Abstract
A range of ionic currents have been suggested to be involved in distinct aspects of epileptogenesis. Based on pharmacological and genetic studies, potassium currents have been implicated, in particular the transient A-type potassium current (KA). Epileptogenic activity comprises a rich repertoire of characteristics, one of which is synchronized activity of principal cells as revealed by occurrences of for instance fast ripples. Synchronized activity of this kind is particularly efficient in driving target cells into spiking. In the recipient cell, this synchronized input generates large brief compound EPSPs. The fast activation and inactivation of KA lead us to hypothesize a potential role in suppression of such EPSPs. In this work, using computational modeling, we have studied the activation of KA by synaptic inputs of different levels of synchronicity. We find that KA participates particularly in suppressing inputs of high synchronicity. We also show that the selective suppression stems from the current's ability to become activated by potentials with high slopes. We further show that KA suppresses input mimicing the activity of a fast ripple. Finally, we show that the degree of selectivity of KA can be modified by changes to its kinetic parameters, changes of the type that are produced by the modulatory action of KChIPs and DPPs. We suggest that the wealth of modulators affecting KA might be explained by a need to control cellular excitability in general and suppression of responses to synchronicity in particular. We also suggest that compounds changing KA-kinetics may be used to pharmacologically improve epileptic status.
Keywords: epileptogenesis, fast ripples, synchronicity, dendritic potentials, transient A-type potassium current, Kv4.2
Introduction
In epileptogenesis, a range of ionic currents have been implicated, among these potassium currents (Timofeev et al., 2004; Birnbaum et al., 2004). For instance, it is well known that application of the K-channel blocker 4-AP induces seizures (Voskuyl and Albus, 1985), but as glutamate also is released the interpretation is complicated. However, more specific support that in particular the transient A-type potassium current (KA) may be involved comes from studies where seizures were induced by application of the selective KA blocker scorpion toxin PiTx-Kα (Juhng et al., 1999). Furthermore, in the methylazoxymethanol model of cortical malformations and epilepsy (Castro et al., 2001), the hippocampal heterotopia showed loss of the KA-current. Moreover, the heterotopia also lacked expression of functional Kv4.2 potassium channels, which is one of the genes coding for KA currents in brain (Yuan et al., 2005, Chen et al., 2006). Finally, temporal lobe epilepsy has been reported in a patient with a genetic Kv4.2 truncation mutation (Singh et al., 2006).
KA has fast activation and relatively fast inactivation. As viewed from the soma, it belongs to the threshold currents activated near the spiking threshold and in this respect it produces the well known delay of an action potential. KA is present in pyramidal cell dendrites (Hoffman et al., 1997; Korngren and Sakmann, 2000), where it affects the backward spread of action potentials, as well as integration of EPSPs (Hoffman et al. 1997, Ramakers and Storm 2002, Kim et al. 2007). Importantly, it also has the property to suppress rapid large compound EPSPs (Urban et al., 1998). Such EPSPs may be present during fast ripples. Fast ripples are discrete high frequency oscillations (HFOs) in the range 200-600 Hz, which have been recorded in human mesial temporal epilepsy (Bragin et al., 1990), and will be further discussed below. Epileptogenic activity, and in particular fast ripples are characterized by spiking at high synchronicity levels (Bragin et al. 2000, 2002, 2005, Lasztóczi et al. 2004). Such activity will at target cells produce synchronous compound EPSPs characterized by fast rise times, large amplitudes and short duration. We hypothesized that the properties of KA, with its fast activation, would be suitable to suppress this voltage profile more than one from less synchronous input. Less synchronized activity would due to its higher DC-level and smaller and slower EPSP-fluctuations also trigger the relatively slower KA inactivation and thereby prevent the current from suppressing the EPSP. In vivo, action potentials most commonly arise from brief dendritic depolarizations reflecting synchronous synaptic inputs (Azouz and Gray, 1999, 2003). KA may therefore reduce how effective synchroneous input is in generating action potentials in a neuron. Availability and integrity of dendritic KA currents may thus be an important component regulating recruitment of neurons into seizure activity.
Fast ripples are field oscillations between 200-600 Hz with a broad peak in the power spectrum around e.g. 250 Hz (Engel et al. 2009) or 350 Hz (Dzhala and Staley 2004). These events, recorded from human focal epileptic brain, occur mostly in regions of primary epileptogenesis and rarely in regions of secondary spread (Jirsch et al., 2006). The phenomena is suggested to depend on simultaneous onset of activity in excitatory synaptic circuits (Bragin et al. 2000, Grenier et al., 2003, Dzhala and Staley 2004). It is evident that principal neurons can not sustain firing at these high rates, as found by Lasztóczi et al. (2004) and disussed by Foffani et al. (2007) and one suggestion has been that there may exist populations of neurons firing at lower subharmonics and that the populations alternate and thereby together generate this high frequency (Staley 2007). Moreover, the frequency of fast ripples may not be the main factor distinguishing a fast ripple from e.g. a ripple (Engel et al. 2009) and frequencies of fast ripples may reach into the interval commonly attributed to ripples. Furthermore, increases in synchronicity appear to precede increases in frequency (e.g. transitions into fast ripples) shortly before onset of epileptogenic activity (Lasztóczi et al. 2004). Moreover, neurons are more synchronized in fast ripples than in ripples (Lasztóczi et al. 2004, Engel et al. 2009). We have for these reasons focused on the increase in synchronicity and studied whether KA is differentially more activated by fast ripples than ripples. To evaluate whether such a dynamic perspective would work quantitatively, we performed biophysical simulations. It may be noted that we will not deal with the synchronizing mechanism per se, in which inhibition from interneurons have been suggested to play a major role, but investigate the response of a neuron to synchronized input, and thereby the mechanism of recruitment of principal neurons into seizure activity.
Methods
Neuron model
The model used in this work was based on the model by Migliore et al (1999) which was downloaded from the ModelDB (Hines et al., 2004) database http://senselab.med.yale.edu/modeldb. This model has been used by Hoffman, Migliore, Poolos et al. in work on CA1 pyramidal cell dendrite processing, specifically the role of the KA and h-currents in backpropagating action potentials. During initial tests, it was found that the Na-current conductance had to be reduced in the dendritic compartments by 1/8 and enhanced in the soma compartments by 5.6 (giving a relative conductance difference of 45) to ensure that synaptically induced action potentials were induced in the soma and not in the dendrites. This is consistent with combined experimental and computational findings where an approximately 50 times higher Na-channel density at the site of initiation of action potentials has been found (Meeks and Mennerick 2007; Kole et al. 2008). Moreover, to simplify our analysis of dendritic processing, we did not make the distinction as in the original model between distal and proximal characteristics of KA, but used only the distal model throughout the dendrite. In a subsequent sensitivity test where effects of additional currents were analyzed, the influence of another threshold current, the h-current, as well as the influence of a persistent sodium current, were studied. The model had a resting potential of -65mV which well corresponds to values found in vivo (Crochet et al., 2006). The EPSP amplitude in the soma remaining for a synaptic input at 171μm was 41%. This corresponds well to experimental findings of 38% remainder for lacunosum moleculare inputs on CA1 pyramidal cells (Inoue et al., 2001) as well as 78%-29% remainder in CA1 cells for inputs at 100μm and 300μm respectively (Magee, 2000).
