Abstract
The effects of extremely low frequency electromagnetic fields (EMF) on intracellular Ca2+ mobilization and cellular function in RBL 2H3 cells were investigated. Exposure to EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h did not produce any cytotoxic effects in RBL 2H3 cells. Melittin, ionomycin and thapsigargin each dose-dependently increased the intracellular Ca2+ concentration. The increase of intracellular Ca2+ induced by these three agents was not affected by exposure to EMF (60 Hz, 1 mT) for 4 or 16 h in RBL 2H3 cells. To investigate the effect of EMF on exocytosis, we measured beta-hexosaminidase release in RBL 2H3 cells. Basal release of beta-hexosaminidase was 12.3±2.3% in RBL 2H3 cells. Exposure to EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h did not affect the basal or 1 µM melittin-induced beta-hexosaminidase release in RBL 2H3 cells. This study suggests that exposure to EMF (60 Hz, 0.1 or 1 mT), which is the limit of occupational exposure, has no influence on intracellular Ca2+ mobilization and cellular function in RBL 2H3 cells.
Keywords: EMF, Ca2+ mobilization, Exocytosis, Beta-hexosaminidase
INTRODUCTION
Many epidemiologic studies suggested the possibility that exposure to extremely low frequency electromagnetic fields (EMF) may be related to the risk of acute lymphoblastic leukemia in children [1-3]. There is a public concern about the possible adverse health effects associated with exposure to EMF. However, the mechanism of the interaction between EMF and cellular systems is still unclear.
We previously reported that there are no significant changes in phospholipase activity such as PLA2, PLC and PLD in RAW 264.7 cells and RBL 2H3 cells exposed to EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h [4]. However, cellular function may be affected by a variety of signaling pathways in addition to the phospholipase pathway. Considering that many cellular functions are closely related to the increase of intracellular Ca2+ concentration, it is plausible that EMF exposure may cause alterations in Ca2+ mobilization via both extracellular Ca2+ influx and intracellular Ca2+ release.
Ca2+ is a universal messenger that controls a variety of cell functions, including secretion. The increase of intracellular Ca2+ is caused by the influx of extracellular Ca2+ via Ca2+ channel, intracellular Ca2+ release via inositol-1,4,5-triphosphate (IP3) [5,6] and inhibition of the Ca2+ pump that lowers the intracellular Ca2+ concentration. Intracellular Ca2+ mobilization can be regulated by a variety of exogenous stimulants. Melittin-induced increase of intracellular Ca2+ is related to PLC-mediated inositol triphosphate (IP3) accumulation with receptor operated Ca2+ channels (ROCC) [7]. Ionomycin is a well-known ionophore and increases intracellular Ca2+ concentration through Ca2+-release activated Ca2+ (CRAC) channels [8]. Thapsigargin also increases intracellular Ca2+ concentration via the inhibition of sarco/endoplasmic reticulum Ca2+ ATPase [9]. In this study, we investigated the effect of EMF on intracellular Ca2+ mobilization stimulated by melittin, ionomycin and thapsigargin and on beta-hexosaminidase release in RBL 2H3 cells.
METHODS
Materials
Melittin, ionomycin, thapsigargin and ρ-nitrophenyl-N-acetyl-β-glucosaminide were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Dulbecco's modified Eagle's minimum essential medium (DMEM) and fetal bovine serum (FBS) were purchased from Invitrogen Co. (Grand Island, NY, USA). Fura-2/AM was purchased from Enzo Life Sciences (Plymouth, PA, USA). Other reagents were purchased from Sigma Chemical Co. (USA).
Cell culture
Rat basophilic leukemia (RBL 2H3) cells were grown in Dulbecco's modified Eagle minimum essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotic-antifungal mix (100 IU/ml penicillin G, 100 µg/ml of streptomycin and 0.25 µg/ml of amphotericin B) at 37℃ in 5% CO2.
EMF exposure system
EMF generation equipment was designed and constructed by Korea Electrotechnology Research Institute (Korea). Monitoring of magnetic field was conducted under observation of the current injected to the exposure system, because the magnetic field is proportional to the injected current. The field generator consisted of four square-shaped coils and one cage with three testing floors (top, middle and bottom floor).
