Abstract
Mucosa-associated lymphoid tissue (MALT) is the initial inductive site for mucosal immunity. It is present in the different layers of the mucosal wall and consists of organized lymphoid tissue which may occur as isolated or aggregated lymphoid follicles (LFs) and interfollicular areas. It is present in many organs, including the pig stomach. Gastric MALT has been intensely studied in experimentally infected pigs but few data are available in healthy, non-gnotobiotic or germ-free animals. In the present study we described the gastric MALT in conventional piglets in the cardiac mucosa of the gastric diverticulum, in the pyloric mucosa, and in the sites of transition from cardiac to oxyntic and from cardiac to pyloric mucosa by means of histological and immunohistochemical stains. The majority of LFs were located in the cardiac mucosa and in the transition from the cardiac to the oxyntic mucosa. Here the LFs were mainly located in the submucosa and reached the mucosa; we called these submucosal lymphoid follicles (SLFs). In the pyloric mucosa and in the transition sites from the cardiac to the pyloric mucosa, LFs were located in the mucosa; we called these mucosal lymphoid follicles (MLFs). In SLFs, a compartmental organization of T and B lymphocytes was present; by contrast, in the MLFs, the T and B cells were intermingled, suggesting the possibility of different roles for the two types of follicles. In the epithelium overlying the lymphoid tissue, numerous T lymphocytes and some cells immunoreactive to cytokeratin-18 were observed. Following the application of the fluorescent tracer DiI into the SLFs of the diverticulum, enteric neurones located in the submucosal plexus were labelled, confirming the interplay between the immune and the enteric nervous system.
Keywords: 1,1′-didodecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; cytokeratin-18; gastric MALT; piglet
Introduction
Mucosa-associated lymphoid tissue (MALT) is the initial inductive site for mucosal immunity and plays a major role in the protection of the mucosal barrier and in allergic reactions. It is an important entry site for antigen uptake and the induction of immune responses. MALT is present in the thickness of the mucosal wall in close contact with the mucosal surface; it consists of organized lymphoid tissue which may exist as isolated (ILFs) or aggregated lymphoid follicles, and inter-follicular areas. About half of the lymphocytes of the immune system are located in MALT (Croitoru & Bienenstock, 1994). Such tissue is more precisely classified in relation to the site in which it is found, e.g. gastric MALT, gut-associated lymphoid tissue (GALT), nasopharynx-associated lymphoid tissue (NALT), bronchus-associated lymphoid tissue (BALT), conjunctiva-associated lymphoid tissue (CALT), lacrimal drainage-associated lymphoid tissue (LDALT), larynx-associated lymphoid tissue (LALT) and salivary-gland or duct-associated lymphoid tissue (SALT/DALT) (Brandtzaeg et al. 2008; Beyaz et al. 2010). The palatine tonsils, adenoids, aggregated lymphoid nodules (Peyer's patches, PPs), appendix and colonic lymphoglandular complexes are all part of this organized lymphoid tissue (Cesta, 2006; Liebler-Tenorio & Pabst, 2006). MALT has been extensively investigated in small laboratory animals and the data obtained extrapolated to humans. In recent years, however, the importance of pigs as an alternative animal model has been proposed in the study of the immune system, due to the many morpho-functional similarities to humans (Butler & Šinkora, 2007).
In many species, numerous studies have been conducted on GALT. In the intestines, which are exposed to numerous potential pathogens, food-borne antigens and commensal microorganisms throughout the life of an animal, the GALT is well developed and is functionally divided into inductive and effector sites. The inductive sites consist of PPs and ILFs which ensure the secretion of IgA and the production of clonal B cells, whereas the effector sites consist of the epithelium and lamina propria containing numerous lymphocytes (Mowat, 2003; Fagarasan & Honjo, 2004; Lorenz & Newberry, 2004; Newberry, 2008). PPs are easily observable macroscopically through the mucosal and serous surfaces on the anti-mesenteric wall of the small intestine (Newberry, 2008) and are covered with follicle-associated epithelium (FAE), which contains morphologically distinct cells (microfold cells or M cells) specialized in the uptake of antigens from the lumen to the mucosal lymphoid tissue in which the processing and initiation of immune responses occurs (Kyd & Cripps, 2008; Rothkötter, 2009; Valpotić et al. 2010). Similar to PPs, ILFs contain germinal centres with segregated B- and T-cell areas and an overlying FAE complete with M cells (Hamada et al. 2002; Newberry, 2008).
Furthermore, in the small intestines of rats (Mayrhofer & Brooks, 1995; Mayrhofer et al. 1999; Hitotsumatsu et al. 2005) and human (Moghaddami et al. 1998), a novel organized lymphoid entity, lymphocyte-filled villi (LFV), has been described; this structure consists of packed lymphocytes located in the lamina propria of the villi. Moreover, lymphoid structures called cryptopatches (CPs) have been described only in the intestine of mice (Ishikawa et al. 1999; Hamada et al. 2002; Lügering & Kucharzik, 2006; Burkey et al. 2009). It has been hypothesized that CPs have a primary generative function, in which the extrathymic generation of intraepithelial lymphocytes occurs (Saito et al. 1998; Eberl & Littman, 2004). CPs are absent in the mouse stomach (Newberry & Lorenz, 2005), and in human, rat and porcine small intestines (Pabst et al. 2005; Burkey et al. 2009).
Few studies regarding mucosal immunity have been carried out on gastric MALT, probably because mucosal immune responses in the stomach have been considered of little importance to gut host diseases or because of the inhospitable microbial environment. Moreover, it is believed that the normal stomach of many mammals, including humans, is devoid of lymphoid tissue, which only develops after a bacterial infection or other types of gastritis. Gastric MALT has been described in pigs; it appears in foetal life and its evolution varies according to gestational age; in gnotobiotic animals, lymphoid follicles (LFs) and diffuse infiltrate are present in the diverticulum and in the corpus at birth (Driessen et al. 2002). Moreover, studies on gastric MALT in pigs have especially focused on the histopathological features caused by Helicobacter pylori (Green et al. 1997; Cantet et al. 1999; Poutahidis et al. 2001; Koga et al. 2002; Krakowka & Eaton, 2002; Park et al. 2004; Hellemans et al. 2007; Haesebrouck et al. 2009) and, in some cases, on other infectious agents such as Campylobacter pylori (Krakowka et al. 1987) and Gastrospirillum suis (Mendes et al. 1991). Furthermore, the majority of authors used gnotobiotic (Krakowka et al. 1987; Green et al. 1997) or germ-free pigs (Koga et al. 2002; Krakowka & Eaton, 2002) for their studies, apart from Poutahidis et al. (2001) who used conventional pigs purchased from a commercial farm.
