Abstract
Our study demonstrates that substrates fabricated using a ‘reactive’ layer-by-layer approach promote well-defined cell-substrate interactions of human corneal epithelial cells. Specifically, crosslinked and amine-reactive polymer multilayers were produced by alternating ‘reactive’ deposition of an azlactone-functionalized polymer [poly(2-vinyl-4,4-dimethylazlactone)] and a primary amine-containing polymer [branched poly(ethylene imine)]. Advantages of our system include a 5 to 30-fold decrease in deposition time compared to traditional polyelectrolyte films and direct modification of the films with peptides. Our films react with mixtures of an adhesion-promoting peptide containing Arg-Gly-Asp (RGD) and the small molecule d-glucamine, a chemical motif which is non-fouling. Resulting surfaces prevent protein adsorption and promote cell attachment through specific peptide interactions. The specificity of cell attachment via immobilized RGD sequences was verified using both a scrambled RDG peptide control as well as soluble-RGD competitive assays. Films were functionalized with monotonically increasing surface densities of RGD which resulted in both increased cell attachment and the promotion of a tri-phasic proliferative response of a human corneal epithelial cell line (hTCEpi). The ability to treat PEI/PVDMA films with peptides for controlled cell-substrate interactions enables the use of these films in a wide range of biological applications.
Keywords: corneal epithelial cell, layer-by-layer, RGD, d-glucamine, attachment, proliferation
Introduction
Creating surfaces that enable precise control over cell-substrate interactions is an important material design consideration in the development of many biotechnology applications, including corneal prosthetics and other implantable devices, stent grafts, cell-based bioassays, and biosensors. Although the desired level of control varies with each application, most approaches to the design of functional surfaces have focused on methods that can be used to (1) present a sufficient density of biomolecular functionality (e.g., adhesion ligands) required for cell attachment and survival, while at the same time (2) introduce functionality that resists protein adsorption and the non-specific attachment of cells and microbes. One of the most common biological motifs used to promote specific cell attachment to surfaces is the short peptide arginine-glycine-aspartic acid (RGD), a sequence found at the binding sites of many extracellular matrix proteins and a structural motif that has been demonstrated to specifically bind to integrin transmembrane receptors.1,2 In addition to promoting cell attachment, RGD has been observed to regulate a number of other cell functions, including cell proliferation,3–9 differentiation,3,6,9 and protein expression.9 Of particular relevance to the work presented here, RGD functionalized surfaces and has been recently been used to promote increased rates of corneal wound healing.10
Many methods have been developed to incorporate RGD motifs onto the surfaces of materials, including the design of functional self-assembled monolayers (SAMs),11–14 interpenetrating networks (IPNs),4,15–17 hydrogels,3,18–23 peptide amphiphiles,9,24–26 and the use of layer-by-layer methods for the assembly of thin, polyelectrolyte-based films.7,8,27,28 Although all of these methods have been demonstrated to be useful in both fundamental and applied studies, methods based on layer-by-layer assembly provide several practical advantages because (i) they can be used to deposit thin or ultrathin films conformally on the surfaces of a wide range of different materials and (ii) they provide a means for controlling film thickness, film composition, and mechanical properties, often with nanometer-scale precision. In addition to specific binding of cells to peptides on the surface, we note that several groups have developed approaches to prevent nonspecific protein adsorption29. Anti-fouling properties in combination with selected peptides can then be utilized to promote specific cellular interactions. Of particular relevance to this current study, Berg et al. demonstrated that polyelectrolyte multilayers (PEMs) fabricated from poly(acrylic acid) and polyacrylamide could be patterned with controlled densities of RGD and used to promote the attachment and spreading of NR6 fibroblast cells.27 In addition, Chua et al. have demonstrated that PEMs fabricated using hyaluronic acid and chitosan could be used to immobilize RGD and promote osteoblast adhesion and proliferation while inhibiting bacterial growth. In the above studies, the structures of the polymers used to fabricate these PEM’s required the initial treatment of the films with either a heterobifunctional linker or an activating compound to render the surfaces of the films reactive to promote the covalent immobilization of the peptide.
The approach to surface immobilization of RGD reported here makes use of methods and materials developed recently for the ‘reactive’ layer-by-layer assembly of azlactone-containing polymer multilayers.30,31 This approach to assembly preserves many of the general advantages of layer-by-layer assembly, but it differs substantially from more widely-used methods for the aqueous assembly of polyelectrolyte-based materials in three important ways: (i) film growth is mediated by the formation of covalent bonds between mutually reactive polymer species (rather than by electrostatic or other weak interactions), and therefore leads to thin films that are covalently crosslinked, (ii) film growth mediated by reactions between azlactone and amine functionalities is significantly more rapid than conventional electrostatic layer-by-layer deposition (individual layers can be deposited within seconds), and (iii) the films contain residual reactive azlactone functionality that can be used to functionalize the films directly by exposure to amine-functionalized molecules. Past studies have demonstrated that reactive layer-by-layer assembly of branched poly(ethylene imine) (PEI) and poly(2-vinyl-4,4’-dimethylazlactone) (PVDMA) leads to conformal, amine-reactive films30,31 and that these films can be modified to manipulate surface properties (e.g., to modulate water contact angles by treatment with hydrophobic amines, etc.).32,33 Past work has also demonstrated that treatment of PEI/PVDMA films with d-glucamine (a small-molecule chemical motif demonstrated to prevent the adsorption of proteins and the adhesion of cells) can be used to design coatings that prevent protein adsorption and cell attachment,30 and that methods that tune the density of immobilize glucamine can provide additional control over non-specific cell attachment and growth.34 Finally, PEI/PVDMA multilayers can be fabricated conformally on the surfaces of planar objects as well as the surfaces of topographically complex objects,31,35,36 providing a versatile platform for the investigation of cell-substrate interactions in a variety of contexts.
In this study, we demonstrate that RGD-containing peptides can be immobilized directly on the surfaces of these amine-reactive films, and that treatment with solutions containing mixtures of RGD peptide and d-glucamine can be used to design film-coated surfaces that promote interactions with cells though specific RGD-integrin interactions. An immortalized human corneal epithelial cell line, hTCEpi, used widely as a model for human corneal epithelial cells,37 was chosen to examine the impact of controlled RGD densities. This approach to the design of multilayer-coated surfaces presents reproducible and tunable densities of RGD in an inert background of d-glucamine. When combined with the versatility of the layer-by-layer processes used to fabricate these films as described above, this reactive approach to the immobilization of RGD motifs could prove useful for the design and investigation of biomaterials in a variety of fundamental and applied contexts.
Materials and Methods
Materials
Branched poly(ethylene imine) (PEI, Mn = 10,000 g/mol, Mw = 25,000 g/mol) and solvents were purchased from Sigma-Aldrich (Milwaukee, WI). 2-Vinyl-4,4-dimethylazlactone (VDMA) monomer was a kind gift from Steve Heilmann at 3M. Poly(2-vinyl-4,4-dimethylazlactone) (PVDMA, Mn = 18,000, PDI = 3.1) was synthesized according to methods described in Buck et al.35 The peptides GGGRGDSP (“RGD”) and GGGRDGSP (“RDG”) were synthesized at the Biotechnology Center at the University of Wisconsin (Madison, WI). d-Glucamine was purchased from TCI America (Portland, OR). Cell culture and staining reagents were purchased from Invitrogen (Carlsbad, CA) unless otherwise noted.
