Abstract
The timing of when the embryonic left-right (LR) axis is first established and the mechanisms driving this process are subjects of strong debate. While groups have focused on the role of cilia in establishing the LR axis during gastrula and neurula stages, many animals appear to orient the LR axis prior to the appearance of, or without the benefit of, motile cilia. Because of the large amount of data available in the published literature and the similarities in the type of data collected across labs, I have examined relationships between the studies that do and do not implicate cilia, the choice of animal model, the kinds of LR patterning defects observed, and the penetrance of LR phenotypes. I found that treatments affecting cilia structure and motility had a higher penetrance for both altered gene expression and improper organ placement compared to treatments that affect processes in early cleavage stage embryos. I also found differences in penetrance that could be attributed to the animal models used; the mouse is highly prone to LR randomization. Additionally, the data were examined to address whether gene expression can be used to predict randomized organ placement. Using regression analysis, gene expression was found to be predictive of organ placement in frogs, but much less so in the other animals examined. Together, these results challenge previous ideas about the conservation of LR mechanisms, with the mouse model being significantly different from fish, frogs and chick in almost every aspect examined. Additionally, this analysis indicates that there may be missing pieces in the molecular pathways that dictate how genetic information becomes organ positional information in vertebrates; these gaps will be important for future studies to identify, as LR asymmetry is not only a fundamentally fascinating aspect of development but also of considerable biomedical importance.
Keywords: left-right asymmetry, cilia, ion flux, meta-analysis, animal model, regression analysis
Introduction
While the exterior structures of the vertebrate body plan are largely symmetrical across the midline, the internal placement and development of organs are asymmetrical between the left and right sides (Levin, 2005). In most vertebrates, this includes the placement of the heart, stomach, liver, gall bladder and spleen, as well as the size and shape of paired organs such as the lungs (Casey, 1998). Birth defects involving the asymmetric development of the internal organs affect approximately 1 in 8000 births, and can have serious consequences for the individual largely due to improper connections of blood vessels (Casey and Hackett, 2000; Kosaki and Casey, 1998; Ramsdell, 2005). The understanding of the mechanisms needed to generate left-right (LR) asymmetry is therefore important for the fields of perinatal medicine and developmental biology.
Problems with asymmetry can be classified into distinct subtypes (Casey and Hackett, 2000; Kosaki and Casey, 1998): situs inversus, the complete mirror inversion of all body organs; isomerisms, symmetrical or midline placement of organs that are normally biased toward one side of the body, or mirror-image duplication of organs that are normally found on one side of the body; dextrocardia and other single organ inversions; and heterotaxia, a loss of concordance in which the laterality of each organ is determined independently. While many treatments and mutations can induce these phenotypes, very little is known about the mechanisms responsible for generating each one. Humans and mammals develop all of these problems (Lander et al., 1998), but other animals such as Xenopus rarely if ever demonstrate isomerisms. Additionally, some phenotypes, such as heterotaxia, are quite detrimental to the health of humans and mammals, as evidenced by perinatal lethality of heterotaxic mutants [for example, (Tan et al., 2007)], while heterotaxic tadpoles appear quite healthy and can live for several months (Morokuma et al., 2008a). These observations suggest that there may be some fundamental differences in how animals with very different embryonic architectures establish LR asymmetry (Speder et al., 2007; Palmer, 2004).
There are three widely accepted steps necessary for the establishment of LR asymmetry. First, a mechanism is needed to orient the LR axis with the dorsal-ventral and anterior-posterior axes (Brown and Wolpert, 1990); the LR axis is always defined in relation to the other two. The orientation of this axis must occur reliably and reproducibly for there to be a bias in asymmetry; otherwise the subsequent offspring could each individually be LR asymmetric but in an unbiased direction, generating a population of mixed mirror-image asymmetries. In the second step, chiral information established in the first step is translated to asymmetric gene expression. Several genes, including nodal, lefty and pitx2, have well characterized asymmetric expression patterns that have been observed in multiple species; the positive- and negative-feedbacks among members of these signaling pathways are well understood (Burdine and Schier, 2000; Schlueter and Brand, 2007; Duboc and Lepage, 2008). Finally, in the third step, information from asymmetric gene expression is amplified and transmitted to several organ systems, and differential migration, proliferation, tension, and adhesion of cells allows for asymmetric development and position of organs (Yost, 1991; Yost, 1992; Gros et al., 2009; Tabin, 2006).