To our knowledge, one aspect of gastric MALT which has received little attention is its possible innervation, although, in recent decades, many studies in neuroscience and immunology have established the anatomical and cellular basis for a dynamic interplay between the immune and the nervous systems (Tracey, 2009). The gastrointestinal system is used as a model to study the interactions between the nervous and the immune systems in both healthy and infected animals; however, studies focused on the gut rather than the stomach. Moreover, although the role of the enteric nervous system (ENS) in controlling secretion, blood flow regulation and motility for propulsive and mixing movements is supported by morphological, physiological and pharmacological studies carried out in the small and large intestines of many mammals, few studies have focused on the role of the ENS in immune responses. Changes in the ENS neurones can occur transiently, in response to an acute stimulus, or permanently, following chronic pathological damage (Ekblad & Bauer, 2004). Consistent changes in the number of enteric cholinergic and galanin-expressing neurones were observed in stomachs of pigs affected by swine dysentery (Pidsudko et al. 2008; Kaleczyc et al. 2010), suggesting that these neurones have a specific role in local neural circuits in infected swine.
The aim of the present study was to describe gastric MALT in conventional piglets by means of histological staining, paying particular attention to the distribution and histological aspect of the lymphoid tissue. To distinguish between T and B lymphocytes, immunohistochemical methods were used, using polyclonal anti-human CD3 antibody, which is considered to be an excellent marker of swine T lymphocytes when used on paraffin sections (Mason et al. 1989; Chianini et al. 2001; Pérez et al. 2002), and monoclonal antibody anti-CD79α, which has been described as a useful pan-B marker in paraffin-embedded tissue (Tanimoto & Ohtsuki, 1996; Faldyna et al. 2007). The anti-cytokeratin-18 antibody, which several studies in pigs indicate as the optimal marker of M cells in the FAE (Gebert et al. 1994; Rothkötter, 2009), has also been tested. In addition, in fixed tissue, crystals of 1,1′-didodecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) were inserted into the follicles and into the lamina propria to determine whether ENS neurones are involved in the innervation of gastric MALT. This anterograde/retrograde fluorescent tracer applied onto nerve endings is transported along the nerve fibres by means of passive diffusion, movement of approximately 1 cm during 6 months of incubation in fixed tissue (Baker & Reese, 1993), and has been successfully used to study neuronal projections, including ileal PPs (Chiocchetti et al. 2008).
Materials and methods
Tissue preparation
Large White pigs (n = 7), purchased from Suidea (Reggio Emilia, Italy) and weaned at 45 days were used. For the entire period of the experiment the animals were fed a standard balanced diet for weaning pigs. The pigs were housed individually in pens with a mesh floor in a temperature-controlled room; tap water was freely available. The procedure was conducted according to Italian law pertaining to experimental animals and was approved by the Ethic Scientific Committee for Experiments on Animals of the University of Bologna. All efforts were made to minimize the number of animals used and their suffering. The piglets were deeply anaesthetized with sodium thiopental (10 mg kg body weight, Zoletil 100; Vibrac) and slaughtered by an intracardiac injection of Tanax® (0.5 mL kg BW; Intervet Italia). The stomach was gently removed from each piglet, opened along the great curvature from the diverticulum to the pyloric sphincter and, after a brief washing with 0.01 m phosphate buffer saline (PBS), eight samples were collected in the sites 1–8 as shown in Fig. 1. The tissue samples were pinned tightly to balsa wood and fixed in 10% buffered formalin for 24 h at room temperature (RT). The specimens were then dehydrated in a graded series of ethanol and embedded in paraffin. From each sample, 40 serial transverse (5 μm thick) and 40 serial tangential sections (7 μm thick) were obtained and mounted on poly-l-lysine coated slides and then processed for histology and immunohistochemistry.
Fig. 1.
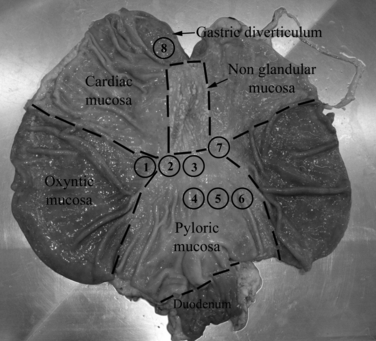
The stomach of a piglet opened along the greater curvature, from the diverticulum to the pyloric sphincter to show the sites of sampling (1–8).
Histology and immunohistochemistry
Masson's trichrome stain was used for histological observation (McManus & Mowry, 1960). For immunohistochemistry, the avidin-biotin-peroxidase complex (ABC) method was used, as described elsewhere (Bosi et al. 2006). Briefly, paraffin sections were deparaffinized and rehydrated; to unmask the antigenic sites, the slides were heated in sodium citrate buffer (pH 6.0) in a microwave.
Endogenous peroxidase was blocked with 1% aqueous hydrogen peroxide solution for 30 min at RT, and subsequently incubated for 30 min in PBS containing 10% normal goat serum, 1% normal swine serum and 10% bovine serum albumin to prevent nonspecific binding of the antibodies. The sections were then incubated overnight at 4 °C with the following antibodies: mouse anti-cytokeratin-18 1 : 15 000 (Sigma-Aldrich, CY90), mouse anti-α subunit of H+/K+-ATPase 1 : 4000 (Chemicon, MAB 3188), mouse anti-CD79α 1 : 2000 (clone HM47/A9) and polyclonal rabbit antiserum anti-CD3 1 : 2000 (Sigma-Aldrich, C7930). After washing, the sections were incubated at RT for 1 h with the appropriate biotin-conjugated secondary antibody [goat anti-mouse IgG and goat anti-rabbit IgG, both diluted 1 : 500 (Vector)] and then treated with ABC complex (Vector elite kit, Vector Laboratories). The immune reactions were visualized applying a 3,3′-diaminobenzidine chromogen solution (Vector DAB kit, Vector Laboratories).
Tissue preparation and DiI tracing in fixed tissue
Small crystals of DiI (Molecular Probes, Eugene, OR, USA) were diluted at 3% in 100% ethanol and evaporated onto small glass beads (about 200 μm; Sigma). The glass beads were placed in the middle of the gastric follicles to detect if neurones projecting to the follicles were present. In other samples, after gently removing the overlying epithelium by the use of entomological forceps, glass beads were inserted into the lamina propria to identify whether neurones projecting to the mucosal layers were present.
Adult (3 months) domestic pigs were used. Adult material was obtained from the slaughterhouse of the Faculty of Veterinary Medicine of Bologna. Segments of the stomach diverticulum were pinned out with the mucosa facing upwards in a Sylgard-lined Petri dish and fixed in 4% phosphate-buffered paraformaldehyde for at least 2 h at RT. Subsequently, pieces of the gastric wall were cut and, using a stereomicroscopy and ophthalmology tweezers, fluorescent tracer DiI was applied to the follicles to identify neurones projecting into them. In other samples, after gently removing the overlying epithelium with entomological forceps, glass beads were inserted into the lamina propria to identify neurones projecting into the mucosal layer. The specimens were then re-incubated in the fixative for 6–8 months at 37 °C. After incubation, the specimens were rinsed in PBS and the tissues were subsequently transferred to a mixture of PBS-sucrose-azide and OCT compound (Tissue Tek, Sakura Finetek Europe, the Netherlands) at a ratio of 1 : 1 for an additional 24 h before being embedded in 100% OCT and fixed in liquid nitrogen. For evaluation of the specimens, transverse and tangential cryosections (20 μm thick) were obtained.