Fabrication of PEI/PVDMA multilayers
Reactive polymer multilayers were fabricated on glass substrates using a layer-by-layer approach similar to that previously described.30 Glass slides (12.5 mm × 37.5 mm) were rinsed with acetone, ethanol, methanol, and deionized water, and then 1) submerged in a solution of PEI in acetone (20 mM with respect to molecular weight of the polymer repeat unit) for 30 seconds; 2) submerged in two consecutive acetone rinse solutions, each for 30 seconds; 3) submerged in a solution of PVDMA in acetone (20 mM with respect to the polymer repeat unit) for 30 seconds; and finally 4) submerged in two additional acetone rinse baths for 30 seconds each. This cycle (steps 1 through 4) was repeated ten times for each substrate, with each cycle forming one layer pair of PEI and PVDMA (referred to hereafter as a PEI/PVDMA ‘bilayer’). After the final cycle of polymer deposition, film-coated substrates were immediately dried with compressed nitrogen and stored in a vacuum desiccator prior to use. Films maintain a homogenous appearance without the presence of cracks, peeling or delamination.
Fabrication of RGD/d-glucamine and RDG/d-glucamine functionalized films
Small spots (diameters ~ 3mm) functionalized with varying amounts of peptide surrounded by areas functionalized to prevent protein adsorption were patterned onto the surfaces of film-coated substrates by treatment with DMSO solutions containing varying concentrations of d-glucamine and either the cell attachment-promoting peptide (RGD) or a negative control scrambled peptide (RDG). Seven solutions were prepared by mixing defined ratios of these components in the following manner. The total sum concentration of the mixture components was held constant at 110 mM. Mixtures contained 1, 3, 5, 7, 9, 15, and 20 mole % RGD (or RDG), with the remainder composed of d-glucamine (e.g., solutions containing 1.1 mM peptide were supplemented with 108.9 mM d-glucamine, etc.). PEI/PVDMA films were then patterned by treatment with 0.5 µL spots of each peptide/d-glucamine solution for one hour, followed by rinsing with DMSO and ethanol, and drying with compressed nitrogen. To completely passivate remaining areas of the films surrounding these peptide/d-glucamine-treated areas, the substrates were completely covered with 100 µL of a stock solution of d-glucamine (110 mM in DMSO) and allowed to sit for one hour. Finally, the substrates were rinsed with deionized water and ethanol, dried with compressed nitrogen and stored up to 3 days in a vacuum desiccator prior to use.
Human corneal epithelial cell culture
Telomerase-immortalized human corneal epithelial (hTCEpi) cells were a generous gift from Dr. James Jester at the University of California, Irvine.37 hTCEpi cells were cultured in epithelial medium containing a 3:2 ratio of Ham’s F12:Dulbelco’s Modified Eagles medium (DMEM), supplemented with 2.5% (v/v) fetal bovine serum (FBS), 0.4 µg/mL hydrocortisone, 8.4 ng/mL cholera toxin, 5 µg/mL insulin, 24 µg/mL adenine, 10 ng/mL epidermal growth factor, 100 units penicillin, and 100 µg/mL streptomycin.38,39 hTCEpi cells were plated into 100 mm tissue culture plates containing a mitomycin-c treated Swiss 3T3 fibroblast layer. hTCEpi cells, between passages 45 and 60, were incubated at 37 °C and 5% CO2 until they reached approximately 70% confluence.
Plating of cells on RGD/d-glucamine-functionalized substrates
In preparation for cell seeding, a single peptide array substrate was placed into each well of a 6-well tissue culture plate (BD Falcon, CA), and all samples were exposed to UV light for 15 min in a sterile laminar flow hood. hTCEpi cells were plated at a density of 25,000 cells per cm2. All cells were incubated for 24 h after plating to allow for attachment and spreading. For proliferation studies, cells were incubated over a 5 day time period. All experiments were prepared in triplicate and performed a minimum of three times.
Characterization of cell attachment
To investigate the attachment of hTCEpi cells to peptide–functionalized substrates, the number of cells attached to the surface 24 hours after plating was characterized using a nucleic acid dye. hTCEpi cells were rinsed with phosphate-buffered saline (PBS, pH 7.2) and incubated at 37 °C with a 2 mM solution in epithelial medium of SYTO-11 green fluorescent nucleic acid stain (Molecular Probes, Inc., Eugene, OR) for 30 minutes. Cells were rinsed twice with warm DMEM, replenished with epithelial medium and imaged. For quantification of hTCEpi cell attachment, the substrates were imaged using either a Zeiss Axiovert 100M (Thornwood, New York) or an Olympus IX70 (Center Valley, PA) fluorescence microscope. Images acquired at 4X magnification with the Olympus IX70 microscope were used to create a montage of the images of hTCEpi cell attachment over the entire substrate. Fluorescence microscopy images acquired at 10X magnification with the Zeiss Axiovert 100M were analyzed for the total number of hTCEpi cells per image. ImageJ software (NIH) was used to count the total number of cells per image. The average number of cells per 10X image from a representative experiment is reported.
Competitive RGD binding assay using soluble peptide
RGD and RDG were individually dissolved in sterile deionized water to yield 100 mM stock solutions. Either sterile water or one of the prepared 100 mM peptide solutions was added to the medium to cells that had been allowed to attach for 24 hours resulting in final concentrations of 0.1 or 1 mM peptide. Phase contrast microscopy images (10X magnification) of cells were acquired at 0 (before incubation with peptide), 1, 4, and 24 hours after the addition of soluble peptides. All experiments were performed in triplicate, and data are reported as the percentage of cells remaining on the surface.
Characterization of hTCEpi cell proliferation
The proliferation of hTCEpi cells plated onto peptide array substrates was characterized 1, 3 and 5 days after seeding. On days 1 (24 hours) and 3 (72 hours) after plating, 10X phase images were taken. On day 5, the cells were stained with SYTO-11 as previously described and 10X fluorescence images were taken. For each RGD spot on the peptide array substrates, 3 images (covering the entire RGD-patterned spot) were taken. Total cell number was determined and normalized to the total cell number from day 1. Representative data from one of four experiments are reported.
Statistical analysis
Experiments were analyzed using analysis of variance (ANOVA). When variability was determined to be significant (P < 0.05), the Bonferroni multiple comparison test was used to determine significance (P < 0.05) between groups. Significance was further divided into “statistically significant” (0.01 ≤ P < 0.05), “very significant” (0.001 ≤ P < 0.01), and “extremely significant” (P < 0.001).
Results
PEI/PVDMA films were functionalized in a manner that promoted hTCEpi cell attachment and proliferation and limited non-specific interactions between cells and proteins that adsorb to the surface. In a series of initial experiments, glass slides were coated with ten PEI/PVDMA bilayers approximately 100 nm thick.30,31 This thickness was chosen based on a previous report that demonstrated stability and longevity on glass slides in cell culture conditions.30 Film coated glass slides were then functionalized by exposure to solutions containing different molar ratios of an RGD-containing peptide and d-glucamine (Figure 1). The primary amine groups in the peptide and d-glucamine permit covalent immobilization of these motifs by reaction with residual azlactone groups in the PEI/PVDMA films.30,31,35 The covalent binding of RGD to the PEI/PVDMA films was confirmed using polarization-modulation infrared reflectance-absorbance spectroscopy (PM-IRRAS, see supplementary material).
Figure 1.
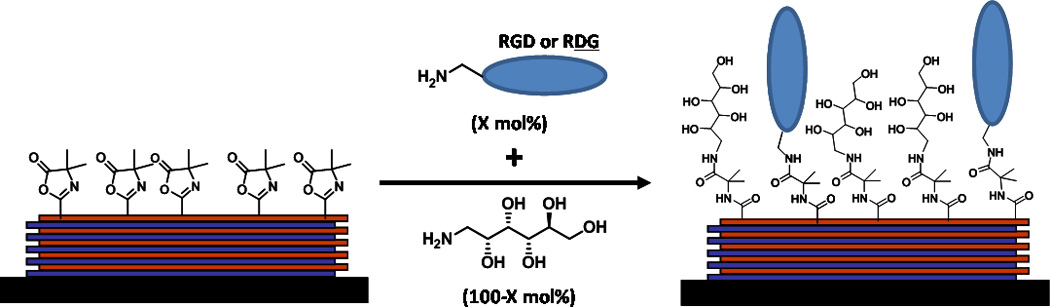
A schematic demonstrating functionalization of PEI/PVDMA multilayer films with mixed solutions of RGD and d-glucamine. Films were allowed to react with solutions (total concentration of 110 mM) that ranged from 0% RGD (100% d-glucamine) to 20% RGD (80% d-glucamine).
