Abstract
Calcineurin (CaN) is activated in diabetes and plays a role in glomerular hypertrophy and extracellular matrix (ECM) accumulation. Here, kidneys from diabetic model mice were investigated for the expression of the regulator of CaN 1 (RCAN1) isoform 4 (RCAN1.4) which had been shown to be transcriptionally upregulated by CaN activation. We found the increased immunoreactivity for RCAN1 in the glomerular cells of db/db mice and streptozotocin-induced diabetic mice. In concordance, the expression of RCAN1 protein and RCAN1.4 mRNA were elevated in the whole kidney sample from db/db mice. Interleukin-1β (IL-1β), tumor necrosis factor-α, and glycated albumin (AGE-BSA) were identified as inducers of RCAN1.4 in mesangial cells. Pretreatment of cyclosporine A blocked the increases of RCAN1.4 stimulated by IL-1β or AGE-BSA, suggesting that activation of CaN is required for the RCAN1.4 induction. Stable transfection of RCAN1.4 in Mes-13 mesangial cells upregulated several factors relevant to ECM production and degradation. These results suggested that RCAN1.4 might act as a link between CaN activation and ECM turnover in diabetic nephropathy.
Keywords: RCAN1, Calcineurin, Mesangial cell, Diabetic nephropathy, Extracellular matrix
INTRODUCTION
Diabetic nephropathy is characterized histologically by an accumulation of excessive extracellular matrix protein (ECM) in the glomerular interstitium [1,2]. This abnormality could be caused by functional changes in diabetic glomeruli, particularly in glomerular mesangial cells, which were found to be capable of producing ECM proteins. Elevated blood glucose concentrations [3], advanced glycation endproducts (AGEs) [4], oxidation of renal glycoproteins [5], and a range of proteins implicated in inflammation and ECM turnover are involved in this pathological abnormality [6-8]. However, only limited information is available about the role of intracellular signaling molecules. Studies in humans and in animal models of renal hypertrophy have indicated that early hypertrophy and ECM accumulation are potentially reversible [9]. Therefore, an increased understanding of the changes in signaling pathways involved in diabetes may facilitate the development of therapies for preventing or reversing renal hypertrophy.
Calcineurin (CaN) is a calcium-dependent, serine/threonine phosphatase that has been implicated in ECM accumulation in diabetic nephropathy. CaN activation and nuclear localization of its substrate, the nuclear factor of activated T cells (NFAT), were observed in kidney tissues of streptozotocin (STZ)-induced diabetic rats [10]. Inhibition of CaN by cyclosporine A (CsA) injections, beginning at the time of STZ treatment, prevented renal hypertrophy and glomerular ECM accumulation. Moreover, insulin-like growth factor-I [11] and transforming growth factor β (TGF-β) [12] require activation of the CaN in ECM production in cultured mesangial cells. This suggested that CaN may be a common signaling mechanism for some fibrogenic stimuli in the pathogenesis of diabetic nephropathy.
CaN activity is negatively regulated by several endogenous inhibitory mechanisms [13]. The best studied is the human regulator of calcineurin 1 (RCAN1) protein, which binds to the catalytic subunit of CaN and inhibits its phosphatase activity [14]. The RCAN1 gene was localized near the Down Syndrome Critical Region on chromosome 21, and it generates four different transcripts (isoforms 1 through 4) [15]. However, only RCAN isoform 1 (RCAN1.1) and isoform 4 (RCAN1.4) proteins have been detected in tissues [16]. Expression is differently regulated for these two isoforms. The expression of RCAN1.1 was shown to be constitutive, but the transcription of RCAN1.4 could be induced by diverse mitogens and inflammatory cytokines, including vascular endothelial growth factor (VEGF) [17], transforming growth factor-β1 (TGF-β1) [18], interleukine-1 (IL-1), tumor necrosis factor-α (TNF-α) [19], and reactive oxygen species [20]. These molecules have also been causatively implicated in diabetic nephropathy [6]; therefore, we were interested in the role of RCAN1.4 in the development of this disease. The RCAN1.4 protein is a highly expressed in fetal and adult kidneys [16]. Thus, the first aim of our study was to examine whether expression of RCAN1.4 might be altered in the kidneys of diabetic mouse models; our second aim was to identify any molecules that affected the expression of RCAN1.4. In addition, we investigated the effects of chronic overexpression of RCAN1.4 on ECM production by establishing a stable Mes-13 cell line transfected with the RCAN1.4 expression vector.