Results
Histological and immunohistochemical description
No histological gastric inflammatory lesions consisting of submucosal oedema and neutrophilic infiltration were observed. Gastric MALT was identified in all the examined sites (1–8) shown in Fig. 1, although with different aspects and distributions. In sites 1 and 7, the gastric mucosa had a transitional aspect (the transition from cardiac to oxyntic mucosa takes place gradually), the tunica mucosa was thicker and an increasing number of parietal cells were present. In sites 2 and 3, the mucosa had a transitional aspect between the cardiac and the pyloric mucosa and no parietal cells were seen. In sites 4, 5 and 6, the mucosa had a typical pyloric aspect with an increase in the depth of the gastric pits. Site 8 showed typical cardiac mucosa with short glands, coiled at their bases.
Gastric MALT was populated by small- to medium-sized lymphocytes arranged in the LFs and diffuse lymphoid tissue infiltrating the lamina propria between the glands; numerous intra-epithelial lymphocytes (IELs), dispersed at the base of the overlying epithelium, were also seen. Encapsulated lymphoid follicles were located within the submucosa; they crossed the muscularis mucosae extending into the lamina propria to reach the overlying epithelium; we called these submucosal lymphoid follicles (SLFs) (Figs 2A and 3A). The lymphocytes were sometimes densely packed in the lamina propria and the structures formed were confined to the mucosa without crossing the muscularis mucosae in any of the 40 sections examined; these we termed mucosal lymphoid follicles (MLFs) (Figs 2B and 3D). A subepithelial dome region was not always evident because of the infiltration of the lymphocytes up to the basal lamina of the epithelium (Fig. 3D).
Fig. 2.
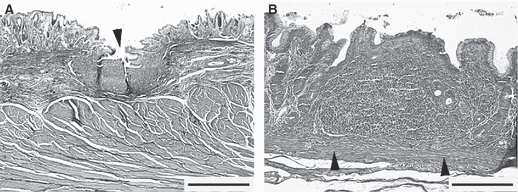
Transverse section of piglet gastric mucosa stained by Masson's trichrome. (A) A single ovoid submucosal lymphoid follicle (arrowhead) crossing the muscularis mucosae. (B) A single mucosal lymphoid follicle confined to the lamina propria without crossing the muscularis mucosae (arrowheads). Bars: (A) 800 μm; (B) 200 μm.
Fig. 3.
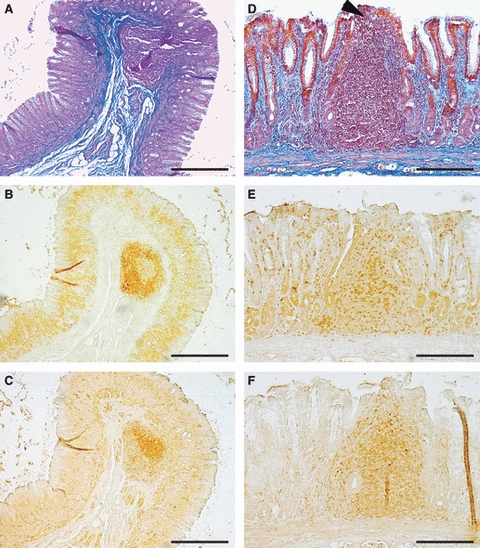
Serial transverse section of a submucosal (A–C) and a mucosal (D–F) lymphoid follicle stained with Masson's trichrome (A,D), CD3 (B,E) and CD79α (C,F). Note the compartmental organization of the lymphocytes in submucosal follicles; the T lymphocytes were confined to the mantle zone (B) and the B lymphocytes to the germinal centre (C). In the mucosal follicles, the T and B lymphocytes were intermingled (E,F) and a sub-epithelial dome region was evident (D, arrowhead). Bars: (A–C) 400 μm; (D–F) 200 μm.
In sites 1, 7 and 8, the majority of LFs were of the SLF type. Conversely, in sites 2, 3, 4, 5 and 6, the majority of LFs were of the MLF type (Table 1). LFs had various shapes (round, oval, ellipsoidal) and a columnar feature was occasionally observed in the lamina propria for both the SLFs and the MLFs. The overlying epithelium was sometimes invaginated in the gastric MALT and formed diverticula which entered into the lymphoid tissue (Figs 2A and 4). They were of the SLF type and were the easiest to identify macroscopically. The LFs appeared as a single lymphoid structure or, at least as observed in the tangential sections, aggregated to form PP-like structures (Fig. 4E,F). In the majority of SLFs, a clear compartmental organization of the lymphocytes was observed. The T lymphocytes were confined to the mantle zone and the B lymphocytes to the germinal centre (Fig. 3B,C); in some follicles, the T lymphocytes were densely packed at the base of the lymphoid follicles (Fig. 5A). In contrast, in MLFs, the T and B lymphocytes were intermingled without any clearly defined compartmental organization (Fig. 3E,F). Numerous T lymphocytes were found in the overlying epithelium for both SLFs (Fig. 5B) and MLFs.
Table 1.
Distribution of submucosal lymphoid follicles (SLFs) and mucosal lymphoid follicles (MLFs) in the piglet gastric mucosa in the eight selected sites
| Selected sites | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pig No. | SLF | MLF | SLF | MLF | SLF | MLF | SLF | MLF | SLF | MLF | SLF | MLF | SLF | MLF | SLF | MLF |
| 1 | 1 | – | 1 | – | – | 1 | 1 | 1 | 1 | 1 | – | 1 | 2 | – | 4 | 2 |
| 2 | 4 | 2 | 3 | 2 | – | 3 | – | 2 | 1 | 2 | – | 1 | 2 | 1 | 7 | 6 |
| 3 | – | 2 | – | 1 | – | – | – | 2 | – | 2 | – | 1 | 4 | 3 | 3 | 3 |
| 4 | 2 | – | – | 2 | – | – | – | – | – | 1 | – | 1 | 1 | – | 4 | 1 |
| 5 | – | – | – | – | – | – | – | 1 | – | – | – | – | 1 | – | 2 | 2 |
| 6 | 3 | – | – | 1 | – | 1 | – | 1 | – | 1 | 1 | – | 3 | 2 | 6 | 4 |
| 7 | – | 1 | 1 | – | – | 1 | – | 2 | – | – | – | 2 | 2 | 1 | 2 | 3 |
| Total no. | 10 | 5 | 5 | 6 | 0 | 6 | 1 | 9 | 2 | 7 | 1 | 6 | 15 | 7 | 28 | 21 |
Fig. 4.
