Abstract
Osteoarthritis (OA) is an age-related joint disease that is characterized by degeneration of articular cartilage and chronic pain. Oxidative stress is considered one of the pathophysiological factors in the progression of OA. We investigated the effects of grape seed proanthocyanidin extract (GSPE), which is an antioxidant, on monosodium iodoacetate (MIA)-induced arthritis of the knee joint of rat, which is an animal model of human OA. GSPE (100 mg/kg or 300 mg/kg) or saline was given orally three times per week for 4 weeks after the MIA injection. Pain was measured using the paw withdrawal latency (PWL), the paw withdrawal threshold (PWT) and the hind limb weight bearing ability. Joint damage was assessed using histological and microscopic analysis and microcomputerized tomography. Matrix metalloproteinase-13 (MMP13) and nitrotyrosine were detected using immunohistochemistry. Administration of GSPE to the MIA-treated rats significantly increased the PWL and PWT and this resulted in recovery of hind paw weight distribution (P < 0.05). GSPE reduced the loss of chondrocytes and proteoglycan, the production of MMP13, nitrotyrosine and IL-1β and the formation of osteophytes, and it reduced the number of subchondral bone fractures in the MIA-treated rats. These results indicate that GSPE is antinociceptive and it is protective against joint damage in the MIA-treated rat model of OA. GSPE could open up novel avenues for the treatment of OA.
Keywords: antioxidants, grape seed proanthocyanidins, inflammation, interleukin-1β, osteoarthritis
Introduction
Osteoarthritis (OA) is a degenerative disorder of the joints that causes significant pain and functional disability. OA involves not only the articular cartilage, but also the subchondral bone, ligaments, synovial membrane and periarticular muscle (Chen et al., 2010; Henrotin and Kurz, 2007; Krasnokutsky et al., 2008). The cause of OA is unknown, but several studies have suggested that this joint degeneration results from the combination of mechanical stress and biochemical factors, and mainly reactive oxygen species (ROS) and matrix metalloproteinases (MMPs) (Henrotin and Kurz, 2007).
ROS such as the superoxide anion (O2-), hydrogen peroxide (H2O2) and hydroxyl radicals (OH) act as a physiological defense system against microbial infection and they are involved in maintaining normal cellular functions and intracellular signal transduction (Filippin et al., 2008). However, as ROS are capable of damaging lipids, proteins, DNA and the extracellular matrix, excess ROS are removed by antioxidants (Dröge, 2002).
Ultimately, oxidative stress refers to a cellular state in which the production of ROS is elevated and/or the levels of antioxidants are reduced. There is much evidence indicating that oxidative stress is involved in the pathogenesis of OA. Increased ROS promote cartilage degradation by inhibiting cartilage matrix synthesis and inducing chondrocyte apoptosis and cartilage matrix breakdown (Henrotin et al., 2005). The serum total antioxidant capacity, the thiol level and the catalase activity of OA patients are low (Altindag et al., 2007). The cartilage from patients with OA contains less extracellular antioxidants such as superoxide dismutase than does normal cartilage (Regan et al., 2005). The levels of nitrated type II collagen peptides in the sera of patients with OA are elevated, which is indicative of the formation of peroxynitrite (ONOO-), which is a marker of oxidative stress (Deberg et al., 2005).
Intra-articular injection of monosodium iodoacetate (MIA) induces disorganization of the articular cartilage by inhibiting the activity of glyceraldehyde-3-phosphate dehydrogenase in chondrocytes, and this results in disruption of glycolysis and eventual cell death. These chondrocytes have similar histopathology to that of human OA and the MIA induced OA animal model closely mimics both the pain and structural changes associated with the disease (Janusz et al., 2001; Bove et al., 2003).
Grape seed extract contains lipids, proteins, carbohydrates and polyphenols. Proanthocyanidins are potent natural antioxidants, and they are the most abundant phenolic compounds in grape seeds and they are high molecular weight polymers comprised of dimers or trimers of (+)-catechin and (-)-epicatechin (Fine, 2000). It has been suggested that grape seed proanthocyanidin extract (GSPE) is an effective therapy for oxidation-related diseases in animal models of tumors, atherosclerosis, gastric ulcers, cataracts, diabetic retinopathy and rheumatoid arthritis (Ray et al., 2001; Sato et al., 2001; Pataki et al., 2002; Durukan et al., 2006; Cho et al., 2009; Prasain et al., 2009). However, its effects on the chronic pain and structural damage relevant to the pain in OA have not been investigated. We examined whether GSPE relieves pain and if it prevents the structural changes known to cause pain in a rat model of OA produced by MIA injection.
Results
Effects of GSPE on the pain in the MIA-induced OA model
The mechanical paw withdrawal thresholds to von Frey stimuli were measured for 4 weeks after MIA injection using a dynamic plantar esthesiometer. Four days after the injection of MIA, the paw withdrawal latency (PWL) and paw withdrawal threshold (PWT) were lower in the MIA-treated group than in the nonarthritis group, which had received a saline injection instead of MIA. However, the PWL and PWT were significantly higher in the GSPE-treated groups (100 mg/kg: data not shown, 300 mg/kg) than in the MIA-treated group (P < 0.01). Seven days after the injection of MIA, the PWL and PWT in the GSPE-treated groups had recovered to a greater extent than that in the MIA-treated group (P < 0.05) and this trend was maintained for 4 weeks (Figures 1A and 1B).
