Abstract
Quercetin (3,3',4',5,7-pentahydroxyflavone) is an attractive therapeutic flavonoid for cancer treatment because of its beneficial properties including apoptotic, antioxidant, and antiproliferative effects on cancer cells. However, the exact mechanism of action of quercetin on ion channel modulation is poorly understood in bladder cancer 253J cells. In this study, we demonstrated that large conductance Ca2+-activated K+ (BKCa) or MaxiK channels were functionally expressed in 253J cells, and quercetin increased BKCa current in a concentration dependent and reversible manner using a whole cell patch configuration. The half maximal activation concentration (IC50) of quercetin was 45.5±7.2 µM. The quercetin-evoked BKCa current was inhibited by tetraethylammonium (TEA; 5 mM) a non-specific BKCa blocker and iberiotoxin (IBX; 100 nM) a BKCa-specific blocker. Quercetin-induced membrane hyperpolarization was measured by fluorescence-activated cell sorting (FACS) with voltage sensitive dye, bis (1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC4(3); 100 nM). Quercetin-evoked hyperpolarization was prevented by TEA. Quercetin produced an antiproliferative effect (30.3±13.5%) which was recovered to 53.3±10.5% and 72.9±3.7% by TEA and IBX, respectively. Taken together our results indicate that activation of BKCa channels may be considered an important target related to the action of quercetin on human bladder cancer cells.
Keywords: Bladder cancer cell, Proliferation, Quercetin, BKCa, Voltage sensitive dye
INTRODUCTION
Quercetin (3,3',4',5,7-pentahydroxyflavone) belongs to a class of polyphenolic flavonoid compounds present in fruits and vegetables and is associated with a reduced risk of cancer [1]. Therefore, quercetin has received much attention as a promising anticancer drug with little toxicity when administered orally or intravenously [2,3]. The antitumor activity of quercetin has been tested in several cancers including breast [4], colon [5], gastric [6], leukemic [7] and bladder cells [8]. Anticancer actions were attributed to several cellular mechanisms including apoptosis by triggering the generation of ROS [9], and regulation of the cell cycle by modulating several molecular targets including p21 [10], cyclin B [11], p27 [12], and topoisomerase II [13,14]. However the ion channel-related cancer prevention cellular mechanism of quercetin is still not thoroughly understood, especially in bladder cancer cells. Although various expression and ion channel activity has been suggested to regulate proliferation, establishment, and progression of cancer [15], it would be worthwhile to investigate the precise ion channel-related mechanism of quercetin in bladder cancer cells.
Quercetin has been suggested as an activator or inhibitor in Ca2+ channels [16,17], Ca2+-activated K (KCa) channels [18,19], voltage-dependent K (KV) channels [20], CFTR (cystic fibrosis transmembrane regulator) chloride channels [21], and neurotransmitter-induced current [22]. Among these paths, the large conductance Ca2+-activated K+ (BKCa) channel has been theorized as having relevance to cell proliferation, but this assumption is still controversial [23-27].
Since adjuvant therapy is needed following resection of superficial bladder cancer, quercetin could be useful as adjuvant nutrition in bladder cancer treatment. Hence, the study of ion channels in relation to cancer is recognized as a potential breakthrough for cancer diagnosis and therapy.
Thus, we investigated the relevance of BKCa and quercetin in the proliferation of bladder cancer cells. We found that quercetin-evoked BKCa channels participated in growth inhibition of human bladder cancer cells.
METHODS
Cell cultures
Cell cultures from the Department of Urology at Chungbuk National University. The cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 units per ml penicillin, and 100 µg per ml streptomycin in a humidified incubator at 37℃ with 5% CO2.
Electrophysiology
Electrophysiological recording was performed in whole cell configurations [28] using a patch clamp amplifier (Axopatch 200B, Axon Instruments, Inc., Foster City, CA, USA). The patch pipettes were pulled from borosilicate capillaries (Harvard Apparatus Ltd., Edenbridge, Kent, UK) using a Narishige puller (PP-830, Tokyo, Japan). The patch pipettes used for whole cell configuration had a resistance of 2~3 megaohms when filled with the pipette solutions. All recordings were performed at room temperature (22~24℃). The normal Tyrode's (NT) bath solution contained (mM); 143 NaCl, 5.4 KCl, 0.5 NaH2PO4, 0.5 MgCl2, 1.8 CaCl2, 5 HEPES, and 10 glucose, adjusted to pH 7.4 with sodium hydroxide (NaOH). When whole cell configuration was performed, the high-K+ pipette solution contained (mM); 150 KCl, 1 MgCl2, 5 Mg-ATP, and 2 EGTA, titrated to pH 7.2 with potassium hydroxide (KOH). Quercetin solutions were made in test medium from 1,000 times concentrated stock solution in dimethyl sulfoxide (DMSO) in final concentrations up to 100 µM.