METHODS
Chemicals and antibodies
STZ, TGF-β1, angiotensin II (ANG II), VEGF, IL-1β, TNF-α, monocyte chemotactic protein-1 (MCP-1), glycated bovine albumin (AGE-BSA), mannitol, and D-glucose were purchased from Sigma (St. Louis, MO, USA). CsA was obtained from A.G.Scientific (San Diego, CA, USA), and H2O2 was from Junsei (Tokyo, Japan). An antibody specific for RCAN1was purchased from Novus Biologicals (Littleton, CO, USA).
Animal models
All animal procedures were approved by the Ethics Committee of the Catholic University of Korea (CUMC-2011-0038-02) and carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the U.S. Institutes of Health (National Institutes of Health Publication No. 85~23, revised 1996).
To obtain the type 1 diabetic mouse model, 8 to 9 week-old, male, C57BL/6 mice (Koatec, Kyungki, Korea) were injected intraperitoneally with STZ dissolved in sterile citrate buffer (0.05 mol/l sodium citrate, pH 4.5, 60 mg/kg) for 5 consecutive days during the first week of the study. Control mice were injected with citrate buffer alone. Mice with a blood glucose level >280 mg/dl were considered diabetic [21]; these mice were sacrificed for kidney isolation at 16 weeks after STZ treatment. For the type 2 diabetic mouse model, we purchased male, C57BL/KsJ-leprdb/leprdb, 6-week old diabetic mice (db/db) from Jackson Laboratories (Bar Harbor, ME, USA). We also purchased their non-diabetic heterozygote littermates (db/m) to serve as controls. This group of mice was sacrificed at 20 weeks of age.
Cell culture and treatments
Mes-13 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in a 3 : 1 mixture of Dulbecco's Modified Eagle's Medium and Ham's F12 medium supplemented with 14 mM HEPES and 5% fetal bovine serum and maintained under an atmosphere of 5% CO2 at 37℃.
Isolation of mesangial cells from the male C57BL/6 mice was based on a method in which the glomeruli were digested with collagenase [22]. Briefly, the kidneys were removed and dissected free of the capsule and medulla. The cortex was homogenized with a scalpel, passed over successive sieves with pores of 230~104 µm (Sigma, St. Louis, MO, USA), and collected on a final sieve with a pore size of 73.7 µm. The tissue (containing the glomeruli) was collected, washed with phosphate buffered saline (PBS), and digested with 2.5% collagenase (184 U/ml; Worthington type CLS IV) in PBS for 15~25 min at 37℃. The glomeruli were washed in growth medium (DMEM, 55%; Ham's F12, 20%; l-glutamine, 2 mM; trace elements, 1%; transferrin, 5 mg/ml; insulin, 125 U/ml; FBS, 20%; 500 U/ml penicillin G/500 U/ml streptomycin sulfate/2 mg/ml amphotericin B), seeded in culture dishes, and incubated in growth medium at 37℃ for 10~14 days. For experiments, cells (5×105 per well) were plated in a six-well culture dish and incubated for 24 h in the complete medium and then another 24 h in serum-reduced condition (0.1% FBS) to attain quiescence. For RCAN1.4 induction experiments, the cells were stimulated with TGFβ1 (4 ng/ml), ANG II (2 µM), VEGF (10 ng/ml), IL-1β (20 ng/ml), TNF-α (20 ng/ml), MCP-1 (100 ng/ml), H2O2 (300 µM), AGE-BSA (50 µg/ml), BSA (50 µg/ml; as a control of AGE-BSA), or cultured for 48h in high glucose condition (33 mM) or in osmotically balanced control medium (5.9 mM glucose plus 27 mM mannitol).
Creation of stable cell lines
Parallel cultures of Mes-13 cells were transfected with vectors (pcDNA3.1/NT-GFP-TOPO; Invitrogen, CA, USA) that contained the genes encoding green fluorescent protein (GFP) fused to RCAN1.4 or a vector with GFP alone [23]. Transfections were performed with Lipofectamine 2,000 (Invitrogen, USA) according to the manufacturer's instructions. After selection against 400 µg/ml G418 (Roche Applied Science, Germany) for 2 weeks, GFP-positive populations were purified on a FACS Calibur machine (BD Biosciences, USA). The selected cells were maintained in complete medium supplemented with 100 µg/ml G418. Transcripts of RCAN1.4 constructs in these cells were confirmed by reverse transcription-PCR amplification (data not shown).
