Abstract
Protein arginine methylation is important for a variety of cellular processes including transcriptional regulation, mRNA splicing, DNA repair, nuclear/cytoplasmic shuttling and various signal transduction pathways. However, the role of arginine methylation in protein biosynthesis and the extracellular signals that control arginine methylation are not fully understood. Basic fibroblast growth factor (bFGF) has been identified as a potent stimulator of myofibroblast dedifferentiation into fibroblasts. We demonstrated that symmetric arginine dimethylation of eukaryotic elongation factor 2 (eEF2) is induced by bFGF without the change in the expression level of eEF2 in mouse embryo fibroblast NIH3T3 cells. The eEF2 methylation is preceded by ras-raf-mitogen-activated protein kinase kinase (MEK)-extracellular signal-regulated kinase (ERK1/2)-p21Cip/WAF1 activation, and suppressed by the mitogen-activated protein kinase (MAPK) inhibitor PD98059 and p21Cip/WAF1 short interfering RNA (siRNA). We determined that protein arginine methyltransferase 7 (PRMT7) is responsible for the methylation, and that PRMT5 acts as a coordinator. Collectively, we demonstrated that eEF2, a key factor involved in protein translational elongation is symmetrically arginine-methylated in a reversible manner, being regulated by bFGF through MAPK signaling pathway.
Keywords: cyclin-dependent kinase inhibitor p21; fibroblast growth factor 2; mitogen-activated protein kinases; N,N-dimethylarginine; peptide elongation factor 2; protein-arginine N-methyltransfe
Introduction
Protein arginine methylation is important in a variety of cellular processes including transcriptional regulation, mRNA splicing, DNA repair, nuclear/cytoplasmic shuttling, signal transduction, and protein-protein interactions (Bedford and Clarke, 2009; Boisvert et al., 2003). However, the arginine methylation of proteins involved in protein biosynthesis and the regulatory mechanisms controlling this are still unknown. The covalent marking of proteins by the addition of a methyl group to arginine residues can promote recognition by binding partners or can modulate biological activity. Protein arginine methyltransferases (PRMTs) catalyze such methylation reactions in eukaryotes. Enzymes that form asymmetric dimethylarginine (aDMA) are designated "type I", and those that form symmetric dimethylarginine (sDMA) are designated "type II" (Gary and Clarke, 1998). PRMT5 appears to be the major type II mammalian enzyme, while the role of PRMT7 is still being established. The reactions of these enzymes display many of the attributes of reversible covalent modifications such as protein phosphorylation and protein lysine methylation. Arginine methylation by PRMTs is regulated by posttranslational modifications of PRMTs or their substrates, interactions of PRMTs with PRMT-binding proteins, and enzymes that counteract PRMT activity (Bedford and Clarke, 2009; Chang et al., 2007; Lee et al., 2005). However, the upstream signal transduction pathways and extracellular signals controlling the regulatory mechanisms described above have not yet been fully elucidated.
Protein elongation occurs through the stepwise lengthening of the polypeptide chain. At each step, ribosomal peptidyl transferase transfers the growing peptide from its carrier tRNA to the α-amino group of the amino acid residue of the aminoacyl-tRNA specified by the following codon. During the elongation cycle on the ribosome, eukaryotic elongation factor 2 (eEF2), a GTPase, facilitates the translocation of the A- and P-site tRNAs to the P and E sites, respectively, with the concomitant advance of the mRNA by one codon (Chinali and Parmeggiani 1982; Nishizuka and Lipmann, 1966). The rate-limiting step in mRNA translation is the initiation step (Hershey and Merrick, 2000). To coordinate overall protein synthesis, the elongation rate needs to increase/decrease according to changes in the initiation rate (Merrick and Nyborg, 2000). However, far less is known about translational regulation of the elongation step of protein biosynthesis compared to that known about the more extensively studied initiation step. Elongation of peptide chains can be regulated independently of translation initiation to avoid missense errors or premature termination due to high levels of elongation. The process of peptide-chain elongation is, at least partially, regulated via eEF2 phosphorylation/dephosphorylation events in response to different stimuli. Signaling events dependent upon activation of mTOR, the mitogen-activated protein kinase kinase (MEK)/extracellular signalregulated kinase (ERK) pathway, and SAPK/p38 MAP kinases result in the phosphorylation of eEF2 and modulation of its activity (Wang et al., 2000; Knebel et al., 2001, 2002; Browne and Proud, 2002). ADP-ribosylation of eEF2 by bacterial toxins on a histidine modified to a diphthamide can affect its translocation activity (Jørgensen et al., 2005). However, the exact mechanisms that initiate and eventually terminate these highly regulated biochemical events are not completely understood. Furthermore, changes in post-translational modifications of eEF2 in diverse cellular processes have not yet been thoroughly elucidated; in particular, little is known about the role of arginine methylation and its regulation.
Basic fibroblast growth factor (bFGF) is expressed in many tissues including brain, kidney, adrenal cortex, and corpus luteum (Slavin, 1995). An immunohistochemical study of skin wounds during healing revealed that bFGF is expressed in the regenerative epidermis, inflammatory cells, newly formed blood vessels, and macrophages (Kibe et al., 2000). bFGF has a wide range of biological effects on cell growth, differentiation, and survival (Akasaka et al., 2004; Chiba et al., 2005; Doniach, 1995). Recently, bFGF was shown to function as a potent stimulator of the reversion of myofibroblasts to fibroblasts in vivo, presumably because of its effects on the down-regulation of α-smooth muscle actin (α-SMA) expression.
In the present study, we investigated the role of bFGF in the regulation of eEF2 arginine methylation, which may affect the characteristics of protein synthesis, thereby providing a mechanistic clue regarding the diverse functions of bFGF. We demonstrate that the symmetric arginine dimethylation of eEF2 is reversibly induced by bFGF through the ras-raf-MEK-ERK1/2-p21Cip/WAF1 pathway in mouse embryo fibroblast NIH3T3 cells, with PRMT7 and PRMT5 acting as the responsible methyltransferases.