Membrane potential measurements by fluorescenceactivated cell sorting (FACS)
Acute changes in plasma membrane potential were measured by flow cytometry using bis (1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC4(3) dye; Invitrogen, Carlsbad, CA, USA). To measure the membrane potential in 253J cells, trypsinized cells was incubated in 100 nM DiBAC4(3) dye with NT solution for 10 min in the dark and then the mean fluorescence intensity (MFI) of the 253J cell population was analyzed with a FACSCalibur (Becton-Dickinson, San Jose, CA, USA) flow cytometer at a wavelength excitation of 488 nm. DiBAC4 (3) was prepared in DMSO according to the manufacturer's instructions. All flow cytometric analyses were accomplished using Cell Quest software (Becton- Dickinson San Jose, CA, USA). Hyperpolarization of membrane potential produced a decrease in MFI or a shift to the left of fluorescence peaks.
Reverse transcription-polymerase chain reaction analysis (RT-PCR)
Total RNA (1 µg) was used as a template with a firststrand cDNA synthesis kit, RT PreMix (Bioneer, Daejeon, Korea) for first-strand cDNA synthesis. RT-PCR was performed using 2 µl of cDNA in a 30 µl reaction containing 0.4 mM of each primer. The cycling conditions were as follows: 40 cycles at 96℃ for 15 s, 55℃ for 30 s, and 72℃ for 1 min. An aliquot (20 µl) of the RT-PCR product was analyzed on a 2% agarose gel prepared in Tris-acetic acid-EDTA (TAE). The identity of each of the PCR products was confirmed by sequence analysis. The sequences for primers were as follows: BKCa (Accession No; U11058), sense 5'acaacatctcccccaacc3', antisense 5'tcatcaccttctttccaattc3' (310 base pair [bp]); SK1 (Accession No; NM002248.3), sense 5'acccctaaatcttggccatcgt3', antisense 5'taggcgggtcctgctttattc3' (283 bp); SK2 (Accession No; NM021614.2), sense 5'cgacaagcacgtcacttacaa3', antisense 5'ctgacatcagaacccggataa3' (214 bp); SK3 (Accession No; NM002249.4), sense 5'aatctccgatagccccattg3', antisense 5'tcgcttcctgtcatctcctctt3' (311 bp); GAPDH (Accession No; J02642), sense 5'aacagcgacacccactcctc3', antisense 5'ggaggggagattcagtctggt3' (258 bp).
Proliferation assay
Cell proliferation was monitored using an XTT cell proliferation assay kit (Biological Industries Israel Beit-Haemek Ltd., Kibbutz Beit-Haemek, Israel). Approximately, 10,000 cells/ml of 253J cells were prepared in a 96-well plate containing 2% FBS, 100 units per ml penicillin, and 100 µg per ml streptomycin. 253J cells treated with quercetin and a K channel blocker were grown in 96-well plates for three days in a final volume of 100 µl/well of culture medium. After the incubation period, 50 µl of the XTT labeling mixture was add to each well and incubated for 4 hr. The absorbance of the samples was measured with a SpectraMax M5e spectrophotometer (Molecular Devices, Sunnyvale, CA, USA) at a wavelength of 450~500 nanometers. Reference absorbance to measure non-specific readings was measured with a wavelength of 630~690 nanometers.