Model of synaptic input
CA1 pyramidal cells have several thousand synapses distributed over the dendritic tree. The proportion of neurons, and thus also synapses, that are active at any point in time is generally low, and this is particularly true for hippocampus (Buzsaki et al. 1983, Frank and Brown 2001). Thus, ten synaptic inputs were included in the neuron model, representing the synapses that become activated at a particular period in time in a restricted part of the dendrite. For each synapse, we generated Poisson distributed synaptic events at 12Hz. The simulation was run for 1.5 s leading to 17 synaptic inputs for the case of full synchronicity. Synaptic inputs of kainate/AMPA-type had a rise time constant of 1.5ms and a decay time constant of 2.5ms and were positioned on the apical dendrite 171μm from the soma. Experimentally, EPSP amplitudes as measured in the soma vary greatly and range from 0.13-2.8mV (Markram et al., 1997) and 0-4mV (Thomson et al., 1995). We used inputs generating 3.1mV in the soma which thus were 7.5mV in the dendrite at the point of input (leading to the reduction factor 0.41 discussed above). For the case with KA present, the synaptic inputs were increased 41% to compensate for the hyperpolarizing effect of KA. The different levels of synchronicity were modeled according to Charcos Lloréns and Fransén (2004). Briefly, different inputs were separated with a Poisson distribution within a time window, and the relative size of the window in relation to the base frequency of 12 Hz determines the degree of synchronicity.
Model of fast ripples
Although much is known about the phenomenology of fast ripples, the mechanisms behind them are not known. We therefore modeled them following two different experimental models, one in vivo and one in vitro.
Case 1. The model is based on studies of fast ripples generated in a slice preparation (Dzhala and Staley 2004). To model fast ripple input to the target neuron, the model uses a sequence of ISI intervals from data of spontaneously generated burst-like discharges in individual neurons evoked in high potassium concentration (Dzhala and Staley 2004) with the following consecutive intervals: 3.8, 4.5, 4.9, 6.8, 7.2, 8.6, 9, 10, 10, 10 ms. The corresponding ISI series from spontaneous burst-type intervals for control levels of potassium concentration were: 5, 7, 9, 10, 10, 10, 10, 10, 10, 10ms (Dzhala and Staley 2004). This input was used to represent normal spiking patterns not associated with epileptogenic activity. If KA works selectively to suppress epileptogenic activity, it is not expected to be significanlty activated by this kind of input. A real neuron is assumed to have many such inputs of the type described above spread over the dendritic tree. In the model, ten synaptic inputs were provided at five different locations of the dendritic tree. In the control case the ten synaptic time series were evenly spaced with a time delay of 2.5 ms, leading to the average delay between bursts of 6ms as reported by Dzhala and Staley (2004), see also Supplementary figure 1. For the fast ripple the delay was decreased to 0.15ms, consistent with the observed decrease towards zero delays in high potassium (Dzhala and Staley 2004).
Case 2. Input from a ripple versus input from a fast ripple. Ripples in awake animals range from 80-200 Hz (Ylinen et al. 1995) and are, contrary to fast ripples, associated with normal brain activity. A ripple episode commonly consist of some 4-16 cycles of oscillations (Ylinen et al., 1995, our estimate). A neuron participates (spikes) on average in 11% of the cycles, and at most in 40% (Ylinen et al., 1995). Focusing on the neurons making up the core of a ripple, we have assumed neurons participate in 25% of the cycles and produce a total of 4 spikes each. Assuming individual neurons fire at 50 Hz during the ripple, a 25% involvement gives an ensemble frequency of 100 Hz containing 16 cycles if neurons fire in four groups 90 degrees out of phase. The target neuron was subjected to 50 synaptic inputs, 10 each on 5 different dendrite locations, with the temporal structure just described, see Supplementary figure 2. Moreover, the 10 inputs at a site were evenly spaced by 1.1ms (Ylinen et al. 1995). Fast ripples are more synchronized than ripples (Lasztóczi et al. 2004; Engel et al. 2009). Assuming a four times higher frequency during a fast ripple compared to a rippe (e.g. 400 Hz and 100 Hz resp.) a spacing of 1.1ms in a ripple would correspond to a spacing of 0.27 ms in a fast ripple. Thus, to test the synchronicity aspect of a fast ripple, a “synchronized” ripple was used. In this case, the time lag between the 10 spikes within one cycle of a ripple was reduced to 0.3 ms. We subsequently studied whether KA would be more activated and thereby to a larger extent suppress activity of a fast ripple.
Results
In order to investigate the potential capacity of KA to differentially suppress EPSPs depending on their level of synchronicity, the neuron model was subjected to 11 runs each of a particular level of synchronicity. In table 1 we show the median time between EPSPs as a function of the synchronicity level. We measured the number of spikes produced by the input as a measure of KA suppression. The rationale behind this choice is that, even though we are considering KA as a postsynaptic dendritic excitability factor, network activity mediated by action potentials is the ultimate component of epileptogenic activity. The neuronal transformation of inputs into spiking is therefore a key element.
Table 1. Time between inputs.
| 100% | 90% | 70% | 40% | Independent | |
|---|---|---|---|---|---|
| median | 0 | 0.67 | 2.01 | 2.68 | 6.71 |
| 10% | 0 | 0.096 | 0.29 | 0.57 | 0.96 |
| 90% | 0 | 2.15 | 6.45 | 12.9 | 21.5 |
The table shows time (in ms) between synaptic inputs for a select number of synchronization levels of figure 1. In the table, median refers to the median value in the distribution of input intervals produced, 10% refers to the shortest intervals and 90% covers almost all of the intervals produced.
Differential suppression
The effect on the postsynaptic dendritic membrane for inputs of three different levels of synchronicity in the absence of KA is shown in figure 1A. It can be seen that with decreasing levels of synchronicity, amplitude and rate of rise of the compound EPSP decreases but variability increases. In 1B we show the soma membrane spiking pattern at these levels of synchronicity in the absence of KA. In figure 1C KA is present. The different panels show number of spikes produced and one can infer how many are suppressed at different levels of synchronicity. In figure 1D we summarize the neuron response with and without KA present. Error bars are based on 20 repetitions of the procedure with different starting random seeds. In general, without KA, higher levels of synchronicity generate more spikes, consistent with the general properties of a neuron as discussed in the introduction. But, with KA present, there is a substantial suppression of efficacy of inputs for high degrees of synchronicity in the interval 90-100%. The level 90% correspond to EPSPs separated by a median of 0.7ms and an interval ranging from 0.1ms to 2.2ms (10% and 90% of the events respectively). The particular shape of the curve, particularly of the peak, depends on the synaptic strength, which is further studied in the supplementary material. Thus, as can be seen from figure 1, whereas KA, as a potassium current, will always be suppressive, it has a selective effect on the synchroneous input that otherwise is so powerful in activating a neuron.
Fig 1.