Real-time RT-PCR
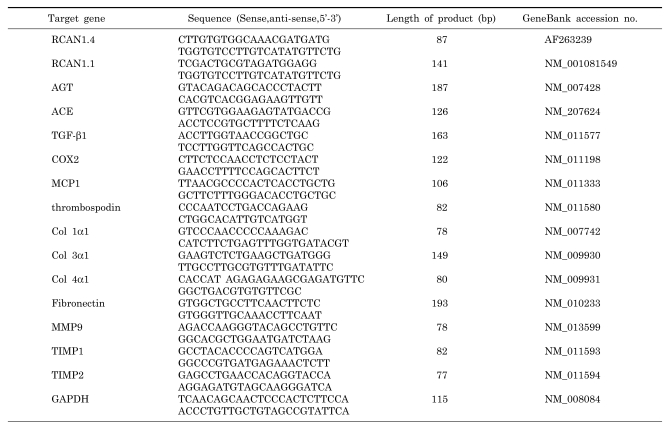
The kidneys of the db/m and db/db mice were homogenized and total RNA was isolated with the RNA queous-Micro kit (Ambion, Austin, TX, USA). The mRNA was prepared from mouse primary mesangial cells and Mes-13 cells with the RNeasy Plus Mini Kit (Qiagen, Germany) according to the manufacturer's instructions. Levels of mRNA were determined by a one-step quantitative RT-PCR protocol with the FullVelocity SYBR® Green QRT-PCR Master Mix (Stratagene, La Jolla, CA, USA) and the Mx3000P system (Stratagene) [24]. The primer sequences are shown in Table 1.
Table 1.
Primer sequences used for real-time RT PCR
Immunohistochemistry
Mice were sacrificed by transaortic perfusion with saline and subsequent 4% paraformaldehyde in PBS. The kidneys were removed, and post-fixed for 4 h in the same fixative, then embedded in Tissue-Tek (Sakura Fine technical, Tokyo, Japan). Free-floating sections (20 µm thick) were incubated overnight at 4℃ with an antibody to RCAN1 raised commercially (1 : 100; Novus Biologicals, Littleton, CO, USA). The sections were subsequently incubated with anti-rabbit IgG (1 : 500; Jackson Immunoresearch, West Grove, PA, USA) for 2 h at room temperature. Finally, all the reactions were visualized with 0.05% diaminobenzidine tetrahydrochloride and 0.005% H2O2 in 0.05 M Tris-HCl (pH 7.4).
Western blot analysis
Tissues were quick frozen in liquid nitrogen, pulverized in a liquid nitrogen-cooled mortar and pestle, and solubilized in lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100, 5 mM sodium fluoride, 1 mM sodium orthovanadate, and protease inhibitor mixture). After insoluble material had been removed by centrifugation, the supernatants were denatured in Laemmli sample buffer. The proteins were separated by SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked for 1 h at room temperature in 1% (w/v) Hammersten grade casein in phosphate-buffered saline containing 0.05% Tween 20. Immunoblotting was performed with anti-RCAN1 in 0.5% casein. The blots were incubated with horseradish peroxidase-conjugated anti-IgG secondary antibody (Vector Laboratories, Burlingame, CA, USA), washed, and then visualized with the Super Signal West Dura chemiluminescence substrate (Pierce Biotechnology, Rockford, IL, USA). The band intensity was analyzed with an LAS-3000 image analyzer (Fujifilm, Tokyo, Japan).
RESULTS
RCAN1.4 was upregulated in the kidneys of db/ db and STZ-induced diabetic mice
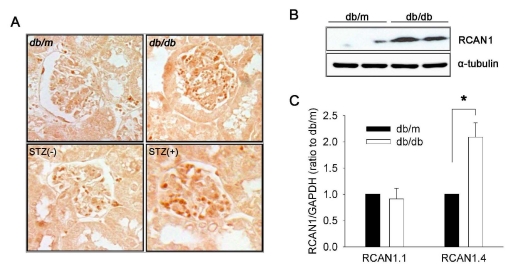
We assessed RCAN1 expression in mouse kidneys by immunohistochemistry with an antibody raised against a portion of the C-terminus of RCAN1. This region is common to all isoforms [16]. Therefore, the antibody was expected to recognize all forms of the RCAN1 protein. Based on the intensity and number of immunoreactive cells (Fig. 1A), the level of RCAN1 expression in the kidneys of db/db [25] and STZ-induced diabetic mice was elevated compared to that of age matched-control db/m and c57BL mice, respectively. The immunoreactive cells were mainly found in the glomeruli. Consistent with the immunohistochemistry, western blot analysis of whole kidney homogenates showed a much higher level of RCAN1 protein expression in db/db mice than db/m mice (Fig. 1B). To determine which isoforms of RCAN1 were expressed, total RNA samples were prepared from the kidneys and subjected to a quantitative real-time PCR analysis with two primers; one recognized a region specific to isoform 1, and the other recognized a region specific to isoform 4. This revealed that the mRNA level of RCAN1.4 was elevated in db/db mice compared to that in db/m mice by approximately two fold. In contrast, there was no significant difference in RCAN1.1 mRNA levels between the two groups (Fig. 1C).