Perhaps the most intriguing question related to LR asymmetry is regarding the initial breaking of symmetry. Several mechanisms have been proposed for the initiation of asymmetry and two major models have emerged. The first, the ciliary model of asymmetry, is the most popular among developmental biologists and is typically cited in textbooks (Gilbert, 2006; Hirokawa et al., 2010; Basu and Brueckner, 2008). This model has two submodels. The first proposes that cilia localized to a small “node” produce a coordinated flow of extra-embryonic fluid (Tabin, 2006). This node is present in mouse, fish (termed the Kupffer’s vesicle, or KV), and frog (a ciliated epithelium at the gastrocoel roof plate, or GRP) (Blum et al., 2009). The flow generated by these cilia is biased directionally due to both the chiral nature of the cilia and their tilt at the node (Nonaka et al., 2005). The cilia are thought to asymmetrically distribute extracellular morphogens, which accumulate on the left side of the embryo and initiate downstream asymmetric gene expression. In the second sub-model, the flow generated by cilia at the node generates a gradient of calcium ions (Shimeld, 2004). This extracellular increase in calcium on the left side of the embryo is sensed by a second group of cilia, which are not motile (Tabin and Vogan, 2003), and stimulates activation of genes such as Notch on the left side (Raya et al., 2004). In both of these sub-models, the original symmetry breaking event is due to biases produced by the motion of cilia at the node. As a whole, the ciliary model is largely based on findings that mutations in ciliary proteins, such as left-right dynein (LRD), cause laterality disorders (Supp et al., 1999); in all “ciliary” mutants, inappropriate orientation of the LR axis is thought to occur because the biased fluid flow is disrupted, the calcium or morphogen gradients are not established, or because the immotile cilia lack the ability to interpret an established calcium gradient (Basu and Brueckner, 2008; Brueckner, 2001).
A second cilia-independent model proposes that asymmetry is generated from a chirally oriented cytoskeleton (Danilchik et al., 2006; Aw et al., 2008) and the asymmetric intracellular localization of motor proteins [i.e. kinesin and LRD (Qiu et al., 2005)] and cell polarity proteins (Bunney et al., 2003a). Because of their LR-biased localizations, these motor and polarity proteins distribute ion transporters in a biased manner between the early blastomeres (Levin, 2006); the asymmetrically distributed cargo includes two proton pumps (Levin et al., 2002; Adams et al., 2006) and two potassium channels (Aw et al., 2008; Morokuma et al., 2008b). Coupled with a network of open gap junctions, the pH gradients and differences in membrane voltage that result from the biased localization of these ion transporters allow charged molecules such as serotonin to be distributed to the right- and ventral-most blastomere (Fukumoto et al., 2005a; Fukumoto et al., 2005b).