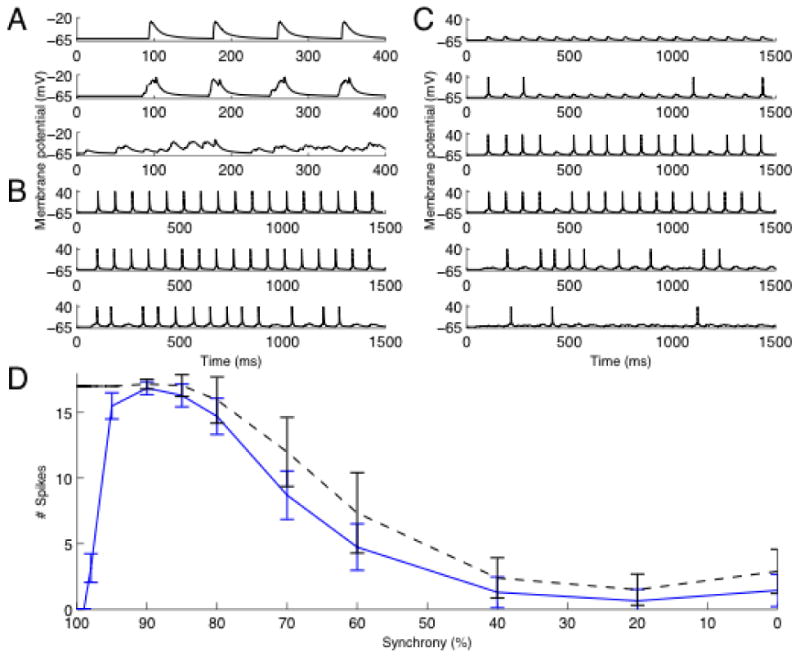
Differential suppression of synchronous input. A. Synaptic input. The figure shows from top the membrane potential at input site with 100%, 90% and independent input respectively. B. The membrane potential in the soma without KA present. The graphs shows from top simulations with 100%, 90% and 70% synchronous input. C. Membrane potential in soma with KA present. The graphs shows from top the simulation with 100%, 98%, 95%, 90%, 70%, and 40 % synchronous input. D. Synchronized input is strongly suppressed by KA. The figure shows the number of spikes produced for different synchronicity levels. The black line represents baseline (control without KA) and the blue line with KA present. Note the pronounced suppression in the interval 100-90%.
KA current amplitude and activation
To further analyze the contribution of KA in this differential suppression, we show in figure 2 the activation, inactivation and current through KA at two different levels of synchronicity. We compare two cases, fully synchronized and 80% level of synchronicity. For comparison, the two instances shown produced the same peak potential, figure 2 (top) around 17ms. As can be seen, KA gives more current (second trace), opens more (third trace) and is less inactivated (bottom trace), at the higher level of synchronicity. Note that this is true even though the case of less synchronized input leads to spiking of the cell (around 18ms). The figure also shows that this is a dynamic effect caused by the interaction between the activation and the inactivation dynamics. More specifically, this is seen by replacing the instantaneous activation and inactivation with their steady-state values (dashed traces) computed using the membrane potential and steady-state activation/inactivation curves (shown in supplementary figure 3). Using steady-state values, differences in kinetics between activation and inactivation are removed. This can be seen most clearly for the relatively slower inactivation (trace D, in particular between 4ms-10ms) which at steady-state balances out activation (trace C) yielding very little current (trace B). Further analysis of the dynamics of KA confirmed that the inactivation is particularly important in the suppression, it must be fast enough but not as fast as the activation. This influence of the inactivation time constant is further studied in relation to modulatory effects on KA described below.
Fig. 2.
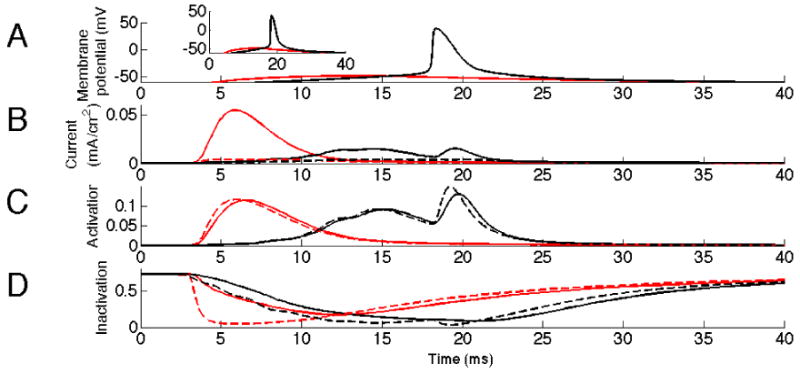
Activation of KA by synchronized versus semi-synchronized input. Synchronous input (100%), in red, activates KA more than semi- synchronous input (80%), in black. The dashed lines represent values of KA steady-state activation and inactivation at the membrane potentials dictated by A. A: Membrane potential in the soma. Inset shows initial slope of EPSP more clearly. B: Current through KA at input site. Note the difference in current around 6 ms. C: Inactivation of KA at input site. The interval 2-10 ms shows that the effect seen in B originates from the dynamics aspects of KA. D: Activation of KA at input site. Note the difference in inactivation around time of input 2-10ms.
Slope of compound EPSP
One major difference between the fully synchronized and the 80% level of synchronicity is the slope (derivative) of the rising phase of the EPSP, inset figure 2 (top) and figure 1A. To further analyze the influence of the derivative on the differential activation of KA, we conducted voltage clamp simulations providing triangular ramps as inputs to the location of the synaptic input, figure 3A. To enable comparisons of cases of different derivatives, we kept the area of the triangular voltages constant as higher potential and longer time always gives more activation and more current. As can be seen in figure 3B, higher derivatives produced higher activation of KA. These differences can be seen both in the amplitude of the current, and in the integral of the current. Thus, in general, inputs of higher synchronicity produce compound EPSPs with higher derivatives, which as shown here activate KA more.
Fig. 3.
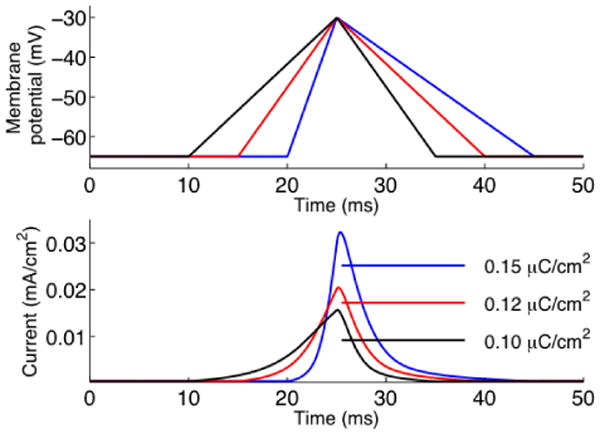
Sensitivity to voltage slope. The top graph shows voltage clamp command of different initial slope but same area. The bottom graph shows the corresponding current through the KA channel. Note the difference in the peak current at 25 ms. The legend indicates the integral of the current for each protocol.
Spatial extent of KA activation
In addition to its sensitivity to the derivative of the voltage, KA as a voltage gated channel, is sensitive to membrane potential. EPSPs propagating in the dendrite decay with distance from the point of synaptic input and we were interested to study the spatial region of activated KA currents. In figure 4A we show the membrane potential at input site. The EPSP appears around the time point 10ms and the backpropagating action potential around 20ms. In figure 4B we show the current of KA at several locations around the point of synaptic input. It can be seen that the peak of KA current is relatively local, about a third of the peak remaining 46μm more proximally, but that points even 100μm away are activated. In the figure, around the time point 20ms, it can also be seen how KA is affected by a backpropagating action potential. Even though the backpropagating action potential amplitude and rate of rise is larger than the EPSP, it is preceded by the EPSP which produces a partial inactivation of KA.
Fig. 4.