Results
The effect of bFGF on arginine methylation of cellular proteins
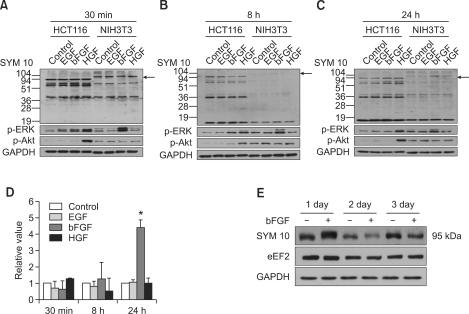
We tested the effects of the growth factors bFGF, epidermal growth factor (EGF), and hepatocyte growth factor (HGH) on the arginine methylation statuses of cellular proteins in NIH3T3 and HCT116 cells (human, colorectal carcinoma). Protein arginine methylation was observed at 30 min, 8 h, and 24 h after the administration of the growth factors via Western blot analysis using anti-symmetric (SYM10 and SYM11) and asymmetric (ASYM24) dimethylarginine antibodies. Many proteins were found to be arginine-methylated, but a significant change (P < 0.001) in arginine methylation was detected by SYM10 for a protein with a molecular weight of about 95 kDa in bFGF-treated NIH3T3 cells, clearly indicating the presence of sDMA. The methylation status of this protein was unchanged until 8 h, but increased at 24 h and was reduced to basal levels within 24 h after the administration of bFGF (Figure 1). Symmetric arginine dimethylation of the 95 kDa protein occurred in NIH3T3 cells but not in HCT116 cells and only when the NIH3T3 cells were stimulated with bFGF, not EGF or HGF, indicating that the methylation of the 95 kDa protein was ligand- and species- or tissue-specific. No change in arginine methylation status in proteins in NIH3T3 and HCT116 cells was detected by SYM11 and ASYM24 following the administration of bFGF, indicating that bFGF did not affect the levels of asymmetric dimethylarginines or symmetric dimethylarginines existing in a different sequence context to that recognized by SYM10 (Supplementary Data Figure S1). ASYM24 also reacted strongly with a protein in the 95-kDa region, but the level of methylation did not change due to treatment with bFGF. Concomitantly, ERK1/2 was significantly activated at 30 min after bFGF administration, and then levels of activated ERK1/2 decreased gradually in bFGF-treated NIH3T3 cells. However, Akt was not activated by bFGF administration when compared to the levels in untreated control NIH3T3 cells.
Figure 1.
Symmetric dimethylation of arginine on a 95-kDa protein is induced by bFGF in NIH3T3 cells. Protein arginine methylation profiles of NIH3T3 and HCT116 cells produced at (A) 30 min, (B) 8 h, and (C) 24 h after the administration of EGF, bFGF, or HGF with concurrent changes in the levels of p-ERK and p-Akt activation. Symmetric dimethylation of arginine on a 95-kDa protein is induced by bFGF 24 h after administration into NIH3T3 cells. ERK1/2 was activated 30 min after bFGF administration, which decreased gradually thereafter, while the level of Akt activation remained unchanged during the period. Cells were cultured in the absence or presence of EGF, bFGF, or HGF, and cell lysates were prepared at 30 min, 8 h, and 24 h. Western blot analysis was performed with anti-symmetric dimethylarginine antibody SYM10 and anti-p-ERK1/2 or anti-p-Akt antibodies. GAPDH was used as a loading control. Protein bands were visualized using enhanced chemiluminescence. (D) Bar graph showing the change in levels of symmetric arginine dimethylation of a 95-kDa protein after the administration of growth factors. Mean and standard deviations were obtained from three independent experiments. Statistically significant differences as determined by the Wilcoxon test were set at *P < 0.001. (E) The change in the levels of symmetric arginine dimethylation of a 95-kDa protein and eEF2 expression in NIH3T3 cells during the three-day period after the administration of bFGF. Cell lysates were obtained at the indicated time points of 1, 2 and 3 days, and Western blots were conducted using SYM10 and anti-eEF2 antibody as in (A). Arrows indicate the symmetrically arginine-dimethylated 95-kDa protein.
Identification of eEF2 as the symmetrically arginine-dimethylated protein induced by bFGF
We performed mass spectrometry (MS) to identify the sDMA-containing 95 kDa proteins in both bFGF-treated and untreated NIH3T3 cell extracts separated on SDS-PAGE; this protein was identified as eEF2 with high confidence in both cases by searching the generated peak list files against NCBI-nonredundant (version 08_06_2010, mammalian entries) database (Supplementary Data Table S1). eEF2 in the untreated cells, which was far less methylated than that in the bFGF-treated cells, had a molecular weight of 95.24 kDa and a pI of 6.5, while the more methylated eEF2 in the bFGF-treated cells had a molecular weight of 95.30 and a pI of 6.4. The molecular weight of the more methylated eEF2 increased by 60 Da compared to the unmethylated eEF2; this corresponds to the molecular weight of four methyl groups, suggesting dimethylation of two arginines per eEF2 molecule. The methylation decreased the pI by 0.1, most likely due to masking of the basic groups of the arginines by methyl groups.
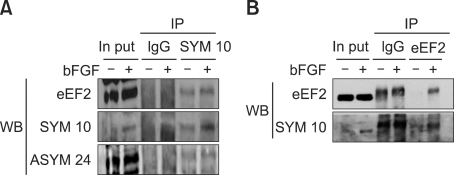
The proteomic data for eEF2 arginine methylation were confirmed through immunoprecipitation experiments. First, we immunoprecipitated NIH3T3 cell lysates with an anti-eEF2 antibody and performed Western blot analysis using anti-eEF2 antibody, SYM10 and ASYM24. Almost the same amount of eEF2 was present in the anti-eEF2 antibody immunoprecipitates and cell lysates from bFGF-treated and untreated cells as in the anti-eEF2 Western blots (Figure 2A). However, the amount of sDMA in the protein in the region corresponding to eEF2 was more abundant in the bFGF-treated cells than in the untreated cells according to a SYM10 Western blot, clearly showing that bFGF does not affect the expression level of eEF2 but induces the synthesis of sDMA in eEF2. These results confirm that eEF2 contains sDMA, and that the level of sDMA is modulated by bFGF. eEF2 also contained aDMA because immunoprecipitated eEF2 strongly reacted with ASYM24 antibody as in the Western blot. However, bFGF did not induce asymmetric arginine dimethylation of eEF2 because the amounts of aDMA in lysates as well as eEF2 immunoprecipitates did not change by bFGF. Next, NIH3T3 cell lysates were immunoprecipitated with SYM10 to isolate sDMA-containing proteins, and immunoprecipitates were analyzed by probing Western blots with anti-eEF2 antibody or SYM10 (Figure 2B). Western blotting using anti-eEF2 antibody clearly showed that eEF2 was present in equal amounts in the whole lysates of bFGF-treated and untreated NIH3T3 cells; however, symmetrically arginine-dimethylated eEF2, which was immunoprecipitated by SYM10, existed exclusively in bFGF-treated cells. When SYM10 was used for Western blot analysis, a much higher level of sDMA-containing protein was observed in the 95-kDa region corresponding to eEF2 in the SYM10 immunoprecipitates as well as in the cell lysates of bFGF-treated cells compared to those in bFGF-untreated cells. Collectively, these results supported the finding that bFGF induces the synthesis of sDMA in eEF2 but does not affect the expression level of eEF2.
Figure 2.
eEF2 contains symmetrically dimethylated arginines induced by bFGF. (A) bFGF induced the level of symmetric arginine dimethylation but neither asymmetric arginine dimethylation nor expression of eEF2. NIH3T3 cell lysates were immunoprecipitated with anti-eEF2 antibody, and immunoprecipitation with normal goat anti-rabbit IgG was performed as a negative control. Western blotting was performed with anti-eEF2 antibody, SYM10 and ASYM24, and protein bands were visualized using enhanced chemiluminescence. GAPDH was used as a loading control. In put, whole cell lysates; IP, immunoprecipitation; WB, western blot. (B) The symmetrically dimethylated arginine-containing 95-kDa protein is eEF2. bFGF induced symmetric arginine dimethylation of eEF2 but did not affect the level of eEF2 expression. NIH3T3 cell lysates were prepared, immunoprecipitated with SYM10, and analyzed using anti-eEF2 antibody and SYM10 Western blots, respectively as in (A).