Fig. 1.
RCAN1.4 expression in the kidneys of diabetic mouse models. (A) Representative photomicrographs of RCAN1 immunohistochemistry in the glomerulus of kidney sections that were obtained from diabetic (db/db) and non-diabetic (db/m) mice at the age of 20 weeks, and from diabetic (STZ+) and non-diabetic (STZ-) C57BL mice at 16 weeks after treatment (400×). (B) Western blot analysis shows increased levels of RCAN1 protein in the whole kidney homogenates of diabetic (db/db) mice compared to non-diabetic (db/m) mice. Alpha-tubulin (α-tubulin) served as a loading control. (C) Specific induction of RCAN1.4 mRNA. The relative amount of mRNAs was estimated by real-time PCR, normalized by GAPDH, and the values for db/db mice (open bars) are relative to the expression observed in db/m mice (filled bars; arbitrarily designated a value of 1). Data are means±SE of five experiments. *p<0.01, db/m vs. db/db.
RCAN1.4 transcription was induced by IL-1β, TNF α, and ACEs
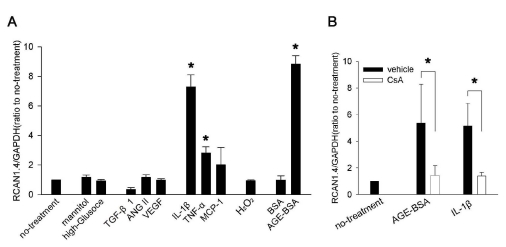
To identify the factors responsible for the induction of RCAN1.4, we stimulated mouse mesangial cells for 24 h with molecules that were causatively implicated in diabetic nephropathy, and then examined RCAN1.4 mRNA levels (Fig. 2A). Among the treatments, IL-1β, TNF-α, and AGE-BSA significantly increased RCAN1.4 mRNA by approximately 7, 3, and 8.5 fold over control, respectively. However, TGFβ1, VEGF, ANG II, MCP-1, H2O2 or culture in high glucose condition did not affect the RCAN1.4 expression. It is well-known that CaN is in the upstream pathway of RCAN1.4 induction [26]; therefore, we examined effect of a CaN inhibitor, CsA, on the RCAN1.4 upregulation by IL-1β and AGE-BSA. As shown Fig. 2B, the pretreatment of cells with CsA significantly inhibited both IL-1β- and AGE-BSA-mediated RCAN1.4 increase.
Fig. 2.
RCAN1.4 mRNA induction by diverse stimuli in mouse primary mesangial cells. (A) Mesangial cells were isolated from glomeruli by differential sieving, and then the cells of passages 7 to 10 in quiescent state were stimulated with the agents indicated below for 24 h or cultured for 48 h in high glucose condition (33 mM) or in osmotically balanced control medium (5.9 mM glucose plus 27 mM mannitol). Effect of the each stimulation was tested for RCAN1.4 mRNA induction, as described in the Methods. Concentrations were: TGF-β1 (4 ng/ml), ANG II (2 µM), VEGF (10 ng/ml), IL-1β (20 ng/ml), TNF-α (20 ng/ml), MCP-1 (100 ng/ml), oxidative stress (300 µM of H2O2), BSA (50 µg/ml), and AGE-BSA (50 µg/ml). *p<0.01 compared to no-treatment. (B) Effect of CsA on IL-1β- or AGE-BSA-mediated RCAN1.4 induction was tested. Quiescent mesangial cells were stimulated with IL-1β (20 ng/ml) or AGE-BSA (50 µg/ml) in presence of CsA (1 µM; open bars) or vehicle (filled bars). After 24 h, mRNAs were isolated to determine RCAN1.4 mRNA induction. All the values are means±SE of five experiments/group. Values are expressed relative to the expression observed in untreated controls (arbitrarily assigned a value of 1). *p<0.01 vs. the corresponding control (vehicle) group.