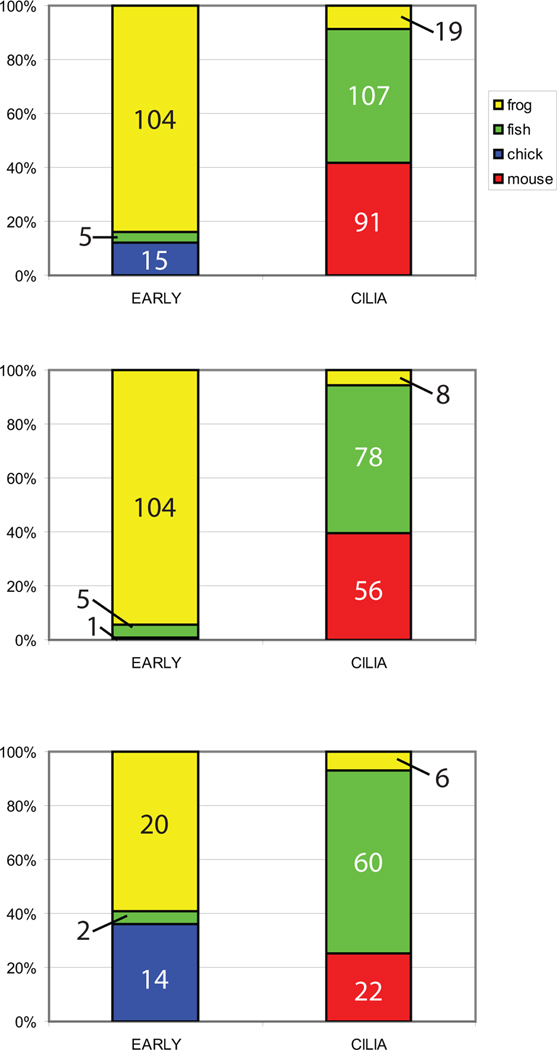
There are two important points to be made from the competing models discussed above. The first is that some overlap in mechanisms has been proposed for these models. For example, LRD has been implicated as important for both models; in the cilia model, mutations in this gene are explained by their effect on extracellular fluid flow (McGrath et al., 2003; Supp et al., 1999) while in the early ion flux model, disruptions in LRD are implicated in intracellular movement of ion transporters and polarity machinery (Qiu et al., 2005; Levin, 2003). The second point is that most studies exploring the role of cilia in the process of symmetry breaking have relied on fish (zebrafish and medaka) and mice to generate data, while studies exploring earlier mechanisms typically use Xenopus and chick (Figure 1). Fish, frogs, mice and chick may establish the LR axis at different times with differently shaped body plans (Hackett, 2002; Speder et al., 2007), yet studies rarely look at any particular mechanism of generating LR asymmetry across species. It is important to understand whether the choice of animal model itself influences the conclusions that are made about the mechanisms involved in LR patterning. A meta-analysis approach, which uses statistics to combine the results of multiple studies, allows for much needed comparisons between animal models to generate a better understanding of the conserved – and non-conserved – mechanisms used to orient the LR axis, and to determine which mechanisms might be the most relevant for biomedicine.
Figure 1. Distribution of studies in CILIA and EARLY treatments by animal model.
A) Distribution of all EARLY and CILIA studies, regardless of endpoint examined. B) Distribution of studies that examined organ situs. C) Distribution of studies that examined Nodal localization. For all panels, the number of treatments examined for each animal model is listed on the graphs. The results clearly indicate that a 2-way ANOVA approach is not an appropriate statistical analysis for this dataset because of minimal overlap of animal models between the EARLY and CILIA groups.
Many treatments affecting LR asymmetry result in 20–40% heterotaxia, a much lower level than the 50–90% expected (depending on the number of organs assessed), an issue that is rarely if ever discussed [see for example (Nagai et al., 2010; Fogelgren et al., 2011; Marszalek et al., 1999; Bajoghli et al., 2007; Amack et al., 2007; Danilchik et al., 2006)]. A considerable number of studies on the mechanisms of LR asymmetry are available and comparable endpoints (i.e. organ situs, developmental defects, expression of nodal, lefty, and pitx2, etc.) have been examined for many of these studies. Therefore, I used this sizable collection of data to address several important questions: First, are there patterns in the penetrance of LR phenotypes in treatments implicated in the early ion flux pathways compared to others implicated in ciliary motion? I have also asked, is there any effect of animal model on the penetrance of LR defects? Importantly, when collecting this data, it was also apparent that many studies examine only asymmetric gene expression and use this data to make conclusions about organ position [see for example (Oki et al., 2010; Antic et al., 2010; Vick et al., 2009; Pathak et al., 2007; Houde et al., 2006; Kramer-Zucker et al., 2005; Zhang et al., 2001)]. A few studies have proposed that gene expression can be used to predict organ laterality, but the mathematical models produced to date have only been applied to extremely limited datasets (Ibanes and Izpisua Belmonte, 2009; Lohr et al., 1997; Mogi et al., 2003). Therefore, I used the available data to ask, can gene expression data be used to reliably predict the effects of a treatment or mutation on organ laterality?
