Abstract
Shilajit, a medicine herb commonly used in Ayurveda, has been reported to contain at least 85 minerals in ionic form that act on a variety of chemical, biological, and physical stressors. The substantia gelatinosa (SG) neurons of the trigeminal subnucleus caudalis (Vc) are involved in orofacial nociceptive processing. Shilajit has been reported to be an injury and muscular pain reliever but there have been few functional studies of the effect of Shilajit on the SG neurons of the Vc. Therefore, whole cell and gramicidin-perfotrated patch clamp studies were performed to examine the action mechanism of Shilajit on the SG neurons of Vc from mouse brainstem slices. In the whole cell patch clamp mode, Shilajit induced short-lived and repeatable inward currents under the condition of a high chloride pipette solution on all the SG neurons tested. The Shilajit-induced inward currents were concentration dependent and maintained in the presence of tetrodotoxin (TTX), a voltage gated Na+ channel blocker, CNQX, a non-NMDA glutamate receptor antagonist, and AP5, an NMDA receptor antagonist. The Shilajit-induced responses were partially suppressed by picrotoxin, a GABAA receptor antagonist, and totally blocked in the presence of strychnine, a glycine receptor antagonist, however not affected by mecamylamine hydrochloride (MCH), a nicotinic acetylcholine receptor antagonist. Under the potassium gluconate pipette solution at holding potential 0 mV, Shilajit induced repeatable outward current. These results show that Shilajit has inhibitory effects on the SG neurons of Vc through chloride ion channels by activation of the glycine receptor and GABAA receptor, indicating that Shilajit contains sedating ingredients for the central nervous system. These results also suggest that Shilajit may be a potential target for modulating orofacial pain processing.
Keywords: Substantia gelatinosa neurons, Shilajit, Patch clamp, Glycine receptor, GABAA receptor
INTRODUCTION
Shilajit (also spelt as Shilajeet or Salajeet) is a blackish-brown exudation of variable consistence, that is obtained from the steep rocks of different formations found in the Himalayas at altitudes between 1,000 to 5,000 meters on the walls of caves embedded in rocks or as rock exudates from Arunachal Pradesh in the East to Kashmir in the West [1-4]. It is also found in Afghanistan, Nepal, Pakistan, China, Tibet and former USSR [5]. Extensive research has been carried out to determine the exact chemical nature of Shilajit in the 1980s, the report showed that the major organic mass of Shilajit comprised humus (60~80%) along with other components such as benzoic acid, hippuric acid, etc [6]. The major physiological action of Shilajit was found to be due to the presence of the bioactive dibenzo-alpha-pyrones along with humic and fulvic acids [1]. Early Ayurvedic writings from the Charaka Samhita [7] described Shilajit as a cure for all disease as well as a Rasayana (rejuvenator) with promises to increase longevity. Shilajit has been used therapeutically for centuries as part of the traditional systems of medicine in many countries, for example, the treatment of genitourinary diseases, diabetes, angina, jaundice, digestive disorders, nervous diseases, chronic bronchitis, anemia, osteoporesis [8]. The substantia gelatinosa (SG), which is the lamina II layer of the trigeminal subnucleus caudalis (Vc), is a critical site for orofacial nociceptive processing because it receives synaptic inputs from the primary myelinated Aδ and unmyelinated C fibers [9]. The SG neurons function as excitatory and inhibitory interneuron, and regulate the output of the projection neurons in lamina I and IV, which transmits noxious information to a higher center [9-13]. Acharya et al. reported the Shilajit-induced analgesic effect using the technique of the hot wire induced tail-flick response. They also reported that Shilajit could play a role in inhibiting the development of analgesic tolerance to morphine [14]. Shilajit is also used as the antinociceptive medicine to moderate the intense muscula and injury pain [15]. These reports suggest that some of the ingredients of Shilajit have beneficial effects on pain control. Unfortunately, despite the huge number of natural remedies that have been in use for ages in Asian countries, there have been few systematic evaluations. In addition, it is unclear if Shilajit affects the activities of the SG neurons of Vc, which is involved in orofacial pain transmission in mice. This study examined the direct membrane currents induced by Shilajit and the related receptors using brain slice patch clamp technique.