Figure 1.
Effects of GSPE on the pain in MIA-induced OA. An experimental model of OA in rats was induced by intraarticular injection of monosodium iodoacetate (MIA) into the knee joint. Oral treatment with GSPE (100 mg/kg or 300 mg/kg given three times per week) or with vehicle was initiated on MIA injection (day 0). (A) The paw withdrawal latency and (B) paw withdrawal threshold were measured by a dynamic plantar aesthesiometer and this was compared with that of the MIA-treated group. △: nonarthritis group, ▲: MIA-treated group, ◇: MIA-treated rats treated with 300 mg/kg GSPE. *P < 0.05, **P < 0.01 compared with the MIA injection group. (C) Weight bearing was measured by an incapacitance tester and this was compared with that of the MIA-treated group at 4 weeks after the injection of MIA. *P < 0.05 compared with the MIA injection group. The experiment was repeated 3 times with similar results.
Hind limb weight bearing is a measure of pain and this was measured using an incapacitance tester. Four weeks after the MIA injection, the hind limb weight bearing of the ipsilateral limb was significantly lower in the MIA-treated group than that in the nonarthritis group. The 300 mg/kg GSPE treated group exhibited significant recovery of hind limb weight bearing (P < 0.05, Figure 1C).
OA is characterized not only by progressive cartilage destruction, but also by alterations in bone such as osteoclast recruitment to subchondral bone and osteophyte formation. Osteophytes are also called bone spurs, and they are smooth outgrowths of bone that build up on the edges of joints due to OA. Therefore, we analyzed the osteoclastic activity and osteophyte formation. At 4 weeks after MIA injection along with GSPE treatment, histological analysis revealed osteoclasts on the subchondral bone fragmented trabeculae of the MIA-treated group (arrow), but GPSE decreased osteoclast generation (Figure 2A). We used micro-CT to examine the direct effect of GSPE on the MIA-induced osteophyte formation. Osteophytes were detected in the MIA-treated group, but not in the GSPE-treated groups (Figure 2B). In our rat model of OA, the histological and micro-CT analyses showed that treatment with 300 mg/kg of GSPE suppressed osteoclasts and the osteophyte formation.
Figure 2.
Osteoclastic activity and osteophyte formation for 4 weeks after MIA injection. Bone and osteophyte formation was evaluated in the hematoxylin and eosin-stained, paraffin-embedded knee joint sections and micro-CT analysis of the MIA injected rats treated with GSPE or vehicle, respectively. (A) Osteoclast generation was assessed using hematoxylin and eosin. The bar graphs represent the mean values ± SD of the cells that stained positive (original magnification × 200). **P < 0.01 compared with the MIA injection group. (B) Osteophyte analysis using Micro-CT.
Effects of GSPE on the articular cartilage damage and the catabolic activity of chondrocytes
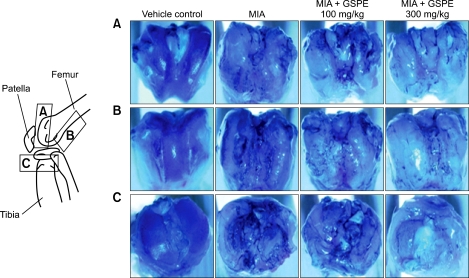
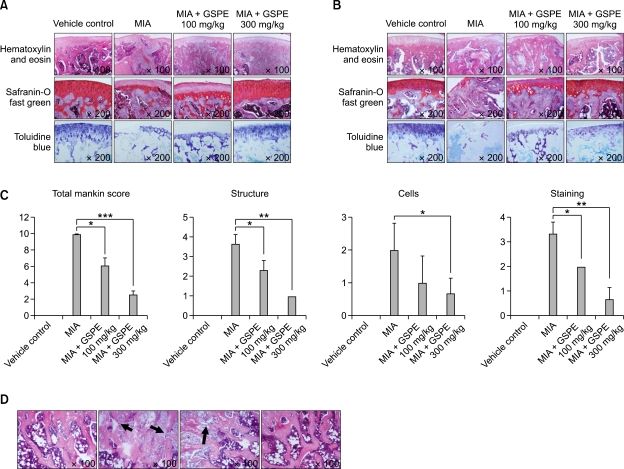
We assessed damage to the articular cartilage surface by using India ink at 4 weeks after the MIA injection. Grossly, the normal joints revealed smooth and shiny articular surfaces. The knees of the MIA-treated group showed irregular abrasions at the articular cartilage surfaces of the femoral condyle and the tibial plateau, but the 300 mg/kg GSPE-treated group had smoother articular surfaces than the MIA-treated group (Figure 3). We used hematoxylin and eosin, Safranin O fast green and toluidine blue staining to examine the structural changes in the joints. The joint from non-induced rat showed smooth articular cartilage surfaces with the underneath layer of flattened chondrocytes in the tangential zone. The chondrocytes were normally distributed in parallel rows in the transitional and radial zones of the articular cartilage. The MIA-treated group showed degeneration of the articular cartilage and disappearance of chondrocytes and proteoglycan in the tangential, transitional and radial zones of the cartilage after 2 weeks, and more severe structural changes after 4 weeks (Figures 4A and 4B). Treatment with 100 mg/kg of GSPE had little effect on the structural changes in the joints, but treatment with 300 mg/kg of GSPE resulted in less severe damage to the articular cartilage compared with that of the MIA-treated group, and chondrocytes and proteoglycan were present in the articular cartilage. The severity of OA lesion was graded using the modified Mankin scoring system. The modified Mankin scores were significantly lower in the GSPE treated group than in the MIA-treated group (Figure 4C). We observed extensive areas of loosely arranged spindle cells contained within a fine stroma instead of subchondral bone marrow in the MIA-treated group. There was a reduction in trabecular connectivity in the MIA-treated group (Figure 4D, arrow). The cartilage of the 300 mg/kg GSPE group was similar to that of the nonarthritis group.