Overexpression of RCAN1.4 increased the production of collagens in Mes-13 cells
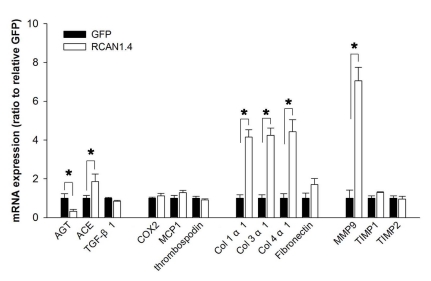
We next addressed whether there is any change of ECM-related gene expression in mesangial cells under the chronic elevation of RCAN1.4. For this, we generated two derivatives of the Mes-13 cell line; one stably expressed the RCAN1.4-GFP fusion protein, and the other expressed GFP alone (control) [23]. These cells were subjected to quantitative real-time PCR for analysis of genes that were previously associated with ECM expansion in the diabetic nephropathy. Among the assayed molecules, significant elevations were observed for the transcripts of angiotensin-converting enzyme (ACE), collagens type I, type III, type IV, and matrix metalloproteinase 9 (MMP-9). On the other hand, expression of angiotensinogen (AGT) was significantly reduced (Fig. 3). RCAN1.4 is an endogenous inhibitor of CaN; therefore, we tested whether the regulation of these genes were mediated by CaN inhibition. For this, Mes-13 cells were treated with CsA (1 µM) for 48 h, and then subjected to analysis of ECM-related gene. Unexpectedly, the expression of the genes was not affected by the CsA treatment (data not shown). These results suggest that chronic elevation of RCAN1.4 induces ECM-related genes in Mes-13 cells; however, the mechanism may not be directly related with CaN inhibition.
Fig. 3.
Expression patterns of ECM-related factors in Mes-13 cells stably transfected with RCAN1.4. Mes-13 cells were stably transfected with an expression vector containing RCAN1.4 construct (RCAN1.4; open bars) or vector alone (GFP; filled bars). From the actively dividing cells in complete medium, mRNAs were prepared and then subjected to analysis for the ECM-related gene expression with specific primers (Table 1). All values are means±SE of at least 5 experiments/group and the values of RCAN1.4-transfected cells are expressed relative to the expression observed in GFP-transfected cells (arbitrarily designated a value of 1). *p<0.01, RCAN1.4 vs. GFP group. Col, collagen.
DISCUSSION
Previous studies have suggested that CaN is an important signaling mediator for ECM production [10-12]. However, RCAN1, the endogenous inhibitor of CaN, has never been examined in the diabetic kidney. In this study we have used STZ-induced diabetic mice and db/db mice as a type 1 and 2 diabetic model, respectively, to study the change of RCAN1.4 expression in the kidney. The findings herein have demonstrated for the first time that RCAN1.4 was upregulated in the glomeruli of the both diabetic models. Farther study showed that the elevation of RCAN1.4 was induced by IL-1β, TNF-α, and AGE-BSA, which were previously implicated in ECM formation in diabetic nephropathy. In the same context, our results showed that RCAN1.4 plays a role in the regulation of several factors responsible for ECM turnover.
Both IL-1β and TNF-α were previously reported to induce RCAN1.4 in neuronal cells [19], however, AGE-BSA is a novel inducer, first reported in this study. AGE-BSA stimulated transcription of RCAN1.4 via a CaN-dependent pathway. This finding was consistent with the previous finding that multiple NFAT-response elements were present in the internal RCAN1.4 promoter between exons 3 and 4 [26]. Our observation was also supported by a recent study that showed AGE-BSA induced a rise in intracellular Ca2+ and subsequent activation of the CaN-NFAT pathway [27]. The same promoter region was involved in the regulation of RCAN1.4 expression in endothelial cells treated with VEGF [28]. However, mesangial cells did not respond to VEGF stimulation in the present study. This appeared to be due to the minimal expression or absence of VEGF receptors on normal mesangial cells [29,30].
We showed that Mes-13 cells stably transfected with RCAN1.4 expressed higher levels of collagen than control Mes-13 cells. Given that RCAN1.4 is an inhibitor of CaN, this result was unexpected, because CaN had previously been reported to mediate ECM production in mesangial cells [10]. One possible explanation for this unexpected result could be that the chronically upregulated RCAN1.4 in Mes-13 cells increased collagen production not by the CaN inhibition mechanism. This hypothesis is supported by the observation that treatment of CsA for 48 h did not affect the expression of collagen in these cells. Upon association of CsA with cyclophilin A, the drug-protein complex binds at the interface between the catalytic and regulatory subunits of CaN [31], while RCAN1 binds at the substrate-docking cleft on the catalytic subunit [32]. Although RCAN1 and CsA utilize distinct binding sites, inhibition of CaN activity by them is the same consequence of the spatial blockage for a substrate to approach the phosphatase active site [32,33].