RESULTS
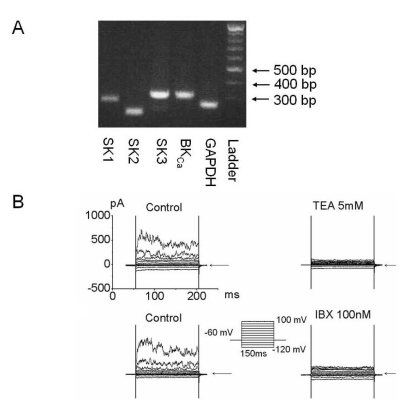
To investigate KCa channel functional expression, we performed RT-PCR and electrophysiological whole cell recording studies in 253J cells (Fig. 1). In RT-PCR analysis, BKCa, or Maxi K, or KCa 1.1, and small conductance Ca2+-activated K channel (SKCa) were expressed (Fig. 1A). In whole cell configuration, the outward current was produced by voltage step pulse from -120 mV to 100 mV for 150 ms with 20 mV increments and 10 sec intervals. The outward current was blocked by K channel blockers such as tetraethylammonium (TEA; 5 mM) and iberiotoxin (IBX; 100 nM) a BKCa channel blocker (Fig. 1B). These results showed that the outward current is mainly BKCa.
Fig. 1.
Functional expression of Ca2+-activated K current in bladder cancer cells. (A) Messenger RNA (mRNA) of ion channels related to the KCa currents was amplified by reverse transcription-polymerase chain reaction (RT-PCR) analysis. BKCa (310 base pair [bp]), SK1 (282 bp), SK2 (214 bp), and SK3 (311 bp) were detected and GAPDH (258 bp) was used as a positive control. (B) Characterization of Ca2+ activated K+ channels (KCa) using whole cell recording in 253J cells. Current held at -60 mV was applied by step pulse from -120 mV to 100 mV in increments of 20 mV for 150 ms. Pipette solutions contained 140 mM KCl, 1 mM MgCl2, 5 mM Mg-ATP, and 2 mM EGTA, and bath solutions contained 143 mM NaCl, 5.4 mM KCl, 0.5 mM NaH2PO4, 0.5 mM MgCl2, 1.8 mM CaCl2, 5 mM HEPES, and 10 mM glucose. Representative outward current was blocked by 5 mM tetraethylammonium (TEA) and 100 nM iberiotoxin (IBX), a MaxiK specific blocker. Arrow indicates zero current.
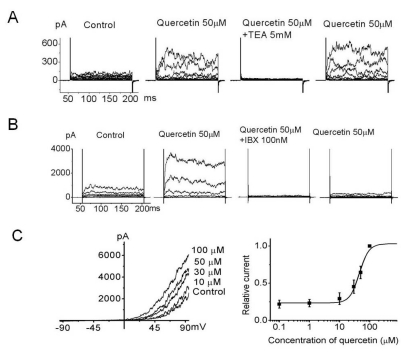
Many flavonoids are known to have anticancer properties; consequently, we screened flavonoids including EGCG, baicalein, curcumin, and quercetin for affects on the functional K channel in 253J cells. Among those analyzed, quercetin (50 µM) provoked a remarkable increase in the outward K current (Fig. 2) which was reversibly recovered with quercetin-free solution. Quercetin-evoked outward current was completely inhibited by TEA and was recovered after removing TEA (Fig. 2A). The quercetin-evoked outward current was also completely inhibited by IBX but was barely recovered with IBX-free solution (Fig. 2B). Other K channel blockers such as 4-aminopyridin (transient outward K channel blocker) and apamin (small conductance KCa blocker) did not affect the quercetin-evoked outward current (data not shown). This result suggests that the quercetin-evoked current was BKCa but not SKCa. We next tested the dose dependency of the effects of quercetin on 253J cells. A graded increase of quercetin concentrations (0.1, 1, 10, 30, 50, and 100 µM) produced an increase of outward current by ramp pulse from -100 to 100 mV at a holding potential of -60 mV (Fig. 2C). Relative current measured at 60 mV was plotted as a function of quercetin. Based upon the reasonable assumption that 100 µM quercetin produces maximal activation of KCa, averaged data from five patches were fitted to a Hill equation of the following form : y=1/(1+(K1/2/[Qu])n), where K1/2 is the apparent concentration of quercetin that produces half-maximal activation (IC50) and n is the Hill coefficient (K1/2=45.5 µM, n=2.7) (Fig. 2C, n=5).
Fig. 2.