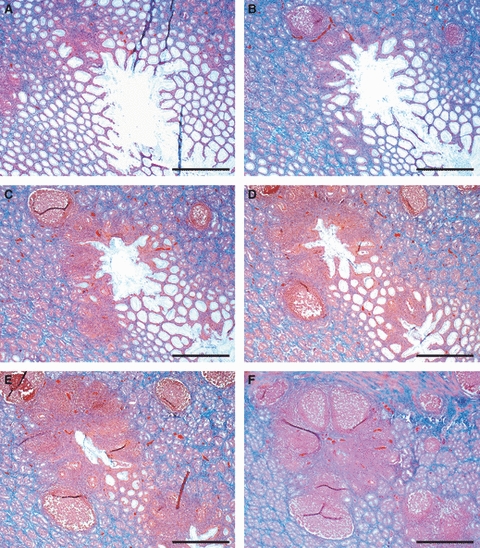
Tangential serial sections of piglet gastric mucosa showing the epithelium forming a diverticulum which enters the lymphoid tissue. (E,F) Lymphoid follicles were aggregated to form PP-like structures. Bars: (A–F) 800 μm.
Fig. 5.
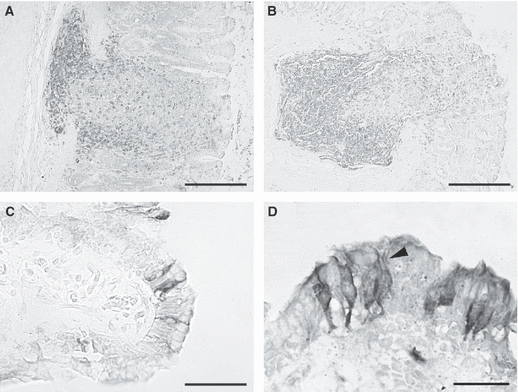
Transverse section of piglet gastric mucosa stained with anti-CD3 (A,B) and anti-cytokeratin 18 antibody (C–D). T lymphocytes were densely packed at the base of a submucosal lymphoid follicle (A) and infiltrated the epithelium overlying a submucosal lymphoid follicle (B). Columnar cytokeratin 18-IR cells either single (C) or grouped (D) were intermingled with epithelial cells. A network of fibrils filled the cytoplasm and surrounded the unlabelled nucleus (D, arrowed). Bars: (A,B) 200 μm; (C) 50 μm; (D) 30 μm.
Some tall columnar cytokeratin-18 immunoreactive (IR) cells were observed interspersed between the mucosal cells; they were isolated (Fig. 5C) or grouped into small clusters of two or three cells (Fig. 5D) and were found throughout the epithelium from the basal lamina to the luminal surface (Fig. 5C,D). The cells were not necessarily confined to the epithelium overlying the LFs; in fact, some lymphocytes were observed in the underlying lamina propria. A network of fibrils filled the cytoplasm and surrounded an unlabelled nucleus (Fig. 5D).
DiI crystals inserted into the follicles
DiI-coated beads were inserted into the follicles of the gastric diverticulum in which the epithelium formed diverticula extending into the lymphoid tissue which were best identifiable at stereomicroscopy; therefore, the data only refer to these SLFs. The DiI-coated beads generally remained in place for the entire period of incubation (up to 8 months) (Fig. 6A); if not, the pieces of tissue were not considered for microscopic observation. DiI tracer was observed throughout the entirety of the follicles to which it was applied, sometimes more concentrated at the level of the connective tissue capsule (Fig. 6B,C,E). Some DiI-labelled neurones were seen in the submucosal plexus (SMP) close to the labelled follicles, sometimes more than 400 μm away (Fig. 6C,E). The neurones varied in shape and generally showed an eccentrically located nucleus (Fig. 6D,G,H). The neuronal process was sometimes seen to be directed to the labelled follicles (Fig. 6B). Round, ovoid, elongated or, occasionally, polyhedral-shaped cells were found, labelled neurones generally exhibited a smoothly contoured soma (Fig. 6D,G,H) and rarely an irregular outline. DiI-labelled nerve fibres, isolated or grouped into small bundles, were seen close to the labelled follicles.
Fig. 6.
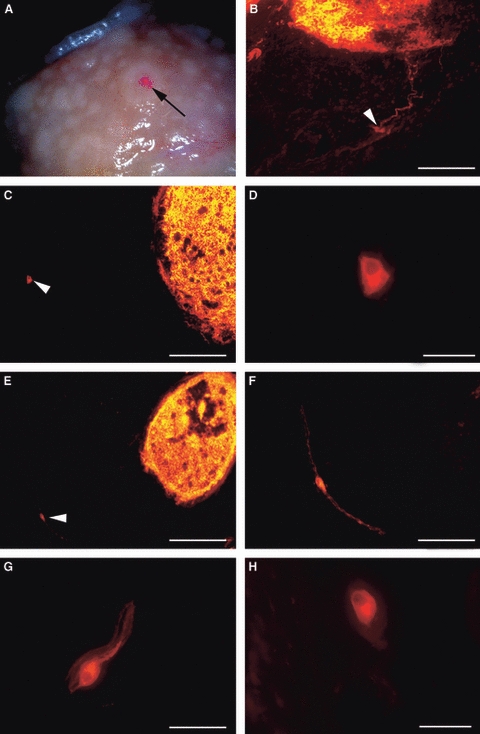
Tangential sections of pig gastric mucosa at the level of the diverticulum. (A) Stereomicroscopy showing DiI-coated beads (arrow) applied to a follicle in fixed tissue after 8 months of incubation. (B,C,E) DiI crystals applied to the follicles; note that the tracer was homogeneously distributed inside the follicles. (B) Labelled neurones (arrowhead) and processes entering the labelled follicle. (C) Polyhedral-labelled neurone (arrowhead) more than 400 μm from a labelled follicle. (D) Higher magnification of the neurone shown in (C). (E) Elongated neurone (about 400 μm from the labelled follicle) along a thin nervous fascicle (arrowhead). (F) Higher magnification of the neurone shown in (E). (G,H) Two labelled neurones with an ovoid shape. Note that the neurones showed smoothly contoured soma and an eccentrically located nucleus (D,G,H,). Bars: (B,F) 100 μm; (C,E) 200 μm; (D,G,H) 30 μm.
DiI crystals inserted into the lamina propria
In the samples in which DiI crystals were inserted into the lamina propria, the muscularis mucosae was also infiltrated. Labelled fibres were seen to form a thin plexus intermingled with adenomers (Fig. 7A); some neurones were seen in the lamina propria with processes in close apposition to the epithelium (Fig. 7B). Labelled fibres were sometimes seen entering into both DiI-labelled and unlabelled follicles (Fig. 7C–E) or resting in the connective capsule (Fig. 7F). Labelled neurones, varying in shape with generally smoothly contoured soma were seen in the SMP near the muscularis mucosae (Fig. 7G). No neurones were found in the myenteric plexus (MP).