Methods
Selection of Studies
To select studies for analysis, PubMed was searched with keywords “left right asymmetry.” Additional studies were found from citation lists at the end of comprehensive review articles (Hirokawa et al., 2006; Levin, 2005; 2006; 2007; Levin and Palmer, 2007; Shiratori and Hamada, 2006). Any study that examined the effects of a genetic mutation, molecular manipulation (morpholino treatment, injection of mRNA, etc.), or pharmaceutical treatment was included. Analyses were limited to studies examining LR asymmetry in Xenopus, fish (zebrafish and medaka), chick or mouse, and further limited to studies published in or before mid 2011. Unpublished data were not sought nor included. A total of 126 studies, encompassing 394 mutations, molecular/genetic manipulations and pharmaceutical treatments that influence patterning of the LR axis, were examined (Supplemental Table 1). Every attempt was made to include all studies amenable to this kind of analysis. Furthermore, the size of this database prevents any single study from dramatically changing the results, so any study missing from this analysis should have minimal effects on the statistical conclusions.
Data Collection
The following endpoints were collected directly from the studies:
Mutant/treatment: what was done to the embryos/animals, including stage-specific information where available.
Organism: The animal model in which experiments were performed.
Measures of organ randomization: % heterotaxia, % situs inversus, % isomerisms, and % midline hearts. Many studies break this information down by category, while others report only “randomized” organ placement without specifics. Other studies report inversion rates for several individual organs, but never provide a total % heterotaxia for all animals. In this case, we selected the worst organ inversion rate for analysis. Two different measures of asymmetric placement of organs were calculated from this data: total laterality problems, calculated as the sum of heterotaxia, situs inversus, and isomerisms, was the most comprehensive assessment of defects in LR asymmetry. Because true isomerisms were only reported in some animal models, % heterotaxia + situs inversus, was also examined.
Number of organs: The number of organs that were examined for inversions.
Sample size (organs): The number of animals that were examined to produce the measures of organ randomization.
Developmental problems: Some authors noted developmental problems in treated animals.
Gene expression randomization: Information was collected on the localization of nodal (Xnr-1, spaw, etc.), lefty (lefty 1, lefty 2, etc.), and pitx2 expression. When available, incorrect expression was separated by right-sided, bilateral and absent localization. Some authors only reported the total value.
Sample size (gene expression): The number of animals that were examined to produce the measures of gene expression randomization.
Assesses cilia: Some studies examined cilia for changes in number, shape, length, or movement.
Significant effects on cilia: Whether an adverse effect was observed for any aspect of cilia development or appearance.
Overview of studies included in analysis
A total of 252 treatments were examined for their effects on organ laterality; 127 examined effects on asymmetry of Nodal (nodal, Xnr-1, Spaw) expression, 56 examined effects on asymmetry of Lefty expression, and 67 examined effects on asymmetry of Pitx2 expression. Treatments were categorized as having effects early in embryogenesis, i.e. during the first few cell cleavages up to blastula stages, prior to when ciliary motion has been observed (Levin and Palmer, 2007), or as having effects during neurula stages when ciliary motion has been implicated in symmetry breaking (Basu and Brueckner, 2008; McGrath and Brueckner, 2003). These two groups will be referred to as EARLY treatments and CILIA treatments, respectively. Treatments were assigned to one of these groups based on information provided in the published manuscripts i.e. when authors proposed that the effects of a mutation were due to alterations in cilia, the treatment was assigned to CILIA, even if there is evidence that the mutation could have an earlier intracellular role. Any treatments that were not proposed to have effects during either of these periods were classified as “other” and were not analyzed for these purposes. Finally, all treatments, including those classified as “other”, were separated by animal model for further analysis.
