Abstract
Cachexia is an irreversible process that can develop in the course of chronic disease. It is characterized by the remodeling of the metabolic, inflammatory, and endocrine pathways. Insulin, growth hormone (GH), and insulin-like growth factor 1 (IGF-1) are involved in glucose, protein, and fat metabolism, which regulates body composition. In body wasting and cachexia, their signaling is impaired and causes anabolic/catabolic imbalance. Important mechanisms include inflammatory cytokines and neurohormonal activation. Remodeled post-receptor insulin, GH, and IGF-1 pathways constitute a potential target for pharmacological treatment in the setting of body wasting and cachexia. Peroxisome proliferator-activated receptor gamma agonists, drugs inhibiting angiotensin II action (angiotensin II antagonists and inhibitors of angiotensin-converting enzyme), and testosterone, which interfere with post-receptor pathways of insulin, GH, and IGF-1, were investigated as pharmacological intervention targets and various clinically important implications were reported. There are several other potential targets, but their treatment feasibility and applicability is yet to be established.
Keywords: Angiotensin II, Cachexia, Growth hormone, Insulin-like growth factor 1, Insulin, PPAR-γ agonist, Testosterone
Introduction
Cachexia is a syndrome associated with weight loss and changes in body composition due to loss of muscle mass, alterations in bone structure, and reduction of fat tissue. Over the years, various definitions have been used [1], which has caused a lack of reliable epidemiological data. After expert consensus meetings, cachexia is more precisely defined as weight loss of at least 5% in 12 months or less and fulfillment of at least three out of five criteria: decreased muscle strength, fatigue, anorexia, low fat-free mass index, and abnormal biochemistry [2, 3]. This definition enables the research community to perform epidemiological and intervention trials [3, 4]. In chronic disease, including chronic heart failure (CHF), chronic obstructive pulmonary disease (COPD), rheumatoid arthritis, or cancer, an ongoing inflammatory process leads to changes in metabolic and hormonal pathways. These can yield alterations in body composition and can eventually cause cachexia [4–6].
The human body regulates its composition through various hormonal effectors, including insulin, insulin-like growth factor 1 (IGF-1), and growth hormone (GH), which are primarily involved in the regulation of protein synthesis and degradation, fat mobilization, and glucose uptake and mobilization. All processes are significantly affected in cachexia. The aim of this article is to review the importance of signaling pathways in body wasting and cachexia development and to discuss some possible targets for pharmacological interventions.
Insulin, GH, and IGF-1 signaling
GH, IGF-1, and insulin are involved in regulating body composition through action on different body compartments. They all act as anabolic agents in skeletal muscle, promoting muscle mass gain. GH primarily regulates liver IGF-1 expression with downstream anabolic effects in skeletal muscle. In the skeleton, GH and IGF-1 induce bone growth and help maintain bone mass. Insulin and GH are involved in fat metabolism: GH induces lipolysis and insulin promotes synthesis of fatty acids in the liver and inhibits their degradation in adipose tissue [7, 8]. Generally, skeletal muscle, bone, and fat tissue are regulated by GH, IGF-1, and insulin, which can induce changes in body composition through distinct and overlapping pathways.
GH or somatotropin is a peptide produced by the pituitary gland. Its secretion is stimulated by hypothalamic GH-releasing hormone and inhibited by somatostatin, another peptide hormone secreted from the hypothalamus. There are also other stimuli that affect GH levels in serum. These include ghrelin—a peptide primarily synthesized not only in the stomach, but also in the hypothalamus and pituitary gland [9]—and other individual factors such as gender, age, diet, exercise, adiposity, and sleep. Negative feedback mechanisms of GH and IGF-1 levels are involved in regulating serum GH concentrations [10, 11].
GH binds to the growth hormone receptor (GHR), which is expressed in skeletal muscle, the liver, adipose tissue, the heart, the kidneys, and other tissues. Activation of GHR induces the synthesis of IGF-1 protein in most tissues, with the liver being the organ that contributes the major part to serum IGF-1 level. Circulating IGF-1 is bound to IGF-binding protein, which prolongs IGF-1 half-life and regulates its availability for target tissues [12, 13].
GH and other factors—for example, exercise—also induce IGF-1 expression locally in the muscle, where it acts as a paracrine modulator [14]. It has been shown that local expression of IGF-1 in CHF can be significantly reduced despite normal serum levels of IGF-1 [15]. IGF-1 binds to the insulin-like growth factor 1 receptor (IGF1R) and insulin receptor, but the affinity for the latter receptor is 100-fold to 1,000-fold lower than insulin affinity. It has to be considered, however, that IGF-1 concentrations in plasma are still 100-fold higher than those of insulin [16]. This ratio may change in the postprandial phase because levels of insulin increase in response to food ingestion, whereas food intake increases IGF-1 levels to a lesser degree [17].
Insulin receptors mainly modify glucose metabolism and can be found in the liver, adipose tissue, and muscle. However, tissues like the brain, the heart, the kidneys, and blood cells also express insulin receptors. Because of the amino acid sequence homology of the insulin receptor and IGF1R, the insulin and IGF-1 half-receptors can heterodimerize, forming an insulin/IGF-1 hybrid receptor that has higher affinity for IGF-1 and, therefore, acts more like IGF-1 than an insulin receptor [18, 19].
GH, IGF-1, and insulin exert their actions on various tissues. Signaling pathways are not the same in all organs and exact mechanisms in different cell types have not been revealed yet. The effects of GH, IGF-1, and insulin on muscle tissue have already received much attention in sports. As naturally occurring substances with anabolic and performance-enhancing effects, they have considerable potential to overcome routine doping-detection procedures and have been misused by elite athletes. Nonetheless, the research community has gained some insight into the effects of supraphysiological levels of GH, IGF-1, and insulin on muscle tissue and into other desirable and undesirable actions [20, 21]. The muscle seems to be the most important tissue affected in body wasting and cachexia processes, and loss of muscle tissue may be the most undesirable way of wasting. Therefore, this discussion focuses on signaling mechanisms of GH, IGF-1, and insulin in skeletal muscle cells.