The voltage fluctuation rate and the harmonic rate of the power quality using a power amp was under 1%. After fixing the magnetic field of the center of the middle floor to 1 mT, the fields at various points were measured. The spatial variation of the magnetic field was less than 3%. This strongly demonstrates that the field generator is well suited for a small-scale in vitro study. Using a water-jet cooling system, the temperature in the incubator at 1 mT was maintained at 37±0.3℃. Also, a magnetic field shielding system using ferrite material was adopted to shield the strong magnetic field in the outer regions of the EMF exposure system. The coils were turned on for at least 30 min before use, and the cells were exposed to 0.1 mT and 1 mT in 60 Hz magnetic field for 4 h and 16 h. All experiments were conducted under the same environmental conditions.
MTT assay
Cell viability was performed with an MTT-based colorimetric assay [10]. Cells in 96-well plates (1×105 cells/well) were exposed to EMF at 37℃ for 4 or 16 h. 20 µl of MTT solution (5 mg/ml in phosphate buffered saline) was added and further incubated for 3 h. After aspirating the supernatant from the wells, 100 µl of dimethyl sulfoxide was added to dissolve the formazan crystals. The absorbance of each well was then read at 520 nm.
Measurement of intracellular Ca2+ mobilization
The intracellular Ca2+ level was measured using Fura-2/AM by monitoring a fluorospectrometer [11]. Briefly, culture medium was replaced and cells were washed 3 times with PBS. After that, cells were detached by trypsin and suspended with 10 ml Krebs solution, then loaded with Fura-2/AM to a final concentration of 2 µM and incubated at 37℃ for 1 h. The loaded cells were washed twice with Krebs solution and centrifuged at 3,000×g for 10 min. The Fura-2 fluorescence was monitored on a Quanta Master (Qm4, Photon Technology International, NJ, USA.) at 37℃ with excitation at 340 and 380 nm and emission at 500 nm. The ratio of F340/F380 was recorded, and the maximum fluorescence ratio (Rmax) was measured by using 0.1% Triton X-100. The minimum fluorescence ratio (Rmin) was measured following depletion of external Ca2+ by addition of 5 mM EGTA/Tris pH8.5.
Measurement of beta-hexosaminidase release
To investigate the effect of EMF on exocytosis, we measured beta-hexosaminidase release in RBL 2H3 cells. RBL 2H3 cells exposed to EMF were washed with PIPES buffer 3 times and then suspended in PIPES buffer. The cells were stimulated with 0.5 µM melittin for 30 min at 37℃ [12]. The cell suspension was centrifuged at 300×g for 10 min. 20 µl of the supernatant was incubated with an equal volume of substrate solution (2 mM ρ-nitrophenyl-N-acetyl-β-glucosaminide, 0.1 M citrate, pH 4.5) in duplicate in 96-well plates for 1 h at 37℃. The reaction was stopped by adding 200 µl of 0.1 M sodium carbonate buffer, pH 10.0 [13]. The absorbance was measured at 405 nm with fluorospectrophotometer (FL600 Microplate Fluorescence Reader, Bio-tek).
Statistical analysis
Results are presented as mean±S.D. and were analyzed statistically by analysis of variance (ANOVA). Differences between groups were determined with the Newman-Keul's test. The level of significance was set at less than 5% (p<0.05).
RESULTS
Effect of EMF on the viability of RBL 2H3 cells
To exclude the possibility that EMF may affect cellular viability, we first confirmed the cytotoxicity of EMF using an MTT assay. The exposure to EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h did not produce any cytotoxic effects in RBL 2H3 cells (Fig. 1).
Fig. 1.
The effect of EMF on cell viability of RBL 2H3 cells. The cells were exposed to EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h and viability was measured with MTT assay. Results are indicated in mean±S.D. from four separate experiments.