Statistical Methods
All data were coded and analyzed with PASW statistical software package 18.0 for Windows (SPSS Inc., Chicago, IL).
1. Analysis of control populations
In a typical meta-analysis, data are normally collected from a treatment group and a control group and analyzed together. Here, control groups were not included in the analysis because background levels of LR defects are incredibly low (typically <1%) and because of this, actual numbers are rarely presented in the published literature.
2. Weighing of studies by sample size
There was a tremendous amount of variability in the number of animals that were utilized to assess laterality defects from study to study. To account for the variance within each study and the size of the studies, data were weighted in SPSS according to the appropriate study size measure (i.e. number of animals examined for measures of organ randomization.) Data shown in Figs 2–4 and Table 1 of the manuscript represent these weighted data. Regression analyses were not weighted because the question being asked was whether one factor was predictive of another when both measures were collected in the same study.
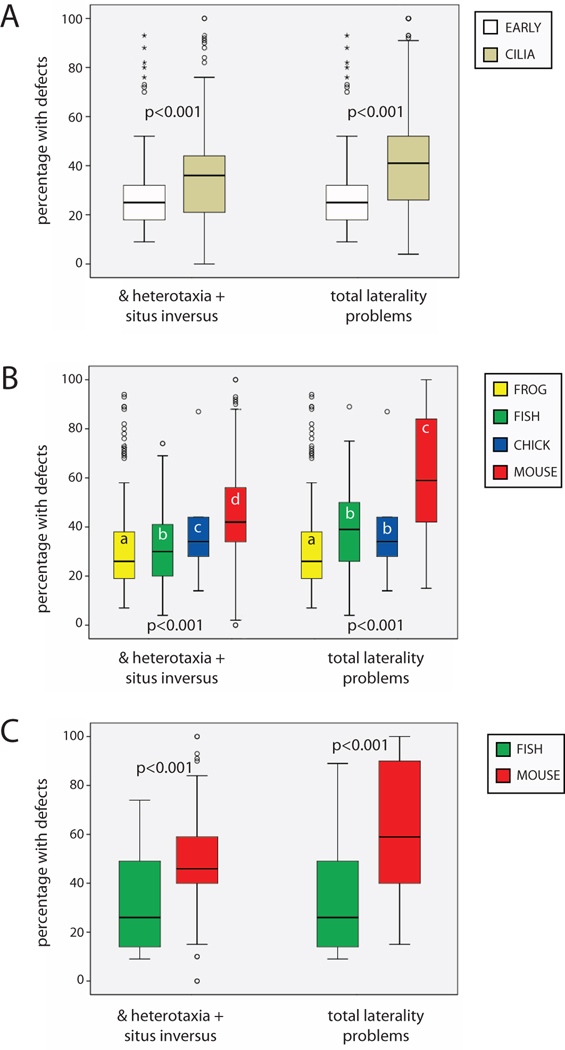
Figure 2. Penetrance of organ situs phenotypes by timing and animal model.
Two different measures of organ situs were calculated for comparisons, % heterotaxia + situs inversus and total laterality problems. A) CILIA treatments produced significantly higher values for both measures of organ situs compared to EARLY treatments. p-values on graph are results of independent samples T-tests using weighted data. B) There were significant differences in % heterotaxia + situs inversus and total laterality problems by animal model, with mice having significantly more penetrant defects compared to the other three animal models. The difference between % heterotaxia + situs inversus and total laterality problems for mice suggests that isomerisms are a uniquely important class of laterality defects in this animal. p-values on graph are ANOVA values using weighted data; different letters indicate significant differences between groups (Dunnett T3 posthoc test, p<0.01). C) When the analysis was limited to mutants only, mice still had more penetrant defects for both measures of organ situs compared to fish, the only other animal with significant numbers of mutants available for analysis. p-values on graph are results of independent sample T-tests using weighted data.