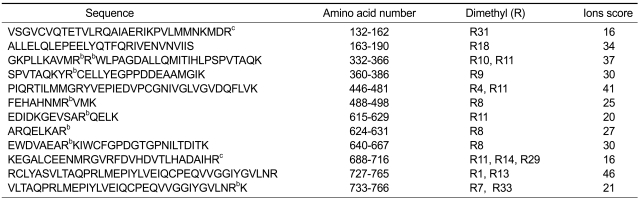
Next, arginine-methylated eEF2 from bFGF-treated NIH3T3 cells was subjected to MS, and arginine methylation sites were identified by searching the generated peak list files against EF-2 protein database. Sequence coverage was 81% (Supplemental Data Table S2). This approach led to the high-confidence identification of several DMA sites with the dimethylated Arg739 (number refers to amino acid position) being most significantly repeatedly observed (Table 1). The identified arginine sites could be either symmetrically or asymmetrically dimethylated. Thus, we compared the DMA sites between eEF2s from bFGF-untreated and treated cells, revealing that dimethylation of arginines including Arg341, Arg342, Arg368, Arg495, Arg625, Arg631, Arg647 and Arg765 was induced by bFGF (indicated by superscript b in Table 1). Consequently, we hypothesized that the arginine sites showing bFGF-inducible dimethylation likely contain bFGF-inducible sDMA.
Table 1.
List of eEF2 peptidesa with dimethylarginines
aeEF2 peptides were obtained by proteolysis of eEF2 from bFGF-treated NIH3T3 cells, and analyzed by mass spectrometry for the presence of dimethylarginine. Thus, the arginine (s) on these peptides can be either symmetrically or asymmetrically dimethylated. bArginines, the dimethylation of which was induced by bFGF. cDimethylarginines, the demethylation of which was induced by bFGF
Mouse (Mus musculus) eEF2 contains one of putative sDMA (GRG and ARG/GRA) or aDMA (RGG) motifs, Arg716. Intriguingly, Arg716 and Arg162 existed as dimethylated form in bFGF-untreated cells, and demethylation of the two dimethylated arginines occurred after the administration of bFGF (indicated by superscript c in Table 1). It was not determined whether the two arginines were symmetrically or asymmetrically dimethylated in the present experiment. Collectively, it appeared that bFGF induces symmetrical dimethylation of some arginines in eEF2 and simultaneous demethylation of part of pre-existing dimethylarginines, generating a diverse combination of dimethylated arginines. There are a total of 45 arginines in eEF2 of mouse (Mus musculus). We found 8 putative arginine sites symmetrically dimethylated and two dimethylarginines demethylated after bFGF treatment of cells in the condition of 81% sequence coverage. Our former proteomic data indicated that bFGF caused the increase of two dimethylarginines in bFGF-treated cells compared to untreated cells. This number appears to be the sum of increased or decreased dimethylations in a diverse combination of dimethylated arginines generated by bFGF.
The ras-raf-MEK-ERK1/2-p21Cip/WAF1 pathway mediates the induction of symmetric arginine dimethylation of eEF2 by bFGF
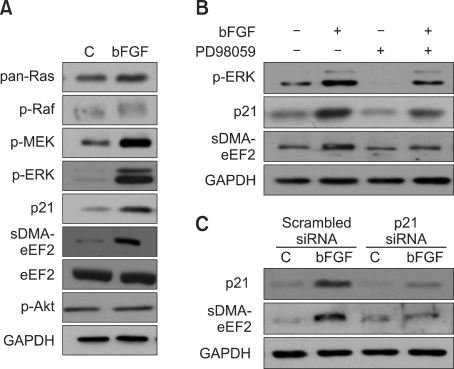
We investigated the components of the bFGF signaling pathway involved in symmetric arginine dimethylation of eEF2. bFGF increases the level of Erk at 30 min after administration but does not affect the level of serine/threonine-protein kinase (Akt), while the amount of sDMA in eEF2 increases significantly during the 24 h period of bFGF administration in NIH3T3 cells (Figure 1). Therefore, we determined the levels of the components upstream and downstream of the Erk pathway in NIH3T3 cells after bFGF administration (Figure 3A). The levels of ras, raf, MEK, Erk1/2, p21Cip/WAF1, and the amount of sDMA in eEF2 increased 24 h after bFGF administration, while the level of eEF2 expression remained unchanged. To confirm the regulatory role of the Erk pathway in symmetric arginine dimethylation of eEF2, we administered the mitogen-activated protein kinase (MAPK) inhibitor, PD98059, to bFGF-treated NIH3T3 cells and investigated whether the stimulatory effects of ERK1/2 on eEF2 arginine methylation were blocked. Indeed, PD98059 almost completely inhibited bFGF-induced symmetric arginine dimethylation of eEF2. In addition, PD98059 suppressed the bFGF-induced up-regulation of p21Cip/WAF1, indicating that p21Cip/WAF1 up-regulation is dependent on MAPK pathway (Figure 3B). Next, we investigated whether p21Cip/WAF1 was indeed involved in the regulation of symmetric arginine dimethylation of eEF2. As expected, treatment of cells with p21Cip/WAF1 short interfering RNA (siRNA) caused the down-regulation of bFGF-induced eEF2 arginine methylation to basal levels (Figure 3C). Collectively, these data clearly demonstrate that the effects of bFGF on symmetric arginine dimethylation of eEF2 are mediated by the ras-raf-MEK-ERK1/2-p21Cip/WAF1 pathway.
Figure 3.
The ras-raf-MEK-ERK1/2-p21Cip/WAF1 pathway mediates the effect of bFGF on symmetric arginine dimethylation of eEF2. (A) The ras-raf-MEK-ERK1/2-p21Cip/WAF1 pathway was activated in the process of bFGF-induced symmetric dimethylation of arginine on eEF2, while the levels of p-Akt and eEF2 expression were not changed. NIH3T3 cells were treated with bFGF, and cell lysates were prepared after 24 h incubation. Western blot analysis was conducted with anti-pan-ras, p-raf, anti-MEK, anti-p-ERK1/2, anti-p21Cip/WAF1, or anti-p-Akt antibodies for signal transduction analysis; SYM10 and anti-eEF2 antibodies were used for eEF2 symmetric arginine dimethylation or expression analysis. Anti-GAPDH antibody was used as a loading control. (B) The inhibition of ERK1/2 by the MAPK inhibitor PD98059 almost completely blocked symmetric arginine dimethylation of eEF2 induced by bFGF with a concurrent decrease in the level of p21Cip/WAF1. NIH3T3 cells were treated with bFGF in the presence or absence of PD98059, and cell lysates were prepared after 24 h incubation. Western blot analysis was conducted with anti-p-ERK, anti-p21Cip/WAF1 and SYM10 antibodies as in (A). (C) The inhibition of p21Cip/WAF1 by siRNA almost completely blocked the bFGF-induced symmetric arginine dimethylation of eEF2 but did not affect bFGF-induced down-regulation of the fibroblast differentiation marker, α-SMA. NIH3T3 cells were transfected with p21Cip/WAF1 siRNA and treated with bFGF. Cell lysates were prepared after 24 h incubation, and Western blot analysis was conducted with anti-p21Cip/WAF1, SYM10 and anti-α-SMA antibodies as in (A).