Mixtures containing varying mole percentages of RGD and d-glucamine (but having constant total molar concentrations of 110 mM) were used to treat substrates coated with PEI/PVDMA films. The percentage of RGD in these solutions varied from 0 to 20 mole %. Surfaces treated with solutions containing 20 mole % of RGD peptide (i.e. 0.20 × 110 mM = 22 mM RGD with the remaining 88 mM composed of d-glucamine) will be referred to as 20% RGD/d-glucamine surfaces. 20% RGD/d-glucamine was the highest level of RGD investigated in this study because we observed no significant increase in cell attachment on surfaces prepared from solutions containing more than 20% RGD in preliminary screening experiments. Each substrate contained seven RGD-functionalized spots of 1, 3, 5, 7, 9, 15, and 20% RGD, surrounded by areas of d-glucamine alone (0% RGD). The substrates with RGD-functionalized spots were used to determine which percentages of RGD in the solution mixtures of RGD/d-glucamine would promote human corneal epithelial (hTCEpi) cell attachment. Twenty-four hours after cell plating, hTCEpi cells were fluorescently stained using SYTO-11 green fluorescent nucleic acid stain and imaged (Figure 2).
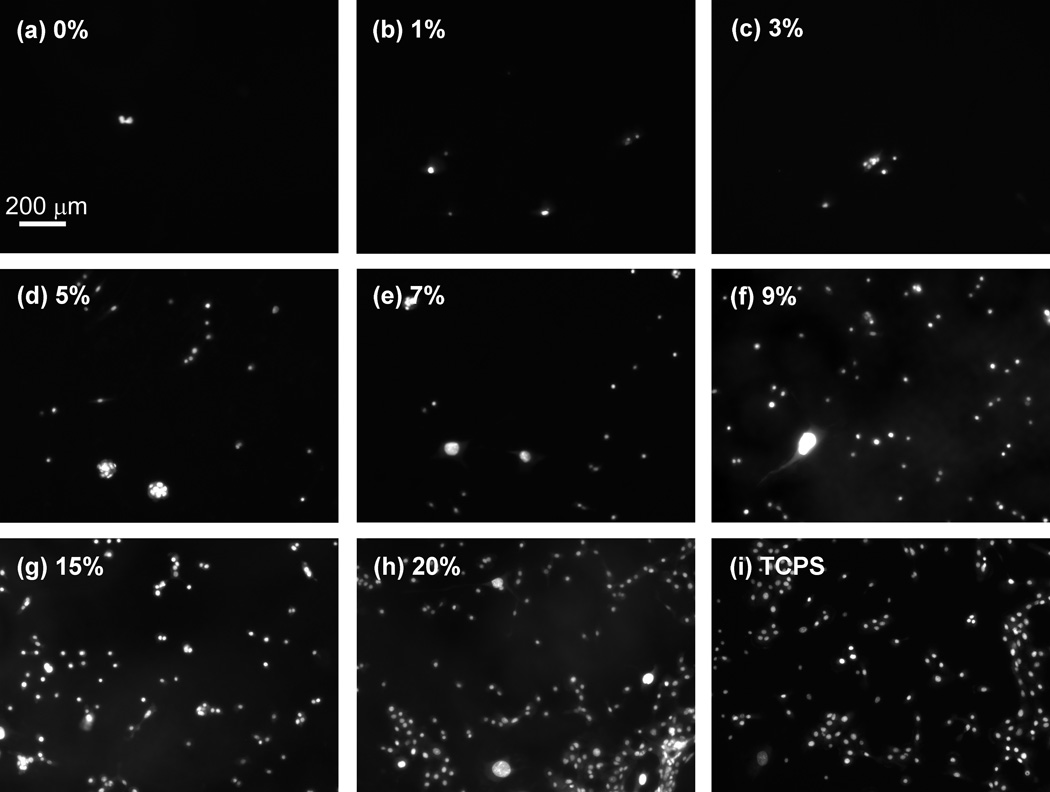
Figure 2.
After 24 hours in culture, hTCEpi cells did not attach significantly to substrates fabricated with 0, and 1% RGD solutions (a–b). However, increased attachment was noted on substrates functionalized with solutions containing 3–20% RGD/d-glucamine (c–h). The cells were stained with SYTO-11 green fluorescent nucleic acid stain.
hTCEpi cell attachment depends on RGD concentration in reaction solution
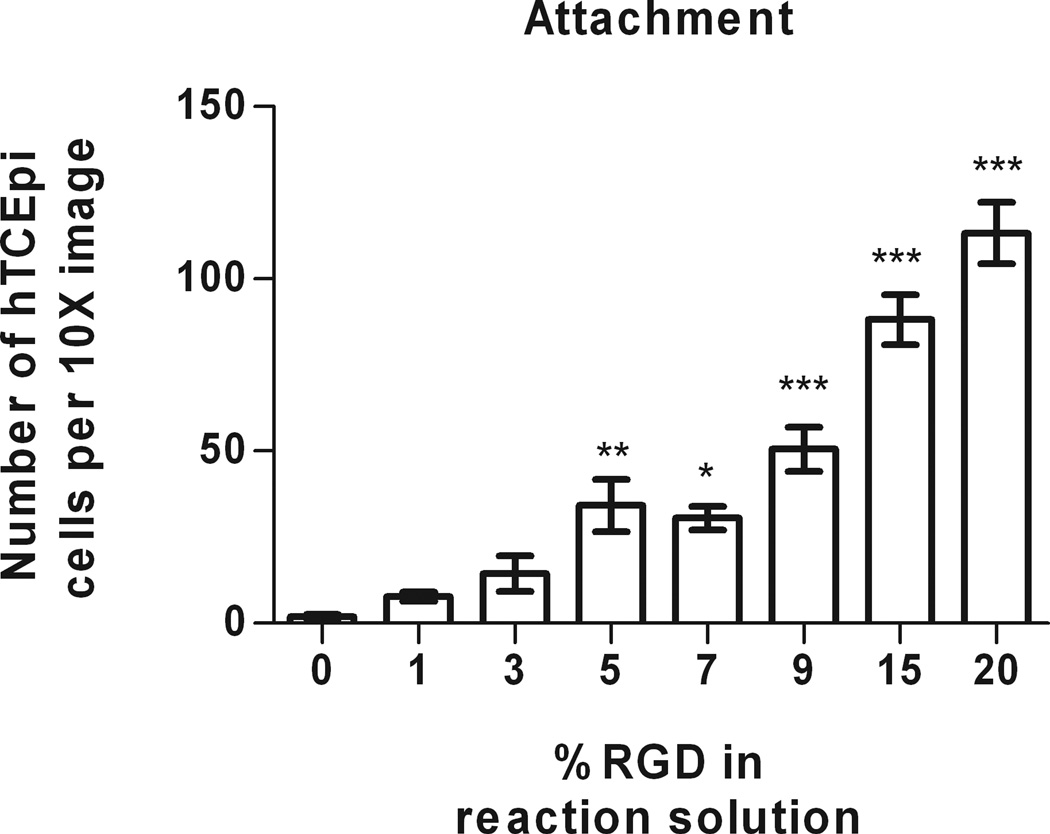
hTCEpi cell attachment to PEI/PVDMA films increased monotonically on surfaces reacted with solutions containing increasing percentages of RGD. At very low concentrations of RGD (0 or 1% RGD/d-glucamine solutions), there was little to no cell attachment (with averages of 1.9 ± 0.6 and 7.8 ± 1.3 cells per image, respectively (Figure 3). Attached cells on these substrates exhibited a rounded morphology, indicative of poor surface anchoring. Cells on 3% RGD/d-glucamine substrates demonstrated increased attachment (14.4 ± 5.1 cells per image); however, limited spreading was observed. Significantly more hTCEpi cells (34.2 ± 7.6 cells per image) attached and spread on spots treated with solutions of 5% RGD/d-glucamine compared to 0% RGD/d-glucamine controls. A clear trend of increased cell attachment and spreading of cells was observed on our 7 to 20% RGD/d-glucamine spots (Figure 3). The most significant (P ≤ 0.001) increases in cell attachment were observed at 9 to 20% RGD/d-glucamine, with 25 to 57 times more cells as compared to 0% RGD/d-glucamine controls.