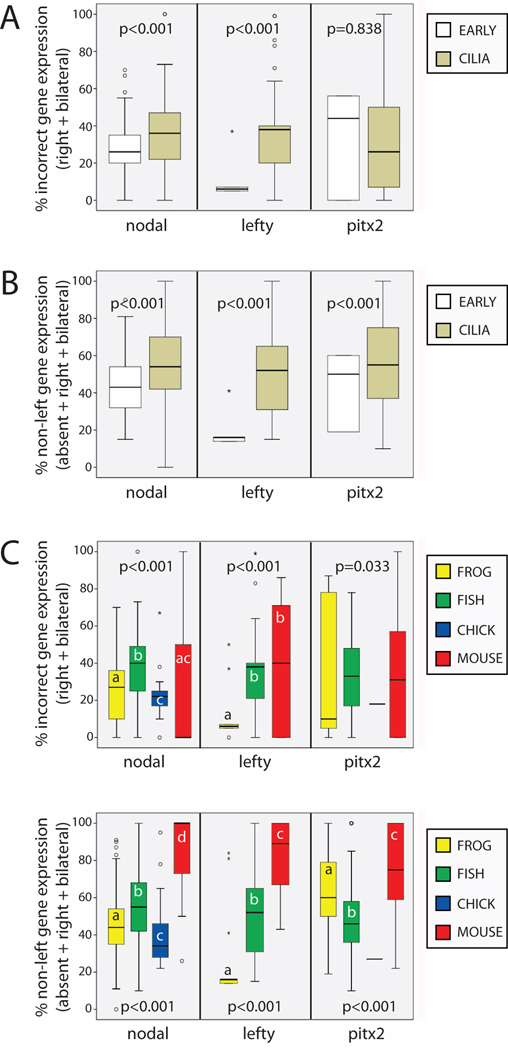
Figure 4. Asymmetric gene expression is influenced by how it is calculated, timing, and animal.
A) When only right and bilateral gene expression were included in calculations, there were significant differences in incorrect gene expression between CILIA and EARLY treatments two of the three genes examined. B) When right, bilateral and absent gene expression were included in calculations, there were significant differences in all non-left gene expression between CILIA and EARLY treatments for all three genes examined. C) Incorrect gene expression was influenced by animal model for all three genes examined, although the differences in pitx2 did not reach statistical significance. p-values are ANOVA statistics of weighted data; different letters indicate significant differences between groups (Dunnett T3 posthoc test, p<0.01). D) All non-left gene expression was significantly affected by animal model, with mice being the most different. p-values are ANOVA statistics; different letters indicate significant differences between groups (Dunnett T3 posthoc test, p<0.01). For lefty, there were no samples collected in chick. For pitx2, there was only 1 sample for chick, so this group was excluded from analysis.
Table 1.
Influence of timing and animal model on the incidence of developmental problems.
| All developmental problems |
embryonic / perinatal lethality |
kidney problems |
edema | heart problems |
encephaly | neural tube / notochord problems |
facial defects |
bent axes | polydactyly | |
|---|---|---|---|---|---|---|---|---|---|---|
| EARLY | 4% | 1% | 0% | 0% | 3% | 0% | 0% | 0% | 3% | 0% |
| CILIA | 45% | 8% | 14% | 12% | 7% | 8% | 6% | 6% | 28% | 1% |
| X Square | 7658 | 1114 | 2679 | 2396 | 376 | 1543 | 1228 | 1226 | 4538 | 150 |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Xenopus | 4% | 1% | 0% | 0.1% | 2% | 0% | 0% | 0.2% | 2% | 0% |
| fish | 44% | 1% | 16% | 12% | 5% | 5% | 3% | 6% | 35% | 0% |
| chick | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| mouse | 65% | 53% | 6% | 18% | 22% | 26% | 28% | 7% | 0% | 6% |
Details are provided indicate the frequency of developmental problems by timing and animal model. Developmental problems occurred more frequently in CILIA treatments compared to EARLY treatments, with the single exception of heart problems. Overall, fish and mouse had more developmental problems compared to Xenopus or chick. In fish, the most common problems were bent axes and kidney malformations. In mice, the most common problems were embryonic / perinatal lethality, neural tube / notochord malformations, and encephaly.