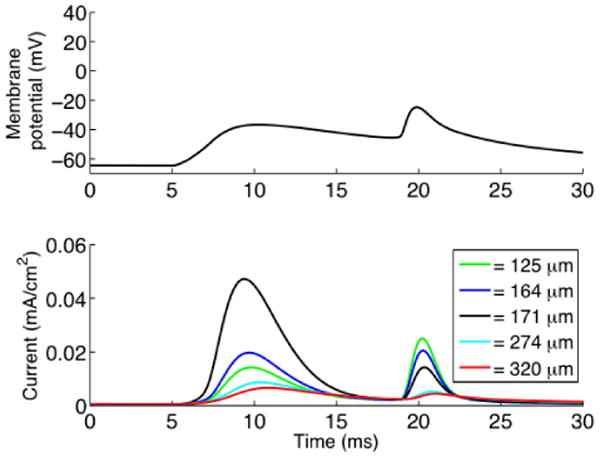
Distance-dependent KA activation. The top graph shows the membrane potential at input site (171μm from soma) with a 92% level of synchronous input. The first event is the EPSP and second is the backpropagating action potential. The bottom graph shows the current through the KA channel. The current was measured at five different locations 125μm, 164μm, 171μm, 247μm and 340μm from the soma.
Selectivity of the suppression of synchronous input
KA is modulated by a range of factors, affecting the conductance of the current as well as its activation and inactivation characteristics, and we will return to this in the discussion. One potential consequence of such changes are changes in the suppression profile of different synchronicity levels. We have studied the effects on the inactivation time constant produced by auxillary subunits of KA, KChIPs and DPPs (Nakamura et al., 2001a; Gutman et al., 2005; Jerng et al. 2004, 2007). In figure 5 we show the effect on suppression by using different KA inactivation time constants. As can be seen in figure 5, both the selectivity of suppression with regard to high levels of synchronicity (interval of high suppression, position of the peak of activation), as well as the degree of suppression of lower levels of synchronicity (level of suppression at independent input, rate of decline at intermediate levels of input) are attenuated. We will return to the modulatory action on inactivation kinetics in our analysis of suppression of fast ripples below. Furthermore, auxillary subunits as well as modulators like PKA and PKC produce shifts in the steady-state activation and to a lesser extent also the inactivation curves (Hoffman and Johnston, 1998; Nakamura et al., 2001a; Gutman et al., 2005; Jerng et al. 2004, 2007). When auxillary subunits produce a hyperpolarizing shift, the produced KA current is increased (data not shown). Conversely, PKA and PKC produce shifts in the depolarizing direction, leading to an effective reduction of KA current and as a consequence a loss of differential suppression (data not shown). The results thus show that the suppressive characteristics of KA may be changed, and we argue potentially may be adjusted to functional demands of the network.
Fig. 5.
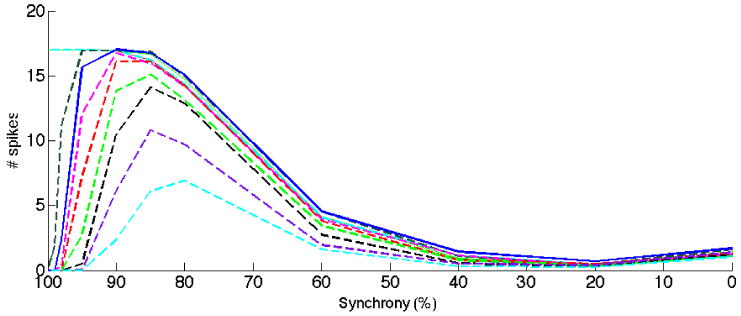
The selectivity to synchronized input varies when KA dynamics is modified. The inactivation time constant was changed representing modulatory influences of e.g. auxillary proteins. As in figure 1, the solid blue curve is the result with standard values of KA. The magenta, red, light green, black purple and cyan curves represent decreasing inactivation time constant in steps of 1ms. Dark green and solid cyan curves represent increasing inactivation time constant in steps of 1ms. Note the significant difference in reduction of synchronized input, effectively covering the interval 95-80%.
Suppression of epileptogenic activity
As discussed in the introduction, experimental observations indicate the appearance of fast ripples at or before the onset of seizures. We have investigated to what extent KA is able to suppress a fast ripple. In the first study, we generated input to a neuron from in vitro data on spontaneous bursts in individual neurons (Dzhala and Staley 2004) generated either in control or high concentrations of potassium, the latter used as a model of epiletogenic activity. Figure 6 shows the result comparing activity produced in control levels of potassium to those of “fast ripples” generated in high levels of potassium, the latter case tested both with and without KA present. As can be seen, KA does not suppress input in the control condition, but it does so for the fast ripple case. The fast ripple case without KA shows that without KA the cell spikes as in the control condition. Thus, a neuron containing KA would to a lesser extent participate in the fast ripple event, and spread of the fast ripple event might therefore be reduced or prevented.
Fig. 6.
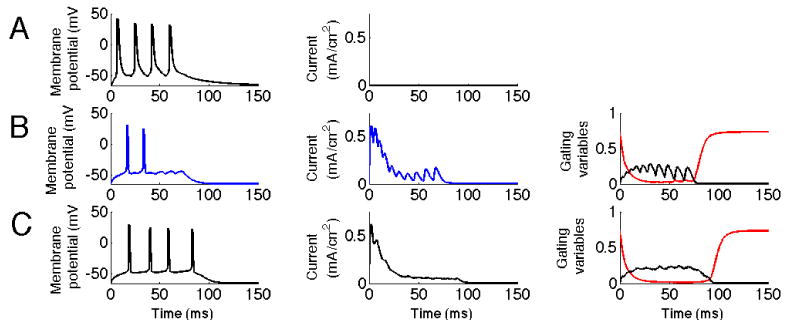
KA reduces response to fast ripple input. The first column represents the membrane potential of the soma. The second column represents the current through the KA channel measured at the location of the synaptic input. The third column represents the gating variables of the KA channel. The black line represents activation and the red line inactivation. A. Fast ripple with no KA present. Input represents higher synchronicity levels present during high potassium concentration conditions. B. Fast ripple with KA present. Note the reduction in number of action potentials generated due to the increase in KA current. The plot of gating variables show that this input leads to a larger degree of activation, particularly at its peaks and to a lesser degree of inactivation, particularly for later inputs. C. Control input representing lower synchronicity present in control concentration of potassium.
In the second case, we started from a model based on in vivo data on ripples (Ylinen et al. 1995). From this we generated fast ripples by increasing the synchronicity, consistent with the observation by Lasztóczi et al. (2004) and Engel et al. (2009). We tested both input at a frequency of 50 Hz and of 100 Hz, effectively representing oscillations of higher harmonics like 100 and 200 Hz as suggested by Staley (2007) and discussed in the introduction. As can be seen in figure 7, the control case with input replicating a ripple does not get suppressed, but input from the more synchronized fast ripple is suppressed if KA is present. These results are also consistent with the findings of figure 1 showing differential suppression for comparable spike ISIs in the window 90% (no suppression)-100 % (total suppression). We were also interested to study the modulatory influence by e.g. KChIPs and DPPs on the suppression of fast ripples found. In Table 2 we summarize our findings. Enhanced modulation (reduced inactivation time constant) leads to a reduced current as the inactivation kinetics approaches that of activation (as discussed in figure 2) and thereby to reduced suppression of fast ripples. Decreased modulation on the other hand leads to increased current and as a result suppression that also affects ripple activity.
Fig. 7.