Skeletal muscle
Insulin signaling in skeletal muscle
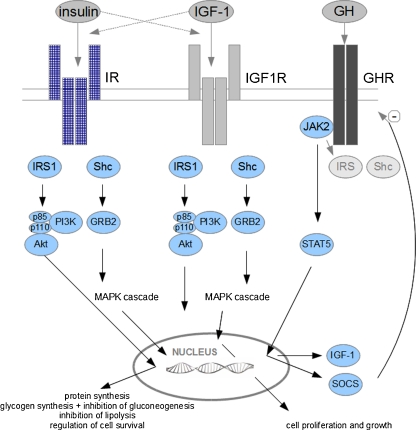
The insulin receptor consists of two α-subunits and two β-subunits, linked together with disulfide bonds. Binding of insulin to the α-subunit induces a conformational change that enables adenosine triphosphate (ATP) binding to the intracellular domain of the β-subunit. Following ATP binding, the receptor autophosphorylates, activating its protein kinase function. The insulin receptor can phosphorylate various substrates, and one of the main signaling pathways starts with the phosphorylation of insulin receptor substrate (IRS) proteins. There are six different IRS proteins (IRS-1 to IRS-6) [22], among which IRS-1 plays the main role in skeletal muscle [23]. Phosphatidylinositol 3-kinase (PI3K) recognizes phosphorylated IRS with the p85 regulatory subunit and further catalyzes phosphorylation of serine/threonine kinases with the p110 subunit (Fig. 1). The main downstream effector of this pathway is Akt kinase, which, when phosphorylated, translocates to the nucleus. There it regulates lipid, protein, and glycogen synthesis and cell survival [16].
Fig. 1.
Schematic presentation of GH, IGF-1, and insulin signaling. IGF-1 insulin-like growth factor 1, GH growth hormone, IR insulin receptor, IGF1R insulin-like growth factor 1 receptor, GHR growth factor receptor, IRS-1 insulin receptor substrate 1, Shc Shc protein, GRB2 growth factor receptor-bound protein 2, PI3K phosphatidylinositol 3-kinase, Akt Akt protein, JAK2 Janus kinase 2, STAT5 signal transducer and activator of transcription 5, SOCS suppressor of cytokine signaling
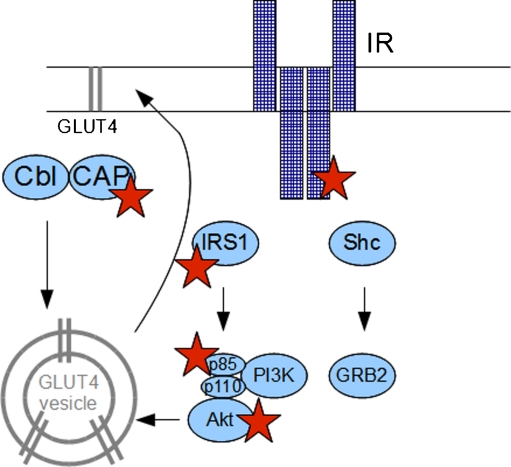
Another important action of insulin is insulin-dependent glucose transport facilitated through glucose transporter type 4 (GLUT4) translocation to the membrane; this process can be stimulated by insulin or by other stimulatory factors like muscle contraction [24, 25]. Insulin induces GLUT4 translocation through the PI3K-dependent pathway and through the PI3K-independent pathway associated with Cbl-associated protein (CAP)/Cbl complex (Fig. 2). Herein, its role in GLUT4 transport remains questionable, especially in skeletal muscle [26, 27].
Fig. 2.
Stars indicate the proteins of insulin signaling cascade affected by PPAR-γ agonists. Cbl Cbl protein, CAP Cbl-associated protein, IRS-1 insulin receptor substrate 1, Shc Shc protein, GRB2 growth factor receptor-bound protein 2, PI3K phosphatidylinositol 3-kinase, Akt Akt protein, GLUT4 glucose transporter 4, IR insulin receptor
IGF-1 signaling in muscle
IGF-1 mainly acts through binding to IGF1R. This receptor is a transmembrane tyrosine kinase that autophosphorylates after IGF-1 binding. Phosphorylation creates a docking site for its substrates: IRS-1 and Shc protein. Again, IRS-1 can activate the p85 regulatory subunit of PI3K, resulting in the activation of the PI3K/Akt pathway, which inhibits cell apoptosis and promotes protein synthesis and cell differentiation. Alternatively, phosphorylation of Shc protein leads to the activation of a mitogen-activated protein kinase (MAPK) cascade, ending in induced cell proliferation [28].
GH signaling in muscle
As discussed earlier, GH exerts its effects through GHR, a transmembrane receptor, which undergoes dimerization after binding of GH. The phosphorylation of receptor-associated Janus kinase 2 (JAK2) leads to the formation of a docking site for members of the signal transducers and activators of transcription (STAT) family of transcription factors [29]. Phosphorylation of STAT5 leads to its dissociation from the receptor and translocation into the nucleus, where it regulates the expression of various genes that enable physiological actions of GH [30]. Among these genes, the expression of suppressors of cytokine signaling (SOCSs) is induced. This family of proteins negatively modulates cytokine-mediated signal transduction pathways. SOCSs, in turn, inhibit GH signaling through a negative feedback mechanism [29]. The JAK/STAT signaling pathway is also responsible for the induction of IGF-1 mRNA expression [31], although Jørgensen et al. found this to be regulated like this only in fat tissue and not in muscle [32].
There are two additional pathways in GH signaling that are triggered by JAK2 phosphorylation. First, there is the MAPK pathway, similar as in IGF-1 signaling, and second, the PI3K/Akt pathway, starting with phosphorylation of IRS proteins by JAK2 [33].
The exact mechanisms of GH signaling remain to be investigated, especially the distinction of signaling pathways in adipose tissue and muscle. Although the JAK2/STAT5 pathway seems to be fully activated with GH administration, the MAPK and PI3K/Akt pathway response to GH is questionable [29, 32].
The role of insulin, GH, and IGF-1 in cachexia
Insulin and GH resistance
In patients with chronic diseases such as CHF and cancer, increased levels of GH accompanied by comparatively low serum concentrations of IGF-1 have been observed. If GH is the main stimulus for IGF-1 secretion, this condition points to unresponsive peripheral tissues and GH resistance [34]. Similarly, insulin signaling becomes impaired in chronic disease and insulin resistance develops. Indeed, in patients with CHF, insulin resistance and higher insulin levels have been observed [35]. With these changes in metabolic signaling, two important anabolic stimuli that induce protein synthesis and inhibit protein degradation in muscle cells are lost. Although GH and insulin seem to have synergistic actions in promoting protein synthesis, GH actually induces insulin resistance. The exact mechanism is not known, nor is the distinction between influences of GH on insulin signaling in the liver, adipose tissue, and muscle. Increased SOCS3 expression and uncoupling of PI3K and its downstream effectors are some of mechanisms that have been suggested [29, 36].