Inhibition of quercetin-evoked outward current by a K channel blocker in 253J cells. Voltage steps of 150 ms duration were applied from -120 mV to 100 mV in 20 mV increments every 10 sec. (A) Outward current activated by 50 µM quercetin was inhibited by TEA and recovered after TEA washout. (B) The specific BKCa channel inhibitor, IBX, inhibited ~97% of the quercetin-evoked whole cell outward current and the current was almost irreversible with IBX-free solution including quercetin in bladder cancer cells. (C) Dosedependent effects of quercetin on bladder cancer cells at 60 mV were plotted (n=5 cells per concentration). Quercetin substantially activated the outward current dose dependently. Quercetin-evoked current continually recorded for 150 ms ramp pulse from -100 to 100 mV at a holding potential of -60 mV. The bath solution contained normal Tyrode's (NT) and 0 µM, 1 µM, 10 µM, 30 µM, 50 µM, and 100 µM quercetin. Each point represents the mean ± standard deviation of the mean (SD) (n=5).
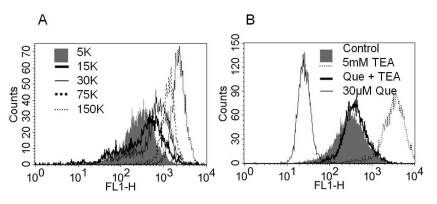
To observe changes in membrane potential with a K channel antagonist or quercetin, we investigated variations of MFI by FACS analysis using voltage sensitive dye DiBAC4(3) (100 nM) (Fig. 3). DiBAC4(3), the anionic bis-oxonol dye is known to accumulate in the cytoplasm of depolarized cells [29]. Therefore, in FACS analysis, depolarization causes an increase in MFI or a shift to the right of fluorescence peak. To test whether the dye could reflect a change in resting membrane potential, we applied various K concentrations. MFI was increased with increasing extracellular K concentrations (5 K, 15 K, 30 K, 75 K, and 150 K) (Fig. 3A). We also observed the effects of pharmacological drugs with DiBAC4(3) (Fig. 3B). MFI in TEA-treated cells increased compared to no blocker-treated cells (Fig. 3B). In contrast, MFI in quercetin-treated (30 µM) cells was decreased, indicating a hyperpolarization of membrane potential. The quercetin-evoked fluorescent peak was shifted right by TEA, indicating a depolarization compared to cells treated with quercetin alone (Fig. 3B). These results suggested hyperpolarization by quercetin might involve K channel activation.
Fig. 3.
The changes of membrane potential using voltage sensitive dye, bis (1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC4(3)) with various K+ concentrations, K+ channel blockers, and quercetin. Fluorescence-activated cell sorting (FACS) assay results from 253J cells loaded with DiBAC4(3) (100 nM) are shown. (A) As graded increase of KCl concentration mean fluorescence intensity (MFI) was increased (the peak shifted right) (B) Treatment with quercetin (30 µM) decreased DiBAC4(3) MFI (the peak shifted left). The application of TEA (5 mM, dotted line) in quercetin-free solution augmented MFI more than control (shaded bar), whereas co-treatment with TEA (5 mM) and quercetin (thick solid line) prevented quercetin-induced hyperpolarization (thin solid line).
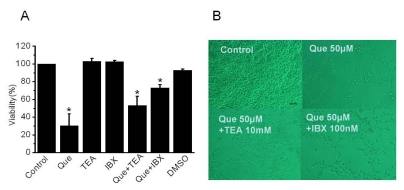
To explore the involvement of K channel on cell proliferation, we performed a proliferation test (XTT assay) in 253J cells (n=32). Cell viability was reduced to 30.3±13.5% (mean±standard error [SE]) in the presence of quercetin and quercetin-induced decreased cell viability was prevented by adding TEA (52.3±10.5%, mean±SE) or IBX (72.9±3.7%, mean±SE) in the presence of quercetin (Fig. 4). On the contrary, application of a K channel blocker in quercetin-free solution had little affect on cell viability.
Fig. 4.