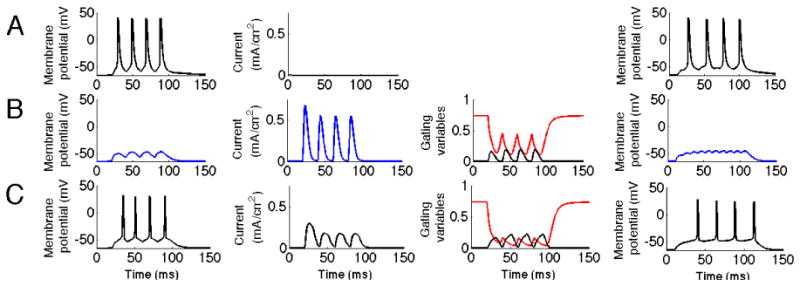
KA reduces response to fast ripple input. The three first columns correspond to the simulation when the input frequency was 50 Hz and the fourth column a case with 100Hz input. The first column represents the membrane potential of the soma. The second column represents the current through the KA channel measured at the location of the synaptic input. The third column represents the gating variables of the KA channel. The black line represents the activation and the red line inactivation.A. Fast ripple input with no KA present. B. Fast ripple input with KA present. Note the significant reduction in spike activity. It can be seen that the inactivation is lower than in the control case C, particularly in its troughts. C. Ripple input. KA does not suppress input representing ripple activity.
Table 2. Effects on fast ripples from changes in inactivation kinetics.
| 50Hz | 100Hz | |||||
|---|---|---|---|---|---|---|
| - | c | + | - | c | + | |
| Without KA | 4 | 4 | 4 | 4 | 4 | 4 |
| Fast ripple input | 0 | 0 | 3 | 0 | 0 | 3 |
| Ripple input | 3 | 4 | 4 | 2 | 4 | 4 |
Number of spikes produced following fast ripple input (case 2). c control, standard value of the inactivation time constant i.e. same data as in figure 7 first and fourth column, + enhanced modulation by reducing the inactivation time constant by 4 ms representing enhanced KChIP/DPP modulation, - reduced modulation by increasing the inactivation time constant by 4 ms representing reduced KChIP/DPP modulation.
As mentioned, the genesis of fast ripple events is not known. If a group of neurons would receive several inputs within a small window of time (due to random fluctuations or some particular process), it is conceivable that they might spike and that the synchroneous activity thereby could spread to other neurons. We investigated whether KA would oppose such a single synchroneous input. Analyzing data from figure 1 at the synchronicity level 98%, the probability of the first spike of the input is 0.15, the probability of a spike following the second input is 0.35 and for the third it is 0.3. Thus, the first input is more suppressed than the following two. Thus, KA is not only able to suppress trains of inputs, but also single ones. KA may therefore also be part in preventing the initiation of fast ripples and seizures.
Discussion
In this work, we have shown that the KA current selectively suppresses synchronized inputs. This property may be particularly important in the context of epiletogenesis, where synchronized activity e.g. in the form of fast ripples is observed at the onset of seizure activity. Preventing the spread of highly synchronized input may be an important mechanism in the control of seizures.
Neocortical pathological ripples
We have here used a model of a CA1 pyramidal cell. However, KA is also present in neocortical pyramidal cells (Korngreen et al., 2000; Schaefer et al., 2003; Yuan et al., 2005). Moreover, although there are differences between the pathological high frequency ripples observed in neocortex commonly denoted “neocortical ripples” compared to those observed in hippocampal strucures termed “fast ripples”, there are also commonalities. Grenier et al. (2003) present evidence that neocortical ripples occur at the time of transition to ictal events. There is furthermore a strong correlation between neuronal excitation and the intensity of neocortical ripples (Grenier et al., 2003). More specifically, neocortical ripples during seizures involve coordinated action potentials in a majority of neurons synchronized on the millisecond scale (Grenier et al., 2003). Our results based on hippocampal fast ripples may therefore be of more general nature.
Separate roles for inhibition and KA
In this work the focus is on the spread of synchroneous activity among excitatory neurons. We have thus studied the response of a neuron to synchronized input and not dealt with the synchronizing mechanism per se, in which inhibition from interneurons has been suggested to play a major role (Maglóczky and Freund 2005). Moreover, activity of interneurons may also play a minor role in the generation of ripples and fast ripples. More specifically, during ripples, interneurons were reported to fire at higher frequencies than that of the ripples, and there was no clear modulation of firing by ripples (Grenier et al., 2003), eg the interneurons are not directly pacing the ripples. On the other hand, neocortical ripples during nonseizure states depend on IPSPs (Grenier et al., 2003). Thus, whereas inhibition from interneurons may play a major role during seizures, synchronized activity of principal cells may be more important for the initial spread of activity.
Synchronization in non-rhythmic events
Synchronous activity is not only present during oscillations, but may also be present during non-rhythmic events relevant for epilepsy. Large field potential spikes, for instance, reflect epileptic network activity (Ulbert et al., 2004). Comparisons of human intracranial EEG recordings and spontaneous events in in vitro slice preparations taken from the same patient show they have spike and waves events resembling each other (Cohen et al 2002). In these slices, intracellular recordings show high degrees of synchronization during the spike and wave events. Thus, in both rhythmic and non-rhythmic events, synchronized neuronal firing is present and this synchronous input may be particularly effective in recruiting additional neurons.
Amplitude of compound EPSP
Our results show that the differential suppressive effect of KA increases with increasing EPSP amplitude. Compound EPSPs generated by multiple inputs in concert may indeed reach large amplitudes. For instance, amplitudes of sharp waves of 8mV have been recorded intracellularly (Maier et al., 2003). In pathological circuits comprising the start of epileptic activities, synaptic efficacies may be even larger. KA may therefore be particularly important in preventing hyperexcitability under those circumstances.
Role of modulators of KA
A number of different substances modulate KA, for instance PKA (Hoffman and Johnston, 1998), PKC (Hoffman and Johnston, 1998), MAPK (Yuan et al., 2002), arachidonic acid (Colbert et al., 1999; Ramakers and Storm, 2002), adenyl cyclase activating peptide (Han et al., 2005; 2006) and frequentin (Nakamura et al., 2001b), for a review, see Jerng et al. (2004). Furthermore, potassium channels colocalize with membrane proteins, notably the Kv channel-interacting proteins (KChIP) and dipeptidyl peptidase-like proteins (DPP), e.g. DPP6 and DPP10 (for a review, see Jerng et al. 2004). The presence of KChIPs and DPPs in neurons enhances the surface expression of KA. Moreover, it has been shown that KChIPs produce substantial changes in KA characteristics, e.g. shifting the steady-state activation and inactivation curves 10′s of millivolts as well as changing inactivation kinetics (Nakamura et al., 2001a; Gutman et al., 2005; Jerng et al. 2007). Moreover, DPPs produce a hyperpolarizing shift of steady-state curves and a more rapid inactivation kinetics (Jerng et al. 2007). There may be several reasons for this complex regulation of KA. 1) General excitability, the control of the threshold for action potential generation, latency of spike, or number of spikes produced by somatic input. 2) Backpropagation of action potentials, specifically a control of spread of backpropagating action potentials. 3) Interaction with incoming EPSPs and potentially synaptic plasticity. 4) Modulation of efficacy of synchronized input, in particular, modulating the degree of specificity of this suppression, as shown in figure 5. Our results also suggest that effects from PKA and PKC would work in the detrimental direction whereas KChIPs and DPPs would enhance the suppressive effects of KA on hypersynchroneous activity.
A vicious circle involving KA
We have in this work discussed how reduction of KA may be a risk factor in epilepsy. However, the opposite may also be true. In hippocampal tissue after pilocarpine induced seizures, CA1 pyramidal neuron dendrites showed decreased availability of the A-type potassium channel due to transcriptional and posttranslational processes (Bernard et al., 2004). Furthermore, after convulsant drug pentylenetetrazole treatment (Tsaur et al., 1992; Francis et al., 1997), Kv1.2 and Kv4.2 mRNAs were reduced. Thus, there seems to be a bidirectional relationship between seizures on the one hand and expression and electrophysiological properties of KA on the other, and thus a possibility of a vicious circle.