Although RCAN1 has been best characterized as an inhibitor of CaN, RCAN1 also directly interacts with several proteins, including Raf-1 [34], NIK [35], CREB [36], SCFβ-TrCP ubiquitin ligase [37], and Tollip [38]. In addition, RCAN1 has been demonstrated to modulate the activity of nuclear factor κB [23] and ERK pathways [39]. The speculation of RCAN1.4-mediated ECM production, in which CaN inhibition was not involved, dose not conflict with the results of a previous report that showed treatment with CaN inhibitors prevented ECM accumulation in a diabetic model [10]; in that case, we hypothesize that the treatment with CsA might have suppressed RCAN1.4 expression by CaN inhibition, and thereby inhibited the RCAN1.4- mediated ECM production. However, our data did not address the mechanism by which chronic elevation of RCAN1.4 affected the expression of collagens. Thus, this point remains to be elucidated in future studies.
In summary, we demonstrated that RCAN1.4 expression was elevated in a diabetic nephropathy mouse model, and we identified causative factors. In an attempt to elucidate a role for RCAN1.4, we found that overexpression of RCAN1.4 affected the expression levels of several proteins relevant to ECM production and degradation in diabetic nephropathy, such as collagens, MMP-9, ACE, and AGT. Although the mechanism and overall influence of these changes on the pathogenesis of diabetic nephropathy are difficult to determine at this stage, the current study showed that RCAN1.4 might play a role in ECM production and degradation.
ACKNOWLEDGEMENTS
This research was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Minister of Education, Science, and Technology (awarded to C.W.P; R01-2009-0073171) and also by a grant from the Seoul St. Mary's Clinical Medicine Research Program, in the year of 2009, through the Catholic University of Korea (awarded to C.W.P).
ABBREVIATIONS
- ECM
extracellular matrix
- CaN
calcineurin
- RCAN1
regulator of calcineurin 1
- RCAN1.4
regulator of calcineurin 1 isoform 4
- CsA
cyclosporine A
- STZ
streptozotocin
- IL-1β
Interleukin-1β
- TNF-α
tumor necrosis factor-α
- AGEs
advanced glycation endproducts
- VEGF
vascular endothelial growth factor
- TGF-β
transforming growth factor β
- NFAT
nuclear factor of activated T cells
- AGT
angiotensinogen
- ANG II
angiotensin II
- MMP-9
matrix metalloproteinase 9
- ACE
angiotensin-converting enzyme
References
- 1.Iidaka K, McCoy J, Kimmelstiel P. The glomerular mesangium: a quantitative analy-sis. Lab Invest. 1968;19:573–579. [PubMed] [Google Scholar]
- 2.Kawano K, Arakawa M, McCoy J, Porch J, Kimmelstiel P. Quantitative study of glomeruli. Focal glomerulonephritis and diabetic glomerulosclerosis. Lab Invest. 1969;21:269–275. [PubMed] [Google Scholar]
- 3.Ayo SH, Radnik RA, Garoni JA, Glass WF, 2nd, Kreisberg JI. High glucose causes an increase in extracellular matrix proteins in cultured mesangial cells. Am J Pathol. 1990;136:1339–1348. [PMC free article] [PubMed] [Google Scholar]
- 4.Doi T, Vlassara H, Kirstein M, Yamada Y, Striker GE, Striker LJ. Receptor-specific increase in extracellular matrix production in mouse mesangial cells by advanced glycosylation end products is mediated via platelet-derived growth factor. Proc Natl Acad Sci USA. 1992;89:2873–2877. doi: 10.1073/pnas.89.7.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol. 2003;14:1358–1373. doi: 10.1097/01.asn.0000065640.77499.d7. [DOI] [PubMed] [Google Scholar]
- 6.Flyvbjerg A. Putative pathophysiological role of growth factors and cytokines in experimental diabetic kidney disease. Diabetologia. 2000;43:1205–1223. doi: 10.1007/s001250051515. [DOI] [PubMed] [Google Scholar]
- 7.Chiarelli F, Gaspari S, Marcovecchio ML. Role of growth factors in diabetic kidney disease. Horm Metab Res. 2009;41:585–593. doi: 10.1055/s-0029-1220752. [DOI] [PubMed] [Google Scholar]