Figure 3.
Microscopic analysis of the damaged cartilage in the femoral and tibial articular cartilage of an OA rat model using India ink staining at 4 weeks after MIA injection. The gross morphological changes of the femoral condyles and tibial plateau were photographed using a digital camera with application of India ink to contrast the cartilage lesions.
Figure 4.
Histologic analysis of the OA joints after treatment with GSPE in MIA-induced arthritis. The knee joints from the OA rats treated with GSPE or vehicle were stained with hematoxylin and eosin , Safranin O fast green, and toluidine blue (original magnification ×100 for hematoxylin and eosin and ×200 for Safranin O fast green and toluidine blue). (A) Two weeks after the injection of MIA and (B) 4 weeks after the injection of MIA. (C) The OA lesion was graded on a scale of 0-13 using the modified Mankin scoring system with a combined score of structure, cellular abnormalities and matrix staining. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the MIA injection group. (D) Femoral condyle 14 days after the MIA injection (original magnification ×100).
We analyzed the MMP13 expression, which is a key catabolic enzyme, to assess the effects of GSPE on the catabolic activity of chondrocytes. Positive immunohistochemical reactivity to MMP13 was visualized as brown cytoplasmic and nuclear staining. No positive staining for MMP13 was observed in the normal cartilage. The strong expression of cytoplasmic and nuclear MMP13 was observed in the MIA-treated group. The MMP13 expression in the cartilage was decreased by GSPE (Figure 5). This result suggests that GSPE can reduce the activity of the catabolic enzyme MMP13 and this enzyme has been implicated in cartilage breakdown.
Figure 5.
The MMP13 expression in synovium and cartilage in the OA model using immunohistochemistry at 4 weeks after MIA injection. Immunohistochemistry was performed on representative sections of the joints from the MIA-injected rats treated with GSPE or with vehicle. The positive cells are brown; the nuclei were counterstained with hematoxylin. The bar graphs represent the mean values ± SD of the positively stained cells (original magnification ×400). *P < 0.05, **P < 0.01 compared with the MIA injection group.
Immunohistochemical assessment of the IL-1β and nitrotyrosine expressions after treatment with GSPE in the OA rat model
The cytokines produced by the chondrocytes, and especially IL-1β, play a significant role in the degradation of cartilage. IL-1β is the main cytokine instigator of cartilage degeneration in arthritis, and it induces MMP in chondrocytes. To confirm the cartilage protective activity of GSPE, we immunostained for the IL-1β expression in cartilage and subchondral bone. We observed an increased IL-1β expression on the cartilage of the MIA-treated group. However, IL-1β was markedly decreased in the GSPE treated group. We performed immunohistochemical staining for nitrotyrosine, which is a marker for oxidant stress, to determine whether antioxidant activity is responsible for the antinociceptive effect of GSPE and its protective effect against structural damage to knee joints. Nitrotyrosine was not detected in the cartilage or synovium of the nonarthritis group, but it was detected in the cartilage and chondrocytes of the MIA-treated group (Figure 6). The nitrotyrosine staining of cartilage and synovium was reduced in the high-dose GSPE group.
Figure 6.
Immunohistochemical analysis of the IL-1β and nitrotyrosine expressions after treatment with GSPE in the MIA-induced arthritis models at 4 weeks after MIA injection. The positive cells are brown; the nuclei were counterstained with hematoxylin. The slides of the representative sections showed the expression of IL-1β or nitrotyrosine (original magnification × 400).