METHODS
Brain slice preparation
All experiments were approved by the Experimental Animal Care and Ethics Committee of Chonbuk National University. The mice (Damul Science, Suwon, Korea) were housed under 12 h light: 12 h dark cycles (lights on at 07:00 h) with access to food and water ad libitum. Immature (postnatal days 5~15) male and female mice were decapitated between 10:00 and 12:00 h. The brains were removed rapidly and placed in ice-cold bicarbonate-buffered artificial cerebrospinal fluid (ACSF) with the following composition (in mM): 126 NaCl, 2.5 KCl, 2.4 CaCl2, 1.2 MgCl2, 11 D-glucose, 1.4 NaH2PO4 and 25 NaHCO3 (pH 7.4, bubbled with 95% O2 and 5% CO2). Coronal slices (150 µm thickness) containing the rostral part of the Vc (1~2 mm from obex) were then cut in ice-cold ACSF using a vibratome (Microm, Germany). The slices were allowed to recover in oxygenated ACSF for at least 1 hr at room temperature.
Whole-cell and gramicidin-perforated patch clamp recording
The brain slices were transferred to the recording chamber, held submerged and superfused continuously with ACSF at 4~5 ml/min. The slices were viewed using an upright microscope (BX51WI, Olympus, Tokyo, Japan) and Nomarski differential interference contrast optics. The patch pipettes were pulled from the thin-wall borosilicate glass-capillary tubing (PG52151-4, WPI, Sarasota, USA) on a Flaming puller (P-97, Sutter Instruments Co., Novato, CA). For whole cell recording, the pipette solution containing (in mM) 130 KCl (or 130 K-gluconate), 5 NaCl, 0.4 CaCl2, 1 MgCl2, 10 HEPES, 4 Mg-ATP and 1.1 EGTA (pH 7.3 with KOH) was passed through a disposable 0.22 µm filter. For perforated recording, the pipette solution was composed of (in mM) 130 KCl, 5 NaCl, 0.4 CaCl2, 1 MgCl2, 10 HEPES, 1.1 EGTA (pH 7.3 with KOH). Gramicidin (Sigma, St. Louis, USA) was first dissolved in dimethylsulfoxide (Sigma) to a concentration of 2.5~5 mg/ml. The solution was diluted to a final concentration of 2.5~5 µg/ml in the pipette solution immediately before use and sonicated for 10 min. A glass-capillary electrode was loaded with this pipette solution. The whole-cell and gramicidin-perforated patch clamp recordings were performed using an Axopatch 200B amplifier (Axon Instruments, Foster City, USA). The tip resistance of the electrode was 4~6 MΩ. The membrane currents and membrane potential changes were sampled online using a Digidata 1322A interface (Axon Instruments) connected to an IBM PC. The signals were filtered (2 kHz, Bessel filter of Axopatch 200B) before digitizing at a rate of 1 kHz. The acquisition and subsequent analysis of the acquired data were performed using Clampex9 software (Axon Instruments, USA). The traces were plotted using Origin7 software (MicroCal Software, Northampton, USA). All recordings were performed at room temperature.
Chemicals and statistics
Shilajit was purchased from Dekha Herbals (Lalitpur, Nepal). The test compounds were dissolved in an ACSF solution and tested by adding perfusing ACSF at known concentrations. AP5 (d,l-2-amino-5-phosphonopentanoic acid), strychnine, picrotoxin, tetrodotoxin, mecamylamine hydrochloride and the chemicals for ACSF were purchased from Sigma (USA). All values are expressed as the mean±S.E.M. A paired t-test or one sample t-test was used to examine the difference.