Loss of lean mass
Loss of lean mass is a result of either increased protein degradation or decreased protein synthesis. Protein degradation/synthesis homeostasis is maintained through various mediators, including insulin, GH, and IGF-1. In human cells, protein degradation follows various proteolytic pathways, where the main five are the ATP-dependent ubiquitin–proteasome system, the calcium-dependent (calpains) pathway, the caspase system, matrix proteinases, and the lysosomal (cathepsins) pathway [5]. The ubiquitin–proteasome system seems to be importantly involved in cachectic muscle wasting because its overexpression and overactivation have been shown in various diseases related to cachexia [37, 38]. More specifically, this system is responsible for increased myosin degradation [39]. The ubiquitin protein ligases muscle atrophy F-box (MAFbx) and muscle ring finger protein 1 (MuRF1) are responsible for linking ubiquitin to proteins and targeting them for degradation in this proteasome system [38]. These ligases are regulated by the Akt/PI3K pathway, which lies downstream of IGF-1 and the insulin receptor. Moreover, it has been shown that IGF-1 and insulin also directly suppress the expression of MAFbx [40]. Protein synthesis is also regulated by the Akt/PI3K pathway because Akt forms a complex with mTORC1, a serine/threonine protein kinase that induces protein synthesis.
Inflammatory processes
Body wasting and cachexia are associated with (over)activation of the inflammatory system [5, 41, 42]. The exact interference mechanisms with metabolic and endocrine pathways are multifactorial but they remain poorly understood. Inflammatory cytokines are able to regulate cellular responses and are, therefore, also involved in the modulation of GH, IGF-1, and insulin signaling.
Tumor necrosis factor alpha
Tumor necrosis factor alpha (TNF-α) seems to be an important link between various forms of cachexia [5]. Indeed, its inhibitory action on heart and muscle protein synthesis has been shown [43]. TNF-α contributes to GH resistance by downregulating the expression of GHR [44]. Similarly, a connection between TNF-α and reduced expression of GLUT4, leading to insulin resistance, has been proved [45]. TNF-α also reduces phosphorylation of IRS-1 and IRS-2 by IGF1R, thus inhibiting signaling of IGF-1 in muscle cell development [46]. Moreover, it inhibits IGF-1 expression locally in muscle. Activation of transcription factor nuclear factor-κB and increased expression of MAFbx ubiquitin ligase are another two TNF-α actions; these result in the induction of protein breakdown and inhibition of myogen differentiation [47, 48].
Interleukin-1
Interleukin (IL)-1 is another cytokine involved in catabolic processes [49]. In hepatocytes, IL-1β and TNF-α inhibit GH-stimulated IGF-1 gene expression. On the other hand, IL-1β and TNF-α had no influence on basal IGF-1 expression [50]. IL-1β also prevents IGF-1 from promoting protein synthesis [46].
Interleukin-6
Interleukin (IL)-6 is a cytokine recognized to play an important role in cachexia [51]. It is produced in most cell types; the major contributor being skeletal muscle, where it is formed in response to exercise [41]. Investigating IL-6 interference with GH, IGF-1, and insulin signaling has led to various conclusions. The effects of IL-6 on insulin sensitivity in skeletal muscle show different patterns over time. They are thought to be positive in the short-term and negative after chronic exposure [52]. IL-6 not only stimulates basal IGF-1 gene expression [53], but also the expression of SOCS3, which induces ubiquitin–proteasome system-mediated degradation of IRS-1 and thereby impairs insulin/IGF1 signaling [54]. This dual role of IL-6 may not be surprising because cytokines are generally involved in regulating various pathways.
GH/IGF-1/insulin signaling: potential targets for cachexia treatment
An ideal drug for treating cachexia would have anabolic, anti-inflammatory, and appetite-stimulating actions. Unfortunately, an effective remedy for this devastating condition has not been found yet. GH, IGF-1, and insulin signaling pathways have already been identified as important contributors and we believe that these pathways could be clinically relevant targets for pharmacological treatment.
GH/IGF-1/insulin administration
The application of GH, insulin, and IGF-1 has already been misused by star athletes, exploiting their anabolic actions [21, 55]. To achieve an anabolic effect in cachectic patients, use of high doses of GH would be required because GH resistance is a common condition in this population [34]. Safety concerns arise when using GH for cachexia treatment because increased mortality has been associated with GH administration in critically ill patients [56]. Insulin treatment is similarly limited through insulin resistance, which is often present in cachectic patients [5]. To avoid these issues, targeting post-receptor pathways could be effective. Insulin, GH, and IGF-1 have their own receptors and signaling through specific pathways, but most of them use similar effector molecules. All three receptors (GHR, IGF1R, and IR) are tyrosine kinases, sharing the PI3K/Akt and MAPK pathway, which could be considered a pharmacological target.
However, one must bear in mind the oncogenic potential of interference with pathways promoting cell growth. Abnormalities in PI3K/Akt signaling are common in cancers and this has been widely exploited for targeted cancer treatment [57]. This raises the need for more targeted intervention, which might be offered by pharmacological entities, as subsequently discussed in this article.
Peroxisome proliferator-activated receptor gamma agonists
Peroxisome proliferator-activated receptor gamma (PPAR-γ) agonists are involved in insulin signaling (Fig. 2). Their primary action is binding to PPAR-γ receptors and stimulating transcription of genes, leading to improvement of insulin sensitivity. One potential target of PPAR-γ agonists is GLUT4. Reduced GLUT4 in skeletal muscle was shown to contribute to insulin resistance in CHF [58] and rosiglitazone was shown to induce GLUT4 translocation to the membrane in mouse skeletal muscle [59]. Rosiglitazone also stimulates the transcription of the CAP gene [60] and pioglitazone has been shown to stimulate the expression of IRS proteins in adipocytes [61]. It is, therefore, likely that glitazones interfere with IGF-1 and GH actions. Because they act on the post-receptor pathways of these receptors, they are putative agents for treating diseases with insulin and GH resistance. Indeed, glitazones are effective in reducing GH-induced liver and skeletal muscle insulin resistance [62].
PPAR-γ agonists also affect the inflammatory component of chronic diseases, which is closely connected to the metabolic pathways shown previously. Rosiglitazone reduced plasma concentrations of C-reactive protein, IL-6, and sTNFα R2 (a cleavage product of the activated tumor necrosis factor TNF-α receptor) in nondiabetic patients with metabolic syndrome [63]. Pioglitazone prevents THF-α-induced insulin resistance by restoring TNF-α-reduced insulin-stimulated 2-deoxyglucose uptake, tyrosine phosphorylation, and protein levels of insulin receptor and IRS-1. It also restores association of p85 with IRS-1 and PI3K activity [61]. Anti-inflammatory actions of PPAR-γ agonists are generally recognized and could be used in treating cardiovascular diseases [64]. Unfortunately, PPAR-γ agonists can lead to fluid retention, and overexpression of PPAR-γ receptors has been related to cardiac dysfunction in mice [65]. Accordingly, PPAR-γ agonists are contraindicated or require careful monitoring in patients with heart failure [66].
PPAR-γ agonists have been extensively studied in the light of their insulin-sensitizing actions, but so far, the effects of PPAR-γ agonists on muscle mass in patients with cachexia have not been investigated. There were some studies performed in patients with CHF, but the weight gain in these patients remained mostly undefined with respect to fluid retention [67].