Discussion
Oxidative stress is associated with the development of OA and the beneficial therapeutic effects of antioxidants such as vitamin E and vitamin C have been demonstrated in clinical trials and in animal studies of OA (Canter et al., 2007). However, the effects of antioxidants on the symptoms and structural changes of OA are controversial (Henrotin and Kurz, 2007). Henrotin and Kurz suggested that drugs with high antioxidant activity could be used as therapeutic modalities for OA (Henrotin and Kurz, 2007). GSPE has higher antioxidant activity than other well-known antioxidants such as vitamin C, vitamin E and gallic acid (Ariga, 2004). Further, the polyphenols in GSPE have already shown beneficial antioxidant effects on other oxidative stress-associated diseases (Cho et al., 2009). Thus, our present data show that GSPE treatment led to a significant reduction of the pain and structural changes in MIA induced OA rats. To the best of our knowledge, this is the first report to demonstrate the effects of GSPE on MIA-induced OA of the knee joint in rats.
We showed that GSPE treatment resulted in a significant decrease in chondrocyte damage and proteoglycan loss in MIA-induced OA. These results are similar to those of a recent study by Miller et al. in which they demonstrated that a proanthocyanidin-rich Amazonian genonutrient has chondroprotective effects in human chondrocytes (Miller et al., 2007). As mentioned earlier, the degradation of cartilage results from the imbalance between the mechanical stresses and catabolic activity, and the latter is mainly controlled by ROS and MMPs. Thus, our results can be explained that the antioxidant defense of GSPE decreased the chondrocyte damage by scavenging ROS and MMPs, by preventing their formation or by repairing the damage.
We utilized nitrotyrosine to investigate the role of GSPE and especially to probe the relationship between the antioxidant activity and antinociceptive effect of GSPE. Nitrotyrosine is an accepted marker of (NOS)-mediated oxidative damage. Nemirovskiy et al. demonstrated a modest increase in the levels of the plasma total and free 3-nitrotyrosine, which has been used as a measure of oxidative stress in an MIA rat model of OA (Nemirovskiy et al., 2009). Our present data indicates that treatment with high dose GSPE decreased nitrotyrosine production in the cartilage and synovium, and this suggests that the antioxidant activity of GSPE may contribute to reducing pain in the MIA rat model of OA.
We then analyzed the MMP13 expression to assess the effects of GSPE on the catabolic activity of chondrocytes. MMP13 is one of the most important metalloproteases involved in the osteoarthritic pathological process (Reboul et al., 1996). The MMP13 expression was significantly upregulated in the cartilage of MIA-treated rats (Barve et al., 2007). Del Carlo et al. demonstrated that pretreatment of articular chondrocytes with N-acetyl-L-cysteine, which is an antioxidant, prevented fibronectin fragment- stimulated MMP13 production (Del Carlo et al., 2007). Baragi et al. noted that MMP13 inhibitors exerted chondroprotective effects and they could potentially reduce joint pain in MIA-treated rats (Baragi et al., 2009). Our immunohistochemical staining results demonstrated that the GSPE-treated rats had fewer MMP13-producing cells in their arthritic joints than did the MIA-treated rats. These results support the fact that GSPE has a chondroprotective effect by balancing the production of ROS and MMP.
Although articular cartilage is primarily affected in OA, it does not have nerve fibers. Suri et al. suggested that vascularization of the damaged articular cartilage is accompanied by innervation, which contributes to pain (Suri et al., 2007). GSPE has been reported to be effective in inhibiting angiogenesis (Lu et al., 2009). Therefore, it is possible that GSPE relieves pain by inhibiting vascularization after MIA treatment.
Pain fibers are present in the synovium, ligaments, bone, muscle and meniscus of the knee. In the MIA rat model of OA, synovial inflammation occurs during the first week after MIA treatment and it resolves 1 week later (Beyreuther et al., 2007). Our results demonstrate that treatment with GSPE increased the PWL and PWT on day 4 after MIA treatment and the hind paw weight distribution was recovered on day 28 after MIA treatment, Our results support that GSPE exerted an antinociceptive effect by reducing synovial inflammation, as acute synovial inflammation induced by MIA injection causes peripheral and central sensitization (Bove et al., 2003).
The process of sensitization is thought to be the basis of arthritic pain. Peripheral sensitization is caused by inflammatory mediators such as substance P, calcitonin gene-related peptide, vasoactive intestinal peptide (VIP), eicosanoids, ion channel ligands and cytokines (McDougall, 2006). McDougall et al. suggested that VIP is a modulator of joint pain in the MIA-induced rat model of OA (McDougall et al., 2006). Kochukov et al. reported the TNF-α enhanced the functional thermal and chemical responses of temperature-sensitive transient receptor potential (TRP) cation channels in human synoviocytes (Kochukov et al., 2009). Our research group previously reported that GSPE inhibited the TNF-α expression in synoviocytes in the joints of the CIA mouse (Cho et al., 2009).
In this way, GSPE may exert its therapeutic effects on the MIA rat model of OA not only through its antioxidant activity, but also through its anti-inflammatory activity. Dumond et al. reported that the expressions of proinflammatory genes such as IL-1β, iNOS and COX2 in chondrocytes were enhanced at an early stage of MIA-induced OA (Dumond et al., 2004). Li et al. demonstrated that GSPE has anti-inflammatory effects as it inhibited the production of inflammatory cytokines such as nitric oxide, IL-1β, TNF-α and prostaglandin E2 (Li et al., 2001).