Fig. 7.
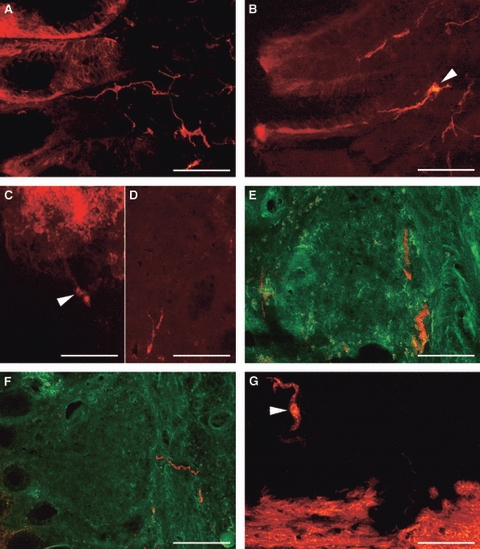
Transverse sections of pig gastric mucosa at the level of the diverticulum after the insertion of DiI crystals in the lamina propria. (A) Note a thin plexus of labelled fibres between the adenomers. (B) A neurone (arrowhead) in the lamina propria projecting to the epithelium. (C) Labelled neurones (arrowhead) projecting into a labelled follicle. (D,E) Labelled fibres in unlabelled follicles. (F) Labelled fibres resting on the connective follicular capsule. (G) Labelled, ovoid neurone (arrowhead) near the labelled muscularis mucosae. (E,F) Merging with the green filtre cube to best show the lymphoid follicle structure. Bars: (A,E,F) 200 μm; (B,C,G) 100 μm; (D) 50 μm.
Discussion
This article describes pig gastric MALT, with particular attention given to the distribution of lymphoid structures in the cardiac mucosa of the diverticulum, the pyloric mucosa and the transitional sites from the cardiac to the oxyntic mucosa and from the cardiac to the pyloric mucosa. We did not consider the oxyntic mucosa of the corpus because we very rarely observed LFs in our previous studies designed to test the effects of different diets on weaned piglet gastric mucosa (Bosi et al. 2006; Mazzoni et al. 2008). Similarly to Driessen et al. (2002), we found many LFs in the cardiac mucosa of the gastric diverticulum (site 8) and in the corpus (sites 1 and 7), i.e. in the sites in which the transition from cardiac to oxyntic mucosa occurred. In these sites, LFs were mainly situated in the submucosa and reached the mucosa. In the lesser curvature (sites 2 and 3) and in the pyloric antrum (sites 4–6), we found a smaller number of LFs that were mainly situated in the lamina propria without crossing the muscularis mucosae in any of 40 sections examined. The different extension of the LFs therefore appears to be strictly related to the site of sampling, which could explain the differences in observations compared with those Green et al. (1997), who found lymphoid nodules mainly located in the lamina propria of the lesser curvature rather than in the diverticulum.
In SLFs, a compartmental organization of the T and B lymphocytes was present, resembling the PPs extensively described by many authors (Newberry, 2008; Burkey et al. 2009) and analogously to what had been observed in the gastric MALT of humans, (Carney, 2010), and normal (Driessen et al. 2002) and infected pigs (Mendes et al. 1991; Green et al. 1997; Hellemans et al. 2007).
In contrast, in MLFs, the T and B lymphocytes packed in the lamina propria were intermingled, resembling the LFV described by Moghaddami et al. (1998) in the human small intestine. In fact, as the LFV as well as pig MLFs were confined to the mucosal layer and did not contain organized follicles with a germinal centre, similarly to human LFV, the overlying IELs were infiltrated by T cells. However, we did not observe the lymphocytes to be more densely packed at the base of the pig MLFs; this could reflect an interspecific difference. The lack of a germinal centre could suggest that MLFs are relatively inactive under normal conditions and may be activated under conditions of an increased mucosal antigenic stimulus. Another possible suggestion is that, similar to the murine CPs, and the rat LFV, in which clusters of undifferentiated cells expressing c-Kit were present (Hitotsumatsu et al. 2005), pig MLFs represent extrathymic progenitors of intraepithelial lymphocytes.
The epithelium overlying the gastric lymphoid tissue showed similarities to FAE; in fact, it contained large numbers of IELs, and cells of cytokeratin-18-IR were found interspersed between the epithelial cells. This marker has been tested in pigs to demonstrate M cells (Gebert et al. 1994; Rothkötter, 2009); however, the specificity of this antibody as a marker of M cells may be in doubt because porcine epithelial cell lines (Schierack et al. 2006) and goblet cells [unpublished communications of Post & Rothkötter (Rothkötter, 2009)] may also express cytokeratin-18. M cells have generally been described in the FAE overlying PPs or ILFs intermingled with enterocytes (Hamada et al. 2002; Lorenz et al. 2003; Newberry & Lorenz, 2005; Pabst et al. 2005; Burkey et al. 2009; Hondo et al. 2011). Relatively little is known about M cells outside the gut, although they have been found in the upper and lower airways and in the conjunctiva of the eye (Gebert & Pabst, 1999). Although M cells have a typical ultrastructural appearance with an invaginated basal membrane and apical disorganized microvilli under light microscopy, it is difficult to distinguish them from other epithelial cells. However, on the basis of the similarity between gastric MALT and PPs, it is plausible to think that stomach cells, like M cells, which are specialized in the uptake and transport of antigens, are present and that immunoreactivity to cytokeratin-18 also functions as an M-cell marker in the pig stomach.
DiI
Nerve fibres innervating the lymphoid tissue, besides constituting the root for infections of the central nervous system by ingested neurotropic viruses or prion proteins (van Keulen et al. 2002), can modulate immune responses. Interplay between the immune and the nervous systems has been extensively studied. Catecholamine and peptide neuronal and non-neuronal transmitters, such as neuropeptide Y, substance P, vasoactive intestinal peptide, galanin and calcitonin gene-related peptide, represent the peptides most involved in neuroimmune modulation (Mignini et al. 2003); both T and B lymphocytes, as well as other cells of the immune system (such as macrophages, dendritic cells, mast cells), express receptors for these transmitters. Besides neuroimmune modulation, neuropeptides are involved in lymphocyte maturation. Galanin, for example, probably acting through R1 and R2 receptors, is involved in the control of thymus growth and exerts anti-proliferative and pre-apoptotic effects on immature rat thymocytes (Trejter et al. 2002).