The effect of proliferation by quercetin and K channel blockers in 253J cells (A) Cells were treated for three days with quercetin (50 µM) or various K channel blockers in the presence or absence of quercetin. After treatment, proliferation was detected by XTT assay. Error bars represent mean±standard error [SE] for 32 separate experiments. Dimethyl sulfoxide (DMSO) was used with the same volume of quercetin. Asterisks indicate values which are different from the respective control (t-test, p<0.05). (B) Effect of quercetin and K channel blocker on 253J cell growth and viability. Cells were grown in 2% serum culture media and captured 24 hrs after addition of channel blockers and quercetin using a Nikon microscope at 10×20 magnification. Scale bar, 200 µm
DISCUSSION
In the present study, we described functional expression of quercetin-evoked Ca2+-activated K+ channel (KCa) current. The current is IBX-sensitive BKCa which influences the proliferation of bladder cancer 253J cells.
Cellular mechanisms that link specific membrane K+ channels have been suggested to regulate cell growth. Maintaining the proper membrane voltage during proliferation is very important and mainly controlled by K+ channels [30]. Intracellular K+ depletion through K+ efflux by K+ channel activation is strongly related to apoptosis and proliferation by cell shrinkage [30].
Therefore, based on the FACS analysis and XTT assay (Fig. 3B, 4), our study demonstrated that quercetin-evoked hyperpolarization is due to K+ efflux through BKCa activation and may eventually trigger apoptosis or inhibition of tumor cell proliferation, at least in bladder cancer cells.
The involvement of BKCa in cell growth however is still controversial [26,27], although there is a close link between BKCa channels and cell proliferation [31,32] and several published data have shown involvement of BKCa channels in tumor growth in various malignant cell lines [33-36]. Abdullaev and colleagues [26] suggested that the BKCa channel lacked participation in cell proliferation in glioma cells. Roger and colleagues [27] proposed that the BKCa channel did not participated in cell proliferation under basal conditions in which the intracellular free Ca2+ is too low to activate BKCa. A latter report supports our present results with quercetin-untreated cells in that BKCa did not influence cell growth (Fig. 4) because TEA and IBX did not affect tumor cell proliferation in normal quercetin-free culture conditions. In addition, the suggestion that activation of BKCa in the presence of a Ca2+stimulant such as ATP could inhibit cell proliferation [27] was also similar to our results showing quercetin-evoked BKCa being involved in cell growth inhibition. Therefore, we assume that there are TEA- or IBX-mediated antiproliferative mechanisms present based on the fact that blockade of cancer BKCa channels produced cell cycle arrest or apoptosis only in the presence of a BKCa channel stimulant such as quercetin.
Bladder cancer is the seventh most common cancer in Korean men and a majority of bladder cancers are transitional cell carcinoma (TCC) [37]. Since most cases are superficial tumors which are limited to the mucosa, intravesical administration of BCG (bacillus Calmette-Guerin) after transurethral resection is by far the most effective treatment [38,39]. However, side effects with BCG therapy are common [39,40]. Therefore, it is desirable to develop a non-toxic adjuvant treatment for patients with a high risk of recurrence after tumor resection and intravesical chemotherapy. Determining the possible use of K+ channel modulators as a new class of anti-neoplastic drugs is considered a worthwhile endeavor.
In summary, large conductance Ca2+-activated K+ channel (BKCa) current influenced the proliferation of bladder cancer cells only in the presence of quercetin. These results suggested that the BKCa channel may represent a valuable target for the anti-proliferative effects of quercetin in 253J cells. Based upon these results we are interested in further confirming a role for K+ channels in mitogenesis and bladder cell growth.
ACKNOWLEDGEMENTS
We thank Hee-Kyung Jang for technical assistance. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No.2011-0001045) and Basic Science Research Program through the NRF funded by the Ministry of Education, Science and Technology (No.2010-0010719).