As medullary hypertension and microfractures in the subchondral bone are considered another source of pain in OA, we analyzed the articular structure by micro-CT (Dieppe and Brandt, 2003). In the present study, the bony trabeculae collapsed and fragmented on day 28. Further, the GSPE-treated rats have fewer osteoclasts and less bony fragmentation in their subchondral bone than did the MIA-treated animals. This is consistent with the results of Guzman et al., who suggested that increased osteoclastic activity during bone remodeling causes pain in MIA-induced OA in rats (Guzman et al., 2003). Our research group previously described the in vitro and in vivo anti-osteoclastogenic effects of GSPE in CIA mice (Cho et al., 2009).
Osteophytes may be a source of pain when they result in stretching of the nerve endings in the periosteum covering the osteophyte. We demonstrated that the number of osteophytes in the GSPE treated rats was lower than the number of osteophytes in the saline treated rats.
Taken together, it seems that the antioxidant property of GSPE mainly contributes to its antinociceptive effects through repairing articular damage via the downregulation of ROS and MMP13 production. Uchida et al. reported that GSPE had a significant antinociceptive effect in mice during the formalin test (Uchida et al., 2008), and Cui et al. showed that GSPE plays a protective role against diabetic peripheral neuropathy by ameliorating nerve damage associated with oxidation (Cui et al., 2008). These results suggest that GSPE has an antinociceptive effect.
In conclusion, we demonstrated that treatment with GSPE attenuates the MIA-induced pain and histologic changes in the knee joint. The antinociceptive and antiarthritic effects of GSPE were mediated by inhibition of cartilage damage, synovitis and subchondral bone fracture, the reduced production of nitrotyrosine and MMP13 and the suppression of osteoclastogenesis. Our data also suggests that the beneficial effects of GSPE treatment in MIA-induced arthritis are secondary to its antioxidant effects. We suggest that GSPE has excellent potential as a therapeutic modality for treating OA.
Methods
Animals
Male Wistar rats (Central Lab. Animal Inc., Seoul, Korea) weighing 270-280 g at the start of the experiment were used. The animals were housed two per cage in a room with controlled temperature conditions (21-22℃) and a reversed light-dark cycle (12 h:12 h) and they had free access to sterile food and water. All the experimental procedures were examined and approved by the Animal Research Ethics Committee at the Catholic University of Korea.
Development of OA and GSPE treatment
The animals were randomized and assigned to treatment groups before the study began. OA was induced by intra-articular injection of 50 µl of 4 mg sodium iodoacetate (Sigma, St. Louis, MO) into the right knee joint using a 26.5 G needle. Control rats were injected with an equivalent volume of saline. The GSPE was kindly provided by Hanlim Pharmaceutical Company (Seoul, Korea). GSPE dissolved in saline was given orally three times per week for 4 weeks after the MIA injection. The GSPE dose was 100 mg/kg or 300 mg/kg.
Pain measurement
Mechanical sensitivity was assessed using a dynamic plantar aesthesiometer (Ugo Basile, Comerio, Italy) and this is an automated version of the von Frey hair assessment procedure. The rats were placed on a metal mesh surface in an acrylic chamber in a temperature-controlled room (-22℃) and they were allowed to acclimatize for 15 min before the testing began. The touch stimulator unit was oriented under the animal. An adjustable angled mirror was used to place the stimulating microfilament (0.5 mm diameter) below the plantar surface of the hind paw. When the instrument was activated, a fine plastic monofilament advanced at a constant speed and touched the paw in the proximal metatarsal region. The filament exerted a gradually increasing force on the plantar surface, starting below the threshold of detection and increasing until the stimulus became painful and the rat removed its paw. The force required to elicit a paw withdrawal reflex was recorded automatically and measured in grams. A maximum force of 50 g and a ramp speed of 20 s were used for all the aesthesiometry tests.
Weight-bearing assessment
Hind limb weight bearing was measured using an incapacitance tester (Linton Instrumentation, Norfolk, UK) that include a dual-channel weight averager. The rats were carefully placed in a plastic chamber and positioned. The force exerted by each hind limb was averaged over a 3 s period. Each data point was the mean of three readings. The percentage of weight distributed onto the treated (ipsilateral) hind limb was calculated using the following equation: (weight on right leg/weight on left leg) ×100.