- 8.Blázquez-Medela AM, López-Novoa JM, Martínez-Salgado C. Mechanisms involved in the genesis of diabetic nephropathy. Curr Diabetes Rev. 2010;6:68–87. doi: 10.2174/157339910790909422. [DOI] [PubMed] [Google Scholar]
- 9.Grønbaek H, Volmers P, Bjørn SF, Osterby R, Orskov H, Flyvbjerg A. Effect of GH/IGF-I deficiency on long-term renal changes and urinary albumin excretion in diabetic dwarf rats. Am J Physiol. 1997;272:E918–E924. doi: 10.1152/ajpendo.1997.272.5.E918. [DOI] [PubMed] [Google Scholar]
- 10.Gooch JL, Barnes JL, Garcia S, Abboud HE. Calcineurin is activated in diabetes and is required for glomerular hypertrophy and ECM accumulation. Am J Physiol Renal Physiol. 2003;284:F144–F154. doi: 10.1152/ajprenal.00158.2002. [DOI] [PubMed] [Google Scholar]
- 11.Gooch JL, Tang Y, Ricono JM, Abboud HE. Insulin-like growth factor-I induces renal cell hypertrophy via a calcineurindependent mechanism. J Biol Chem. 2001;276:42492–42500. doi: 10.1074/jbc.M102994200. [DOI] [PubMed] [Google Scholar]
- 12.Gooch JL, Gorin Y, Zhang BX, Abboud HE. Involvement of calcineurin in transforming growth factor-beta-mediated regulation of extracellular matrix accumulation. J Biol Chem. 2004;279:15561–15570. doi: 10.1074/jbc.M308759200. [DOI] [PubMed] [Google Scholar]
- 13.Rothermel BA, Vega RB, Williams RS. The role of modulatory calcineurin-interacting proteins in calcineurin signaling. Trends Cardiovasc Med. 2003;13:15–21. doi: 10.1016/s1050-1738(02)00188-3. [DOI] [PubMed] [Google Scholar]
- 14.Fuentes JJ, Genescà L, Kingsbury TJ, Cunningham KW, Pérez-Riba M, Estivill X, de la Luna S. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum Mol Genet. 2000;9:1681–1690. doi: 10.1093/hmg/9.11.1681. [DOI] [PubMed] [Google Scholar]
- 15.Fuentes JJ, Pritchard MA, Planas AM, Bosch A, Ferrer I, Estivill X. A new human gene from the Down syndrome critical region encodes a proline-rich protein highly expressed in fetal brain and heart. Hum Mol Genet. 1995;4:1935–1944. doi: 10.1093/hmg/4.10.1935. [DOI] [PubMed] [Google Scholar]
- 16.Fuentes JJ, Pritchard MA, Estivill X. Genomic organization, alternative splicing, and expression patterns of the DSCR1 (Down syndrome candidate region 1) gene. Genomics. 1997;44:358–361. doi: 10.1006/geno.1997.4866. [DOI] [PubMed] [Google Scholar]
- 17.Abe M, Sato Y. cDNA microarray analysis of the gene expression profile of VEGF-activated human umbilical vein endothelial cells. Angiogenesis. 2001;4:289–298. doi: 10.1023/a:1016018617152. [DOI] [PubMed] [Google Scholar]
- 18.Mann KM, Ray JL, Moon ES, Sass KM, Benson MR. Calcineurin initiates smooth muscle differentiation in neural crest stem cells. J Cell Biol. 2004;165:483–491. doi: 10.1083/jcb.200402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho KO, Kim YS, Cho YJ, Kim SY. Upregulation of DSCR1 (RCAN1 or Adapt78) in the peri-infarct cortex after experimental stroke. Exp Neurol. 2008;212:85–92. doi: 10.1016/j.expneurol.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Crawford DR, Leahy KP, Abramova N, Lan L, Wang Y, Davies KJ. Hamster adapt78 mRNA is a Down syndrome critical region homologue that is inducible by oxidative stress. Arch Biochem Biophys. 1997;342:6–12. doi: 10.1006/abbi.1997.0109. [DOI] [PubMed] [Google Scholar]
- 21.Luo JD, Wang YY, Fu WL, Wu J, Chen AF. Gene therapy of endothelial nitric oxide synthase and manganese superoxide dismutase restores delayed wound healing in type 1 diabetic mice. Circulation. 2004;110:2484–2493. doi: 10.1161/01.CIR.0000137969.87365.05. [DOI] [PubMed] [Google Scholar]
- 22.Bradshaw AD, Francki A, Motamed K, Howe C, Sage EH. Primary mesenchymal cells isolated from SPARC-null mice exhibit altered morphology and rates of proliferation. Mol Biol Cell. 1999;10:1569–1579. doi: 10.1091/mbc.10.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YS, Cho KO, Lee HJ, Kim SY, Sato Y, Cho YJ. Down syndrome candidate region 1 increases the stability of the IkappaBalpha protein: implications for its anti-inflammatory effects. J Biol Chem. 2006;281:39051–39061. doi: 10.1074/jbc.M604659200. [DOI] [PubMed] [Google Scholar]