Figure 3.
hTCEpi cells demonstrated significant increase in attachment to films functionalized with 5% RGD/d-glucamine solutions compared to 0 to 3% RGD/d-glucamine solutions. As the percentage of RGD in the reactive solution increases from 5 to 20%RGD/d-glucamine, the number of attached cells per 10X image also increases. (*0.01 ≤ P < 0.05, **0.001 ≤ P < 0.01, ***P < 0.001 compared to 0% RGD control)
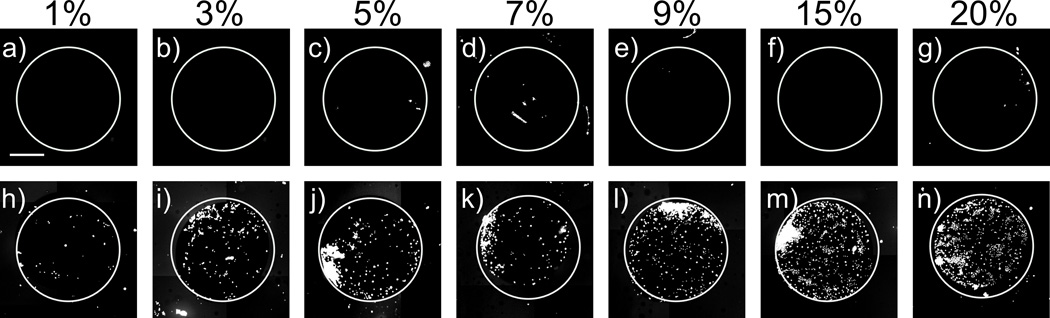
To verify that cell attachment resulted specifically from the presence of RGD on the surface, hTCEpi cells were seeded onto films fabricated using solutions containing 1 to 20% RDG/d-glucamine scrambled peptide control. For all surfaces treated with solutions of RDG/d-glucamine, very low levels of cell attachment were observed (e.g., an average of 7.5 ± 2.1 cells per image). These levels of cell attachment were not significantly different from those observed on 0% RDG (e.g., 100% d-glucamine treated) controls (Figure 4). In contrast, we visualized increased cell attachment as the level of RGD/D-glucamine was increased. Up to 70% coverage of hTCEpi cells was observed on the 20% RGD/D-glucamine.
Figure 4.
Covalent multilayer films were functionalized with either solutions containing RGD or the scrambled negative control peptide, RDG. hTCEpi cells seeded onto RDG surfaces (a–g) were unable to attach. hTCEpi cells seeded onto RGD surfaces (h–n) attached to spots fabricated from 3 to 20% RGD/d-glucamine solutions. Cells were allowed to attach for 24 hours and were then stained with SYTO-11 green fluorescent nucleic acid stain.Scale bar equals 1 mm.
Competitive inhibition of cell attachment to films using soluble RGD
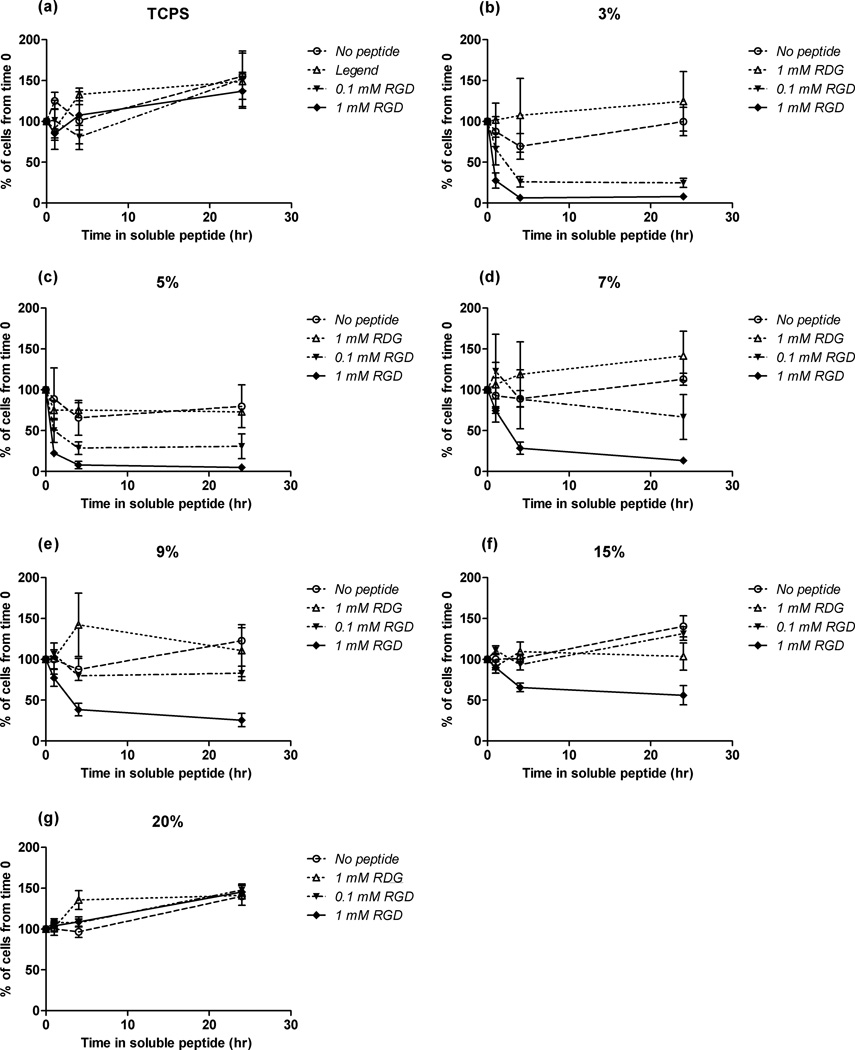
Competitive binding experiments using soluble RGD-containing peptides demonstrated the specificity of hTCEpi cell attachment to surface-associated RGD sequences. First, hTCEpi cells were allowed to attach to RGD/d-glucamine films for 24 hours followed by exposure to one of five different medium conditions containing either no peptide, 0.1 mM RDG, 1 mM RDG, 0.1 mM RGD, or 1 mM RGD. hTCEpi rounding and detachment from the surface in response to free RGD in solution demonstrated specific integrin mediated binding to RGD on the surface.
hTCEpi cells rounded and detached from 3 to 7% RGD/d-glucamine surfaces in response to 0.1 mM soluble RGD. Specifically, addition of 0.1 mM soluble RGD resulted in hTCEpi cell rounding within 1 hour and 75% cell detachment within 4 hours on 3 and 5% RGD/d-glucamine substrates (Figures 5 b, c). On surfaces fabricated with 7% RGD/d-glucamine solutions, addition of 0.1 mM soluble RGD promoted only 25% detachment after 24 hours of incubation (Figure 5 d). No cell detachment was observed on 9 to 20% RGD/d-glucamine substrates under otherwise identical conditions (Figure 5 e–g). hTCEpi cells on substrates exposed to negative controls did not show significant differences in the number of cells attached at 1, 4, and 24 hours compared to number of cells before treatment (open symbols in Figure 5). Cells attached to tissue culture polystyrene (TCPS) (Figure 5 a) showed no detachment response to soluble RGD alone.