We investigated the involvement of p21Cip/WAF1 in bFGF-induced α-SMA suppression because bFGF regulates the level of eEF2 arginine methylation via ERK1/2, and p21Cip/WAF1 is downstream of ERK1/2 in the signal transduction pathway in the present experimental setting. In addition, it is known that bFGF regulates α-SMA expression via ERK1/2 (Ishiguro et al., 2009). Levels of p21Cip/WAF1 were not related to α-SMA expression; p21Cip/WAF1 inhibition by siRNA did not have a significant effect on α-SMA expression, indicating that bFGF/ERK1/2 does not regulate α-SMA expression through p21Cip/WAF1 in contrast to eEF2 arginine methylation (Figure 3C).
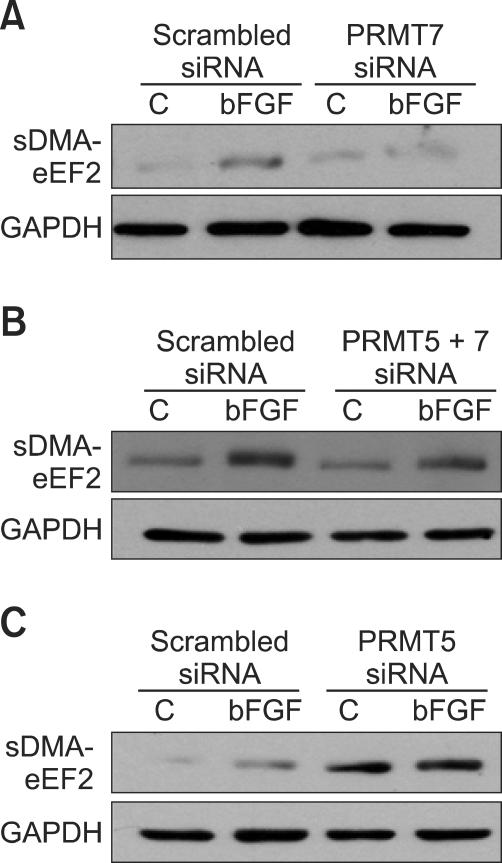
PRMT7 and PRMT5 are responsible for the bFGF-directed regulation of sDMA synthesis in eEF2
The production of sDMA is catalyzed by type II enzymes, and PRMT5 appears to be the major mammalian type II enzyme. The characteristics of PRMT7 are still being established (Bedford and Clarke, 2009). PRMT5 likely interacts with multiple binding partners. It has been suggested that a PRMT5/PRMT7 heterodimer could be the active core of the enzyme complex involved in the Sm protein methylation reaction (Gonsalvez et al., 2007). PRMT5 can also act as a corepressor or coactivator of transcription in the nucleus (Dacwag et al., 2007; Lacroix et al., 2008; Liu et al., 2007).
Thus, we investigated whether PRMT5 and PRMT7 play roles in eEF2 methylation by observing the effects of siRNA inhibition of PRMT5, PRMT7 or both on symmetric arginine dimethylation of eEF2. The efficiency of siRNA inhibition of PRMT5 and PRMT7 mRNA levels was validated using RTqPCR (Supplementary Data Table S3). PRMT7 and PRMT5 were responsible for bFGF-induced symmetric arginine dimethylation of eEF2; inhibition of PRMT7 alone or simultaneous inhibition of PRMT7 and PRMT5 by corresponding siRNAs suppressed the eEF2 methylation (Figures 4A and 4B). Intriguingly, the specific inhibition of PRMT5 by siRNA resulted in a remarkable increase in symmetric arginine dimethylation of eEF2 in untreated as well as bFGF-treated NIH3T3 cells to almost the same extent, notably negating the regulatory effect of bFGF on the eEF2 methylation (Figure 4C). This finding led us to speculate that PRMT5, a major enzyme in symmetric dimethylation of arginine, might function to coordinate bFGF-induced symmetric arginine dimethylation of eEF2 rather than directly methylating eEF2. Thus, we hypothesized that PRMT7 and PRMT5 are clearly required for the bFGF-directed regulation of symmetric arginine dimethylation of eEF2; the putative PRMT7 complex is responsible for the methylation, and PRMT5 may act as a corepressor or coactivator. The mechanism through which PRMTs are regulated by p21Cip/WAF1 from the ras-raf-MEK-ERK1/2-p21Cip/WAF1 pathway remains to be established.
Figure 4.
PRMT7 is responsible for symmetric dimethylation of arginine on eEF2 induced by bFGF, while PRMT5 appears to play a coordinating role. (A) The inhibition of PRMT7 by siRNA resulted in the complete loss of the bFGF-induced symmetric arginine dimethylation of eEF2. The levels of symmetric dimethylation of arginine on eEF2 and α-SMA expressions were not correlated to each other. NIH3T3 cells were transfected with PRMT7 siRNA for 4 days and treated with bFGF for 24 h. Cell lysates were prepared, and the RNA level of PRMT7 was analyzed using real-time quantitative PCR. Western blot analysis was conducted with SYM10 and anti-α-SMA antibodies. Anti-α-SMA Western blot was used to determine the interrelationship between the levels of symmetric dimethylation of arginine on eEF2 and the expression of the fibroblast differentiation marker, α-SMA. Anti-GAPDH antibody was used as a loading control. (B) PRMT5 inhibition remarkably increases the amount of sDMA on eEF2 and negates the regulatory effect of bFGF on the symmetric arginine dimethylation of eEF2. NIH3T3 cells were transfected with PRMT5 siRNA for 4 days and treated with bFGF for 24 h. Cell lysates were prepared, and Western blot analysis was conducted as in (A). (C) The simultaneous inhibition of PRMT7 and PRMT5 suppressed the bFGF-induced symmetric arginine dimethylation of eEF2. NIH3T3 cells were co-transfected with PRMT7 and PRMT5 siRNAs for 4 days and treated with bFGF for 24 h. Cell lysates were prepared, and Western blot analysis was conducted as in (A).
bFGF has been shown to down-regulate α-SMA expression through ERK1/2 activation, and this process is closely associated with the differentiation of fibroblasts into myofibroblasts (Ishiguro et al., 2009; Kawai-Kowase et al., 2004; Maltseva et al., 2001). Because bFGF also has an impact on eEF2 methylation through ERK1/2 activation, which is a key component of the protein translation elongation process, we tested if there was a direct correlation between the levels of sDMA in eEF2 and α-SMA expressions. The bFGF treatment did decrease the levels of α-SMA expression and increased the levels of sDMA in eEF2 in NIH3T3 cells (Figure 4). However, neither decreased levels of sDMA in eEF2 caused by siRNA inhibition of PRMT7 nor increased levels of sDMA in eEF2 generated by the siRNA inhibition of PRMT5 affected a bFGF-induced change in the level of expression of α-SMA. Thus, it does not appear that there is a direct connection between the levels of sDMA in eEF2 and α-SMA expressions.