Effect of EMF on intracellular Ca2+ mobilization
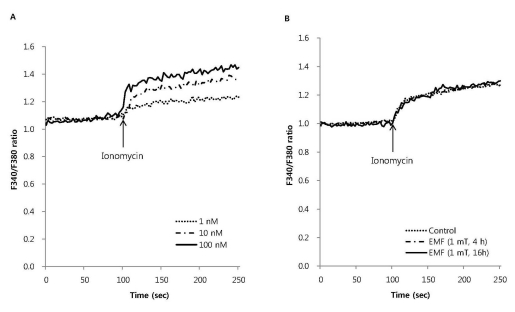
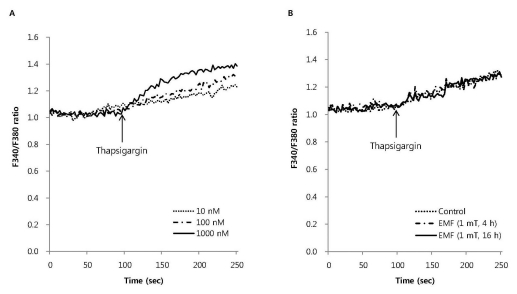
To investigate the effect of EMF on the influx of extracellular Ca2+ via receptor operated Ca2+ channels (ROCC), we measured melittin-induced intracellular Ca2+ mobilization in RBL 2H3 cells exposed to EMF (60 Hz, 1 mT) for 4 or 16 h. Melittin dose-dependently increased the intracellular Ca2+ concentration (Fig. 2A). EMF did not affect the 0.5 µM melittin-induced intracellular Ca2+ mobilization (Fig. 2B). As an ionophore, ionomycin increased intracellular Ca2+ concentration in a dose-dependent manner (Fig. 3A), while thapsigargin significantly increased the concentration via inhibition of sarco/endoplasmic reticulum Ca2+ ATPase in RBL 2H3 cells (Fig. 4A). EMF did not influence the intracellular Ca2+ concentration induced by 10 nM ionomycin or 100 nM thapsigargin (Fig. 3B, 4B). EMF (60 Hz, 0.1 mT) for 4 or 16 h did not change the intracellular Ca2+ concentration induced by 0.5 µM melittin, 10 nM ionomycin or 100 nM thapsigargin (data not shown).
Fig. 2.
The effect of EMF on the intracellular Ca2+ mobilization induced by melittin in RBL 2H3 cells. Intracellular Ca2+ mobilization induced by melittin was measured with Quanta Master in Fura- 2AM-loaded RBL 2H3 cells. Melittin dose-dependently increased intracellular Ca2+ mobilization (A) and 0.5 µM melittin-induced intracellular Ca2+ mobilization was not affected by EMF (60 Hz, 1 mT) for 4 or 16 h (B). Results are the representative data of four separate experiments.
Fig. 3.
The effect of EMF on intracellular Ca2+ mobilization induced by ionomycin in RBL 2H3 cells. Ionomycin dose- dependently increased intracellular Ca2+ mobilization (A) and 10 nM ionomycininduced intracellular Ca2+ mobilization was not affected by EMF (60 Hz, 1 mT) for 4 or 16 h (B). Results are the representative data of four separate experiments.
Fig. 4.
The effect of EMF on intracellular Ca2+ mobilization induced by thapsigargin in RBL 2H3 cells. Thapsigargin dose-dependently increased intracellular Ca2+ mobilization (A) and 100 nM thapsigargin-induced intracellular Ca2+ mobilization was not affected by EMF (60 Hz, 1 mT) for 4 or 16 h (B). Results are the representative data of four separate experiments.
Effect of EMF on beta-hexosaminidase release in RBL 2H3 cells
To investigate the effect of EMF on exocytosis, we measured beta-hexosaminidase release in RBL 2H3 cells. Basal release of beta-hexosaminidase was 12.3±2.3% in RBL 2H3 cells. Melittin increased beta-hexosaminidase release in a dose-dependent manner, whereas ionomycin and thapsigargin did not cause beta-hexosaminidase release (Table 1). Exposure to EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h did not affect the basal and 1 µM melittin-induced beta-hexosaminidase release in RBL 2H3 cells (Fig. 5).