RESULTS
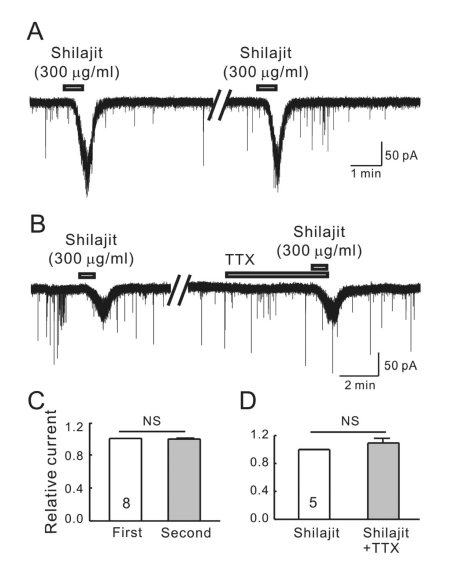
The SG (lamina II) area of the Vc is clearly visible as a translucent band, just medial to the spinal trigeminal tract and travels along the lateral edge of the slice. The whole-cell recordings were obtained from 25 SG neurons at a holding potential of -60 mV. Bath application of 300 µg/ml Shilajit caused inward currents in all neurons tested under the high chloride pipette solution (Fig. 1A). Shilajit (300 µg/ml) was applied successively to determine if the SG neurons are desensitized by the repeated application of Shilajit. In the 8 SG neurons tested, the successive application of Shilajit showed short-lived and repeatable inward currents. The mean inward current (-59.4±14.0 pA, n=8) by the second application of Shilajit was similar to that of the first application (-60.47±14.8 pA). The mean relative inward current induced by the second applied Shilajit was 0.99±0.03 (n=8, Fig. 1C, p>0.05) suggesting that the SG neurons of the Vc are not desensitized by the successive application of Shilajit. Fig. 1B shows a representative trace showing that the Shilajit-induced inward currents were not affected by tetrodotoxin (TTX), a voltage sensitive Na+ channel blocker. The relative inward current induced by Shilajit in the presence of TTX was 1.08±0.13 (n=6, Fig. 1D), suggesting that the Shilajit-induced currents are mediated by the postsynaptic cell actions rather than on the action potential from presynaptic mediated events.
Fig. 1.
Shilajit-induced currents are repeatable and mediated by postsynaptic cell actions on SG neurons. (A) A representative trace showing the repeatable inward currents induced by Shilajit (300 µ g/ml). (B) A representative trace showing the inward current induced by Shilajit (300 µg/ml) alone and in the presence of TTX (0.5 µM). (C) Relative bars showing a comparison of the membrane current changes by the repeated application of Shilajit (300 µg/ml, n=8, paired t-test). (D) Relative bars showing a comparison of the membrane current changes by the Shilajit alone and in the presence of TTX (300 µg/ml, n=5, paired t-test).
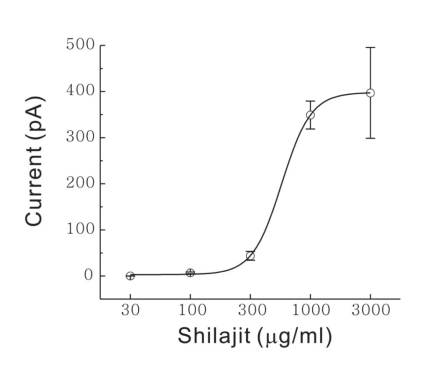
Fig. 2 shows the mean inward currents by Shilajit under each concentration (n=8). A concentration-response relationship existed in terms of the current change of SG neurons that responded to Shilajit (30 µg/ml, 0 pA; 100 µg/ml, -6.82±1.89 pA; 300 µg/ml, -43.8±9.47 pA; 1 mg/ml, -349.2±30.1 pA; 3 mg/ml, -397.1±98.4 pA, p<0.05). The EC50 was estimated to 562 µg/ml. This suggests that Shilajit acts directly on the postsynaptic SG neuron.
Fig. 2.
Shilajit-induced concentration dependent responses on SG neurons. Curve figure showing the response of 30, 100, 300 µg/ml and 1, 3 mg/ml Shilajit (n=8). EC50 was estimated to 562 µg/ml.
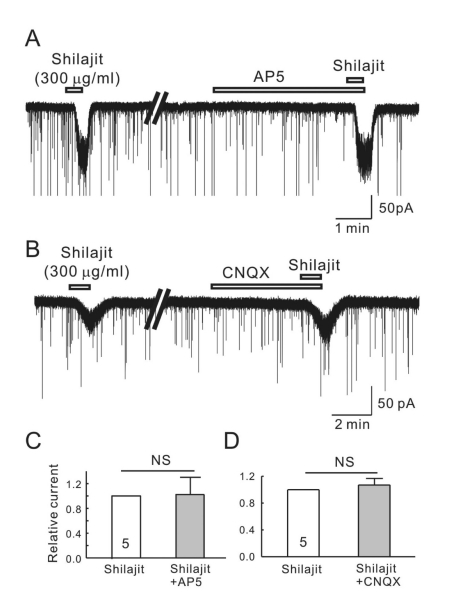
To determine if Shilajit-mediated inward currents are mediated by glutamate receptor activation, Shilajit was applied in the presence of AP5 (20µM), an NMDA receptor antagonist, and CNQX (10 µM), a non-NMDA glutamate receptor antagonist. As shown in Fig. 3A, B, neither AP5 nor CNQX affected the Shilajit-induced inward currents. The mean relative inward currents induced by Shilajit in the presence of AP5 and CNQX were 0.96±0.02 (Fig. 3C, n=5) and 0.94±0.08 (Fig. 3D, n=5), respectively. These results suggest that Shilajit does not target the glutamate receptors on the SG neurons of the Vc.