Anticonvulsants and KA
Preventing or reversing the vicious circle of KA run-down may be a key objective in treatment of seizures. Indeed, several anticonvulsants have been shown to enhance KA currents; lamotrigine (Zona et al., 2002, but see Huang et al. 2004), valproate (Walden et al., 1993) and carbamazepine (Zona et al., 1990). Conversely, GABAB activation reduces potassium currents by shifting their activation in the positive direction (Saint et al., 1990). This interaction could work via the suppressive effect of AA on KA (Ramakers and Storm 2002) activated by phospholipase A2 activation downstream of GABAB. This suppression of KA could be one explanation for less good results of GABA enhancing compounds on some patients. GABAB activation could on the one hand lead to a beneficial direct hyperpolarization of the neuron, but on the other hand also an indirect enhancement of temporal summation capacity and an increased sensitivity to synchroneous activation. In this work, we have investigated the potential link between KA and neural responsiveness to synchroneous synaptic input. Based on the results in this work, the prediction would be a decrease in synchronicity following administration of these compounds. Indeed, lamotrigine, which enhances KA (Zona et al. 2002) reduces population spikes (Langosch et al., 2000) and population spikes have been suggested to be a sign of neuronal synchrony (Andersen et al., 1971). Furthermore interictal spikes are reduced by lamotrigine (Marciani et al., 1996) and the addition of lamotrigine decreases EEG ictal and interictal abnormalities (Akman et al., 2003). A second antiepilepticum, valproate, has been shown to reduce the frequency of extracellularly recorded spontaneous interictal bursts (Albus et al., 1998).
Properties of potassium current enhancers
Observations in epileptic patients of increased excitability due to compromized potassium currents have lead to the recent development of potassium current enhancers (Retigabine and Flupirtine enhancing the M-type (KCNQ/Kv7) channel, Nicorandil enhancing an adenosine triphosphate sensitive potassium (K+ATP) channel and Chlorzoxazone as an opener of a calcium sensitive potassium current), and with their anti-epileptic properties evaluated in Kobayashi et al. (2008). However, K-current enhancing drugs may not always give the desired effect. Resting membrane potential changes induced by a K-current enhancer may be counteracted by homeostatic mechanisms (Marder and Goaillard, 2006) either reducing other K-currents or enhancing depolarizing currents. Likewise, influences on average firing frequency may be balanced out in the long run. Based on the work presented here, pharmacological interventions that affect the neurons response to highly synchronized inputs but less so for intermediate or unsynchronized inputs are suggested.
Supplementary Material
Acknowledgments
This work was supported by Swedish VR 621-2007-4223 and NIH MH061492-06A2. We wish to thank Asad Rustum and Vicente Charcos Lloréns for their work during their masters thesis projects.
References
- Albus H, Williamson R. Electrophysiologic analysis of the actions of valproate on pyramidal neurons in the rat hippocampal slice. Epilepsia. 1998;39:124–39. doi: 10.1111/j.1528-1157.1998.tb01350.x. [DOI] [PubMed] [Google Scholar]
- Andersen P, Bliss TVP, Skrede KK. Unit analysis of hippocampal population spikes. Exp Brain Res. 1971;13:208–221. doi: 10.1007/BF00234086. [DOI] [PubMed] [Google Scholar]
- Azouz R, Gray CM. Cellular mechanisms contributing to response variability of cortical neurons in vivo. J Neurosci. 1999;19:2209–23. doi: 10.1523/JNEUROSCI.19-06-02209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouz R, Gray CM. Adaptive coincidence detection and dynamic gain control in visual cortical neurons in vivo. Neuron. 2003;37:513–23. doi: 10.1016/s0896-6273(02)01186-8. [DOI] [PubMed] [Google Scholar]
- Bernard C, Anderson A, Becker A, Poolos NP, Beck H, Johnston D. Acquired dendritic channelopathy in temporal lobe epilepsy. Science. 2004;305:532–5. doi: 10.1126/science.1097065. [DOI] [PubMed] [Google Scholar]
- Bernard C, Poolos NP, Johnston D. A-type K channel control of dendritic excitability in experimental epilepsy. Soc Neurosci Abstr. 2001;27:559.1. [Google Scholar]
- Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA. Structure and function of Kv4-family transient potassium channels. Physiol Rev. 2004;84:803–33. doi: 10.1152/physrev.00039.2003. [DOI] [PubMed] [Google Scholar]
- Bragin A, Azizyan A, Almajano J, Wilson CL, Engel J., Jr Analysis of chronic seizure onsets after intrahippocampal kainic acid injection in freely moving rats. Epilepsia. 2005;46:1592–8. doi: 10.1111/j.1528-1167.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100-500 Hz) in human epileptic brain and in kainic acid--treated rats with chronic seizures. Epilepsia. 1999;40:127–37. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Mody I, Wilson CL, Engel J., Jr Local generation of fast ripples in epileptic brain. J Neurosci. 2002;22:2012–21. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Engel J., Jr Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: A hypothesis. Epilepsia. 2000;41 6:S144–52. doi: 10.1111/j.1528-1157.2000.tb01573.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Staba RJ, Reddick M, Fried I, Engel J., Jr Interictal high-frequency oscillations (80-500 Hz) in the human epileptic brain: entorhinal cortex. Ann Neurol. 2002;52:407–15. doi: 10.1002/ana.10291. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Lai-Wo L, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Research Reviews. 1983;6:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Cai X, Liang CW, Muralidharan S, Kao JP, Tang CM, Thompson SM. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron. 2004;44:351–64. doi: 10.1016/j.neuron.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Castro PA, Cooper EC, Lowenstein DH, Baraban SC. Hippocampal heterotopia lack functional Kv4.2 potassium channels in the methylazoxymethanol model of cortical malformations and epilepsy. J Neurosci. 2001;21:6626–34. doi: 10.1523/JNEUROSCI.21-17-06626.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 2006;26:12143–51. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akman çigdem Inan, Holmes Gregory L. The effect of lamotrigine on the EEGs of children and adolescents with epilepsy. Epilepsy and Behavior. 2003;4:420–423. doi: 10.1016/s1525-5050(03)00142-2. [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–21. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- Colbert CM, Pan E. Arachidonic acid reciprocally alters the availability of transient and sustained dendritic K(+) channels in hippocampal CA1 pyramidal neurons. J Neurosci. 1999;19:8163–71. doi: 10.1523/JNEUROSCI.19-19-08163.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Carr DB, Ulrich S, Ilijic E, Tkatch T, Surmeier DJ. Dendritic excitability of mouse frontal cortex pyramidal neurons is shaped by the interaction among HCN, Kir2, and Kleak channels. J Neurosci. 2005;25:8776–87. doi: 10.1523/JNEUROSCI.2650-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhala VI, Staley KJ. Mechanisms of fast ripples in the hippocampus. J Neurosci. 2004;24:8896–906. doi: 10.1523/JNEUROSCI.3112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Jr, Bragin A, Staba R, Mody I. High-frequency oscillations: What is normal and what is not? Epilepsia. 2009 doi: 10.1111/j.1528-1167.2008.01917.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Foffani G, Uzcategui YG, Gal B, Menendez de la Prida L. Reduced spike-timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron. 2007;55:930–41. doi: 10.1016/j.neuron.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Francis J, Jugloff DG, Mingo NS, Wallace MC, Jones OT, Burnham WM, Eubanks JH. Kainic acid-induced generalized seizures alter the regional hippocampal expression of the rat Kv4.2 potassium channel gene. Neurosci Lett. 1997;232:91–4. doi: 10.1016/s0304-3940(97)00593-4. [DOI] [PubMed] [Google Scholar]