Discussion
In recent years, it has become clear that methyl groups are as important as phosphate groups in controlling protein function. Protein arginine methylation is involved in diverse biological activities including transcriptional control, DNA repair, mRNA splicing, signal transduction, and protein translocation (Bedford and Clarke, 2009; Boisvert et al., 2003), but it is not associated with protein biosynthesis. In this paper, we demonstrate for the first time that eEF2, a key component in protein translational elongation, is arginine methylated, and that the level of sDMA in eEF2 is modulated by bFGF in a reversible manner through MAPK signaling pathway with PRMT7/PRMT5 as the methyltransferase complex responsible for the methylation.
Boisvert and colleagues generated four arginine methyl-specific antibodies: ASYM24 and ASYM25 (specific for aDMA) and SYM10 and SYM11 (specific for sDMA). Through proteomic analysis using these antibodies, more than 200 proteins that are putatively arginine-methylated were identified from the human HeLa-S3 cell line (Biovest International Inc./National Cell Culture Center, Minneapolis, MN). The major purified protein complexes included components required for pre-mRNA splicing, polyadenylation, transcription, signal transduction, and cytoskeleton and DNA repair, but no factors involved in protein biosynthesis were discovered (Boisvert et al., 2003). In our study, we found that eEF2 is arginine methylated in mouse embryo fibroblast NIH3T3 cells but not in human colorectal carcinoma HCT116 cells, and that symmetric arginine dimethylation of eEF2 is induced by bFGF (Figure 1). It is possible that the ligand-dependent and species- or tissue-specific nature of eEF2 methylation may have delayed the detection of eEF2 arginine methylation; the difference in methylation of eEF2 between human colorectal carcinoma cells and mouse embryo fibroblast cells suggests that eEF2 arginine methylation is a species-specific or even a tissue-specific phenomenon. In addition, eEF2 arginine methylation may be barely detectable without stimulation of cells by signals such as growth factors.
The major regulatory mechanism of eEF2 activity has previously been proposed to be dependent on phosphorylation by eEF2 kinase, a calcium/calmodulin (CaM)-dependent enzyme (Mitsui et al., 1993) that has eEF2 as its only known substrate (Proud, 2000). Phosphorylation of Thr-56 of eEF2 was discovered to regulate the activity of this protein and its ability to bind to ribosomes (Browne and Proud, 2002). Mitogenic and hormonal stimuli that increase elongation rates can induce eEF2 dephosphorylation through activation of mTOR, p38 and MEK pathways, and associated phosphatases like PP2A (Proud, 2000; Sans et al., 2004). eEF2 phosphorylation has been shown to be increased by different stimuli, usually associated with cellular stress, including increases in cytoplasmic calcium level (Nairn et al., 2001), nutrient and amino acid withdrawal (Wang et al., 1998), depletion of cellular ATP (McLeod and Proud, 2002), and increases in cAMP (Gutzkow et al., 2003) and AMP levels (Horman et al., 2002). ADP-ribosylation of eEF2 by bacterial toxins on a histidine modified to a diphthamide inhibits its translocation activity (Jørgensen et al., 2005). No conclusive evidence has yet been provided that bFGF is involved in the phosphorylation or ADP-ribosylation of eEF2, but bFGF turned out to regulate eEF2 methylation in our study. The bFGF-regulated symmetric arginine dimethylation of eEF2 is a novel mechanism for post-translational modification of eEF2. bFGF also appears to cause demethylation of some pre-existing dimethyarginines. However, the functional consequence of this methylation remains unknown in our study, whether it is global protein synthesis or the expression of specific proteins. We propose that the intensity or kind of stimulation might determine how many and which arginines among those in eEF2 are methylated, generating diverse patterns of combination of dimethylarginines with specific functional consequences that need to be determined.
We found that the level of arginine methylation of a specific protein was regulated by a specific extracellular signal, bFGF. PRMTs are known to methylate the arginine residues of certain proteins. In turn, PRMTs are regulated in several ways. First, PRMTs are regulated by posttranslational modifications or through interactions with PRMT-binding proteins and protein complexes. PRMT activity can also be regulated by subcellular compartmentalization. In addition, adjacent posttranslational modifications can mask arginine methylation motifs in PRMT substrates. Finally, enzymes that counteract PRMT activity can reverse or block substrate methylation (Bedford and Clarke, 2009). However, the upstream signaling pathways that control the regulatory mechanisms described above remain to be established. Our data suggest that the bFGF-ras-MAPK pathway extending to p21Cip/WAF1 in the context of symmetric arginine dimethylation of eEF2 is an example of an upstream signaling pathway that ultimately regulates the activities of PRMTs through an as yet unidentified mechanism.
We also suggest that bFGF-regulated eEF2 methylation may allow the identification of additional demethylases because, by 48 h after bFGF treatment, the increased level of sDMA in eEF2 had returned to baseline, while the expression levels of eEF2 remained unchanged throughout the period, suggesting the presence of a demethylation mechanism. Intriguingly, bFGF caused demethylation of some arginines which existed in dimethylated form in the bFGF-untreated cells. This finding indicates the presence of the demethylation mechanism triggered by bFGF. The only known arginine demethylase is the Jumonji domain-containing enzyme, JMJD6, which demethylates H3R2me2 and H4R3me2 (Chang et al., 2007). Further research is required in this field, especially in terms of characterizing additional proteins in the Jumonji-only family of enzymes or other types of arginine demethylases.
Our results show that p21Cip/WAF1 is downstream of ERK1/2 in the context of symmetric arginine methylation of eEF2 but not for α-SMA expression. In fibroblasts, bFGF causes ERK1/2 activation, which is implicated in the suppression of α-SMA expression through inhibition of Smad nuclear translocation, contributing to increased fibrosis (Ishiguro et al., 2009; Leivonen et al., 2005). The intermediary signaling pathway connecting ERK1/2 and p21Cip/WAF1 in the process of eEF2 arginine methylation has yet to be elucidated.