ABBREVIATIONS
- KCa
Ca2+-activated K+
- DiBAC4(3)
bis (1,3-dibutylbarbituric acid) trimethine oxonol
- IBX
iberiotoxin
- ROS
reactive oxygen species
- KV
voltage-dependent K
- CFTR
cystic fibrosis transmembrane regulator
- FACS
fluorescence-activated cell sorting
- MFI
mean fluorescence intensity
References
- 1.Hirpara KV, Aggarwal P, Mukherjee AJ, Joshi N, Burman AC. Quercetin and its derivatives: synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bio-availability. Anticancer Agents Med Chem. 2009;9:138–161. doi: 10.2174/187152009787313855. [DOI] [PubMed] [Google Scholar]
- 2.Okamoto T. Safety of quercetin for clinical application (Review) Int J Mol Med. 2005;16:275–278. [PubMed] [Google Scholar]
- 3.Ruiz MJ, Fernández M, Picó Y, Mañes J, Asensi M, Carda C, Asensio G, Estrela JM. Dietary administration of high doses of pterostilbene and quercetin to mice is not toxic. J Agric Food Chem. 2009;57:3180–3186. doi: 10.1021/jf803579e. [DOI] [PubMed] [Google Scholar]
- 4.Avila MA, Velasco JA, Cansado J, Notario V. Quercetin mediates the down-regulation of mutant p53 in the human breast cancer cell line MDA-MB468. Cancer Res. 1994;54:2424–2428. [PubMed] [Google Scholar]
- 5.Kim HJ, Kim SK, Kim BS, Lee SH, Park YS, Park BK, Kim SJ, Kim J, Choi C, Kim JS, Cho SD, Jung JW, Roh KH, Kang KS, Jung JY. Apoptotic effect of quercetin on HT-29 colon cancer cells via the AMPK signaling pathway. J Agric Food Chem. 2010;58:8643–8650. doi: 10.1021/jf101510z. [DOI] [PubMed] [Google Scholar]
- 6.Wang HY, Guo LM, Chen Y, Zhao XH, Cheng CL, Wu MY, He LY. Quercetin inhibits growth and induces apoptosis of human gastric carcinoma cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2006;22:585–587. [PubMed] [Google Scholar]
- 7.Monasterio A, Urdaci MC, Pinchuk IV, López-Moratalla N, Martínez-Irujo JJ. Flavonoids induce apoptosis in human leukemia U937 cells through caspase- and caspase-calpain-dependent pathways. Nutr Cancer. 2004;50:90–100. doi: 10.1207/s15327914nc5001_12. [DOI] [PubMed] [Google Scholar]
- 8.Ma L, Feugang JM, Konarski P, Wang J, Lu J, Fu S, Ma B, Tian B, Zou C, Wang Z. Growth inhibitory effects of quercetin on bladder cancer cell. Front Biosci. 2006;11:2275–2285. doi: 10.2741/1970. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Swarts SG, Yin L, Liu C, Tian Y, Cao Y, Swarts M, Yang S, Zhang SB, Zhang K, Ju S, Olek DJ, Jr, Schwartz L, Keng PC, Howell R, Zhang L, Okunieff P. Antioxidant properties of quercetin. Adv Exp Med Biol. 2011;701:283–289. doi: 10.1007/978-1-4419-7756-4_38. [DOI] [PubMed] [Google Scholar]
- 10.Moon SK, Cho GO, Jung SY, Gal SW, Kwon TK, Lee YC, Madamanchi NR, Kim CH. Quercetin exerts multiple inhibitory effects on vascular smooth muscle cells: role of ERK1/2, cell-cycle regulation, and matrix metalloproteinase-9. Biochem Biophys Res Commun. 2003;301:1069–1078. doi: 10.1016/s0006-291x(03)00091-3. [DOI] [PubMed] [Google Scholar]
- 11.Choi JA, Kim JY, Lee JY, Kang CM, Kwon HJ, Yoo YD, Kim TW, Lee YS, Lee SJ. Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. Int J Oncol. 2001;19:837–844. doi: 10.3892/ijo.19.4.837. [DOI] [PubMed] [Google Scholar]
- 12.Beniston RG, Campo MS. Quercetin elevates p27(Kip1) and arrests both primary and HPV16 E6/E7 transformed human keratinocytes in G1. Oncogene. 2003;22:5504–5514. doi: 10.1038/sj.onc.1206848. [DOI] [PubMed] [Google Scholar]
- 13.Mittra B, Saha A, Chowdhury AR, Pal C, Mandal S, Mukhopadhyay S, Bandyopadhyay S, Majumder HK. Luteolin, an abundant dietary component is a potent anti-leishmanial agent that acts by inducing topoisomerase II-mediated kinetoplast DNA cleavage leading to apoptosis. Mol Med. 2000;6:527–541. [PMC free article] [PubMed] [Google Scholar]