Extensive studies regarding GALT innervation have been carried out but, to our knowledge, no studies have focused on the innervation of gastric lymphoid follicles. There are conflicting data available on PPs. Studies on pigs (Krammer & Kühnel, 1993; Vulchanova et al. 2007; Kaleczyc et al. 2010) and cattle (Balemba et al. 1999) have failed to reveal any nerve fibres within the PPs; by contrast, nerve fibres have been found inside PP follicles in cattle (Defaweux et al. 2007), pigs (Kulkarni-Narla et al. 1999), mice (Ottaway et al. 1987; Defaweux et al. 2005; Ma et al. 2007) and sheep (Heggebø et al. 2003; Lalatta-Costerbosa et al. 2007; Chiocchetti et al. 2008). In the present study, we observed intrinsic innervations of the gastric follicles coming from neurones located in the SMP. The majority of labelled neurones showed ovoid or elongated shapes and generally smoothly contoured soma resembling type II primary sensory neurones; only a few neurones showed an irregular outline. It is generally assumed that, in the stomach, contrary to what has been observed in the gut, few neurones are present in both the SMP and the MP, and that primary sensory neurones throughout the enteric reflexes are very rare. The intrinsic reflexes are therefore poorly developed (Furness, 2008; Schemann et al. 2008) and stomach activity is most influenced by extrinsic innervation coming from the brain stem rather than the intrinsic reflex. However, studies of the gastric ENS have mainly been conducted on small laboratory animals. Recent studies of Kaleczyc et al. (2010) have revealed that, in the pig stomach, a well-developed ganglionated SMP is present and it can be subdivided into inner (near the muscularis mucosae) and outer SMP (near the circular muscle layer), as observed in other areas of the intestines of large mammals. In the present study, DiI-labelled neurones were found in the SMP, generally located in the outer portion of the SMP; however, labelled fibres were found in the lamina propria after DiI application. Therefore, we cannot exclude that the neurones located in the lamina propria or in the inner SMP may also innervate the gastric follicles. Although it was not possible to define immunohistochemically the neurochemical code of the labelled neurones, they resembled type II sensory neurones with a smoothly contoured soma and could constitute the afferent sensory arc which detects the molecular products resulting from injury. In fact, the inflammatory neural reflex, which has recently been described by Tracey (2009), consists of a sensory afferent arc and an efferent arc, which transmit signals modulating the immune responses.
In conclusion, in pig gastric MALT, two morphologically, and probably functionally, different types of LFs were present: SLFs and MLFs. Both types were present in all the sites examined, although the number varied. The ENS neurones located in the SMP were involved in the innervation of lymphoid follicles; however, we cannot exclude that neurones in the lamina propria or in the inner SMP could also contribute to this innervation. The labelled neurones resembled type II sensory neurones; however, additional studies using DiI tracer in organotypic cultures are necessary to best define the neurochemical code of these follicle-innervating neurones and phenotype them.
Acknowledgments
The research leading to these results has received funding from the European Community's Seventh Framework Program (FP7/2007–2013) under the grant agreement no. 227549.
References
- Baker GE, Reese BE. Using confocal laser scanning microscopy to investigate the organization and development of neuronal projections labeled with DiI. Methods Cell Biol. 1993;38:325–344. doi: 10.1016/s0091-679x(08)61009-2. [DOI] [PubMed] [Google Scholar]
- Balemba OB, Mbassa GK, Semuguruka WD, et al. The topography, architecture and structure of the enteric nervous system in the jejunum and ileum of cattle. J Anat. 1999;195:1–9. doi: 10.1046/j.1469-7580.1999.19510001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyaz F, Ergün E, Bayraktaroğlu AG, et al. Identification of intestinal M cells in isolated LF and Peyer's patches of the Angora rabbit. Cell Tissue Res. 2010;341:417–427. doi: 10.1007/s00441-010-1005-5. [DOI] [PubMed] [Google Scholar]
- Bosi P, Mazzoni M, De Filippi S, et al. A continuous dietary supply of free calcium formate negatively affects the parietal cell population and gastric RNA expression for H+/K+-ATPase in weaning pigs. J Nutr. 2006;136:1229–1235. doi: 10.1093/jn/136.5.1229. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Kiyono H, Pabst R, et al. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008;1:31–37. doi: 10.1038/mi.2007.9. [DOI] [PubMed] [Google Scholar]
- Burkey TE, Skjolaas KA, Minton JE. Board-invited review: porcine mucosal immunity of the gastrointestinal tract. J Anim Sci. 2009;87:1493–4501. doi: 10.2527/jas.2008-1330. [DOI] [PubMed] [Google Scholar]
- Butler JE, Šinkora M. The isolator piglet: a model for studying the development of adaptive immunity. Immunol Res. 2007;39:33–51. doi: 10.1007/s12026-007-0062-7. [DOI] [PubMed] [Google Scholar]
- Cantet F, Magras C, Marais A, et al. Helicobacter species colonizing pig stomach: molecular characterization and determination of prevalence. Appl Environ Microbiol. 1999;65:4672–4676. doi: 10.1128/aem.65.10.4672-4676.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney JA. Gastric mucosal lymphoid follicles: histology, distribution, frequency, and etiologic features. Am J Surg Pathol. 2010;34:1019–1024. doi: 10.1097/PAS.0b013e3181e1acb0. [DOI] [PubMed] [Google Scholar]
- Cesta MF. Normal structure, function, and histology of mucosa-associated lymphoid tissue. Toxicol Pathol. 2006;34:599–608. doi: 10.1080/01926230600865531. [DOI] [PubMed] [Google Scholar]
- Chianini F, Majó N, Segalés J, et al. Immunohistological study of the immune system cells in paraffin-embedded tissues of conventional pigs. Vet Immunol Immunopathol. 2001;82:245–255. doi: 10.1016/S0165-2427(01)00364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiocchetti R, Mazzuoli G, Albanese V, et al. Anatomical evidence for ileal Peyer's patches innervations by enteric nervous system: a potential route for prion neuroinvasion? Cell Tissue Res. 2008;332:185–194. doi: 10.1007/s00441-008-0583-y. [DOI] [PubMed] [Google Scholar]