PRMT7 has been shown to have type II activity toward peptide and protein substrates (Lee et al., 2005) or type III activity that monomethylates only arginine-containing peptides (Miranda et al., 2004). Neither of these activities is particularly robust. Even though PRMT7 is involved in the methylation of the C-terminal tails of Sm proteins in conjunction with PRMT5 and is required for normal snRNP biogenesis in human cells (Gonsalvez et al., 2007), more work is needed to characterize the enzymatic activities of PRMT7 and to establish its interaction partners. In our study, we found that PRMT7 siRNA clearly suppresses sDMA synthesis in eEF2 induced by bFGF in NIH3T3 cells, strongly indicating that PRMT7 is likely the core enzyme in the putative complex catalyzing eEF2 methylation. PRMT5 is an enzyme that appears to function in several types of complexes in both the cytoplasm and the nucleus, acting as a coordinator of specific biological functions as well as a major enzyme in the arginine methyltransferase complex. In the cytoplasm, PRMT5 is involved in snRNP biogenesis through its ability to methylate a number of Sm proteins (Neuenkirchen et al., 2008). In the nucleus, similar to CARM1, PRMT5 can complex with hSWI/SNF ATP-dependent chromatin remodeling proteins to function as a transcriptional coactivator (Dacwag et al., 2007). PRMT5 also associates with regulators of transcriptional elongation (Liu et al., 2007). Binding of PRMT5 to COPR5 (cooperator of PRMT5) can result in the preferential methylation of H4R3 over H3R8 (Lacroix et al., 2008). Moreover, COPR5 binding appears to be responsible for PRMT5 transcriptional corepressor activity. In our study, PRMT7 and PRMT5 appear to play critical roles in the putative enzyme complex involved in the bFGF-induced sDMA synthesis in eEF2. Intriguingly, our results based on siRNA transfection experiments indicate that PRMT5 likely acts as a corepressor of this process, with PRMT7 responsible for methylation, even though PRMT5 ordinarily acts as a major enzyme in the complex involved in the production of sDMA; siRNA inhibition of PRMT5 markedly increased eEF2 methylation, negating the regulatory role of bFGF in the methylation. There is also a possibility that there are other factors associated with the PRMT7/PRMT5 complex, e.g., FBXO11, the characteristics of which are still being established in the context of sDMA synthesis (Cook et al., 2006).
In conclusion, we have demonstrated bFGF-induced symmetric arginine dimethylation of mouse embryo fibroblast eEF2 with its signaling pathway and responsible enzymes. This opens new investigative directions regarding the role and regulatory mechanism of arginine methylation in the elongation process of protein biosynthesis.
Methods
Antibodies
Anti-sDMA antibodies, namely SYM10, SYM11, and the anti-aDMA antibody, ASYM24 (antibodies that bind to sDMA or aDMA within sequences KR*GR*GR*GR*G, KAAILLAQVAAR*GR*GR*GMGR*G and KGR*GR*GR*GR *GPPPPPR*GR*GR*GR*G, respectively; R* designates dimethylarginine), were purchased from Upstate Biotechnology (Lake Placid, NY). Anti-eEF2 antibody was purchased from Abcam (Cambridge, MA). Anti-p-ras, p-raf and PRMT7 antibodies were obtained from Millipore (Billerica, MA). Anti-MEK, p-ERK1/2, and p-Akt antibodies were purchased from Cell Signaling Biotechnology (Beverly, MA). Anti-p21cip/waf and GAPDH antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-α-SMA antibody was purchased from Sigma (St. Louis, MO).
siRNA sequences
siRNAs were purchased from Genolution Pharmaceuticals, Inc. (Seoul, Korea). The mouse p21Cip/WAF1 (GenBank accession number NM_007669) target sequences were 5'-CCAGCCUGACAGAUUUCUAUU-3' (sense) and 5'-UAGAAAUCUGUCAGGCUGGUU-3' (antisense). The PRMT 5 (GenBank accession number NM_013768.3) target sequences were CCCTTAATCAGGAAGATAA (sense) and UUAUCUUCCUGAUUAAGGG (antisense). The PRMT7 (GenBank accession number NM_145404.1) target sequences were CGGAGCAGGUGUUUACAGU (sense) and ACUGUAAACACCUGCUCCG (antisense). Scrambled siRNAs with sequences 5'-CACUUACGCUGAGUACUUCUU-3' (sense) and 5'-GAAGUACUCAGCGUAAGUGUU-3' (antisense) were used as negative controls.
Cell culture and transfection
NIH3T3 and HCT116 cells were obtained from ATCC and were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS), 100 IU/ml penicillin, and 100 µg/ml streptomycin (Invitrogen, Carlsbad, CA) at 37℃ under 5% CO2 tension in the presence or absence of 10 ng/ml bFGF (Invitrogen), 20 ng/ml EGF (Sigma, St. Louis, MO), or 20 ng/ml HGF (Sigma). For signal transduction assays, NIH3T3 cells were treated with 20 µM PD98059 (2'-amino-3'-methoxyflavone) (Merck KGaA, Darmstadt, Germany). For transfection experiments, NIH3T3 cells were plated at a density of 5 × 105 cells per 60 mm culture dish and transfected with p21Cip/WAF1, PRMT7, or PRMT5 siRNAs using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions.
RTqPCR
NIH3T3 cells were transfected with 20 nM PRMT5 or PRMT7 siRNAs for five days, and then the RNA level of each PRMT was analyzed using real-time quantitative PCR (Cepheid, CA) with CYBRgreen method (TAKARA, Kyoto, Japan) according to the manufacturer's instructions with actin as control. The PRMT5 primers were 5'-TCACCTCAGTGGAGTGCTTG-3' (forward) and 5'-TCACCTCAGTGGAGTGCTTG-3' (reverse). The PRMT7 primers were 5'-TACTGCAGGGGCTGACTTCT-3' (forward) and 5'-TCACCTCAGTGGAGTGCTTG-3' (reverse).
Western blot analysis and immunoprecipitation assays
For Western blot analysis, NIH3T3 and HCT116 cells were lysed with 1 × Laemmli buffer (0.14 M Tris, pH 6.8, containing 2.4 M glycerol, 0.21 M sodium dodecyl sulfate, and 0.3 mM bromophenol blue). Cell lysates were heated at 100℃ for 5-10 min and separated via electrophoresis in 8-12% SDS-polyacrylamide gels. After electrophoretic transfer of proteins onto nitrocellulose membrane, membranes were blocked with 5% nonfat dry milk in PBS containing 0.1% (v/v) Tween 20 for 30 min at 25℃ and then incubated with SYM10, SYM11, or ASYM24 for arginine methylation analysis. Anti-pan-ras, anti-p-raf, anti-MEK, anti-p-ERK1/2, anti-p21Cip/WAF1 or anti-p-Akt antibodies were used for signal transduction experiments; and anti-α-SMA antibody was used for fibroblast differentiation analysis. Anti-GAPDH antibody was used as a loading control. Protein bands were visualized using enhanced chemiluminescence (ECL; Amersham Inc., Buckinghamshire, UK). Statistically significant differences as determined by the Wilcoxon test were noted at P < 0.001 (*). For immunoprecipitation experiments, NIH3T3 cells were lysed with RadioImmunoPrecipitation Assay (RIPA) buffer (Upstate Biotechnology, Lake Placid, NY). Four hundred micrograms of cell lysate was immunoprecipitated with SYM 10 or anti-eEF2 (1:25 vol/vol) (clone number EP880Y) antibody, and 50 µl protein A sepharose CL-4B (GE Healthcare, Fairfield, CT) in the presence of 0.05% BSA overnight at 4℃. As a negative control, we performed immunoprecipitation with normal goat anti-rabbit IgG (Upstate Biotechnology).