Angiotensin II antagonists
The role of angiotensin II in vasoconstriction has been recognized and extensively investigated in arterial hypertension, whereas its role in metabolic processes and involvement in muscle wasting has been increasingly recognized in recent years. Angiotensin II has been shown to cause insulin resistance in skeletal muscle by inhibiting insulin-stimulated GLUT4 translocation, and various mechanisms have been proposed [68, 69]. Its role in muscle wasting has also been confirmed: angiotensin II was shown to promote protein degradation by lowering IGF-1 in skeletal muscle [70] and through induction of the ubiquitin–proteasome pathway [71].
There are two main pharmacologic approaches to target the effects of angiotensin II: inhibiting the formation of angiotensin II (angiotensin convertase inhibitors, ACEI) and blocking the receptor of angiotensin II (angiotensin II receptor antagonists). Because alternative ACE-independent pathways of angiotensin II formation exist, the use of angiotensin II R antagonists (“sartans”) could be more specific in targeting angiotensin II-mediated muscle wasting.
The impact of inhibitors of angiotensin-converting enzyme (ACEI) and angiotensin II R antagonists on muscle wasting in chronic diseases has already been observed because these drugs are widely used in clinical practice. Enalapril was shown to reduce the risk of weight loss in CHF patients [72] and ACEI helped maintain weight but not muscle strength in patients with congestive heart failure or hypertension [73]. Elderly patients without heart failure on antihypertensive treatment with ACEI were associated with larger muscle mass than patients receiving other hypertensive therapy [74]. In addition, insulin sensitivity was improved by losartan and lisinopril in hypertensive patients [75]. On the contrary, the TRAIN study reported no significant modifications in muscle strength after 6 months of fosinopril therapy in older persons with high cardiovascular risk profile [76].
Due to the proven clinical indications of ACEI and angiotensin II R antagonists, these studies were performed only in patients with CHF or hypertension. Moreover, the lack of cachexia definition left the patients from these studies unclassified regarding their cachectic state. Further studies are, therefore, needed to describe the role of ACEI and angiotensin II R antagonists in cachexia.
Testosterone
Testosterone, a naturally occurring anabolic hormone, has already been recognized as a substance with the potential to prevent muscle wasting and cachexia [5]. Moreover, its involvement in insulin, GH, and IGF-1 signaling has been recognized and investigated.
The influence of testosterone on insulin signaling is exerted by affecting GLUT4 and IRS-1 expression and Akt phosphorylation. This effect is dose-dependent: low doses of testosterone improve insulin sensitivity (especially in testosterone deficiency conditions) and high doses cause insulin resistance [77, 78].
IGF-1 is also associated with testosterone signaling, but the mechanism is still not clarified. IGF-1 signaling in skeletal muscle is not obligatory to mediate the anabolic effects of testosterone [79], and thus, testosterone induction of IGF-1 expression in the androgenic anabolism process is likely, but remains unproven [80, 81].
Relatively low serum levels of both testosterone and GH have been observed in elderly men. It is not surprising that application of both substances alone or in combination improved muscle protein synthesis in this population. However, a disruption in GH and testosterone signaling was suggested in elderly men [82]. It is, therefore, possible that, in cachexia, similar changes in post-receptor processes hinder the signaling of both hormones, leading to decreased production of IGF-1 in skeletal muscle along with loss of other signals important for muscle protein synthesis.
Use of testosterone or other anabolic steroids to treat muscle wasting in cachexia has been tested in different populations. In COPD patients, muscle wasting was reversed by oxandrolone, an anabolic steroid, and muscle mass and strength were increased by testosterone [83, 84]. The latter two parameters were also improved in CHF patients that received testosterone replacement [85, 86].
Evidence from clinical studies in humans
CHF, COPD, and cancer are the main conditions driving the incidence of body wasting and cachexia [4]. To cope with the increasing burden, it is plausible to focus clinical trial efforts on these conditions. Only a few trials have been completed, and most of them have demonstrated skeletal muscle/body size benefits (Table 1). Whether this translates into better outcomes remains to be established.
Table 1.
Observed effects of selected drugs on muscle function and weight in humans with CHF, COPD, or cancer
| Drug | CHF | COPD | Cancer |
|---|---|---|---|
| PPAR-γ agonists | ↑ body weight [67] | No data available | No data available |
| Ang II R antagonists and ACEI | Maintenance of weight, but not muscle strength [73] | No changes in body weight and no effect on exercise parameters [89] | No data available |
| ↓ risk of weight loss [72, 87] | |||
| ACEI/digoxin/diuretic combination increases muscle bulk and subcutaneous fat [88] | |||
| Testosterone and other anabolic steroids | ↑ functional capacity and muscle strength in elderly women [90] | ↑ LBM and muscle strength [93] | Less severe weight loss [95] |
| ↑ functional capacity, improved symptoms; no changes in skeletal muscle bulk and hand-grip strength [91] | ↑ body weight, ↑ fat-free mass [94] | ↑ hand-grip strength [96] | |
| Improved exercise capacity and muscle strength [86] | Restored weight (primarily LBM) after weight loss [83] | ↑ body weight (but inferior to dexamethasone and MA) [97] | |
| No effect on skeletal muscle bulk or strength, improved exercise capacity [92] |
CHF chronic heart failure, COPD chronic obstructive pulmonary disease, PPAR-γ peroxisome proliferator-activated receptor gamma, Ang II R angiotensin II receptor, ACEI angiotensin-converting enzyme inhibitor, LBM lean body mass, MA megestrol acetate
Clinical implications and future research
In cachexia, insulin, GH, and IGF-1 signaling is impaired. Although the action of these three signaling molecules on muscle tissue is essential for preserving muscle mass and function, targeting their signaling pathways should be considered in the search for new compounds for cachexia treatment. Due to the lack of response to the basic stimuli of insulin, IGF-1, and GH in muscle cells in cachexia, two approaches seem reasonable: (1) targeting post-receptor pathways—for example, with PPAR-γ agonists, or (2) using alternative pathways in muscle cells to reach the same targets inside the cell (angiotensin II R antagonists/ACEI and testosterone). Several studies have addressed this issue, but the results do not fully support implementation in clinical practice. Potential pharmacological targets can be found among the effector molecules involved in overlapping pathways of GH, IGF-1, and insulin signaling.
Acknowledgement
Mitja Lainscak received the Heart Failure Association Research Fellowship. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [98].