- Croitoru K, Bienenstock J. Characteristics and functions of mucosa-associated lymphoid tissue. In: Ogra PL, Lamm ME, McGhee JR, Mestecky J, Strober W, Bienenstock J, editors. Handbook of Mucosal Immunology. San Diego: Academic Press; 1994. pp. 141–149. [Google Scholar]
- Defaweux V, Dorban G, Antoine N, et al. Neuroimmune connections in jejunal and ileal Peyer's patches at various bovine ages: potential sites for prion neuroinvasion. Cell Tissue Res. 2007;329:35–44. doi: 10.1007/s00441-007-0396-4. [DOI] [PubMed] [Google Scholar]
- Defaweux V, Dorban G, Demonceau C, et al. Interfaces between dendritic cells, other immune cells, and nerve fibres in mouse Peyer's patches: potential sites for neuroinvasion in prion diseases. Microsc Res Tech. 2005;6:1–9. doi: 10.1002/jemt.20135. [DOI] [PubMed] [Google Scholar]
- Driessen A, Van Ginneken C, Creemers J, et al. Histological and immunohistochemical study of the lymphoid tissue in the normal stomach of the gnotobiotic pig. Virchows Arch. 2002;441:589–598. doi: 10.1007/s00428-002-0651-8. [DOI] [PubMed] [Google Scholar]
- Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- Ekblad E, Bauer AJ. Role of vasoactive intestinal peptide and inflammatory mediators in enteric neuronal plasticity. Neurogastroenterol Motil. 2004;16(Suppl. 1):123–128. doi: 10.1111/j.1743-3150.2004.00487.x. [DOI] [PubMed] [Google Scholar]
- Fagarasan S, Honjo T. Regulation of IgA synthesis at mucosal surfaces. Curr Opin Immunol. 2004;16:277–283. doi: 10.1016/j.coi.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Faldyna M, Samankova P, Leva L, et al. Cross-reactive anti-human monoclonal antibodies as a tool for B-cell identification in dogs and pigs. Vet Immunol Immunopathol. 2007;119:56–62. doi: 10.1016/j.vetimm.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Furness JB. The enteric nervous system: normal functions and enteric neuropathies. Neurogastroenterol Motil. 2008;20(Suppl. 1):32–38. doi: 10.1111/j.1365-2982.2008.01094.x. [DOI] [PubMed] [Google Scholar]
- Gebert A, Pabst R. M cells at locations outside the gut. Semin Immunol. 1999;11:165–170. doi: 10.1006/smim.1999.0172. [DOI] [PubMed] [Google Scholar]
- Gebert A, Rothkötter HJ, Pabst R. Cytokeratin 18 is an M-cell marker in porcine Peyer's patches. Cell Tissue Res. 1994;276:213–221. doi: 10.1007/BF00306106. [DOI] [PubMed] [Google Scholar]
- Green WB, Eaton K, Krakowka S. Porcine gastric mucosa associated lymphoid tissue (MALT): stimulation by colonization with the gastric bacterial pathogen, Helicobacter pylori. Vet Immunol Immunopathol. 1997;56:119–131. doi: 10.1016/s0165-2427(96)05736-4. [DOI] [PubMed] [Google Scholar]
- Haesebrouck F, Pasmans F, Flahou B, et al. Gastric helicobacters in domestic animals and nonhuman primates and their significance for human health. Clin Microbiol Rev. 2009;22:202–223. doi: 10.1128/CMR.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Hiroi T, Nishiyama Y, et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol. 2002;168:57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- Heggebø R, Gonzales L, Press CM, et al. Disease associated PrP in the enteric nervous system of scrapie-affected sheep. J Gen Virol. 2003;84:1327–1338. doi: 10.1099/vir.0.18874-0. [DOI] [PubMed] [Google Scholar]
- Hellemans A, Chiers K, De Bock M, et al. Prevalence of ‘Candidatus Helicobacter suis’ in pigs of different ages. Vet Rec. 2007;161:189–192. doi: 10.1136/vr.161.6.189. [DOI] [PubMed] [Google Scholar]
- Hitotsumatsu O, Hamada H, Naganuma M, et al. Identification and characterization of novel gut-associated lymphoid tissues in rat small intestine. J Gastroenterol. 2005;40:956–963. doi: 10.1007/s00535-005-1679-8. [DOI] [PubMed] [Google Scholar]
- Hondo T, Kanaya T, Takakura I, et al. Cytokeratin 18 is a specific marker of bovine intestinal M cell. Am J Physiol Gastrointest Liver Physiol. 2011;300:G442–G453. doi: 10.1152/ajpgi.00345.2010. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Saito H, Suzuki K, et al. New gut associated lymphoid tissue ‘cryptopatches’ breed murine intestinal intraepithelial T cell precursors. Immunol Res. 1999;20:243–250. doi: 10.1007/BF02790407. [DOI] [PubMed] [Google Scholar]
- Kaleczyc J, Podlasz P, Winnicka A, et al. Characterization of autonomic nerve markers and lymphocyte subsets in the ileal Peyer's patch of pigs infected experimentally with Brachyspira hyodysenteriae. J Comp Pathol. 2010;143:248–257. doi: 10.1016/j.jcpa.2010.04.003. [DOI] [PubMed] [Google Scholar]
- van Keulen LJ, Vromans ME, van Zijderveld FG. Early and late pathogenesis of natural scrapie infection in sheep. APMIS. 2002;110:23–32. doi: 10.1034/j.1600-0463.2002.100104.x. [DOI] [PubMed] [Google Scholar]
- Koga T, Shimada Y, Sato K, et al. Experimental Helicobacter pylori gastric infection in miniature pigs. J Med Microbiol. 2002;51:238–246. doi: 10.1099/0022-1317-51-3-238. [DOI] [PubMed] [Google Scholar]
- Krakowka S, Eaton KA. Helicobacter pylori-specific immunoglobulin synthesis in gnotobiotic piglets: evidence for the induction of mucosal immunity in the stomach. Vet Immunol Immunopathol. 2002;88:173–182. doi: 10.1016/s0165-2427(02)00164-2. [DOI] [PubMed] [Google Scholar]
- Krakowka S, Morgan DR, Kraft WG, et al. Establishment of gastric Campylobacter pylori infection in the neonatal gnotobiotic piglet. Infect Immun. 1987;55:2789–2796. doi: 10.1128/iai.55.11.2789-2796.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer HJ, Kühnel W. Topography of the enteric nervous system in Peyer's patches of the porcine small intestine. Cell Tissue Res. 1993;272:267–272. doi: 10.1007/BF00302732. [DOI] [PubMed] [Google Scholar]
- Kulkarni-Narla A, Beitz AJ, Brown DR. Catecholaminergic, cholinergic and peptidergic innervation of gut-associated lymphoid tissue in porcine jejunum and ileum. Cell Tissue Res. 1999;298:275–286. doi: 10.1007/s004419900096. [DOI] [PubMed] [Google Scholar]
- Kyd JM, Cripps AW. Functional differences between M cells and enterocytes in sampling luminal antigens. Vaccine. 2008;26:6221–6224. doi: 10.1016/j.vaccine.2008.09.061. [DOI] [PubMed] [Google Scholar]
- Lalatta-Costerbosa G, Mazzoni M, Clavenzani P, et al. NOS-immunoreactivity and NADPH-d histochemistry in the enteric nervous system of Sarda breed sheep with different PrP genotypes in wholemount and cryostat preparations. J Histochem Cytochem. 2007;55:387–401. doi: 10.1369/jhc.6A7052.2007. [DOI] [PubMed] [Google Scholar]
