
- Wingfield A, Stine EAL, Lahar CJ, Aberdeen JS. Does the capacity of working memory change with age? Experimental Aging Research. 1988;14:103–107. doi: 10.1080/03610738808259731. [DOI] [PubMed] [Google Scholar]
- Zacks RT, Hasher L, Li KZH. Human memory. In: Craik FIM, Salthouse TA, editors. Handbook of Aging and Cognition. 2. Mahwah, NJ: Erlbaum; 2000. pp. 293–357. [Google Scholar]
- Zacks RT, Radvansky GA, Hasher L. Studies of directed forgetting in older adults. Journal of Experimental Pyschology: Learning, Memory, and Cognition. 1996;22:143–156. doi: 10.1037//0278-7393.22.1.143. [DOI] [PubMed] [Google Scholar]