Figure 5.
hTCEpi cells detached from PEI/PVDMA surfaces treated with RGD/d-glucamine when exposed to soluble RGD. Soluble RGD was added to the medium 24 hours after cell seeding onto surfaces. In the presence of 0.1 and 1 mM soluble RGD, hTCEpi cells detached from surfaces treated with 3 and 5% RGD/d-glucamine solutions (b and c) within 4 hours. Cells on surfaces fabricated from 7 to 20% RGD/d-glucamine solutions (d–g) demonstrated little to no detachment in the presence of 0.1 mM soluble RGD. However, in the presence of 1 mM soluble RGD, hTCEpi cells on 7 to 15% RGD/d-glucamine surfaces (d–f) demonstrated surface RGD-density dependent detachment. Cells on TCPS (a) and substrates fabricated from 20% RGD solutions (g) did not demonstrate detachment in the presence of 0.1 and 1 mM soluble RGD. Each graph is representative of the remaining cells attached to TCPS or a given %RGD/d-glucamine-modified surface. Symbols: ○- no peptide added to culture medium; △-1 mM RDG added to culture medium; ●- 0.1 mM RGD added to culture medium;▲- 1 mM RGD added to culture medium. Error bars denote the standard error of the mean. Graphs are representative of experiments performed in triplicate.
hTCEpi cells attached to surfaces prepared by treatment with solutions containing 9 to 20% RGD/d-glucamine were observed to round up and/or detach only at substantially higher concentrations of soluble RGD. A ten-fold increase in soluble RGD of 1mM promoted significantly greater cell detachment compared to 0.1 mM soluble RGD. Under these conditions, approximately 80% of the cells detached from surfaces produced using solutions of 3 and 5% RGD/d-glucamine within the first hour of incubation and 95% of the cells were detached after 4 hours. In addition, 20 to 30% of cells detached from 7 and 9% RGD/d-glucamine surfaces after 4 hours of incubation with 1 mM RGD. After 24 hours, there was almost 100% detachment of cells on the 3, 5, and 7% RGD/d-glucamine functionalized surfaces, a 75% decrease in cell attachment on surfaces prepared with solutions of 9% RGD/d-glucamine, and a 50% decrease in cell attachment on surfaces prepared with solutions of 15% RGD/d-glucamine. Cells attached to 20% RGD/d-glucamine surfaces did not demonstrate detachment throughout the entire 24 hours of incubation with both 0.1 and 1 mM RGD. The data indicate that hTCEpi cell response to soluble RGD is dependent upon both time and surface RGD density, suggesting cells are interacting specifically through the immobilized RGD peptides on the PEI/PVDMA film surface.
hTCEpi cell proliferation on RGD/d-glucamine-functionalized substrates
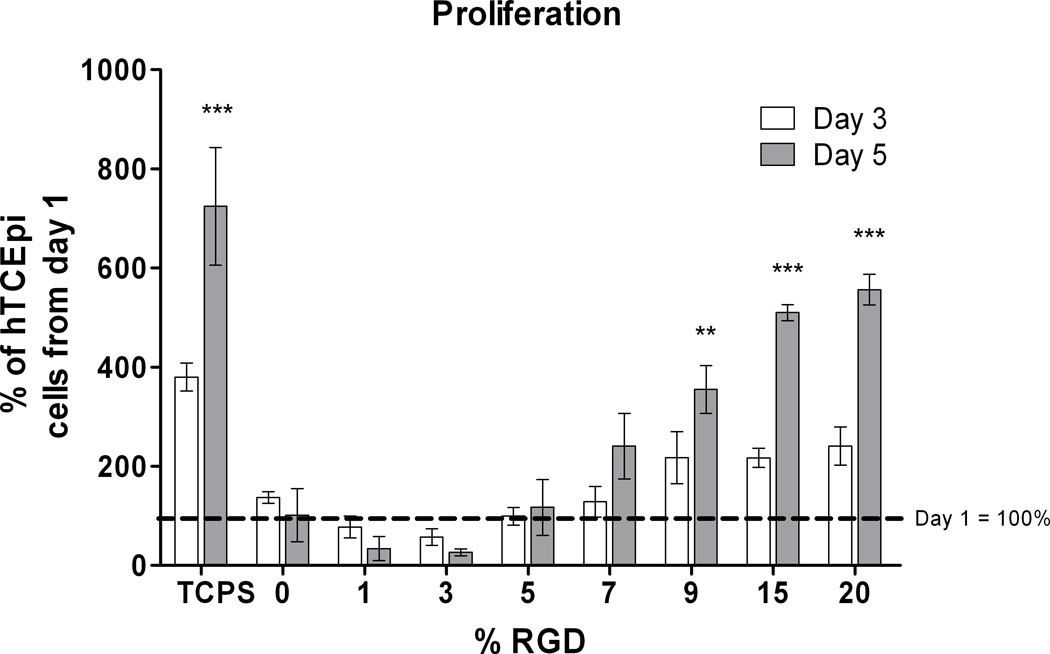
In addition to determining the specificity of hTCEpi cell attachment to RGD/d-glucamine-functionalized PEI/PVDMA multilayer films, we investigated the impact of surface bound RGD on the proliferation of hTCEpi cells. The rate of hTCEpi cell proliferation demonstrated three distinctive phases that depended on the relative amount of surface-bound RGD. First, no attachment or proliferation was observed over a 5 day period for hTCEpi cells on films treated with solutions of RGD ranging from 0 to 3% RGD/d-glucamine. Cells seeded on films functionalized using 3% RGD/d-glucamine solutions initially attached to the surface; however, by day 5, the hTCEpi cell number reduced by 75%, suggesting that the amount of immobilized RGD was not sufficient to sustain long-term attachment and subsequent proliferation (Figure 6). Second, films fabricated using solutions of 5 and 7% RGD/d-glucamine supported cell attachment but not proliferation. On 5% RGD/d-glucamine films, the total cell numbers per image remained constant over 5 days. From day 1 to day 5, a 2.4 fold increase in cell number was observed on 7% RGD/d-glucamine films, but this increase was not statistically different from cell proliferation on 5% RGD/d-glucamine films. Third, hTCEpi cells showed a monotonic increase in the rate of proliferation on surfaces fabricated from solutions containing 9 to 20% RGD/d-glucamine. A 3.5-fold increase in cell number was observed from day 1 to day 5 on films functionalized using solutions containing 9% RGD/d-glucamine. The highest rates of proliferation were observed on 15 and 20% RGD/d-glucamine films with average increases in cell number of 5 to 5.5-fold over 5 days, respectively, and were approaching rates observed for cells on TCPS. The data demonstrate that treatment with a solution containing a minimum of 9% RGD/d-glucamine is needed to produce substrates that will support hTCEpi proliferation.