Fig. 3.
Shilajit-activated currents were not mediated by the NMDA and non-NMDA glutamate receptors on the SG neurons. (A, B) Representative traces showing the inward current induced by Shilajit application (300 µg/ml). The Shilajit induced inward current persisted in the presence AP5 (NMDA receptor antagonist) and CNQX (non-NMDA receptor antagonist). (C, D) Relative responses of Shilajit in the presence of AP5 or CNQX compared to Shilajit alone (n=5).
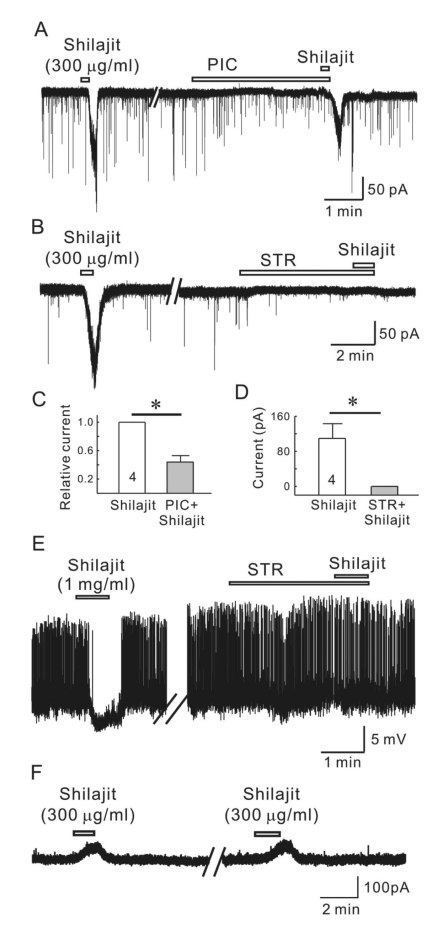
Shilajit was applied in the presence of picrotoxin, a GABAA receptor antagonist to determine if the Shilajit-induced inward currents are mediated by GABAA receptor activation. As shown in Fig. 4A, picrotoxin (50µM) partially blocked the Silajit-induced inward currents. The mean relative inward current induced by Shilajit in the presence of picrotoxin was 0.37±0.09 (n=4) that of Shilajit alone (p<0.05), suggesting Shilajit may target the GABAA receptors on the SG neurons of the Vc (Fig. 4C). In addition, Shilajit was applied in the presence of strychnine, a glycine receptor antagonist to determine if the Shilajit-mediated inward currents are mediated by glycine receptor activation. As shown in Fig. 4B and D, the Shilajit-induced inward currents were totally blocked by strychnine (2µM). In gramicidin-perforated current clamp mode, Shilajit (1 mg/ml) induced membrane hyperpolarization (-5.34±0.69 mV, n=3) with cessation of action potentials but failed to induce membrane hyperpolarization in the presence of strychnine in all the 3 neurons tested (at resting state, RMP, -53.91±1.97 mV) (Fig. 4E). To confirm the Shilajitinduced responses are mediated chloride ion movement, Shilajit was applied on SG neurons under the potassium gluconate pipette solution at VH=0 mV. As shown Fig. 4F, Shilajit induced outward currents in 3 of 3 neurons tested.
Fig. 4.