3. Statistical comparisons of groups
For comparisons between EARLY and CILIA treatments, independent sample T-tests of weighted data were performed. For comparisons between animal models, 1-way ANOVA followed by Dunnett T3 posthoc tests were performed on weighted data. This posthoc analysis was selected because it is appropriate for data with unequal variances. 2-way ANOVA could not be performed because of minimal overlap between animal models represented in EARLY and CILIA groups (see Figure 1). Finally, for comparisons of organ situs and gene expression data, regression analysis with ANOVA was performed. In all analyses, differences were considered significant when p<0.01.
4. Presentation of data
Data in figures presented as stem-and-leaf plots were created with SPSS. In these graphs, the central line marks the 50th percentile, the outer bounds of the box represent the 25th and 75th percentiles, and the stems represent the 10th and 90th percentiles. Regression data figures were created in Excel. The regression lines on these figures represent best-fit lines, and the R2 values and ANOVA p-values are presented on each graph.
Results
Both timing of treatments and animal model influence penetrance of organ laterality defects
To determine whether the penetrance of LR organ laterality measures was affected by timing of treatment, I began by calculating total laterality problems and % heterotaxia + situs inversus in EARLY and CILIA treatments. Both measures were significantly greater in CILIA versus EARLY treatments (Figure 2A), although each group displayed a large amount of variability. Because animals manifest laterality defects differently (i.e. the high incidence of isomerisms in mice), I next examined the penetrance of both measures of laterality defects by animal model. For both % heterotaxia + situs inversus and total laterality problems, there were significant differences by animal model, and LR defects were most penetrant in the mouse (Figure 2B). Thus, it is concluded that the timing of treatments (CILIA versus EARLY) and animal model each significantly influence the penetrance of LR phenotypes.
Because there is the possibility that the type of treatment (i.e. mutations versus molecular or pharmaceutical manipulations) could influence the penetrance of LR defects, I separately examined only those treatments involving mutations. Because mutants are limited to the mouse and fish models, comparisons were limited to these two animals, but no further limitations (i.e. distinctions between natural and induced mutants) were made. When this comparison was performed, the striking differences between both % heterotaxia + situs inversus and total laterality problems remained; the penetrance was significantly higher in mouse mutants compared to fish mutants (Figure 2C). From these results, it is concluded that the mouse is significantly different from the other animal models examined, even when comparisons are limited to genetic mutants.
The presence of other developmental problems does not influence laterality defects
Previous studies have shown that in Xenopus, developmental defects that affect patterning of the other axes can influence orientation of the LR axis (Lohr et al., 1997; Lohr et al., 1998; Vandenberg and Levin, 2010a). To determine whether this is the case for all treatments, I first determined the frequency of developmental problems in EARLY and CILIA treatments. Treatments affecting CILIA had significantly higher frequencies of kidney malformations, lethality, edemas, encephaly, heart deformities, malformations of the neural tube or notochord, facial defects, bent axes, and polydactyl digits compared with EARLY treatments (Table 1). When comparing the incidence of developmental defects by animal model, 65% of mouse treatments and 44% of fish treatments induced at least one developmental defect, while only 4% of frog treatments and 0% of chick treatments had these effects (Table 1). It should be noted that these numbers may be conservative estimates because many studies make no mention of whether the animals were otherwise normal, or not.