Figure 6.
hTCEpi cells demonstrated proliferation rates dependent on the surface density of RGD. On 0 to 3% RGD/d-glucamine films, cells did not attach well. Those cells that initially attached to the surface ultimately detached over the course of 5 days. Cells on surfaces fabricated with 5% RGD/d-glucamine did not proliferate, while cells on surfaces fabricated with 7% RGD/d-glucamine showed a slight increase in cell numbers. The highest rates of proliferation were observed on surfaces fabricated from 9–20% RGD/d-glucamine solutions. (*0.01 ≤ P < 0.05, **0.001 ≤ P < 0.01, ***P < 0.001 compared to 0, 1, 3, and 5 % RGD from day 5)
Discussion
The results presented here demonstrate that amine-reactive PEI/PVDMA films can be functionalized with RGD/d-glucamine solutions, and that control over the relative surface densities of RGD in a background of d-glucamine can be used to mediate human corneal cell-substrate interactions. The relative amount of RGD on the surface is controlled by treatment of PEI/PVDMA films with solutions containing mixtures of a peptide containing the RGD sequence and d-glucamine. The primary amine groups present in these two molecules can react with azlactone-groups on the surface of the PEI/PVDMA films to promote covalent immobilization.30,31,35 We note that these two species compete for available azlactone-groups on the surface and potentially within the bulk of these films, but may not react at equal rates. Therefore, it was necessary at the outset of these studies to identify solutions of RGD and d-glucamine with optimal molar ratios of surface-bound RGD to elicit specific hTCEpi-substrate interactions. Using hTCEpi cell attachment, proliferation and competitive inhibition experiments as measurable end points, we demonstrate that cell-substrate interactions are mediated by the relative amount of RGD bound to the surface.
The monotonic increase in hTCEpi cell attachment we observed on films functionalized with solutions containing increasing percentages of RGD demonstrates that the relative RGD surface density is also monotonically increasing. Our observations are consistent with recent studies using different materials systems to vary the surface density of RGD in non-protein adsorbing backgrounds.3,7,11–13,27,40 Self-assembled monolayers (SAMs) of alkanethiols on gold substrates formed using mixtures of RGD-containing molecules and poly(ethylene glycol) promoted little to no cell attachment and spreading with RGD surface densities of 0.01 fmol/cm2 and less.11,40 However, a 10-fold increase in surface density of RGD promotes a 30-fold increase in cell attachment11 and doubles the number of fully spread cells.40 Likewise, cell attachment to polyelectrolyte multilayer (PEM) films presenting 4 to 25 pmol/cm2 RGD-containing ligands in an inert background of either poly(allylamine hydrochloride) (PAH) or hyaluronic acid (HA) demonstrated monotonic increases in cell attachment in response to incremental addition of surface-bound RGD.7,27 The results of these studies support our findings with hTCEpi cell attachment and demonstrate that a minimum surface density of RGD is required for cells to attach and that PEI/PVDMA multilayer films can be functionalized with increasing RGD-surface densities with a d-glucamine background by increasing the mole percentage of RGD in the solution mixtures.
The observation of increasing proliferation of hTCEpi cells on surfaces treated with solutions containing increasing RGD concentrations further demonstrates that PEI/PVDMA films can be functionalized with increasing surface densities of RGD. Similar proliferation data have been presented in previous reports demonstrating that increasing the surface density of RGD in a protein-resistant background will increase proliferation rates of many cell lines, including C2C12 skeletal myoblasts,3 human dermal fibroblasts,18 mouse osteoblast MC3T3-E1,7 and calvarial osteoblasts.4,6 As demonstrated in these reports, a minimum level of surface bound RGD is required to allow for cell proliferation. Similar to our observations on hTCEpi cells, further increases in the surface density of RGD result in increased rates of proliferation. Our results imply that PEI/PVDMA films prepared from reaction mixtures of RGD/d-glucamine have comparable increasing surface densities of RDG. The ability to tune the proliferation rate of corneal epithelial cells by varying the surface density of RGD on multilayer films that can conformally coat a range of topographically diverse materials provides opportunities to further optimize the design of biomaterials for cell culture systems and prosthetics.
The rate of hTCEpi cell rounding and detachment under competitive inhibition of RGD-cell binding depended on the amount of RGD used to treat the film surface, provided additional evidence that the relative surface density of RGD bound to PEI/PVDMA films can be modulated by changing the amount of RGD in the reaction mixture. Studies that support our findings have been demonstrated in other cell-types including endothelial cells,11 myoblasts3 and fibroblasts22,41 where cells attached to surfaces with higher densities of RGD required either longer incubation times or higher concentrations of soluble RGD to demonstrate specificity.
In addition to showing that the immobilization of RGD and d-glucamine to PEI/PVDMA films can be tuned to sufficiently support hTCEpi cell attachment and proliferation, our data also demonstrate that hTCEpi cells interact specifically with surface-bound RGD. First, hTCEpi cells did not attach to films functionalized with solutions containing mixtures of the scrambled peptide (RDG) and d-glucamine. Reports have demonstrated that cells do not attach to surfaces presenting RDG-containing peptides at surface densities comparable to surface densities of RGD that support cell attachment,14,17,21 indicating cells are specifically bound to RGD. Second, competitive inhibition of hTCEpi attachment to RGD-functionalized PEI/PVDMA films in the presence of soluble RGD indicates that the cells are specifically interacting with surface-bound-RGD. For example, when enough soluble RGD is presented to the cells, cell receptors will bind to the free peptides allowing for cell detachment. These results are similar to observations made in several other studies using RGD bound to SAMs,11,41 polyelectrolyte multilayers,27 and hydrogels3,22 that demonstrate specific cell-RGD attachment.
It is interesting to note that the quickest detachment of hTCEpi cells occurred on substrates that allow for initial cell attachment and not proliferation. This observation suggests that sufficient RGD-cell adhesion points and/or strong cell-substrate binding are essential for hTCEpi proliferation on these films. One indication that RGD-integrin binding, rather than just adhesion, plays a crucial role in cell proliferation is the observation in a study from Schuler et al. that osteoblasts proliferated at higher rates on RGD-containing surfaces compared to surfaces that present non-integrin binding42 Although proliferation responses can be dependent on cell type, it is likely that corneal epithelial cell proliferation is significantly regulated via pathways activated by RGD-integrin binding. This may explain why insignificant proliferation was observed for hTCEpi cells on surfaces that were functionalized with the minimum level of RGD to promote the onset of cell attachment.
As noted above, other approaches have been used to design materials that elicit controlled cell-substrate interactions upon immobilization of small molecule and/or macromolecular inhibitors of protein adsorption and cell adhesion. For example, surfaces that inhibit protein adsorption have been prepared using 1) self-assembled monolayers (SAMs) of oligo- and poly(ethylene glycol) (PEG) functionalized alkanethiols on gold,11,40 2) hydrogels comprised of poly(ethylene glycol),21,23,43 and 3) interpenetrating network (IPN) polymers in which the outermost surface of the film is poly(ethylene glycol).4,15,44 Several of these past studies have either measured or estimated the densities of non-fouling moieties presented on the surfaces of these materials. For example, in the case of self-assembled monolayers, quasi-crystalline packing of the alkane portion of the SAM results in a very high surface density (~100 pmol/cm2) of the PEG moieties. Although additional characterization will be required to understand the molecular-level structures of our PEI/PVDMA films, it is unlikely that our films present reactive azlactone groups at such a high density, or, as a result, that immobilized d-glucamine can self-organize to produce such highly dense surface structures.
In contrast to reports on SAMs, Hern and Hubbell have reported in past studies that the onset of cell attachment to RGD-functionalized PEG hydrogels occurs at surface densities on the order of pmol/cm2,21 providing a wide range of possible surface densities of RGD that promote cell attachment. We used several different spectroscopic methods (including X-ray photoelectron spectroscopy (XPS), polarization modulation infrared reflection adsorption spectroscopy (PM-IRRAS), and near edge X-ray absorption fine structure (NEXAFS) spectroscopy) in attempts to characterize the density of RGD groups presented in the surfaces of our treated films. Unfortunately, quantitative interpretations of the results of these experiments (e.g., based on changes in elemental composition or differences in amide functionality) were complicated by the presence of the underlying PEI/PVDMA films due to the presence of amide functionality in the films and the presence of abundant C, H, N, and O atoms. Additional quantitative experiments, such as the treatment of these reactive films with radiolabeled RGD motifs, will be required to characterize ligand densities on our films. However, the results of our current study demonstrate that post-fabrication treatment can be used to functionalize multilayer-coated surfaces with densities of both RGD and anti-fouling D-glucamine motifs that are appropriate to both (i) direct and (ii) modulate the attachment and proliferation of hTCEpi cells.