Table 1.
Dose-response of beta-hexosaminidase release by melittin, ionomycin and thapsigargin in RBL 2H3 cells
*Significantly different from control (p<0.05).
Fig. 5.
The effect of EMF on basal (A) and 1 µM melittin-induced beta-hexosaminidase release (B) in RBL 2H3 cells. Both basal and 1 µM melittin-induced beta-hexosaminidase release were not affected by exposure to EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h. Results indicate mean±S.D. from four separate experiments.
DISCUSSION
The aim of this study was to investigate the effect of EMF on intracellular Ca2+ mobilization and exocytosis of beta-hexosaminidase in RBL 2H3 cells. Before measuring the exocytosis of beta-hexosaminidase, it was very important to confirm the cytotoxicity of EMF. Exposure to EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h did not show any cytotoxic activity in RBL 2H3 cells. It has been reported that 0.5 mT EMF has no significant effect on proliferation of human peripheral blood mononuclear cells [14], but 20 mT EMF for up to 23 days could inhibit the growth of human mesenchymal stem cells [15]. The cytotoxicity of EMF may be dependent on the intensity of EMF. The current data did not reveal any cytotoxic activity in RBL 2H3 cells, suggesting that exocytosis of beta-hexosaminidase may be independent on cell membrane rupture by EMF.
Ca2+ is a universal messenger that controls various cellular functions, including secretion, contraction, proliferation and differentiation. Intracellular Ca2+ is increased by a variety of pathways, such as the influx of extracellular Ca2+, release of intracellular Ca2+ stores [5,6] and inhibition of the Ca2+ pump that reduces intracellular Ca2+ concentration. In this experiment, melittin, ionomycin and thapsigargin each dose-dependently increased intracellular Ca2+ concentration. The increase of intracellular Ca2+ induced by these three agents was not affected by exposure to EMF (60 Hz, 1 mT) for 4 or 16 h in RBL 2H3 cells. The melittin-induced increase of intracellular Ca2+ was related to PLC-mediated inositol triphosphate (IP3) accumulation with receptor operated Ca2+ channels (ROCC) [7]. Ionomycin is a well-known ionophore and increases intracellular Ca2+ concentration through Ca2+-release activated Ca2+ (CRAC) channels [8]. Thapsigargin also increases intracellular Ca2+ concentration via the inhibition of sarco/endoplasmic reticulum Ca2+ ATPase [9]. These data suggest that EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h does not influence intracellular Ca2+ mobilization via the influx of extracellular Ca2+, the release of intracellular Ca2+ store or the Ca2+ pump in RBL 2H3 cells. However, there has been much debate regarding the effect of EMF on intracellular Ca2+ concentration: EMF has been shown to increase intracellular Ca2+ concentration [16,17], to decrease it [18] and to have no effect on it [19,20]. Such different effects of EMF on intracellular Ca2+ concentration may be due to the cell types used and the experimental conditions.
To investigate the effect of EMF on exocytosis, we measured beta-hexosaminidase release in RBL 2H3 cells. Exposure to EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h did not affect the basal and 1 µM melittin-induced beta-hexosaminidase release in RBL 2H3 cells. This data agreed with the previous report that single exposure to EMF did not cause the degranulation of mast cells [21].
Guidelines on 50/60 Hz electromagnetic fields were issued by IRPA/INIRC in 1990. The basic hypothesis suggested that 50/60 Hz magnetic fields from external sources such as powerlines could be linked to an increased risk of childhood leukemia [1]. However, the mechanism underlying the interaction between EMF and cellular functions remains elusive. It is therefore necessary to assess the changes of intracellular Ca2+ and cellular function caused by EMF in order to understand the possible adverse effects of EMF in leukocytes. In this study, we used a kind of leukocyte, RBL 2H3 cells (rat basophilic leukemia cells), and exposed the cells to EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h. It has been reported that the limit of EMF for general public exposure and occupational exposure are 0.2 mT and 1 mT, respectively [22]. The intensity of EMF used in this experiment is the limit of occupational exposure. Although further studies are necessary to identify the changes in intracellular Ca2+ mobilization and cellular function by EMF exposure, we suggest that EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h may have no influence on intracellular Ca2+ mobilization and cellular function in RBL 2H3 cells.