- Frank LM, Brown EN, Wilson MA. A comparison of the firing properties of putative excitatory and inhibitory neurons from CA1 and the entorhinal cortex. J Neurophysiol. 2001;86:2029–40. doi: 10.1152/jn.2001.86.4.2029. [DOI] [PubMed] [Google Scholar]
- Gasior M, French A, Joy MT, Tang RS, Hartman AL, Rogawski MA. The anticonvulsant activity of acetone, the major ketone body in the ketogenic diet, is not dependent on its metabolites acetol, 1,2-propanediol, methylglyoxal, or pyruvic acid. Epilepsia. 2007;48:793–800. doi: 10.1111/j.1528-1167.2007.01026.x. [DOI] [PubMed] [Google Scholar]
- Graham BP. Pattern recognition in a compartmental model of a CA1 pyramidal neuron. Network: Computation in Neural Systems. 2001;12:473–492. [PubMed] [Google Scholar]
- Grenier F, Timofeev I, Steriade M. Neocortical very fast oscillations (ripples, 80-200 Hz) during seizures: intracellular correlates. J Neurophysiol. 2003;89:841–52. doi: 10.1152/jn.00420.2002. [DOI] [PubMed] [Google Scholar]
- Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stuhmer W, Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- Han P, Lucero MT. Pituitary adenylate cyclase activating polypeptide reduces A-type K+ currents and caspase activity in cultured adult mouse olfactory neurons. Neuroscience. 2005;134:745–56. doi: 10.1016/j.neuroscience.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Han P, Lucero MT. Pituitary adenylate cyclase activating polypeptide reduces expression of Kv1.4 and Kv4.2 subunits underlying A-type K(+) current in adult mouse olfactory neuroepithelia. Neuroscience. 2006;138:411–9. doi: 10.1016/j.neuroscience.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Hines ML, Morse T, Migliore M, Carnevale NT, Shepherd GM. ModelDB: A Database to Support Computational Neuroscience. J Comput Neurosci. 2004;17:7–11. doi: 10.1023/B:JCNS.0000023869.22017.2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Johnston D. Downregulation of transient K+ channels in dendrites of hippocampal CA1pyramidal neurons by activation of PKA and PKC. J Neurosci. 1998;18:3521–8. doi: 10.1523/JNEUROSCI.18-10-03521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–75. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Horimoto N, Nabekura J, Ogawa T. Arachidonic acid activation of potassium channels in rat visual cortex neurons. Neuroscience. 1997;77:661–71. doi: 10.1016/s0306-4522(96)00490-3. [DOI] [PubMed] [Google Scholar]
- Huang CW, Huang CC, Liu YC, Wu SN. Inhibitory effect of lamotrigine on A-type potassium current in hippocampal neuron-derived H19-7 cells. Epilepsia. 2004;45:729–36. doi: 10.1111/j.0013-9580.2004.58403.x. [DOI] [PubMed] [Google Scholar]
- Inoue M, Hashimoto Y, Kudo Y, Miyakawa H. Dendritic attenuation of synaptic potentials in the CA1 region of rat hippocampal slices detected with an optical method. Eur J Neurosci. 2001;13:1711–21. doi: 10.1046/j.0953-816x.2001.01550.x. [DOI] [PubMed] [Google Scholar]
- Jerng HH, Lauver AD, Pfaffinger PJ. DPP10 splice variants are localized in distinct neuronal populations and act to differentially regulate the inactivation properties of Kv4-based ion channels. Mol Cell Neurosci. 2007;35:604–24. doi: 10.1016/j.mcn.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerng HH, Pfaffinger PJ, Covarrubias M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci. 2004;27:343–69. doi: 10.1016/j.mcn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- Johnston D, Hoffman DA, Poolos NP. Potassium channels and dendritic function in hippocampal pyramidal neurons. Epilepsia. 2000;41:1072–3. doi: 10.1111/j.1528-1157.2000.tb00304.x. [DOI] [PubMed] [Google Scholar]
- Juhng KN, Kokate TG, Yamaguchi S, Kim BY, Rogowski RS, Blaustein MP, Rogawski MA. Induction of seizures by the potent K+ channel-blocking scorpion venom peptide toxins tityustoxin-K(alpha) and pandinustoxin-K(alpha) Epilepsy Res. 1999;34:177–86. doi: 10.1016/s0920-1211(98)00111-9. [DOI] [PubMed] [Google Scholar]
- Keene DL. A systematic review of the use of the ketogenic diet in childhood epilepsy. Pediatr Neurol. 2006;35:1–5. doi: 10.1016/j.pediatrneurol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Kim J, Jung SC, Clemens AM, Petralia RS, Hoffman DA. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron. 2007;54:933–47. doi: 10.1016/j.neuron.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Nishizawa Y, Sawada K, Ogura H, Miyabe M. K(+)-channel openers suppress epileptiform activities induced by 4-aminopyridine in cultured rat hippocampal neurons. J Pharmacol Sci. 2008;108:517–528. doi: 10.1254/jphs.08214fp. [DOI] [PubMed] [Google Scholar]
- Kole MH, Ilschner SU, Kampa BM, Williams SR, Ruben PC, Stuart GJ. Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci. 2008;11:178–86. doi: 10.1038/nn2040. [DOI] [PubMed] [Google Scholar]
- Korngreen A, Sakmann B. Voltage-gated K+ channels in layer 5 neocortical pyramidal neurones from young rats: subtypes and gradients. J Physiol. 2000;3:621–39. doi: 10.1111/j.1469-7793.2000.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langoscha JM, Zhoua XY, Grunzeb H, Waldena J. New Insights into the Mechanisms Sites of Action of Lamotrigine. Neuropsychobiology. 2000;42(suppl 1) doi: 10.1159/000054848. [DOI] [PubMed] [Google Scholar]
- Lasztóczi B, Antal K, Nyikos L, Emri Z, Kardos J. High-frequency synaptic input contributes to seizure initiation in the low-[Mg2+] model of epilepsy. Eur J Neurosci. 2004;19:1361–72. doi: 10.1111/j.1460-9568.2004.03231.x. [DOI] [PubMed] [Google Scholar]
- Lipowsky R, Gillessen T, Alzheimer C. Dendritic Na+ channels amplify EPSPs in hippocampal CA1 pyramidal cells. J Neurophysiol. 1996;76:2181–91. doi: 10.1152/jn.1996.76.4.2181. [DOI] [PubMed] [Google Scholar]
- Magee J. Dendritic integration of excitatory synaptic input. Nat Rev Neurosci. 2000;1:181–90. doi: 10.1038/35044552. [DOI] [PubMed] [Google Scholar]
- Magloczky Z, Freund TF. Impaired and repaired inhibitory circuits in the epileptic human hippocampus. Trends Neurosci. 2005;28:334–40. doi: 10.1016/j.tins.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Marciani MG, Spanedda F, Bassetti MA, et al. Effect of lamotrigine on EEG paroxysmal abnormalities and background activity: a computerized analysis. Br J Clin Pharmacol. 1996;42:621–627. doi: 10.1111/j.1365-2125.1996.tb00057.x. [DOI] [PubMed] [Google Scholar]