Joint histology and immunohistochemistry
The animals were perfused via the ascending aorta with 10% neutral buffered formalin (pH 7.4). The knee joints, including the patella and joint capsule, were resected and kept in the same fixative for an additional 48 h at 4℃. The fixed specimens were decalcified with 5% formic acid decalcifier for 6 days at 4℃. After decalcification, the specimens were embedded in paraffin. Standardized 7 µm serial sections were obtained at the medial and lateral midcondylar level in the sagittal plane and they were stained with H&E and Safranin O or Toluidine blue for evaluation of the proteoglycan content. The slides for immunohistochemistry were deparaffinized and rehydrated using a graded series of ethanol solutions. The sections were depleted of endogenous peroxidase activity by adding methanolic H2O2 and then they were blocked with normal goat serum for 30 min. After overnight incubation at 4℃ with anti-nitrotyrosine antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA), anti IL-1β antibody (Santa Cruz Biotechnology) or anti-MMP13 antibody (Abcam, Cambridge, UK), the samples were incubated with the respective secondary antibodies (biotinylated anti-mouse IgG or rabbit IgG) for 20 min with a streptavidin- peroxidase complex (Vector) for 1 h, and finally they were incubated with 3,3'-diaminobenzidine (Dako, Glostrup, Denmark). The sections were counter-stained with Mayer's hematoxylin and they were photographed using an Olympus photomicroscope (Japan).
A modified Mankin system (Mankin et al., 1971) was also used to score cartilage change, as follows: the structure was scored on a scale of 0-6, where 0 = normal, 1 = irregular surface, including fissures into the radial layer, 2 = pannus, 3 = absence of superficial cartilage layers (≥ 6), 4 = slight disorganization (an absent cellular row and some small superficial clusters), 5 = fissures into the calcified cartilage layer and 6 = disorganization (chaotic structure, clusters and osteoclastic activity); cellular abnormalities were scored on a scale of 0-3 where 0 = normal, 1 = hypercellularity, including small superficial clusters, 2 = clusters and 3 = hypocellularity; the matrix staining was scored on a scale of 0-4 where 0 = normal/slight reduction of staining, 1 = staining reduced in the radial layer, 2 = staining reduced in the interterritorial matrix, 3 = staining present only in the pericellular matrix and 4 = staining absent.
Microscopic assessment of the OA joints
India ink staining was used to assess the extent and location of cartilage degradation. The tibia and femur bones were separated and all excess soft tissue was removed by careful dissection under a dissecting microscope. The articular surface of each specimen was rinsed with PBS and painted with India ink diluted in saline. The excess ink was removed by gentle wiping with moist cotton wool. The cartilage surfaces of the femoral condyle and the tibial plateau were examined using a microscope.
Microcomputerized tomography (Micro-CT)
Micro-CT was used to develop three-dimensional images of bone. A SkyScan Desktop Micro CT 1172 (Aartselaar, Belgium) with a source voltage of 60 kV and a current of 167 µA was used to acquire the X-ray radiographs. The specimens were attached to a stage that rotated 180° and the images were acquired every 0.4°. After scanning, the cross-sectional slices were reconstructed and three-dimensional analyses were performed using CTAn SkyScan software. Each scan result was reconstructed using the same threshold values to distinguish bone and air. The bone mass and microarchitecture parameters, including the fraction of bone volume (BV), were evaluated using the built-in software of the micro-CT.
Acknowledgements
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A092258) and by the Institute of Clinical Medicine Research of Bucheon St. Mary's Hospital, Research Fund, BCMC 10IA01.
Abbreviations
- GSPE
grape seed proanthocyanidin extract
- MIA
monosodium iodoacetate
- MMP
matrix metalloproteinase
- O2-
superoxide anion
- OA
osteoarthritis
- OH
hydroxyl radicals
- ONOO-
peroxynitrite
- PWL
paw withdrawal latency
- PWT
paw withdrawal threshold
- ROS
reactive oxygen species
- TRP
transient receptor potential
- VIP
vasoactive intestinal peptide
References
- 1.Altindag O, Erel O, Aksoy N, Selek S, Celik H, Karaoglanoglu M. Increased oxidative stress and its relation with collagen metabolism in knee osteoarthritis. Rheumatol Int. 2007;27:339–344. doi: 10.1007/s00296-006-0247-8. [DOI] [PubMed] [Google Scholar]
- 2.Ariga T. The antioxidative function, preventive action on disease and utilization of proanthocyanidins. Biofactors. 2004;21:197–201. doi: 10.1002/biof.552210140. [DOI] [PubMed] [Google Scholar]