Silajit-induced inward currents are mediated by the activation of glycine and GABAA receptors. (A) Representative trace showing the inward current induced by Shilajit (300 µg/ml). Shilajit-induced inward current was reduced in the presence of picrotoxin (PIC, GABAA receptor antagonist). (B) A representative trace showing the inward current induced by Shilajit application (300 µg/ml) in whole-cell recording. The Shilajit-induced inward current was reduced in the presence of strychnine. (C) Relative response of Shilajit in the presence of PIC compared to Shilajit alone (n=4), *p<0.05. (D) Membrane current change in Shilajit alone and in the presence of strychnine (STR, a glycine receptor antagonist, n=4) *p<0.05. (E) A representative trace showing hyperpolarization induced by Shilajit application (1 mg/ml) in gramicidin-perforated recording (RMP=.54 mV). Shilajit-induced hyperpoalrization was blocked in the presence of strychnine. Membrane potential change in Shilajit alone and in the presence of strychnine (STR, n=3) *p<0.05. (F) A representative trace showing the repeatable outward currents induced by Shilajit (300 µg/ml) under potassium gluconate pipette solution at VH=0 mV.
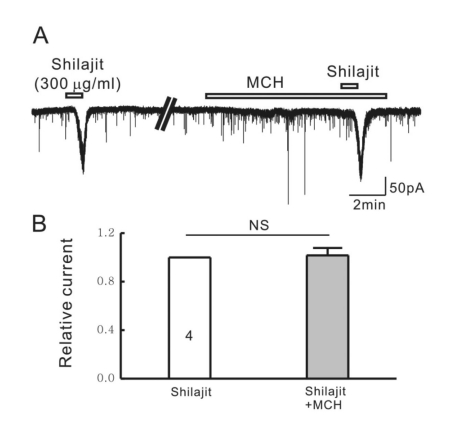
To exclude the possibility of the glycine release by activation of nicotinic acetylcholine receptors (nAchR), we applied Shilajit in the presence of mecamylamine hydrochloride (MCH, 10 µM), a non-selective nAChR antagonist. As shown in Fig. 5. MCH did not affect the Shilajit-induced inward currents.
Fig. 5.
Silajit-induced inward currents are not mediated by the activation of nACh receptors. (A) Representative trace showing the inward current induced by Shilajit (300 µg/ml). Shilajit-induced inward current was not affected in the presence of MCH (nACh receptor antagonist). (B) Relative response of Shilajit in the presence of MCH compared to Shilajit alone (n=4), *p<0.05.
DISCUSSION
These results demonstrate that Shilajit affects the SG neuronal activities by activating the glycine- and/or GABAA receptors. To our knowledge, this is the first report of the direct membrane effects of Shilajit in brain slices using the patch clamp technique. Bath application of Shilajit induced the reproducible and short lasting inward currents on the SG neurons following a dose manner under the high Cl- pipette solution. The inward currents persisted in the presence of TTX, a Na+ channel blocker, suggesting that Shilajit acts on the SG neuronal cells directly rather than on the presynaptic-mediated action potential mechanisms. The inward currents by Shilajit were suppressed by the GABAA receptor antagonist and glycine receptor antagonist, however not affected by the nACh receptors antagonist under the high Cl- pipette solution. In addition, Shilajit induced repeatable outward currents under potassium gluconate pipette solution at VH=0 mV suggesting that Shilajit exhibits GABA-, and glycine-mimetic actions through the GABAA and glycine receptors.
GABA and glycine are the chief inhibitory neurotransmitters in the mammalian central nervous system that regulate the excitability throughout the nervous system. The GABA actions are mediated by two classes of GABA receptors, the GABAA receptor, which is a ligand-gated chloride channel, and the GABAB receptors, which are G-protein coupled metabotropic receptors [16-18]. The action of GABA through the GABAA receptors depends on the membrane potential and intracellular chloride concentration [17]. In addition, it has been suggested that GABA agonists and GABA transporter inhibitor are very effective in alleviating mechanical allodynia in rats [19]. In whole cell mode, Shilajit evoked inward currents, which were blocked partly by the GABAA receptor antagonist, picrotoxin. Under this condition, intracellular Cl- should be very high because the membrane was ruptured and the patch pipette Cl- freely diffused into the cell [20]. Glycine mediates its effects through the chloride current by activation of the glycine receptors [21]. This is one of the most widely distributed inhibitory receptors in the central nervous system and plays important roles in a variety of physiological processes, particularly in mediating inhibitory neurotransmission in the spinal cord and brainstem [22]. Further, activation of the nAchR can induce the release of glycine and acts on glycine receptors [23]. In this study, the Shilajit-induced inward currents were blocked totally by the glycine receptor antagonist, strychnine, and maintained in the presence of nAChR antagonist MCH, suggesting that Shilajit activates the chloride ion channels through the glycine receptors. In addition, gramicidin-perforated and whole-cell (under the potassium gluconate pipette solution) patch clamp recoding were performed to confirm the involvement of chloride ion movement in the Shilajit-mediated response on SG neurons. The GABAA and glycine receptors are primarily permeable to chloride ions [24], and the use of gramicidin will allow recordings that leave the intracellular [Cl-] undisturbed [25,26]. In this case, Shilajit-induced membrane hyperpolarization was also blocked by strychnine. Overall, the Shilajit response on SG neurons occurs through chloride ion movement by the activation of GABAA and/or glycine receptors.