MS analysis
Analysis of arginine methylation was performed as previously described (Kirino et al., 2010). A 95-kDa arginine-methylated protein gel band was excised from SDS-PAGE gel. The protein band was subjected to reduction in 10 mM dithiothreitol for 45 min at 37℃ and alkylation in 55 mM iodoacetamide for 15 min at room temperature in the dark and was digested overnight at 37℃ with 12.5 ng/µl trypsin (Promega, Madison, WI). The digested peptides were acidified to 0.1% trifluoroacetic acid and desalted onto C18 Ziptips (Millipore, Billerica, MA) according to the manufacturer's instruction. The desalted peptides were analyzed in an LCQ Deca mass spectrometer (Thermo Fisher Scientific, Waltham, MA) coupled online to an Agilent 1100 HPLC system. An in-house pulled 0.075 × 130 mm emitter column was prepared by packing with Zorbax 300SB-C18, 5 µm (Agilent, Santa Clara, CA) using a slurry packer (Alltech, Lexington, KY). Peptides were separated with a linear acetonitrile gradient: 0 % solvent A (5% acetonitrile, 0.1% formic acid) to 40% solvent B (95% acetonitrile, 0.1% formic) over 80 min and then 90% solvent B in 15 min at a flow rate of 0.2 µl/min. For tandem mass spectrometry, MS and MS/MS data were obtained in a data-dependent mode. The full mass scan range mode was m/z = 250 - 2000 Da for the selection of a precursor ion. An exclusion dynamic mode was applied to exclude the most intense ion from further selection over a 2-min period. The subsequent MS/MS data were acquired using a 2 m/z unit ion isolation window in the automated gain control (AGC) mode where AGC values of 5.00e + 05 and 1.00e + 04 were set for full MS and MS/MS, respectively. The normalized CID was set at 35.0.
Database searches
The individual spectra from MS/MS were processed using SEQUEST software (Thermo Fisher Scientific, Waltham, MA). The generated peak list files were searched against NCBI-nonredundant (version 08_06_2010, mammalian entries) or EF-2 protein databases using the MASCOT 2.1 program with the following parameters; oxidation on methionine, carbamidomethylation or propionamidylation of cysteine, arginine dimethylation as variable modifications, semi-tryptic specificity, peptide mass tolerance at 2 Da, MS/MS ion mass tolerance at 1 Da, allowance of missed cleavage at 3, and charge states of +1, +2, and +3. Peptides with ions scores of 20 and higher were initially considered, and individual dimethylated peptides were manually validated.
Supplemental data
Supplemental data include a figure and three tables and can be found with this article online at http://e-emm.or.kr/article/article_files/SP-43-10-02.pdf.
Acknowledgements
This work was supported by a grant from the National Nuclear R&D Program of the Ministry of Science and Technology of Korea, Basic Atomic Energy Research Institute and Korea University Grant (K1032471).
Abbreviations
- aDMA
asymmetric dimethylarginine
- Akt
phospho-serine/threonine-protein kinase
- bFGF
basic fibroblast growth factor
- eEF2
eukaryotic elongation factor 2
- HGF
hepatocyte growth factor
- MEK
mitogen-activated protein kinase kinase
- PRMT
protein arginine methyltransferase
- sDMA
symmetric dimethylarginine
- siRNA
short interfering RNA
Supplementary Material
Supplemental Data
References
- 1.Akasaka Y, Ono I, Yamashita T, Kimbow K, Ishii T. Basic fibroblast growth factor promotes apoptosis and suppresses granulation tissue formation in acute incisional wounds. J Pathol. 2004;203:710–720. doi: 10.1002/path.1574. [DOI] [PubMed] [Google Scholar]
- 2.Bedford MT, Clarke SG. Protein arginine methylation in mammals: Who, what, and why. Mol Cell. 2009;33:1–13. doi: 10.1016/j.molcel.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boisvert FM, Cote J, Boulanger MC, Richard S. A proteomic analysis of arginine-methylated protein complexes. Mol Cell Proteomics. 2003;2:1319–1330. doi: 10.1074/mcp.M300088-MCP200. [DOI] [PubMed] [Google Scholar]
- 4.Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem. 2002;269:5360–5368. doi: 10.1046/j.1432-1033.2002.03290.x. [DOI] [PubMed] [Google Scholar]
- 5.Chang B, Chen Y, Zhao Y, Bruick RK. JMJD6 is a histone arginine demethylase. Science. 2007;318:444–447. doi: 10.1126/science.1145801. [DOI] [PubMed] [Google Scholar]
- 6.Chiba S, Kurokawa MS, Yoshikawa H, Ikeda R, Takeno M, Tadokoro M, Sekino H, Hashimoto T, Suzuki N. Noggin and basic FGF were implicated in forebrain fate and caudal fate, respectively, of the neural tube-like structures emerging in mouse ES cell culture. Exp Brain Res. 2005;163:86–99. doi: 10.1007/s00221-004-2148-y. [DOI] [PubMed] [Google Scholar]
- 7.Chinali G, Parmeggiani A. Differential modulation of the elongation-factor-G GTPase activity by tRNA bound to the ribosomal A-site or P-site. Eur J Biochem. 1982;125:415–421. doi: 10.1111/j.1432-1033.1982.tb06699.x. [DOI] [PubMed] [Google Scholar]
- 8.Cook JR, Lee JH, Yang ZH, Krause CD, Herth N, Hoffmann R, Pestka S. FBXO11/PRMT9, a new protein arginine methyltransferase, symmetrically dimethylates arginine residues. Biochem Biophys Res Commun. 2006;342:472–481. doi: 10.1016/j.bbrc.2006.01.167. [DOI] [PubMed] [Google Scholar]
- 9.Dacwag CS, Ohkawa Y, Pal S, Sif S, Imbalzano AN. The protein arginine methyltransferase Prmt5 is required for myogenesis because it facilitates ATP-dependent chromatin remodeling. Mol Cell Biol. 2007;27:384–394. doi: 10.1128/MCB.01528-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doniach T. Basic FGF as an inducer of anteroposterior neural pattern. Cell. 1995;83:1067–1070. doi: 10.1016/0092-8674(95)90133-7. [DOI] [PubMed] [Google Scholar]
- 11.Gary JD, Clarke S. RNA and protein interactions modulated by protein arginine methylation. Prog Nucleic Acid Res Mol Biol. 1998;61:65–131. doi: 10.1016/s0079-6603(08)60825-9. [DOI] [PubMed] [Google Scholar]
- 12.Gonsalvez GB, Tian L, Ospina JK, Boisvert FM, Lamond AI, Matera AG. Two distinct arginine methyltransferases are required for biogenesis of Sm-class ribonucleoproteins. J Cell Biol. 2007;178:733–740. doi: 10.1083/jcb.200702147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutzkow KB, Lahne HU, Naderi S, Torgersen KM, Skalhegg B, Koketsu M, Uehara Y, Blomhoff HK. Cyclic AMP inhibits translation of cyclin D3 in T lymphocytes at the level of elongation by inducing eEF2-phosphorylation. Cell Signal. 2003;15:871–881. doi: 10.1016/s0898-6568(03)00038-x. [DOI] [PubMed] [Google Scholar]