- 3.Baragi VM, Becher G, Bendele AM, Biesinger R, Bluhm H, Boer J, Deng H, Dodd R, Essers M, Feuerstein T, Gallagher BM, Jr, Gege C, Hochgurtel M, Hofmann M, Jaworski A, Jin L, Kiely A, Korniski B, Kroth H, Nix D, Nolte B, Piecha D, Powers TS, Richter F, Schneider M, Steeneck C, Sucholeiki I, Taveras A, Timmermann A, Van Veldhuizen J, Weik J, Wu X, Xia B. A new class of potent matrix metalloproteinase 13 inhibitors for potential treatment of osteoarthritis: Evidence of histologic and clinical efficacy without musculoskeletal toxicity in rat models. Arthritis Rheum. 2009;60:2008–2018. doi: 10.1002/art.24629. [DOI] [PubMed] [Google Scholar]
- 4.Barve RA, Minnerly JC, Weiss DJ, Meyer DM, Aguiar DJ, Sullivan PM, Weinrich SL, Head RD. Transcriptional profiling and pathway analysis of monosodium iodoacetate-induced experimental osteoarthritis in rats: relevance to human disease. Osteoarthritis Cartilage. 2007;15:1190–1198. doi: 10.1016/j.joca.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Beyreuther B, Callizot N, Stohr T. Antinociceptive efficacy of lacosamide in the monosodium iodoacetate rat model for osteoarthritis pain. Arthritis Res Ther. 2007;9:R14. doi: 10.1186/ar2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, Schrier DJ, Kilgore KS. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage. 2003;11:821–830. doi: 10.1016/s1063-4584(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 7.Canter PH, Wider B, Ernst E. The antioxidant vitamins A, C, E and selenium in the treatment of arthritis: a systematic review of randomized clinical trials. Rheumatology (Oxford) 2007;46:1223–1233. doi: 10.1093/rheumatology/kem116. [DOI] [PubMed] [Google Scholar]
- 8.Chen B, Qin J, Wang H, Magdalou J, Chen L. Effects of adenovirus-mediated bFGF, IL-1Ra and IGF-1 gene transfer on human osteoarthritic chondrocytes and osteoarthritis in rabbits. Exp Mol Med. 2010;42:684–695. doi: 10.3858/emm.2010.42.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho ML, Heo YJ, Park MK, Oh HJ, Park JS, Woo YJ, Ju JH, Park SH, Kim HY, Min JK. Grape seed proanthocyanidin extract (GSPE) attenuates collagen-induced arthritis. Immunol Lett. 2009;124:102–110. doi: 10.1016/j.imlet.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Cui XP, Li BY, Gao HQ, Wei N, Wang WL, Lu M. Effects of grape seed proanthocyanidin extracts on peripheral nerves in streptozocin-induced diabetic rats. J Nutr Sci Vitaminol (Tokyo) 2008;54:321–328. doi: 10.3177/jnsv.54.321. [DOI] [PubMed] [Google Scholar]
- 11.Deberg M, Labasse A, Christgau S, Cloos P, Bang Henriksen D, Chapelle JP, Zegels B, Reginster JY, Henrotin Y. New serum biochemical markers (Coll 2-1 and Coll 2-1 NO2) for studying oxidative-related type II collagen network degradation in patients with osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2005;13:258–265. doi: 10.1016/j.joca.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Del Carlo M, Schwartz D, Erickson EA, Loeser RF. Endogenous production of reactive oxygen species is required for stimulation of human articular chondrocyte matrix metalloproteinase production by fibronectin fragments. Free Radic Biol Med. 2007;42:1350–1358. doi: 10.1016/j.freeradbiomed.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dieppe P, Brandt KD. What is important in treating osteoarthritis? Whom should we treat and how should we treat them. Rheum Dis Clin North Am. 2003;29:687–716. doi: 10.1016/s0889-857x(03)00054-1. [DOI] [PubMed] [Google Scholar]
- 14.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 15.Dumond H, Presle N, Pottie P, Pacquelet S, Terlain B, Netter P, Gepstein A, Livne E, Jouzeau JY. Site specific changes in gene expression and cartilage metabolism during early experimental osteoarthritis. Osteoarthritis Cartilage. 2004;12:284–295. doi: 10.1016/j.joca.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Durukan AH, Evereklioglu C, Hurmeric V, Kerimoglu H, Erdurman C, Bayraktar MZ, Mumcuoglu T. Ingestion of IH636 grape seed proanthocyanidin extract to prevent selenite-induced oxidative stress in experimental cataract. J Cataract Refract Surg. 2006;32:1041–1045. doi: 10.1016/j.jcrs.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 17.Filippin LI, Vercelino R, Marroni NP, Xavier RM. Redox signalling and the inflammatory response in rheumatoid arthritis. Clin Exp Immunol. 2008;152:415–422. doi: 10.1111/j.1365-2249.2008.03634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fine AM. Oligomeric proanthocyanidin complexes: history, structure, and phytopharmaceutical applications. Altern Med Rev. 2000;5:144–151. [PubMed] [Google Scholar]
- 19.Guzman RE, Evans MG, Bove S, Morenko B, Kilgore K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: an animal model of osteoarthritis. Toxicol Pathol. 2003;31:619–624. doi: 10.1080/01926230390241800. [DOI] [PubMed] [Google Scholar]
- 20.Henrotin Y, Kurz B. Antioxidant to treat osteoarthritis: dream or reality? Curr Drug Targets. 2007;8:347–357. doi: 10.2174/138945007779940151. [DOI] [PubMed] [Google Scholar]