- 14.Vidya Priyadarsini R, Senthil Murugan R, Maitreyi S, Ramalingam K, Karunagaran D, Nagini S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur J Pharmacol. 2010;649:84–91. doi: 10.1016/j.ejphar.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z. Roles of K+ channels in regulating tumour cell proliferation and apoptosis. Pflugers Arch. 2004;448:274–286. doi: 10.1007/s00424-004-1258-5. [DOI] [PubMed] [Google Scholar]
- 16.Saponara S, Sgaragli G, Fusi F. Quercetin as a novel activator of L-type Ca(2+) channels in rat tail artery smooth muscle cells. Br J Pharmacol. 2002;135:1819–1827. doi: 10.1038/sj.bjp.0704631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saponara S, Sgaragli G, Fusi F. Quercetin antagonism of Bay K 8644 effects on rat tail artery L-type Ca2+ channels. Eur J Pharmacol. 2008;598:75–80. doi: 10.1016/j.ejphar.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Nishida S, Satoh H. Possible Involvement of Ca activated K channels, SK channel, in the quercetin-induced vasodilatation. Korean J Physiol Pharmacol. 2009;13:361–365. doi: 10.4196/kjpp.2009.13.5.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhlmann CR, Schaefer CA, Kosok C, Abdallah Y, Walther S, Lüdders DW, Neumann T, Tillmanns H, Schäfer C, Piper HM, Erdogan A. Quercetin-induced induction of the NO/cGMP pathway depends on Ca2+-activated K+ channel-induced hyperpolarization-mediated Ca2+-entry into cultured human endothelial cells. Planta Med. 2005;71:520–524. doi: 10.1055/s-2005-864152. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Ma JH, Zhang PH, Zou AR, Tu DN. Quercetin activates human Kv1.5 channels by a residue I502 in the S6 segment. Clin Exp Pharmacol Physiol. 2009;36:154–161. doi: 10.1111/j.1440-1681.2008.05061.x. [DOI] [PubMed] [Google Scholar]
- 21.Pyle LC, Fulton JC, Sloane PA, Backer K, Mazur M, Prasain J, Barnes S, Clancy JP, Rowe SM. Activation of the cystic fibrosis transmembrane conductance regulator by the flavonoid quercetin: potential use as a biomarker of ΔF508 cystic fibrosis transmembrane conductance regulator rescue. Am J Respir Cell Mol Biol. 2010;43:607–616. doi: 10.1165/rcmb.2009-0281OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee BH, Hwang SH, Choi SH, Shin TJ, Kang J, Lee SM, Nah SY. Quercetin Inhibits α3β4 Nicotinic acetylcholine receptormediated ion currents expressed in Xenopus oocytes. Korean J Physiol Pharmacol. 2011;15:17–22. doi: 10.4196/kjpp.2011.15.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouadid-Ahidouch H, Roudbaraki M, Ahidouch A, Delcourt P, Prevarskaya N. Cell-cycle-dependent expression of the large Ca2+-activated K+ channels in breast cancer cells. Biochem Biophys Res Commun. 2004;316:244–251. doi: 10.1016/j.bbrc.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 24.Han X, Xi L, Wang H, Huang X, Ma X, Han Z, Wu P, Ma X, Lu Y, Wang G, Zhou J, Ma D. The potassium ion channel opener NS1619 inhibits proliferation and induces apoptosis in A2780 ovarian cancer cells. Biochem Biophys Res Commun. 2008;375:205–209. doi: 10.1016/j.bbrc.2008.07.161. [DOI] [PubMed] [Google Scholar]
- 25.Cambien B, Rezzonico R, Vitale S, Rouzaire-Dubois B, Dubois JM, Barthel R, Karimdjee BS, Mograbi B, Schmid-Alliana A, Schmid-Antomarchi H. Silencing of hSlo potassium channels in human osteosarcoma cells promotes tumorigenesis. Int J Cancer. 2008;123:365–371. doi: 10.1002/ijc.23511. [DOI] [PubMed] [Google Scholar]