- Marder E, Goaillard J. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–74. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- Margulis M, Tang CM. Temporal integration can readily switch between sublinear and supralinear summation. J Neurophysiol. 1998;79:2809–13. doi: 10.1152/jn.1998.79.5.2809. [DOI] [PubMed] [Google Scholar]
- Markram H, Lübke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J Physiol. 1997;500:409–40. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks JP, Mennerick S. Action potential initiation and propagation in CA3 pyramidal axons. J Neurophysiol. 2007;97:3460–72. doi: 10.1152/jn.01288.2006. [DOI] [PubMed] [Google Scholar]
- Migliore M, Hoffman DA, Magee JC, Johnston D. Role of an A-type K+ conductance in the back-propagation of action potentials in the dendrites of hippocampal pyramidal neurons. J Comput Neurosci. 1999;7:5–15. doi: 10.1023/a:1008906225285. [DOI] [PubMed] [Google Scholar]
- Morozov A, Muzzio IA, Bourtchouladze R, Van-Strien N, Lapidus K, Yin D, Winder DG, Adams JP, Sweatt JD, Kandel ER. Rap1 couples cAMP signaling to a distinct pool of p42/44MAPK regulating excitability, synaptic plasticity, learning, and memory. Neuron. 2003;39:309–25. doi: 10.1016/s0896-6273(03)00404-5. [DOI] [PubMed] [Google Scholar]
- Nakamura TY, Nandi S, Pountney DJ, Artman M, Rudy B, Coetzee WA. Different effects of the Ca(2+)-binding protein, KChIP1, on two Kv4 subfamily members, Kv4.1 and Kv4.2. FEBS Lett. 2001;499:205–9. doi: 10.1016/s0014-5793(01)02560-1. [DOI] [PubMed] [Google Scholar]
- Nakamura TY, Pountney DJ, Ozaita A, Nandi S, Ueda S, Rudy B, Coetzee WA. A role for frequenin, a Ca2+-binding protein, as a regulator of Kv4 K+-currents. Proc Natl Acad Sci U S A. 2001;98:12808–13. doi: 10.1073/pnas.221168498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolos N, Migliore M, Johnston D. Pharmacological upregulation of h-channels selectively reduces the excitability of pyramidal neuron dendrites. Nature Neuroscience. 2002;5:767–774. doi: 10.1038/nn891. [DOI] [PubMed] [Google Scholar]
- Ramakers GM, Storm JF. A postsynaptic transient K(+) current modulated by arachidonic acid regulates synaptic integration and threshold for LTP induction in hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 2002;99:10144–9. doi: 10.1073/pnas.152620399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R, Kallenbach U, Singer W, Munk MH. Short- and long-term effects of cholinergic modulation on gamma oscillations and response synchronization in the visual cortex. J Neurosci. 2004;24:10369–78. doi: 10.1523/JNEUROSCI.1839-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint DA, Thomas T, Gage PW. GABAB agonists modulate a transient potassium current in cultured mammalian hippocampal neurons. Neurosci Lett. 1990;118:9–13. doi: 10.1016/0304-3940(90)90236-3. [DOI] [PubMed] [Google Scholar]
- Schaefer AT, Helmstaedter M, Sakmann B, Korngreen A. Correction of conductance measurements in non-space-clamped structures: 1. Voltage-gated K+ channels. Biophys J. 2003;84:3508–28. doi: 10.1016/S0006-3495(03)75086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Ogiwara I, Kaneda M, Tokonami N, Mazaki E, Baba K, Matsuda K, Inoue Y, Yamakawa K. A Kv4.2 truncation mutation in a patient with temporal lobe epilepsy. Neurobiol Dis. 2006;24:245–53. doi: 10.1016/j.nbd.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Sheng M, Tsaur ML, Jan YN, Jan LY. Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron. 1992;9:271–84. doi: 10.1016/0896-6273(92)90166-b. [DOI] [PubMed] [Google Scholar]
- Staley KJ. Neurons skip a beat during fast ripples. Neuron. 2007;55:828–30. doi: 10.1016/j.neuron.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC, Deuchars J. Properties of single axon excitatory postsynaptic potentials elicited in spiny interneurons by action potentials in pyramidal neurons in slices of rat neocortex. Neuroscience. 1995;69:727–38. doi: 10.1016/0306-4522(95)00287-s. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Steriade M. Contribution of intrinsic neuronal factors in the generation of cortically driven electrographic seizures. J Neurophysiol. 2004;92:1133–43. doi: 10.1152/jn.00523.2003. [DOI] [PubMed] [Google Scholar]
- Tsaur ML, Sheng M, Lowenstein DH, Jan YN, Jan LY. Differential expression of K+ channel mRNAs in the rat brain and down-regulation in the hippocampus following seizures. Neuron. 1992;8:1055–67. doi: 10.1016/0896-6273(92)90127-y. [DOI] [PubMed] [Google Scholar]
- Ulbert I, Magloczky Z, Eross L, Czirjak S, Vajda J, Bognar L, Toth S, Szabo Z, Halasz P, Fabo D, Halgren E, Freund TF, Karmos G. In vivo laminar electrophysiology co-registered with histology in the hippocampus of patients with temporal lobe epilepsy. Exp Neurol. 2004;187:310–8. doi: 10.1016/j.expneurol.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Urban NN, Barrionuevo G. Active summation of excitatory postsynaptic potentials in hippocampal CA3 pyramidal neurons. Proc Natl Acad Sci U S A. 1998;95:11450–5. doi: 10.1073/pnas.95.19.11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuyl RA, Albus H. Spontaneous epileptiform discharges in hippocampal slices induced by 4-aminopyridine. Brain Res. 1985;342:54–66. doi: 10.1016/0006-8993(85)91352-6. [DOI] [PubMed] [Google Scholar]
- Walden J, Altrup U, Reith H, Speckmann EJ. Effects of valproate on early and late potassium currents of single neurons. Eur Neuropsychopharmacol1. 1993;3:137–141. doi: 10.1016/0924-977x(93)90265-n. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Hoffman DA, Migliore M, Johnston D. Dendritic K+ channels contribute to spike-timing dependent long-term potentiation in hippocampal pyramidal neurons. PNAS. 2002;99:8366–71. doi: 10.1073/pnas.122210599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nádasdy Z, Jandó G, Szabó I, Sik A, Buzsáki G. Sharp wave-associated high-frequency oscillation. (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Soltész I, Bragin A, Penttonen M, Sik A, Buzsáki G. Intracellular correlates of hippocampal theta rhythm in identified pyramidal cells, granule cells, and basket cells. Hippocampus. 1995;5:78–90. doi: 10.1002/hipo.450050110. [DOI] [PubMed] [Google Scholar]
- Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci. 2002;22:4860–8. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Burkhalter A, Nerbonne JM. Functional Role of the Fast Transient Outward K+ Current IA in Pyramidal Neurons in (Rat) Primary Visual Cortex. J Neurosci. 2005;25:9185–9194. doi: 10.1523/JNEUROSCI.2858-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zona C, Tancredi V, Longone P, D'Arcangelo G, D'Antuono M, Manfredi M, Avoli M. Neocortical potassium currents are enhanced by the antiepileptic drug lamotrigine. Epilepsia. 2002;43:685–90. doi: 10.1046/j.1528-1157.2002.51401.x. [DOI] [PubMed] [Google Scholar]
- Zona C, Tancredi V, Palma E, Pirrone G, Avoli M. Potassium currents in rat cortical neurons in culture are enhanced by the antiepileptic drug carbamazepine. Can J Physiol Pharmacol. 1990;68:545–547. doi: 10.1139/y90-079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