Methods for the layer-by-layer assembly of thin films with controlled cell-substrate interactions have potential use in a wide range of applications, including the development of thin film coatings for medical implants, biological sensors, and other biomaterials. The reactive PEI/PVDMA films used here are particularly interesting because they are physically robust, can be fabricated on a broad range of different substrate materials,31,35 can be removed in a controlled manner to produce reactive free-standing films,36 or modified to control other important surface properties such as hydorphobicity,31 and, as presented here, can be readily functionalized with biologically active molecules. Many bioactive molecules, including peptides, contain primary amines that can react readily with the azlactone-groups of our PEI/PVDMA films. One particular advantage of the azlactone reactive PEI/PVDMA film system used here, relative to other polyelectrolyte-based multilayers used in past studies for the immobilization of RGD peptides,7,27 is that peptides can be immobilized directly by reaction with residual azlactone functionality without the need for pre-treatment or initial modification to render the films reactive. Thus, PEI/PVDMA films can potentially be used to readily fabricate functionalized substrates that can be used to investigate the effects of a wide range of different peptides (in addition to RGD) or combinations of peptides on cell behaviors. This approach is also amenable to investigating the combinatorial effects of controlled surface-cell interactions and biophysical cues, such as topography (e.g., by depositing these reactive films on the surfaces of topographically-patterned substrates to investigate the influences of both chemical and topographic features on cell behavior).
Conclusions
We have demonstrated that amine-reactive PEI/PVDMA multilayers can be functionalized to control hTCEpi cell attachment via surface-bound RGD. Reaction mixtures containing different ratios of RGD to d-glucamine were used to fabricate surfaces that modulated hTCEpi cell attachment and proliferation. A minimum of 5% RGD was necessary to allow for hTCEpi cell attachment. At the minimum surface RGD density that allowed for cell attachment, cells did not proliferate. A minimum of 7–9% RGD was necessary to promote hTCEpi proliferation. We demonstrated, using two methods, that cells specifically attach to surface-bound RGD: (1) cells did not bind to surfaces treated with solutions containing a scrambled negative control peptide, RDG and (2) cells began to round and detach from RGD/d-glucamine-functionalized films upon the addition of soluble RGD to the culture medium. These results demonstrate the ability of RGD-functionalized PEI/PVDMA multilayer films to support controlled cell-substrate interactions for the use in the design of biomaterials and other biological applications.
Supplementary Material
Acknowledgements
The authors would like to thank Yaming Jiang for her technical support. The authors would also like to thank Dr. Maren Buck, Prof. William Murphy, Michelle Wilson and Dr. Christopher Thode for their helpful discussions. Support to P. F. N. and C. J. M. was provided by the NIH-National Eye Institute (1RO1EY017367-01A and 1RO1EY0161134-01A2) and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Eye Institute or the NIH. Support to D. M. L. was provided by the NSF (DMR-0520527) through a grant to the Materials Research Science and Engineering Center (MRSEC) at the University of Wisconsin. A. H. B. is a NSF Graduate Research Fellow.
References
- 1.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 2.Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 3.Rowley JA, Mooney DJ. Alginate type and RGD density control myoblast phenotype. Journal of Biomedical Materials Research. 2002;60(2):217–223. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]
- 4.Harbers GM, Healy KE. The effect of ligand type and density on osteoblast adhesion, proliferation, and matrix mineralization. Journal of Biomedical Materials Research Part A. 2005;75(4):855–869. doi: 10.1002/jbm.a.30482. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Ma L, Yang S, Shao Z, Meng C, Duan D, Li Y. Effect of RGD-modified silk material on the adhesion and proliferation of bone marrow-derived mesenchymal stem cells. Journal of Huazhong University of Science and Technology. Medical sciences. 2009;29(1):80–83. doi: 10.1007/s11596-009-0117-1. [DOI] [PubMed] [Google Scholar]
- 6.Lee KY, Alsberg E, Hsiong S, Comisar W, Linderman J, Ziff R, Mooney D. Nanoscale Adhesion Ligand Organization Regulates Osteoblast Proliferation and Differentiation. Nano Letters. 2004;4(8):1501–1506. doi: 10.1021/nl0493592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chua PH, Neoh KG, Kang ET, Wang W. Surface functionalization of titanium with hyaluronic acid/chitosan polyelectrolyte multilayers and RGD for promoting osteoblast functions and inhibiting bacterial adhesion. Biomaterials. 2008;29(10):1412–1421. doi: 10.1016/j.biomaterials.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Picart C, Elkaim R, Richert L, Audoin F, Arntz Y, Da Silva Cardoso M, Schaaf P, Voegel JC, Frisch B. Primary Cell Adhesion on RGD-Functionalized and Covalently Crosslinked Thin Polyelectrolyte Multilayer Films. Advanced Functional Materials. 2005;15(1):83–94. [Google Scholar]
- 9.Huang Z, Sargeant TD, Hulvat JF, Mata A, Bringas P, Jr, Koh CY, Stupp SI, Snead ML. Bioactive nanofibers instruct cells to proliferate and differentiate during enamel regeneration. Journal of Bone and Mineral Research. 2008;23(12):1995–2006. doi: 10.1359/JBMR.080705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fong E, Tzlil S, Tirrell DA. Boundary crossing in epithelial wound healing. Proc Natl Acad Sci U S A. 2010;107(45):19302–19307. doi: 10.1073/pnas.1008291107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts C, Chen CS, Mrksich M, Martichonok V, Ingber DE, Whitesides GM. Using Mixed Self-Assembled Monolayers Presenting RGD and (EG)3OH Groups To Characterize Long-Term Attachment of Bovine Capillary Endothelial Cells to Surfaces. Journal of the American Chemical Society. 1998;120(26):6548–6555. [Google Scholar]
- 12.Rezania A, Johnson R, Lefkow AR, Healy KE. Bioactivation of Metal Oxide Surfaces. 1. Surface Characterization and Cell Response. Langmuir. 1999;15(20):6931–6939. [Google Scholar]
- 13.Houseman BT, Mrksich M. The microenvironment of immobilized Arg-Gly-Asp peptides is an important determinant of cell adhesion. Biomaterials. 2001;22(9):943–955. doi: 10.1016/s0142-9612(00)00259-3. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y, Mrksich M. The synergy peptide PHSRN and the adhesion peptide RGD mediate cell adhesion through a common mechanism. Biochemistry. 2004;43(50):15811–15821. doi: 10.1021/bi049174+. [DOI] [PubMed] [Google Scholar]
- 15.Bearinger JP, Castner DG, Healy KE. Biomolecular modification of p(AAm-co-EG/AA) IPNs supports osteoblast adhesion and phenotypic expression. Journal of Biomaterials Science, Polymer Edition. 1998;9(7):629–652. doi: 10.1163/156856298x00064. [DOI] [PubMed] [Google Scholar]
- 16.Barber TA, Golledge SL, Castner DG, Healy KE. Peptide-modified p(AAm-co-EG/AAc) IPNs grafted to bulk titanium modulate osteoblast behavior in vitro. Journal of Biomedical Materials Research Part A. 2003;64(1):38–47. doi: 10.1002/jbm.a.10321. [DOI] [PubMed] [Google Scholar]