- 14.Hershey JWB, Merrick WC. Pathway and mechanism of initiation of protein synthesis. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational Control of gene expression. Cold Spring Harber, NY: Cold Spring Harbor Laboratory Press; 2000. pp. 33–88. [Google Scholar]
- 15.Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud C, Rider M. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- 16.Ishiguro S, Akasaka Y, Kiguchi H, Suzuki T, Imaizumi R, Ishikawa Y, Ito K, Ishii T. Basic fibroblast growth factor induces down-regulation of α-smooth muscle actin and reduction of myofibroblast areas in open skin wounds. Wound Repair Regen. 2009;17:617–625. doi: 10.1111/j.1524-475X.2009.00511.x. [DOI] [PubMed] [Google Scholar]
- 17.Jørgensen R, Merrill AR, Yates SP, Marquez VE, Schwan AL, Boesen T, Andersen GR. Exotoxin A-eEF2 complex structure indicates ADP ribosylation by ribosome mimicry. Nature. 2005;436:979–984. doi: 10.1038/nature03871. [DOI] [PubMed] [Google Scholar]
- 18.Kawai-Kowase K, Sato H, Oyama Y, Kanai H, Sato M, Doi H, Kurabayashi M. Basic fibroblast growth factor antagonizes transforming growth factor-beta1-induced smooth muscle gene expression through extracellular signal-regulated kinase 1/2 signaling pathway activation. Arterioscler Thromb Vasc Biol. 2004;24:1384–1390. doi: 10.1161/01.ATV.0000136548.17816.07. [DOI] [PubMed] [Google Scholar]
- 19.Kibe Y, Takenaka H. Kishimoto S. Spatial and temporal expression of basic fibroblast growth factor protein during wound healing of rat skin. Br J Dermatol. 2000;143:720–727. doi: 10.1046/j.1365-2133.2000.03824.x. [DOI] [PubMed] [Google Scholar]
- 20.Kirino Y, Vourekas A, Kim N, de Lima Alves F, Rappsilber J, Klein PS, Jongens TA, Mourelatos Z. Arginine methylation of vasa protein is conserved across phyla. J Biol Chem. 2010;285:8148–8154. doi: 10.1074/jbc.M109.089821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knebel A, Morrice N, Cohen P. A novel method to identify protein kinase substrates: eEF2 kinase is phosphorylated and inhibited by SAPK4/p38delta. EMBO J. 2001;20:4360–4369. doi: 10.1093/emboj/20.16.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knebel A, Haydon CE, Morrice N, Cohen P. Stress-induced regulation of eukaryotic elongation factor 2 kinase by SB 203580-sensitive and -insensitive pathways. Biochem J. 2002;367:525–532. doi: 10.1042/BJ20020916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lacroix M, El Messaoudi S, Rodier G, Le Cam A, Sardet C, Fabbrizio E. The histone-binding protein COPR5 is required for nuclear functions of the protein arginine methyltransferase PRMT5. EMBO Rep. 2008;9:452–458. doi: 10.1038/embor.2008.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JH, Cook JR, Yang ZH, Mirochnitchenko O, Gunderson SI, Felix AM, Herth N, Hoffmann R, Pestka S. PRMT7, a new protein arginine methyltransferase that synthesizes symmetric dimethylarginine. J Biol Chem. 2005;280:3656–3664. doi: 10.1074/jbc.M405295200. [DOI] [PubMed] [Google Scholar]
- 25.Leivonen SK, Häkkinen L, Liu D, Kähäri VM. Smad3 and extracellular signal-regulated kinase 1/2 coordinately mediate transforming growth factor-beta-induced expression of connective tissue growth factor in human fibroblasts. J Invest Dermatol. 2005;124:1162–1169. doi: 10.1111/j.0022-202X.2005.23750.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Zhou Z, Chen G, Bao S. A putative transcriptional elongation factor hIws1 is essential for mammalian cell proliferation. Biochem Biophys Res Commun. 2007;353:47–53. doi: 10.1016/j.bbrc.2006.11.133. [DOI] [PubMed] [Google Scholar]
- 27.Maltseva O, Folger P, Zekaria D, Petridou S, Masur SK. Fibroblast growth factor reversal of the corneal myofibroblast phenotype. Invest Ophthalmol Vis Sci. 2001;42:2490–2495. [PubMed] [Google Scholar]
- 28.McLeod LE, Proud CG. ATP depletion increases phosphorylation of elongation factor eEF2 in adult cardiomyocytes independently of inhibition of mTOR signalling. FEBS Lett. 2002;531:448–452. doi: 10.1016/s0014-5793(02)03582-2. [DOI] [PubMed] [Google Scholar]
- 29.Merrick W, Nyborg J. The protein biosynthesis elongation cycle. In: Sonenberg N, Hershey JW, Mathews M, editors. Translational Control of Gene Expression. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. pp. 89–125. [Google Scholar]
- 30.Miranda TB, Miranda M, Frankel A, Clarke S. PRMT7 is a member of the protein arginine methyltransferase family with a distinct substrate specificity. J Biol Chem. 2004;279:22902–22907. doi: 10.1074/jbc.M312904200. [DOI] [PubMed] [Google Scholar]
- 31.Mitsui K, Brady M, Palfrey HC, Nairn AC. Purification and characterization of calmodulin-dependent protein kinase III from rabbit reticulocytes and rat pancreas. J Biol Chem. 1993;268:13422–13433. [PubMed] [Google Scholar]
- 32.Nairn AC, Matsushita M, Nastiuk K, Horiuchi A, Mitsui K, Shimizu Y, Palfrey HC. Elongation factor-2 phosphorylation and the regulation of protein synthesis by calcium. Prog Mol Subcell Biol. 2001;27:91–129. doi: 10.1007/978-3-662-09889-9_4. [DOI] [PubMed] [Google Scholar]
- 33.Neuenkirchen N, Chari A, Fischer U. Deciphering the assembly pathway of Sm-class U snRNPs. FEBS Lett. 2008;582:1997–2003. doi: 10.1016/j.febslet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Nishizuka Y, Lipmann F. Comparison of guanosine triphosphate split and polypeptide synthesis with a purified E. coli system. Proc Natl Acad Sci USA. 1966;55:212–219. doi: 10.1073/pnas.55.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Proud CG. Control of the elongation phase of protein synthesis. In: Sonenberg N, Hershey JW, Mathews M, editors. Translational Control of Gene Expression. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. pp. 719–739. [Google Scholar]
- 36.Sans MD, Xie Q, Williams JA. Regulation of translation elongation and phophorylation of eEF2 in rat pancreatic acini. Biochem Biophys Res Commun. 2004;319:144–151. doi: 10.1016/j.bbrc.2004.04.164. [DOI] [PubMed] [Google Scholar]
- 37.Slavin J. Fibroblast growth factors: at the heart of angiogenesis. Cell Biol Int. 1995;19:431–444. doi: 10.1006/cbir.1995.1087. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Wang X, Proud CG. Activation of mRNA translation in rat cardiac myocytes by insulin involves multiple rapamycin-sensitive steps. Am J Physiol Heart Circ Physiol. 2000;278:H1056–H1068. doi: 10.1152/ajpheart.2000.278.4.H1056. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Campbell LE, Miller CM, Proud CG. Amino acid availability regulates p70 S6 kinase and multiple translation factors. Biochem J. 1998;334:261–267. doi: 10.1042/bj3340261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data