- 24.Kim YS, Lee HJ, Jang C, Kim HS, Cho YJ. Knockdown of RCAN1.4 increases susceptibility to FAS-mediated and DNAdamage-induced apoptosis by upregulation of p53 expression. Korean J Physiol Pharmacol. 2009;13:483–489. doi: 10.4196/kjpp.2009.13.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153:1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Rothermel B, Vega RB, Frey N, McKinsey TA, Olson EN, Bassel-Duby R, Williams RS. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ Res. 2000;87:E61–E68. doi: 10.1161/01.res.87.12.e61. [DOI] [PubMed] [Google Scholar]
- 27.David KC, Scott RH, Nixon GF. Advanced glycation endproducts induce a proliferative response in vascular smooth muscle cells via altered calcium signaling. Biochem Pharmacol. 2008;76:1110–1120. doi: 10.1016/j.bcp.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Minami T, Horiuchi K, Miura M, Abid MR, Takabe W, Noguchi N, Kohro T, Ge X, Aburatani H, Hamakubo T, Kodama T, Aird WC. Vascular endothelial growth factor- and thrombin-induced termination factor, Down syndrome critical region-1, attenuates endothelial cell proliferation and angiogenesis. J Biol Chem. 2004;279:50537–50554. doi: 10.1074/jbc.M406454200. [DOI] [PubMed] [Google Scholar]
- 29.Simon M, Röckl W, Hornig C, Gröne EF, Theis H, Weich HA, Fuchs E, Yayon A, Gröne HJ. Receptors of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) in fetal and adult human kidney: localization and [125I]VEGF binding sites. J Am Soc Nephrol. 1998;9:1032–1044. doi: 10.1681/ASN.V961032. [DOI] [PubMed] [Google Scholar]
- 30.Cooper ME, Vranes D, Youssef S, Stacker SA, Cox AJ, Rizkalla B, Casley DJ, Bach LA, Kelly DJ, Gilbert RE. Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes. 1999;48:2229–2239. doi: 10.2337/diabetes.48.11.2229. [DOI] [PubMed] [Google Scholar]
- 31.Wang P, Heitman J. The cyclophilins. Genome Biol. 2005;6:226. doi: 10.1186/gb-2005-6-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta S, Li H, Hogan PG, Cunningham KW. Domain architecture of the regulators of calcineurin (RCANs) and identification of a divergent RCAN in yeast. Mol Cell Biol. 2009;29:2777–2793. doi: 10.1128/MCB.01197-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ke H, Huai Q. Structures of calcineurin and its complexes with immunophilins-immunosuppressants. Biochem Biophys Res Commun. 2003;311:1095–1102. doi: 10.1016/s0006-291x(03)01537-7. [DOI] [PubMed] [Google Scholar]
- 34.Cho YJ, Abe M, Kim SY, Sato Y. Raf-1 is a binding partner of DSCR1. Arch Biochem Biophys. 2005;439:121–128. doi: 10.1016/j.abb.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Lee EJ, Seo SR, Um JW, Park J, Oh Y, Chung KC. NFkappaB-inducing kinase phosphorylates and blocks the degradation of Down syndrome candidate region 1. J Biol Chem. 2008;283:3392–3400. doi: 10.1074/jbc.M706707200. [DOI] [PubMed] [Google Scholar]
- 36.Seo SR, Chung KC. CREB activates proteasomal degradation of DSCR1/RCAN1. FEBS Lett. 2008;582:1889–1893. doi: 10.1016/j.febslet.2008.04.059. [DOI] [PubMed] [Google Scholar]
- 37.Asada S, Ikeda A, Nagao R, Hama H, Sudo T, Fukamizu A, Kasuya Y, Kishi T. Oxidative stress-induced ubiquitination of RCAN1 mediated by SCFbeta-TrCP ubiquitin ligase. Int J Mol Med. 2008;22:95–104. [PubMed] [Google Scholar]
- 38.Lee JY, Lee HJ, Lee EJ, Jang SH, Kim H, Yoon JH, Chung KC. Down syndrome candidate region-1 protein interacts with Tollip and positively modulates interleukin-1 receptor-mediated signaling. Biochim Biophys Acta. 2009;1790:1673–1680. doi: 10.1016/j.bbagen.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Lee HJ, Kim YS, Sato Y, Cho YJ. RCAN1-4 knockdown attenuates cell growth through the inhibition of Ras signaling. FEBS Lett. 2009;583:2557–2564. doi: 10.1016/j.febslet.2009.07.023. [DOI] [PubMed] [Google Scholar]