Conflict of interest
The authors declare that they have no conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- ATP
Adenosine triphosphate
- CAP
Cbl-associated protein
- CHF
Chronic heart failure
- COPD
Chronic obstructive pulmonary disease
- GH
Growth hormone
- GLUT4
Glucose transporter type 4
- HOMA
Homeostatic model assessment
- IGF-1
Insulin-like growth factor 1
- IGF1R
Insulin-like growth factor 1 receptor
- IRS-1/2
Insulin receptor substrate 1 and 2
- JAK2
Janus kinase 2
- MAFbx
Muscle atrophy F-box
- mTORC1
Mammalian target of rapamycin complex 1
- MURF1
Muscle ring finger 1
- PI3K
Phosphatidylinositol 3-kinase
- STAT
Signal transducer and activator of transcription 5
- SOCS
Suppressor of cytokine signaling
References
- 1.Lainscak M, Filippatos GS, Gheorghiade M, Fonarow GC, Anker SD. Cachexia: common, deadly, with an urgent need for precise definition and new therapies. Am J Cardiol. 2008;101:8–10. doi: 10.1016/j.amjcard.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 2.Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 4.von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle. 2010;1:1–5. doi: 10.1007/s13539-010-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Haehling S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: a systematic overview. Pharmacol Ther. 2009;121:227–252. doi: 10.1016/j.pharmthera.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 6.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129–134. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ottosson M, Lönnroth P, Björntorp P, Edén S. Effects of cortisol and growth hormone on lipolysis in human adipose tissue. J Clin Endocrinol Metab. 2000;85:799–803. doi: 10.1210/jc.85.2.799. [DOI] [PubMed] [Google Scholar]
- 8.Thomas SH, Wisher MH, Brandenburg D, Sönksen PH. Insulin action on adipocytes. Evidence that the anti-lipolytic and lipogenic effects of insulin are mediated by the same receptor. Biochem J. 1979;184:355–360. doi: 10.1042/bj1840355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner C, Caplan SR, Tannenbaum GS. Interactions of ghrelin signaling pathways with the GH neuroendocrine axis: a new and experimentally tested model. J Mol Endocrinol. 2009;43:105–119. doi: 10.1677/JME-09-0023. [DOI] [PubMed] [Google Scholar]
- 10.Kamegai J, Unterman TG, Frohman LA, Kineman RD. Hypothalamic/pituitary-axis of the spontaneous dwarf rat: autofeedback regulation of growth hormone (GH) includes suppression of GH releasing-hormone receptor messenger ribonucleic acid. Endocrinology. 1998;139:3554–3560. doi: 10.1210/en.139.8.3554. [DOI] [PubMed] [Google Scholar]
- 11.Bermann M, Jaffe CA, Tsai W, de Mott-Friberg R, Barkan AL. Negative feedback regulation of pulsatile growth hormone secretion by insulin-like growth factor I. Involvement of hypothalamic somatostatin. J Clin Invest. 1994;94:138–145. doi: 10.1172/JCI117299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higaki K, Matsumoto Y, Fujimoto R, Kurosaki Y, Kimura T. Pharmacokinetics of recombinant human insulin-like growth factor-I in diabetic rats. Drug Metab Dispos. 1997;25:1324–1327. [PubMed] [Google Scholar]
- 13.Giustina A, Mazzioti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29:535–559. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hambrecht R, Schulze PC, Gielen S, Linke A, Möbius-Winkler S, Erbs S, Kratzsch J, Schubert A, Adams V, Schuler G. Effects of exercise training on insulin-like growth factor-I expression in the skeletal muscle of non-cachectic patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2005;12:401–406. doi: 10.1097/01.hjr.0000173106.68485.b7. [DOI] [PubMed] [Google Scholar]
- 15.Schulze PC, Gielen S, Adams V, Linke A, Mobius-Winkler S, Erbs S, et al. Muscular levels of proinflammatory cytokines correlate with a reduced expression of insulin-like growth factor-I in chronic heart failure. Basic Res Cardiol. 2003;98:267–274. doi: 10.1007/s00395-003-0411-1. [DOI] [PubMed] [Google Scholar]
- 16.Kido Y, Nakae J, Accili D. The insulin receptor and its cellular targets. J Clin Endocr Metab. 2001;86:972–979. doi: 10.1210/jc.86.3.972. [DOI] [PubMed] [Google Scholar]
- 17.Frystyk J, Grøfte T, Skjaerbaek C, Orskov H. The effect of oral glucose on serum free insulin-like growth factor-I and -II in health adults. J Clin Endocrinol Metab. 1997;82:3124–3127. doi: 10.1210/jc.82.9.3124. [DOI] [PubMed] [Google Scholar]
- 18.Pandini G. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem. 2002;277:39684–39695. doi: 10.1074/jbc.M202766200. [DOI] [PubMed] [Google Scholar]
- 19.Soos MA, Whittaker J, Lammers R, Ullrich A, Siddle K. Receptors for insulin and insulin-like growth factor-I can form hybrid dimers. Biochem J. 1990;270:383–390. doi: 10.1042/bj2700383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sönksen PH. Hormones and sport. Insulin, growth hormone and sport. J Endocrinol. 2001;170:13–25. doi: 10.1677/joe.0.1700013. [DOI] [PubMed] [Google Scholar]
- 21.Lainscak M, Osredkar J. Doping and the Olympic games: the good, the bad, and the ugly. Wien Klin Wochenschr. 2009;121:13–14. doi: 10.1007/s00508-008-1121-3. [DOI] [PubMed] [Google Scholar]
- 22.Cai D, Dhe-Paganon S, Melendez PA, Lee J, Shoelson SE. Two new substrates in insulin signaling, IRS5/DOK4 and IRS6/DOK5. J Biol Chem. 2003;278:25323–25330. doi: 10.1074/jbc.M212430200. [DOI] [PubMed] [Google Scholar]
- 23.Previs SF, Withers DJ, Ren JM, White MF, Shulman GI. Contrasting effects of IRS-1 versus IRS-2 gene disruption on carbohydrate and lipid metabolism in vivo. J Biol Chem. 2000;275:38990–38994. doi: 10.1074/jbc.M006490200. [DOI] [PubMed] [Google Scholar]
- 24.Karlsson HKR, Chibalin AV, Koistinen HA, Yang J, Koumanov F, Wallberg-Henriksson H, et al. Kinetics of GLUT4 trafficking in rat and human skeletal muscle. Diabetes. 2009;58:847–854. doi: 10.2337/db08-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauritzen HP, Galbo H, Toyoda T, Goodyear LJ. Kinetics of contraction-induced GLUT4 translocation in skeletal muscle fibers from living mice. Diabetes. 2010;59:2134–2144. doi: 10.2337/db10-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang L, Chiang SH, Saltiel AR. Insulin signaling and the regulation of glucose transport. Mol Med. 2004;10:65–71. doi: 10.2119/2005-00029.Saltiel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitra P, Zheng X, Czech MP. RNAi-based analysis of CAP, Cbl, and CrkII function in the regulation of GLUT4 by insulin. J Biol Chem. 2004;279:37431–37435. doi: 10.1074/jbc.C400180200. [DOI] [PubMed] [Google Scholar]