ACKNOWLEDGEMENTS
This work was supported by the Power Generation & Electricity Delivery of the Korea Institute of Energy Technology and Planning (KETEP) grant funded by the Korean Government Ministry of Knowledge and Economy (No. 2009101030003E).
ABBREVIATIONS
- EMF
extremely low frequency electromagnetic fields
- ROCC
receptor operated Ca2+ channels
- CRAC
Ca2+-release activated Ca2+
References
- 1.Wertheimer N, Leeper E. Electrical wiring configurations and childhood cancer. Am J Epidemiol. 1979;109:273–284. doi: 10.1093/oxfordjournals.aje.a112681. [DOI] [PubMed] [Google Scholar]
- 2.Floderus B, Persson T, Stenlund C, Wennberg A, Ost A, Knave B. Occupational exposure to electromagnetic fields in relation to leukemia and brain tumors: a case-control study in Sweden. Cancer Causes Control. 1993;4:465–476. doi: 10.1007/BF00050866. [DOI] [PubMed] [Google Scholar]
- 3.Matanoski GM, Elliott EA, Breysse PN, Lynberg MC. Leukemia in telephone linemen. Am J Epidemiol. 1993;137:609–619. doi: 10.1093/oxfordjournals.aje.a116718. [DOI] [PubMed] [Google Scholar]
- 4.Song HS, Kim HR, Ko MS, Jeong JM, Kim YH, Kim MC, Hwang YH, Sohn UD, Gimm YM, Myung SH, Sim SS. Effect of extremely low frequency electromagnetic fields (EMF) on phospholipase activity in the cultured cells. Korean J Physiol Pharmacol. 2010;14:427–433. doi: 10.4196/kjpp.2010.14.6.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- 6.Somlyo AV, Bond M, Somlyo AP, Scarpa A. Inositol trisphosphate-induced calcium release and contraction in vascular smooth muscle. Proc Natl Acad Sci USA. 1985;82:5231–5235. doi: 10.1073/pnas.82.15.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen OH, Bouchelouche PN, Berild D. Arachidonic acid and calcium metabolism in rnelittin stimulated neutrophils. Mediators Inflamm. 1992;1:313–317. doi: 10.1155/S0962935192000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahr H, Schindl R, Fritsch R, Heinze B, Hofbauer M, Hack ME, Mörtelmaier MA, Groschner K, Peng JB, Takanaga H, Hediger MA, Romanin C. CaT1 knock-down strategies fail to affect CRAC channels in mucosal-type mast cells. J Physiol. 2004;557:121–132. doi: 10.1113/jphysiol.2004.062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shukla N, Freeman N, Gadsdon P, Angelini GD, Jeremy JY. Thapsigargin inhibits angiogenesis in the rat isolated aorta: studies on the role of intracellular calcium pools. Cardiovasc Res. 2001;49:681–689. doi: 10.1016/s0008-6363(00)00269-8. [DOI] [PubMed] [Google Scholar]
- 10.Woerdenbag HJ, Merfort I, Passreiter CM, Schmidt TJ, Willuhn G, van Uden W, Pras N, Kampinga HH, Konings AW. Cytotoxicity of flavonoids and sesquiterpene lactones from Arnica species against the GLC4 and the COLO 320 cell lines. Planta Med. 1994;60:434–437. doi: 10.1055/s-2006-959526. [DOI] [PubMed] [Google Scholar]
- 11.Tseng PH, Lin HP, Hu H, Wang C, Zhu MX, Chen CS. The canonical transient receptor potential 6 channel as a putative phosphatidylinositol 3,4,5-trisphosphate-sensitive calcium entry system. Biochemistry. 2004;43:11701–11708. doi: 10.1021/bi049349f. [DOI] [PubMed] [Google Scholar]