Shilajit is composed of humus and organic plant material that has been compressed by layers of rock mixed with microbial metabolites. There are four different varieties of Shilajit that have been described in charka samhita, namely savrana (gold Shilajit red in color), rajat (silver Shilajit color in white), tamra (cooper Shilajit color in blue) and lauha (iron-containing Shilajit brownish-black in color) [4]. Shilajit has been reported to contain more than 85 minerals in ionic form and humic substances (mainly fulvic and humic acid) [27]. Clinical research confirms that some ingredients of Shilajit are absorbed quickly through the intestinal tract and once in the systemic circulation, and can penetrate the blood-brain barrier [28]. Therefore, further studies will be needed to determine the actions of the Shilajit on intact SG neurons as well as the biochemical constituents involved.
In conclusion, these results show that Shilajit has inhibitory effects on the SG neurons and affects the SG neuronal activities of the Vc by activating the GABAA and/or glycine receptors. These results suggest that Shilajit may be a potential target for orofacial pain modulation. Further studies will be needed to isolate and identify the compounds associated with these Shilajit-mediated actions.
ACKNOWLEDGEMENTS
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0067150).
ABBREVIATIONS
- SG
substantia gelatinosa
- Vc
trigeminal subnucleus caudalis
- TTX
tetrodotoxin
- MCH
mecamylamine hydrochloride
- ACSF
artificial cerebrospinal fluid
- AP5
d,l-2-amino-5-phosphonopentanoic acid
- nAchR
nicotinic acetylcholine receptor
- PIC
picrotoxin
References
- 1.Ghosal S. Chemistry of shilajit, an immunomodulatory Ayurvedic Rasayana. Pure Appl Chem. 1990;62:1285–1288. [Google Scholar]
- 2.Ali M, Sahrawat I, Singh O. Phytochemical investigation of Shilajit. India J Chem. 2004;43B:2217–2222. [Google Scholar]
- 3.Garedew A, Feist M, Schmolz E, Lamprecht I. Thermal analysis of mumiyo, the legendary folk remedy from the Himalaya region. Thermochimica Acta. 2004;417:301–309. [Google Scholar]
- 4.Agarwal SP, Khanna R, Karmarkar R, Anwer MK, Khar RK. Shilajit: a review. Phytother Res. 2007;21:401–405. doi: 10.1002/ptr.2100. [DOI] [PubMed] [Google Scholar]
- 5.Jaiswal AK, Bhattacharya SK. Effects of Shilajit on memory, anxiety and brain monoamines in rats. Indian J Pharmacol. 1992;24:12–17. [Google Scholar]
- 6.Ghosal S, Singh SK, Srivastava RS. Shilajit part 2. Biphenyl metabolites from Trifolium repens. J Chem Res. 1988;196:165–166. [Google Scholar]
- 7.Charaka S, Sharma PV. Karaprchitiya RasayanaPada. 4th ed. Varanasi: Chowkhambha orientalia Chikitsasthana; 1998. pp. 49–50. [Google Scholar]
- 8.Schepetkin I, Khlebnikov A, Kwon BS. Medical drugs from humus matter: focus on mumie. Drug Dev Res. 2002;57:140–159. [Google Scholar]
- 9.Sessle BJ. Mechanisms of trigeminal and occipital pain. Pain Rev. 1996;3:91–116. [Google Scholar]
- 10.Gobel S. Golgi studies of the neurons in layer II of the dorsal horn of the medulla (trigeminal nucleus caudalis) J Comp Neurol. 1978;180:395–413. doi: 10.1002/cne.901800213. [DOI] [PubMed] [Google Scholar]
- 11.Light AR, Perl ER. Reexamination of the dorsal root projection to the spinal dorsal horn including observations on the differential termination of coarse and fine fibers. J Comp Neurol. 1979;186:117–131. doi: 10.1002/cne.901860202. [DOI] [PubMed] [Google Scholar]