- 17.Park YD, Tirelli N, Hubbell JA. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials. 2003;24(6):893–900. doi: 10.1016/s0142-9612(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 18.Liu SQ, Rachel Ee PL, Ke CY, Hedrick JL, Yang YY. Biodegradable poly(ethylene glycol)-peptide hydrogels with well-defined structure and properties for cell delivery. Biomaterials. 2009;30(8):1453–1461. doi: 10.1016/j.biomaterials.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Gunn JW, Turner SD, Mann BK. Adhesive and mechanical properties of hydrogels influence neurite extension. Journal of Biomedical Materials Research Part A. 2005;72A(1):91–97. doi: 10.1002/jbm.a.30203. [DOI] [PubMed] [Google Scholar]
- 20.Peyton SR, Raub CB, Keschrumrus VP, Putnam AJ. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials. 2006;27(28):4881–4893. doi: 10.1016/j.biomaterials.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. Journal of Biomedical Materials Research. 1998;39(2):266–276. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 22.Halstenberg S, Panitch A, Rizzi S, Hall H, Hubbell JA. Biologically engineered protein-graft-poly(ethylene glycol) hydrogels: a cell adhesive and plasmin-degradable biosynthetic material for tissue repair. Biomacromolecules. 2002;3(4):710–723. doi: 10.1021/bm015629o. [DOI] [PubMed] [Google Scholar]
- 23.Jacob JT, Rochefort JR, Bi J, Gebhardt BM. Corneal epithelial cell growth over tethered-protein/peptide surface-modified hydrogels. Journal of Biomedical Materials Research. Part B Applied Biomaterials. 2005;72B(1):198–205. doi: 10.1002/jbm.b.30131. [DOI] [PubMed] [Google Scholar]
- 24.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294(5547):1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 25.Hosseinkhani H, Hosseinkhani M, Kobayashi H. Design of tissue-engineered nanoscaffold through self-assembly of peptide amphiphile. Journal of Bioactive and Compatible Polymers. 2006;21(4):277–296. [Google Scholar]
- 26.Sargeant TD, Rao MS, Koh CY, Stupp SI. Covalent functionalization of NiTi surfaces with bioactive peptide amphiphile nanofibers. Biomaterials. 2008;29(8):1085–1098. doi: 10.1016/j.biomaterials.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berg MC, Yang SY, Hammond PT, Rubner MF. Controlling mammalian cell interactions on patterned polyelectrolyte multilayer surfaces. Langmuir. 2004;20(4):1362–1368. doi: 10.1021/la0355489. [DOI] [PubMed] [Google Scholar]
- 28.Wan AC, Tai BC, Schumacher KM, Schumacher A, Chin SY, Ying JY. Polyelectrolyte complex membranes for specific cell adhesion. Langmuir. 2008;24(6):2611–2617. doi: 10.1021/la7025768. [DOI] [PubMed] [Google Scholar]
- 29.Reisch A, Voegel JC, Gonthier E, Decher G, Senger B, Schaaf P, Mesini PJ. Polyelectrolyte multilayers capped with polyelectrolytes bearing phosphorylcholine and triethylene glycol groups: parameters influencing antifouling properties. Langmuir. 2009;25(6):3610–3617. doi: 10.1021/la8037846. [DOI] [PubMed] [Google Scholar]
- 30.Buck ME, Breitbach AS, Belgrade SK, Blackwell HE, Lynn DM. Chemical modification of reactive multilayered films fabricated from poly(2-alkenyl azlactone)s: design of surfaces that prevent or promote mammalian cell adhesion and bacterial biofilm growth. Biomacromolecules. 2009;10(6):1564–1574. doi: 10.1021/bm9001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buck ME, Zhang J, Lynn DM. Layer-by-Layer Assembly of Reactive Ultrathin Films Mediated by Click-Type Reactions of Poly(2-Alkenyl Azlactone)s. Advanced Materials. 2007;19(22):3951–3955. [Google Scholar]
- 32.Rasmussen JK, Heilmann SM, Krepski LR, Jensen KM, Mickelson J, Johnson K, Coleman PL, Milbrath DS, Walker MM. Crosslinked, hydrophilic, azlactone-functional polymeric beads: A two-step approach. Reactive Polymers. 1992;16(2):199–212. [Google Scholar]
- 33.Heilmann SM, Rasmussen JK, Krepski LR. Chemistry and technology of 2-alkenyl azlactones. Journal of Polymer Science, Part A: Polymer Chemistry. 2001;39(21):3655–3677. [Google Scholar]
- 34.Buck ME, Lynn DM. Layer-by-Layer Fabrication of Covalently Crosslinked and Reactive Polymer Multilayers Using Azlactone-Functionalized Copolymers: A Platform for the Design of Functional Biointerfaces. Advanced Biomaterials. doi: 10.1002/adem.201080085. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buck ME, Lynn DM. Functionalization of Fibers Using Azlactone-Containing Polymers: Layer-by-Layer Fabrication of Reactive Thin Films on the Surfaces of Hair and Cellulose-Based Materials. ACS Applied Materials & Interfaces. 2010;2(5):1421–1429. doi: 10.1021/am1000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buck ME, Lynn DM. Reactive layer-by-layer assembly of suspended thin films and semipermeable membranes at interfaces created between aqueous and organic phases. Advanced Materials. 2010;22(9):994–998. doi: 10.1002/adma.200903054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robertson DM, Li L, Fisher S, Pearce VP, Shay JW, Wright WE, Cavanagh HD, Jester JV. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Investigative Ophthalmology and Visual Science. 2005;46(2):470–478. doi: 10.1167/iovs.04-0528. [DOI] [PubMed] [Google Scholar]
- 38.Allen-Hoffmann BL, Rheinwald JG. Polycyclic aromatic hydrocarbon mutagenesis of human epidermal keratinocytes in culture. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:7802–7806. doi: 10.1073/pnas.81.24.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabatini LM, Allen-Hoffmann BL, Warner TF, Azen EA. Serial Cultivation of Epithelial Cells from Human and Macaque Salivary Glands. In Vitro Cellular & Developmental Biology. 1991;27A(12):939–948. doi: 10.1007/BF02631121. [DOI] [PubMed] [Google Scholar]
- 40.Massia SP, Hubbell JA. An RGD spacing of 440 nm is sufficient for integrin alpha V beta 3-mediated fibroblast spreading and 140 nm for focal contact and stress fiber formation. Journal of Cell Biology. 1991;114(5):1089–1100. doi: 10.1083/jcb.114.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Massia SP, Hubbell JA. Covalent Surface Immobilization of Arg-Gly-Asp-Containing and Tyr-Ile-Gly-Ser-Arg-Containing Peptides to Obtain Well-Defined Cell-Adhesive Substrates. Analytical Biochemistry. 1990;187(2):292–301. doi: 10.1016/0003-2697(90)90459-m. [DOI] [PubMed] [Google Scholar]
- 42.Schuler M, Hamilton DW, Kunzler TP, Sprecher CM, de Wild M, Brunette DM, Textor M, Tosatti SG. Comparison of the response of cultured osteoblasts and osteoblasts outgrown from rat calvarial bone chips to nonfouling KRSR and FHRRIKA-peptide modified rough titanium surfaces. J Biomed Mater Res B Appl Biomater. 2009;91(2):517–527. doi: 10.1002/jbm.b.31425. [DOI] [PubMed] [Google Scholar]
- 43.Drumheller PD, Hubbell JA. Polymer networks with grafted cell adhesion peptides for highly biospecific cell adhesive substrates. Anal Biochem. 1994;222(2):380–388. doi: 10.1006/abio.1994.1506. [DOI] [PubMed] [Google Scholar]
- 44.Drumheller PD, Elbert DL, Hubbell JA. Multifunctional poly(ethylene glycol) semi-interpenetrating polymer networks as highly selective adhesive substrates for bioadhesive peptide grafting. Biotechnol Bioeng. 1994;43(8):772–780. doi: 10.1002/bit.260430812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.