- 28.Sasaoka T, Ishiki M, Wada T, Hori H, Hirai H, Haruta T, Ishihara H, Kobayashi M. Tyrosine phosphorylation-dependent and -independent role of Shc in the regulation of IGF-1-induced mitogenesis and glycogen synthesis. Endocrinology. 2001;142:5226–5235. doi: 10.1210/en.142.12.5226. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen C, Gormsen LC, Jessen N, Pedersen SB, Møller N, Lund S, et al. Growth hormone signaling in vivo human muscle and adipose tissue: impact of insulin, substrate background, and growth hormone receptor blockade. J Clin Endocrin Metab. 2008;93:2842–2850. doi: 10.1210/jc.2007-2414. [DOI] [PubMed] [Google Scholar]
- 30.Herrington J, Smit LS, Schwartz J, Carter-Su C. The role of STAT proteins in growth hormone signaling. Oncogene. 2000;19:2585–2597. doi: 10.1038/sj.onc.1203526. [DOI] [PubMed] [Google Scholar]
- 31.Frost RA, Nystrom GJ, Lang CH. Regulation of IGF-I mRNA and signal transducers and activators of transcription-3 and -5 (Stat-3 and -5) by GH in C2C12 myoblasts. Endocrinoloy. 2002;143:492–503. doi: 10.1210/en.143.2.492. [DOI] [PubMed] [Google Scholar]
- 32.Jørgensen JOL, Jessen N, Pedersen SB, Vestergaard E, Gormsen L, Lund SA, et al. GH receptor signaling in skeletal muscle and adipose tissue in human subjects following exposure to an intravenous GH bolus. Am J Physiol Endocrinol Metab. 2006;291:899–905. doi: 10.1152/ajpendo.00024.2006. [DOI] [PubMed] [Google Scholar]
- 33.de Castro Barbosa T, de Carvalho JE, Poyares LL, Bordin S, Machado UF, Nunes MT. Potential role of growth hormone in impairment of insulin signaling in skeletal muscle, adipose tissue, and liver of rats chronically treated with arginine. Endocrinology. 2009;150:2080–2086. doi: 10.1210/en.2008-1487. [DOI] [PubMed] [Google Scholar]
- 34.Anker SD, Volterrani M, Pflaum CD, Strasburger CJ, Osterziel KJ, Doehner W, et al. Acquired growth hormone resistance in patients with chronic heart failure: implications for therapy with growth hormone. J Am Coll Cardiol. 2001;38:443–452. doi: 10.1016/S0735-1097(01)01385-7. [DOI] [PubMed] [Google Scholar]
- 35.Suskin N, McKelvie RS, Burns RJ, Latini R, Pericak D, Probstfield J, et al. Glucose and insulin abnormalities relate to functional capacity in patients with congestive heart failure. Eur Heart J. 2000;21:1368–1375. doi: 10.1053/euhj.1999.2043. [DOI] [PubMed] [Google Scholar]
- 36.Takano A, Haruta T, Iwata M, Usui I, Uno T, Kawahara J, et al. Growth hormone induces cellular insulin resistance by uncoupling phosphatidylinositol 3-kinase and its downstream signals in 3T3-L1 adipocytes. Diabetes. 2001;50:1891–1900. doi: 10.2337/diabetes.50.8.1891. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt K, von Haehling S, Doehner W, Palus S, Anker SD, Springer J. IGF-1 treatment reduces weight loss and improves survival in a rat model of cancer cachexia. J Cachexia Sarcopenia Muscle. 2011;2:105–110. doi: 10.1007/s13539-011-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bossola M, Muscaritoli M, Costelli P, Grieco G, Bonelli G, Pacelli F, et al. Increased muscle proteasome activity correlates with disease severity in gastric cancer patients. Ann Surg. 2003;237:384–389. doi: 10.1097/01.SLA.0000055225.96357.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acharyya S, Ladner KJ, Nelsen LL, Damraurer J, Reiser PJ, Swoap S, et al. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114:370–378. doi: 10.1172/JCI20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/S0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lenk K, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular atophysilology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2010;1:9–21. doi: 10.1007/s13539-010-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Filippatos GS, Anker SD, Kremastinos DT. Pathophysiology of peripheral muscle wasting in cardiac cachexia. Curr Opin Clin Nutr Metab Care. 2005;8:249–254. doi: 10.1097/01.mco.0000165002.08955.5b. [DOI] [PubMed] [Google Scholar]
- 43.Lang CH, Frost RA, Nairn AC, MacLean DA, Vary TC. TNF-α impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am J Physiol Endocrinol Metab. 2002;282:336–347. doi: 10.1152/ajpendo.00366.2001. [DOI] [PubMed] [Google Scholar]
- 44.Denson LA, Menon RK, Shaufl A, Bajwa HS, Williams CR, Karpen SJ. TNF-alpha downregulates murine hepatic growth hormone receptor expression by inhibiting Sp1 and Sp3 binding. J Clin Invest. 2001;107:1451–1458. doi: 10.1172/JCI10994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noguchi Y, Yoshikawa T, Marat D, Doi C, Makino T, Fukuzawa K, et al. Insulin resistance in cancer patients is associated with enhanced tumor necrosis factor-alpha expression in skeletal muscle. Biochem Biophys Res Commun. 1998;253:887–892. doi: 10.1006/bbrc.1998.9794. [DOI] [PubMed] [Google Scholar]
- 46.Broussard SR, McCusker RH, Novakofski JE, Strle K, Shen WH, Johnson RW, et al. IL-1beta impairs insulin-like growth factor I-induced differentiation and downstream activation signals of the insulin-like growth factor I receptor in myoblasts. J Immunol. 2004;172:7713–7720. doi: 10.4049/jimmunol.172.12.7713. [DOI] [PubMed] [Google Scholar]
- 47.Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. FASEB J. 1998;12:871–880. doi: 10.1096/fasebj.12.10.971. [DOI] [PubMed] [Google Scholar]
- 48.Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL, Reid MB. TNF-α acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005;19:362–370. doi: 10.1096/fj.04-2364com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Springer J, Filippatos G, Akashi Y, Anker SD. Prognosis and therapy approaches of cardiac cachexia. Curr Opin Cardiol. 2006;21:229–233. doi: 10.1097/01.hco.0000221585.94490.09. [DOI] [PubMed] [Google Scholar]
- 50.Thissen JP, Verniers J. Inhibition by interleukin-1 beta and tumor necrosis factor-alpha of the insulin-like growth factor I messenger ribonucleic acid response to growth hormone in rat hepatocyte primary culture. Endocrinology. 1997;138:1078–1084. doi: 10.1210/en.138.3.1078. [DOI] [PubMed] [Google Scholar]