- 12.Light AR, Kavookjian AM. Morphology and ultrastructure of physiologically identified substantia gelatinosa (lamina II) neurons with axons that terminate in deeper dorsal horn laminae (III-V) J Comp Neurol. 1988;267:172–189. doi: 10.1002/cne.902670203. [DOI] [PubMed] [Google Scholar]
- 13.Pan YZ, Pan HL. Primary afferent stimulation differentially potentiates excitatory and inhibitory inputs to spinal lamina II outer and inner neurons. J Neurophysiol. 2004;91:2413–2421. doi: 10.1152/jn.01242.2003. [DOI] [PubMed] [Google Scholar]
- 14.Acharya SB, Frotan MH, Goel RK, Tripathi SK, Das PK. Pharmacological actions of Shilajit. Indian J Exp Biol. 1988;26:775–777. [PubMed] [Google Scholar]
- 15.Tiwari P, Ramarao P, Ghosal S. Effects of Shilajit on the development of tolerance to morphine in mice. Phytother Res. 2001;15:177–179. doi: 10.1002/ptr.857. [DOI] [PubMed] [Google Scholar]
- 16.Kuffler SW, Edwards C. Mechanism of gamma aminobutyric acid (GABA) action and its relation to synaptic inhibition. J Neurophysiol. 1958;21:589–610. doi: 10.1152/jn.1958.21.6.589. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi A, Onodera K. Effect of bicuculline on the GABA receptor of the crayfish neuromuscular junction. Nat New Biol. 1972;236:55–56. doi: 10.1038/newbio236055a0. [DOI] [PubMed] [Google Scholar]
- 18.Bowery NG, Hill DR, Hudson AL, Doble A, Middlemiss DN, Shaw J, Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980;283:92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Back SK, Lim EJ, Cho GC, Kim MA, Kim HJ, Lee MH, Na HS. Are spinal GABAergic elements related to the manifestation of neuropathic pain in rat? Korean J Physiol Pharmacol. 2010;14:59–69. doi: 10.4196/kjpp.2010.14.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebihara S, Shirato K, Harata N, Akaike N. Gramicidin-perforated patch recording: GABA response in mammalian neurones with intact intracellular chloride. J Physiol. 1995;484:77–86. doi: 10.1113/jphysiol.1995.sp020649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajendra S, Lynch JW, Schofield PR. The glycine receptor. Pharmacol Ther. 1997;73:121–146. doi: 10.1016/s0163-7258(96)00163-5. [DOI] [PubMed] [Google Scholar]
- 22.Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- 23.Bradaïa A, Trouslard J. Nicotinic receptors regulate the release of glycine onto lamina X neurones of the rat spinal cord. Neuropharmacology. 2002;43:1044–1054. doi: 10.1016/s0028-3908(02)00121-1. [DOI] [PubMed] [Google Scholar]
- 24.Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers VB, Haydon DA. Ion transfer across lipid membranes in the presence of gramicidin A. II. The ion selectivity. Biochim Biophys Acta. 1972;274:313–322. doi: 10.1016/0005-2736(72)90179-4. [DOI] [PubMed] [Google Scholar]
- 26.Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J Neurosci Methods. 1995;57:27–35. doi: 10.1016/0165-0270(94)00116-x. [DOI] [PubMed] [Google Scholar]
- 27.Bhattacharya SK, Ghosal S. Effect of Shilajit on rat brain monoamines. Phytotherapy Res. 1992;6:163–164. [Google Scholar]
- 28.Kreuter J, Shamenkov D, Petrov V, Ramge P, Cychutek K, Koch-Brandt C, Alyautdin R. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J Drug Target. 2002;10:317–325. doi: 10.1080/10611860290031877. [DOI] [PubMed] [Google Scholar]