- Liebler-Tenorio EM, Pabst R. MALT structure and function in farm animals. Vet Res. 2006;37:257–280. doi: 10.1051/vetres:2006001. [DOI] [PubMed] [Google Scholar]
- Lorenz RG, Chaplin DD, McDonald KG, et al. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin beta receptor, and TNF receptor I function. J Immunol. 2003;170:5475–5482. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
- Lorenz RG, Newberry RD. Isolated lymphoid follicles can function as sites for induction of mucosal immune responses. Ann N Y Acad Sci. 2004;1029:44–57. doi: 10.1196/annals.1309.006. [DOI] [PubMed] [Google Scholar]
- Lügering A, Kucharzik T. Induction of intestinal lymphoid tissue: the role of cryptopatches. Ann N Y Acad Sci. 2006;1072:210–217. doi: 10.1196/annals.1326.015. [DOI] [PubMed] [Google Scholar]
- Ma B, von Wasielewski R, Lindenmaier W, et al. Immmunohistochemical study of the blood and lymphatic vasculature and the innervation of mouse gut and gut-associated lymphoid tissue. Anat Histol Embryol. 2007;36:62–74. doi: 10.1111/j.1439-0264.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- Mason DY, Cordell J, Brown M, et al. Detection of T cells in paraffin wax-embedded tissue using antibodies against a peptide sequence from the CD3 antigen. J Clin Pathol. 1989;42:1194–1200. doi: 10.1136/jcp.42.11.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer G, Brooks A. Lymphopoiesis in lymphocyte-filled villi in the small intestine of the rat. Clin Immunol Immunopathol. 1995;76:S55. [Google Scholar]
- Mayrhofer G, Moghaddami M, Murphy C. Lymphocyte-filled villi (LFV): non-classical organized lymphoid tissues in the mucosa of the small intestine. Mucosal Immunol Update. 1999;7:9–13. [Google Scholar]
- Mazzoni M, Le Gall M, De Filippi S, et al. Supplemental sodium butyrate stimulates different gastric cells in weaned pigs. J Nutr. 2008;138:1426–1431. doi: 10.1093/jn/138.8.1426. [DOI] [PubMed] [Google Scholar]
- McManus JFA, Mowry RW. Staining Methods; Histologic and Histochemical. New York: Hoeber; 1960. [Google Scholar]
- Mendes EN, Queiroz DM, Rocha GA, et al. Histopathological study of porcine gastric mucosa with and without a spiral bacterium (‘Gastrospirillum suis’) J Med Microbiol. 1991;35:345–348. doi: 10.1099/00222615-35-6-345. [DOI] [PubMed] [Google Scholar]
- Mignini F, Streccioni V, Amenta F. Autonomic innervation of immune organs and neuroimmune modulation. Auton Autacoid Pharmacol. 2003;23:1–25. doi: 10.1046/j.1474-8673.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- Moghaddami M, Cummins A, Mayrhofer G. Lymphocyte-filled villi: comparison with other lymphoid aggregations in the mucosa of the human small intestine. Gastroenterology. 1998;115:1414–1425. doi: 10.1016/s0016-5085(98)70020-4. [DOI] [PubMed] [Google Scholar]
- Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- Newberry RD. Intestinal lymphoid tissues: is variety an asset or a liability? Curr Opin Gastroenterol. 2008;24:121–128. doi: 10.1097/MOG.0b013e3282f4906d. [DOI] [PubMed] [Google Scholar]
- Newberry RD, Lorenz RG. Organizing a mucosal defense. Immunol Rev. 2005;206:6–21. doi: 10.1111/j.0105-2896.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- Ottaway CA, Lewis DL, Asa SL. Vasoactive intestinal peptide-containing nerves in Peyer's patches. Brain Behav Immun. 1987;1:148–158. doi: 10.1016/0889-1591(87)90017-1. [DOI] [PubMed] [Google Scholar]
- Pabst O, Herbrand H, Worbs T, et al. Cryptopatches and isolated lymphoid follicles: dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur J Immunol. 2005;35:98–107. doi: 10.1002/eji.200425432. [DOI] [PubMed] [Google Scholar]
- Park JH, Seok SH, Cho SA, et al. The high prevalence of Helicobacter sp. in porcine pyloric mucosa and its histopathological and molecular characteristics. Vet Microbiol. 2004;104:219–225. doi: 10.1016/j.vetmic.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Pérez J, García PM, Bautista MJ, et al. Immunohistochemical characterization of tumor cells and inflammatory infiltrate associated with cutaneous melanocytic tumors of Duroc and Iberian swine. Vet Pathol. 2002;39:445–451. doi: 10.1354/vp.39-4-445. [DOI] [PubMed] [Google Scholar]
- Pidsudko Z, Kaleczyc J, Wasowicz K, et al. Distribution and chemical coding of intramural neurons in the porcine ileum during proliferative enteropathy. J Comp Pathol. 2008;138:23–31. doi: 10.1016/j.jcpa.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Poutahidis T, Tsangaris T, Kanakoudis G, et al. Helicobacter pylori-induced gastritis in experimentally infected conventional piglets. Vet Pathol. 2001;38:667–678. doi: 10.1354/vp.38-6-667. [DOI] [PubMed] [Google Scholar]
- Rothkötter HJ. Anatomical particularities of the porcine immune system – a physician's view. Dev Comp Immunol. 2009;33:267–272. doi: 10.1016/j.dci.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Saito H, Kanamori Y, Takemori T, et al. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science. 1998;280:275–278. doi: 10.1126/science.280.5361.275. [DOI] [PubMed] [Google Scholar]
- Schemann M, Rohn M, Michel K. Motor control of the stomach. Eur Rev Med Pharmacol Sci. 2008;12(Suppl. 1):41–51. [PubMed] [Google Scholar]
- Schierack P, Nordhoff M, Pollmann M, et al. Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochem Cell Biol. 2006;125:293–305. doi: 10.1007/s00418-005-0067-z. [DOI] [PubMed] [Google Scholar]
- Tanimoto T, Ohtsuki Y. Evaluation of antibodies reactive with porcine lymphocytes and lymphoma cells in formalin-fixed, paraffin-embedded, antigen-retrieved tissue sections. Am J Vet Res. 1996;57:853–859. [PubMed] [Google Scholar]
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejter M, Brelinska R, Warchol JB, et al. Effects of galanin on proliferation and apoptosis of immature rat thymocytes. Int J Mol Med. 2002;10:183–186. [PubMed] [Google Scholar]
- Valpotić H, Kovšca Janjatović A, Lacković G, et al. Increased number of intestinal M cells in levamisole-penetrated weaned pigs experimentally infected with F4ac+ enterotoxigenic Escherichia coli strain. Eur J Histochem. 2010;54:88–91. doi: 10.4081/ejh.2010.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulchanova L, Casey MA, Crabb GW, et al. Anatomical evidence for enteric neuroimmune interactions in Peyer's patches. J Neuroimmunol. 2007;185:64–74. doi: 10.1016/j.jneuroim.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