- 51.Barton BE. IL-6-like cytokines and cancer cachexia. Consequences of chronic inflammation. Immunol Res. 2001;23:41–58. doi: 10.1385/IR:23:1:41. [DOI] [PubMed] [Google Scholar]
- 52.Nieto-Vazquez I, Fernández-Veledo S, de Alvaro C, Lorenzo M. Dual role of interleukin-6 in regulating insulin sensitivity in murine skeletal muscle. Diabetes. 2008;57:3211–3221. doi: 10.2337/db07-1062. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Matthews VB, Allen TL, Risis S, Chan MH, Henstridge DC, Watson N, et al. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia. 2010;53:2431–2441. doi: 10.1007/s00125-010-1865-y. [DOI] [PubMed] [Google Scholar]
- 54.Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277:42394–42398. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- 55.Holt RIG, Sönksen PH. Growth hormone, IGF-1 and insulin and their abuse in sport. Review. Br J Pahrmacol. 2008;154:542–556. doi: 10.1038/bjp.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, Hinds CJ. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341:785–792. doi: 10.1056/NEJM199909093411102. [DOI] [PubMed] [Google Scholar]
- 57.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 58.Doehner W, Gathercole D, Cicoira M, Krack A, Coats AJ, Camici PG, et al. Reduced glucose transporter GLUT4 in skeletal muscle predicts insulin resistance in non-diabetic chronic heart failure patients independently of body composition. Int J Cardiol. 2010;138:19–24. doi: 10.1016/j.ijcard.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 59.Oak S, Tran C, Castillo MO, Thamotharan S, Thamotharan M, Devaskar SU. Peroxisome proliferator-activated receptor-gamma agonist improves skeletal muscle insulin signaling in the pregestational intrauterine growth-restricted rat offspring. Am J Physiol Endocrinol Metab. 2009;297:514–524. doi: 10.1152/ajpendo.00008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bogacka I, Xie H, Bray GA, Smith SR. The effect of pioglitazone on peroxisome proliferator-activated receptor-gamma target genes related to lipid storage in vivo. Diabetes Care. 2004;27:1660–1667. doi: 10.2337/diacare.27.7.1660. [DOI] [PubMed] [Google Scholar]
- 61.Iwata M, Haruta T, Usui I, Takata Y, Takano A, Uno T, et al. Pioglitazone ameliorates tumor necrosis factor-alpha-induced insulin resistance by a mechanism independent of adipogenic activity of peroxisome proliferator-activated receptor-gamma. Diabetes. 2001;50:1083–1092. doi: 10.2337/diabetes.50.5.1083. [DOI] [PubMed] [Google Scholar]
- 62.Sugimoto M, Takeda N, Hattori J, Yoshino K, Nakashima K, Okumura S, et al. Pharmacological treatments for GH-induced insulin resistance. Endocr J. 1999;46:51–53. doi: 10.1507/endocrj.46.Suppl_S51. [DOI] [PubMed] [Google Scholar]
- 63.Samaha FF, Szapary PO, Iqbal N, Williams MM, Bloedon LT, Kochar A, et al. Effects of rosiglitazone on lipids, adipokines, and inflammatory markers in nondiabetic patients with low high-density lipoprotein cholesterol and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:624–630. doi: 10.1161/01.ATV.0000200136.56716.30. [DOI] [PubMed] [Google Scholar]
- 64.Villacorta L, Schopfer FJ, Zhang J, Freeman BA, Chen YE. PPARgamma and its ligands: therapeutic implications in cardiovascular disease. Clin Sci (Lond). 2009;116:205–218. doi: 10.1042/CS20080195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest. 2007;117:2791–2801. doi: 10.1172/JCI30335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10:933–989. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 67.Brink M, Price SR, Chrast J, Bailey JL, Anwar A, Mitch WE, et al. Angiotensin II induces skeletal muscle wasting through enhanced protein degradation and down-regulates autocrine insulin-like growth factor I. Endocrinology. 2001;142:1489–1496. doi: 10.1210/en.142.4.1489. [DOI] [PubMed] [Google Scholar]
- 68.Wei Y, Sowers JR, Clark SE, Li W, Ferrario CM, Stump CS. Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-κB activation via NADPH oxidase. Am J Physiol Endocrinol Metab. 2008;294:345–351. doi: 10.1152/ajpendo.00456.2007. [DOI] [PubMed] [Google Scholar]
- 69.Csibi A, Communi D, Müller N, Bottari SP. Angiotensin II inhibits insulin-stimulated GLUT4 translocation and Akt activation through tyrosine nitration-dependent mechanisms. PLoS One. 2010;5:E10070. doi: 10.1371/journal.pone.0010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song YH, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest. 2005;115:451–458. doi: 10.1172/JCI22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sanders PM, Russell ST, Tisdale MJ. Angiotensin II directly induces muscle protein catabolism through the ubiquitin-proteasome proteolytic pathway and may play a role in cancer cachexia. Br J Cancer. 2005;93:425–434. doi: 10.1038/sj.bjc.6602725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anker SD, Negassa A, Coats AJ, Afzal R, Poole-Wilson PA, Cohn JN, et al. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet. 2003;361:1077–1083. doi: 10.1016/S0140-6736(03)12892-9. [DOI] [PubMed] [Google Scholar]
- 73.Schellenbaum GD, Smith NL, Heckbert SR, Lumley T, Rea TD, Furberg CD, et al. Weight loss, muscle strength, and angiotensin-converting enzyme inhibitors in older adults with congestive heart failure in hypertension. J Am Geriatr Soc. 2005;53:1996–2000. doi: 10.1111/j.1532-5415.2005.53568.x. [DOI] [PubMed] [Google Scholar]
- 74.Di Bari M, van de Poll-Franse LV, Onder G, Kritchevsky SB, Newman A, Harris TB, et al. Antihypertensive medications and differences in muscle mass in older persons: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52:961–966. doi: 10.1111/j.1532-5415.2004.52265.x. [DOI] [PubMed] [Google Scholar]
- 75.Paolisso G, Balbi V, Gambardella A, Varricchio G, Tortoriello R, Saccomanno F, et al. Lisinopril administration improves insulin action in aged patients with hypertension. J Hum Hypertens. 1995;9:541–546. [PubMed] [Google Scholar]