- 12.Huang F, Yamaki K, Tong X, Fu L, Zhang R, Cai Y, Yanagisawa R, Inoue K, Takano H, Yoshino S. Inhibition of the antigen-induced activation of RBL-2H3 cells by sinomenine. Int Immunopharmacol. 2008;8:502–507. doi: 10.1016/j.intimp.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Hemmerling J, Nell S, Kipp A, Schumann S, Deubel S, Haack M, Brigelius-Flohé R. alpha-Tocopherol enhances degranulation in RBL-2H3 mast cells. Mol Nutr Food Res. 2010;54:652–660. doi: 10.1002/mnfr.200900462. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda K, Shinmura Y, Mizoe H, Yoshizawa H, Yoshida A, Kanao S, Sumitani H, Hasebe S, Motomura T, Yamakawa T, Mizuno F, Otaka Y, Hirose H. No effects of extremely low frequency magnetic fields found on cytotoxic activities and cytokine production of human peripheral blood mononuclear cells in vitro. Bioelectromagnetics. 2003;24:21–31. doi: 10.1002/bem.10062. [DOI] [PubMed] [Google Scholar]
- 15.Yan J, Dong L, Zhang B, Qi N. Effects of extremely low- frequency magnetic field on growth and differentiation of human mesenchymal stem cells. Electromagn Biol Med. 2010;29:165–176. doi: 10.3109/01676830.2010.505490. [DOI] [PubMed] [Google Scholar]
- 16.Morabito C, Guarnieri S, Fanò G, Mariggiò MA. Effects of acute and chronic low frequency electromagnetic field exposure on PC12 cells during neuronal differentiation. Cell Physiol Biochem. 2010;26:947–958. doi: 10.1159/000324003. [DOI] [PubMed] [Google Scholar]
- 17.Gaetani R, Ledda M, Barile L, Chimenti I, De Carlo F, Forte E, Ionta V, Giuliani L, D'Emilia E, Frati G, Miraldi F, Pozzi D, Messina E, Grimaldi S, Giacomello A, Lisi A. Differentiation of human adult cardiac stem cells exposed to extremely low- frequency electromagnetic fields. Cardiovasc Res. 2009;82:411–420. doi: 10.1093/cvr/cvp067. [DOI] [PubMed] [Google Scholar]
- 18.Bernabò N, Tettamanti E, Pistilli MG, Nardinocchi D, Berardinelli P, Mattioli M, Barboni B. Effects of 50 Hz extremely low frequency magnetic field on the morphology and function of boar spermatozoa capacitated in vitro. Theriogenology. 2007;67:801–815. doi: 10.1016/j.theriogenology.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Pilger A, Ivancsits S, Diem E, Steffens M, Kolb HA, Rüdiger HW. No effects of intermittent 50 Hz EMF on cytoplasmic free calcium and on the mitochondrial membrane potential in human diploid fibroblasts. Radiat Environ Biophys. 2004;43:203–207. doi: 10.1007/s00411-004-0252-9. [DOI] [PubMed] [Google Scholar]
- 20.Madec F, Billaudel B, Charlet de Sauvage R, Sartor P, Veyret B. Effects of ELF and static magnetic fields on calcium oscillations in islets of Langerhans. Bioelectrochemistry. 2003;60:73–80. doi: 10.1016/s1567-5394(03)00049-5. [DOI] [PubMed] [Google Scholar]
- 21.Rajkovic V, Matavulj M, Johansson O. Combined exposure of peripubertal male rats to the endocrine-disrupting compound atrazine and power-frequency electromagnetic fields causes degranulation of cutaneous mast cells: a new toxic environmental hazard. Arch Environ Contam Toxicol. 2010;59:334–341. doi: 10.1007/s00244-010-9477-6. [DOI] [PubMed] [Google Scholar]
- 22.International Commission on Non-Ionizing Radiation Protection. Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz to 100 kHz) Health Phys. 2010;99:818–836. doi: 10.1097/HP.0b013e3181f06c86. [DOI] [PubMed] [Google Scholar]