- 26.Abdullaev IF, Rudkouskaya A, Mongin AA, Kuo YH. Calciumactivated potassium channels BK and IK1 are functionally expressed in human gliomas but do not regulate cell proliferation. PLoS One. 2010;5:e12304. doi: 10.1371/journal.pone.0012304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roger S, Potier M, Vandier C, Le Guennec JY, Besson P. Description and role in proliferation of iberiotoxin-sensitive currents in different human mammary epithelial normal and cancerous cells. Biochim Biophys Acta. 2004;1667:190–199. doi: 10.1016/j.bbamem.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 29.Yamada A, Gaja N, Ohya S, Muraki K, Narita H, Ohwada T, Imaizumi Y. Usefulness and limitation of DiBAC4(3), a voltage-sensitive fluorescent dye, for the measurement of membrane potentials regulated by recombinant large conductance Ca2+-activated K+ channels in HEK293 cells. Jpn J Pharmacol. 2001;86:342–350. doi: 10.1254/jjp.86.342. [DOI] [PubMed] [Google Scholar]
- 30.Lang F, Föller M, Lang K, Lang P, Ritter M, Vereninov A, Szabo I, Huber SM, Gulbins E. Cell volume regulatory ion channels in cell proliferation and cell death. Methods Enzymol. 2007;428:209–225. doi: 10.1016/S0076-6879(07)28011-5. [DOI] [PubMed] [Google Scholar]
- 31.Huang MH, Wu SN, Chen CP, Shen AY. Inhibition of Ca2+-activated and voltage-dependent K+ currents by 2-mercaptophenyl-1,4-naphthoquinone in pituitary GH3 cells: contribution to its antiproliferative effect. Life Sci. 2002;70:1185–1203. doi: 10.1016/s0024-3205(01)01489-8. [DOI] [PubMed] [Google Scholar]
- 32.Ransom CB, Sontheimer H. BK channels in human glioma cells. J Neurophysiol. 2001;85:790–803. doi: 10.1152/jn.2001.85.2.790. [DOI] [PubMed] [Google Scholar]
- 33.Basrai D, Kraft R, Bollensdorff C, Liebmann L, Benndorf K, Patt S. BK channel blockers inhibit potassium-induced proliferation of human astrocytoma cells. Neuroreport. 2002;13:403–407. doi: 10.1097/00001756-200203250-00008. [DOI] [PubMed] [Google Scholar]
- 34.Weaver AK, Bomben VC, Sontheimer H. Expression and function of calcium-activated potassium channels in human glioma cells. Glia. 2006;54:223–233. doi: 10.1002/glia.20364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coiret G, Borowiec AS, Mariot P, Ouadid-Ahidouch H, Matifat F. The antiestrogen tamoxifen activates BK channels and stimulates proliferation of MCF-7 breast cancer cells. Mol Pharmacol. 2007;71:843–851. doi: 10.1124/mol.106.028290. [DOI] [PubMed] [Google Scholar]
- 36.Bloch M, Ousingsawat J, Simon R, Schraml P, Gasser TC, Mihatsch MJ, Kunzelmann K, Bubendorf L. KCNMA1 gene amplification promotes tumor cell proliferation in human prostate cancer. Oncogene. 2007;26:2525–2534. doi: 10.1038/sj.onc.1210036. [DOI] [PubMed] [Google Scholar]
- 37.Kakehi Y, Hirao Y, Kim WJ, Ozono S, Masumori N, Miyanaga N, Nasu Y, Yokomizo A. Bladder Cancer Working Group report. Jpn J Clin Oncol. 2010;40(Suppl 1):i57–i64. doi: 10.1093/jjco/hyq128. [DOI] [PubMed] [Google Scholar]
- 38.Shang PF, Kwong J, Wang ZP, Tian J, Jiang L, Yang K, Yue ZJ, Tian JQ. Intravesical Bacillus Calmette-Guérin versus epirubicin for Ta and T1 bladder cancer. Cochrane Database Syst Rev. 2011;(5):CD006885. doi: 10.1002/14651858.CD006885.pub2. [DOI] [PubMed] [Google Scholar]
- 39.Friberg S. BCG in the treatment of superficial cancer of the bladder: a review. Med Oncol Tumor Pharmacother. 1993;10:31–36. doi: 10.1007/BF02987766. [DOI] [PubMed] [Google Scholar]
- 40.Lockyer CR, Gillatt DA. BCG immunotherapy for superficial bladder cancer. J R Soc Med. 2001;94:119–123. doi: 10.1177/014107680109400305. [DOI] [PMC free article] [PubMed] [Google Scholar]