- 76.Cesari M, Pedone C, Incalzi RA, Pahor M. ACE-inhibition and physical function: results from the Trial of Angiotensin-Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors (TRAIN) study. J Am Med Dir Assoc. 2010;11:26–32. doi: 10.1016/j.jamda.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen X, Li X, Huang HY, Li X, Lin JF. Effects of testosterone on insulin receptor substrate-1 and glucose transporter 4 expression in cells sensitive to insulin. Zonghua Yi Xue Za Zhi. 2006;86:1474–1477. [PubMed] [Google Scholar]
- 78.Muthusamy T, Murugesan P, Balasubramanian K. Sex steroids deficiency impairs glucose transporter 4 expression and its translocation through defective Akt phosphorylation in target tissues of adult male rat. Metabolism. 2009;58:1581–1592. doi: 10.1016/j.metabol.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 79.Serra C, Bhasin S, Tangherlini F, Barton ER, Ganno M, Zhang A, et al. The role of GH and IGF-1 in mediating anabolic effects of testosterone on androgen-responsive muscle. Endocrinology. 2011;152:193–206. doi: 10.1210/en.2010-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gentile MA, Nantermet PV, Vogel RL, Phillips R, Holder D, Hodor P, et al. Androgen-mediated improvement of body composition and muscle function involves a novel early transcriptional program including IGF1, mechano growth factor, and induction of {beta}-catenin. J Mol Endocrinol. 2010;44:55–73. doi: 10.1677/JME-09-0048. [DOI] [PubMed] [Google Scholar]
- 81.Venken K, Movérare-Skrtic S, Kopchick JJ, Coschigano KT, Ohlsson C, Boonen S, et al. Impact of androgens, growth hormone, and IGF-I on bone and muscle in male mice during puberty. J Bone Miner Res. 2007;22:72–82. doi: 10.1359/jbmr.060911. [DOI] [PubMed] [Google Scholar]
- 82.Marcell TJ, Harman SM, Urban RJ, Metz DD, Rodgers BD, Blackman MR. Comparison of GH, IGF-I, and testosterone with mRNA of receptors and myostatin in skeletal muscle in older men. Am J Physiol Endocrinol Metab. 2001;281:1159–1164. doi: 10.1152/ajpendo.2001.281.6.E1159. [DOI] [PubMed] [Google Scholar]
- 83.Yeh SS, DeGuzman B, Kramer T, M012 Study Group Reversal of COPD-associated weight loss using the anabolic agent oxandrolone. Chest. 2002;122:421–428. doi: 10.1378/chest.122.2.421. [DOI] [PubMed] [Google Scholar]
- 84.Lewis MI, Fournier M, Storer TW, Bhasin S, Porszasz J, Ren SG, et al. Skeletal muscle adaptations to testosterone and resistance training in men with COPD. J Appl Physiol. 2007;103:1299–1310. doi: 10.1152/japplphysiol.00150.2007. [DOI] [PubMed] [Google Scholar]
- 85.Malkin CJ, Jones TH, Channer KS. The effect of testosterone on insulin sensitivity in men with heart failure. Eur J Heart Fail. 2007;9:44–50. doi: 10.1016/j.ejheart.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 86.Caminiti G, Volterrani M, Iellamo F, Marazzi G, Massaro R, Miceli M, Mammi C, Piepoli M, Fini M, Rosano GM. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure a double-blind, placebo-controlled, randomized study. J Am Coll Cardiol. 2009;54:919–927. doi: 10.1016/j.jacc.2009.04.078. [DOI] [PubMed] [Google Scholar]
- 87.Pocock SJ, McMurray JJ, Dobson J, Yusuf S, Granger CB, Michelson EL, et al. Weight loss and mortality risk in patients with chronic heart failure in the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:2641–2650. doi: 10.1093/eurheartj/ehn420. [DOI] [PubMed] [Google Scholar]
- 88.Adigun AQ, Ajayi AA. The effects of enalapril–digoxin–diuretic combination therapy on nutritional and anthropometric indices in chronic congestive heart failure: preliminary findings in cardiac cachexia. Eur J Heart Fail. 2001;3:359–363. doi: 10.1016/S1388-9842(00)00146-X. [DOI] [PubMed] [Google Scholar]
- 89.Andreas S, Herrmann-Lingen C, Raupach T, Lüthje L, Fabricius JA, Hruska N, et al. Angiotensin II blockers in obstructive pulmonary disease: a randomised controlled trial. Eur Respir J. 2006;27:972–979. doi: 10.1183/09031936.06.00098105. [DOI] [PubMed] [Google Scholar]
- 90.Iellamo F, Volterrani M, Caminiti G, Karam R, Massaro R, Fini M, et al. Testosterone therapy in women with chronic heart failure: a pilot double-blind, randomized, placebo-controlled study. J Am Coll Cardiol. 2010;56:1310–1316. doi: 10.1016/j.jacc.2010.03.090. [DOI] [PubMed] [Google Scholar]
- 91.Malkin CJ, Pugh PJ, West JN, van Beek EJ, Jones TH, Channer KS. Testosterone therapy in men with moderate severity heart failure: a double-blind randomized placebo controlled trial. Eur Heart J. 2006;27:57–64. doi: 10.1093/eurheartj/ehi443. [DOI] [PubMed] [Google Scholar]
- 92.Pugh PJ, Jones RD, West JN, Jones TH, Channer KS. Testosterone treatment for men with chronic heart failure. Heart. 2004;90:446–447. doi: 10.1136/hrt.2003.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Casaburi R, Bhasin S, Cosentino L, Porszasz J, Somfay A, Lewis MI, et al. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:870–878. doi: 10.1164/rccm.200305-617OC. [DOI] [PubMed] [Google Scholar]
- 94.Schols AM, Soeters PB, Mostert R, Pluymers RJ, Wouters EF. Physiologic effects of nutritional support and anabolic steroids in patients with chronic obstructive pulmonary disease. A placebo-controlled randomized trial. Am J Respir Crit Care Med. 1995;152:1268–1274. doi: 10.1164/ajrccm.152.4.7551381. [DOI] [PubMed] [Google Scholar]
- 95.Chlebowski RT, Herrold J, Ali I, Oktay E, Chlebowski JS, Ponce AT, et al. Influence of nandrolone decanoate on weight loss in advanced non-small cell lung cancer. Cancer. 1986;58:183–186. doi: 10.1002/1097-0142(19860701)58:1<183::AID-CNCR2820580131>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 96.Szmulewitz R, Mohile S, Posadas E, Kunnavakkam R, Karrison T, Manchen E, et al. A randomized phase 1 study of testosterone replacement for patients with low-risk castration-resistant prostate cancer. Eur Urol. 2009;56:97–103. doi: 10.1016/j.eururo.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loprinzi CL, Kugler JW, Sloan JA, Mailliard JA, Krook JE, Wilwerding MB, et al. Randomized comparison of megestrol acetate versus dexamethasone versus fluoxymesterone for the treatment of cancer anorexia/cachexia. J Clin Oncol. 1999;17:3299–3306. doi: 10.1200/JCO.1999.17.10.3299. [DOI] [PubMed] [Google Scholar]
- 98.von Haehling S, Morley JE, Coats AJ, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8. doi: 10.1007/s13539-010-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]