- 21.Henrotin Y, Kurz B, Aigner T. Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthritis Cartilage. 2005;13:643–654. doi: 10.1016/j.joca.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Janusz MJ, Hookfin EB, Heitmeyer SA, Woessner JF, Freemont AJ, Hoyland JA, Brown KK, Hsieh LC, Almstead NG, De B, Natchus MG, Pikul S, Taiwo YO. Moderation of iodoacetate-induced experimental osteoarthritis in rats by matrix metalloproteinase inhibitors. Osteoarthritis Cartilage. 2001;9:751–760. doi: 10.1053/joca.2001.0472. [DOI] [PubMed] [Google Scholar]
- 23.Kochukov MY, McNearney TA, Yin H, Zhang L, Ma F, Ponomareva L, Abshire S, Westlund KN. Tumor necrosis factor-alpha (TNF-alpha) enhances functional thermal and chemical responses of TRP cation channels in human synoviocytes. Mol Pain. 2009;5:49. doi: 10.1186/1744-8069-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krasnokutsky S, Attur M, Palmer G, Samuels J, Abramson SB. Current concepts in the pathogenesis of osteoarthritis. Osteoarthritis Cartilage. 2008;16(Suppl 3):S1–S3. doi: 10.1016/j.joca.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 25.Li WG, Zhang XY, Wu YJ, Tian X. Anti-inflammatory effect and mechanism of proanthocyanidins from grape seeds. Acta Pharmacol Sin. 2001;22:1117–1120. [PubMed] [Google Scholar]
- 26.Lu J, Zhang K, Chen S, Wen W. Grape seed extract inhibits VEGF expression via reducing HIF-1alpha protein expression. Carcinogenesis. 2009;30:636–644. doi: 10.1093/carcin/bgp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523–537. [PubMed] [Google Scholar]
- 28.McDougall JJ. Arthritis and pain. Neurogenic origin of joint pain. Arthritis Res Ther. 2006;8:220. doi: 10.1186/ar2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDougall JJ, Watkins L, Li Z. Vasoactive intestinal peptide (VIP) is a modulator of joint pain in a rat model of osteoarthritis. Pain. 2006;123:98–105. doi: 10.1016/j.pain.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Miller MJ, Bobrowski P, Shukla M, Gupta K, Haqqi TM. Chondroprotective effects of a proanthocyanidin rich Amazonian genonutrient reflects direct inhibition of matrix metalloproteinases and upregulation of IGF-1 production by human chondrocytes. J Inflamm (Lond) 2007;4:16. doi: 10.1186/1476-9255-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemirovskiy OV, Radabaugh MR, Aggarwal P, Funckes-Shippy CL, Mnich SJ, Meyer DM, Sunyer T, Rodney Mathews W, Misko TP. Plasma 3-nitrotyrosine is a biomarker in animal models of arthritis: Pharmacological dissection of iNOS' role in disease. Nitric Oxide. 2009;20:150–156. doi: 10.1016/j.niox.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Pataki T, Bak I, Kovacs P, Bagchi D, Das DK, Tosaki A. Grape seed proanthocyanidins improved cardiac recovery during reperfusion after ischemia in isolated rat hearts. Am J Clin Nutr. 2002;75:894–899. doi: 10.1093/ajcn/75.5.894. [DOI] [PubMed] [Google Scholar]
- 33.Prasain JK, Peng N, Dai Y, Moore R, Arabshahi A, Wilson L, Barnes S, Michael Wyss J, Kim H, Watts RL. Liquid chromatography tandem mass spectrometry identification of proanthocyanidins in rat plasma after oral administration of grape seed extract. Phytomedicine. 2009;16:233–243. doi: 10.1016/j.phymed.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray SD, Parikh H, Hickey E, Bagchi M, Bagchi D. Differential effects of IH636 grape seed proanthocyanidin extract and a DNA repair modulator 4-aminobenzamide on liver microsomal cytochrome 4502E1-dependent aniline hydroxylation. Mol Cell Biochem. 2001;218:27–33. doi: 10.1023/a:1007272611915. [DOI] [PubMed] [Google Scholar]
- 35.Reboul P, Pelletier JP, Tardif G, Cloutier JM, Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes. A role in osteoarthritis. J Clin Invest. 1996;97:2011–2019. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Regan E, Flannelly J, Bowler R, Tran K, Nicks M, Carbone BD, Glueck D, Heijnen H, Mason R, Crapo J. Extracellular superoxide dismutase and oxidant damage in osteoarthritis. Arthritis Rheum. 2005;52:3479–3491. doi: 10.1002/art.21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato M, Bagchi D, Tosaki A, Das DK. Grape seed proanthocyanidin reduces cardiomyocyte apoptosis by inhibiting ischemia/reperfusion-induced activation of JNK-1 and C-JUN. Free Radic Biol Med. 2001;31:729–737. doi: 10.1016/s0891-5849(01)00626-8. [DOI] [PubMed] [Google Scholar]
- 38.Suri S, Gill SE, Massena de, Wilson D, McWilliams DF, Walsh DA. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann Rheum Dis. 2007;66:1423–1428. doi: 10.1136/ard.2006.063354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uchida S, Hirai K, Hatanaka J, Hanato J, Umegaki K, Yamada S. Antinociceptive effects of St. John's wort, Harpagophytum procumbens extract and Grape seed proanthocyanidins extract in mice. Biol Pharm Bull. 2008;31:240–240. doi: 10.1248/bpb.31.240. [DOI] [PubMed] [Google Scholar]