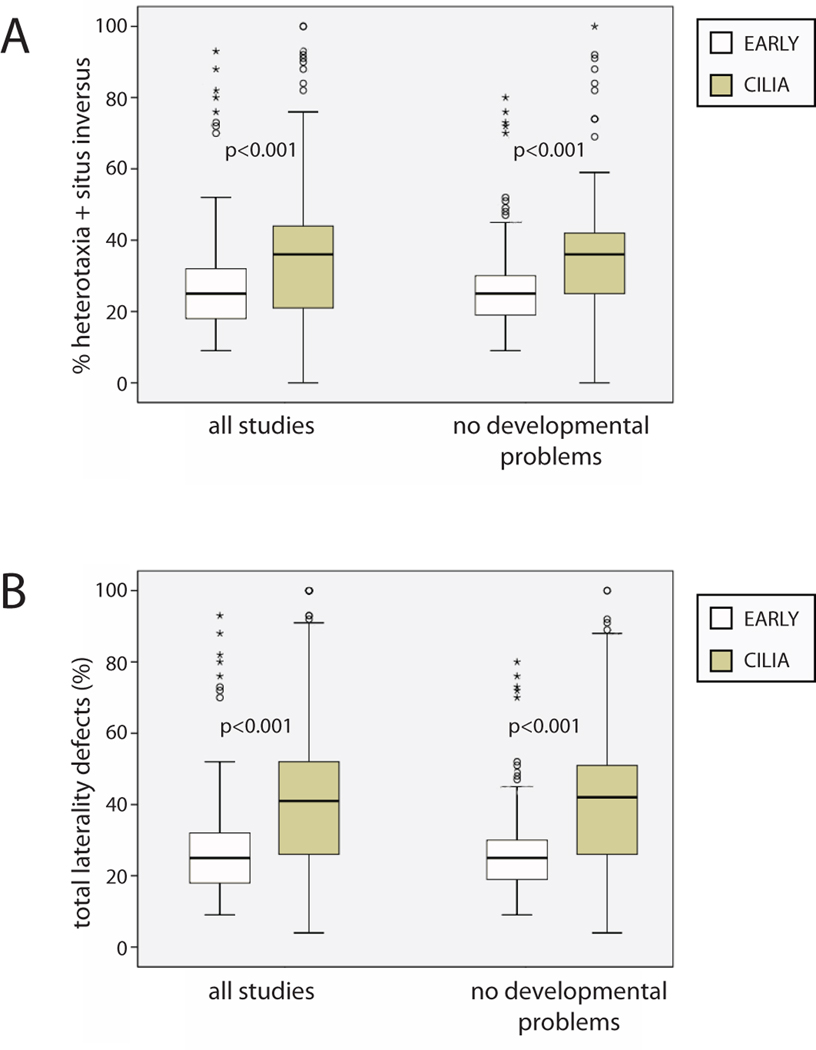
I again calculated the mean % heterotaxia + situs inversus and total laterality problems for EARLY and CILIA treatments, but this time only included those treatments that did not report that they induced other developmental defects. Yet still, even without treatments that induced other malformations, the differences between EARLY and CILIA treatments remained (Figure 3). From these results, it can be concluded that while developmental problems are found more often in CILIA treatments, these abnormalities do not appear to influence the penetrance of LR phenotypes.
Figure 3. Developmental defects have little influence on penetrance of randomized organ situs.
A) % heterotaxia + situs inversus in all studies compared to this same measure after treatments that cause malformations and developmental defects were removed from analysis. B) Total laterality problems, again comparing all studies before and after treatments that cause other defects were removed. For both of these measures, the removal of studies that cause developmental defects has very little impact on either EARLY or CILIA treatments, and significant differences between these groups remain. (In both panels, the data shown for “all studies” is the same as reported in Fig. 2A.) p-values on graph are results of independent samples T-tests using weighted data.
Alterations in asymmetric gene expression are influenced by timing of treatments and animal model
Three genes were examined as measures of asymmetric gene expression that are typically associated with laterality: nodal, lefty, and pitx2. There are several measures of incorrect gene expression that have been reported in the literature (Sampath et al., 1997; Yoshioka et al., 1998; Levin et al., 1995): right-sided and bilateral expression of these genes is incorrect; some treatments also induce absence of gene expression in treated embryos. Absent expression of a typically asymmetric gene is particularly perplexing because there is little discussion in the literature about how lack of expression should affect organ situs. For this reason, whenever possible, I examined incorrect gene expression (the sum of right-sided and bilateral expression) separately from all non-left expression (the sum of right-sided, bilateral and absent gene expression).
In order to determine whether timing of treatments influences asymmetric gene expression, I compared the penetrance of both of these measures of gene expression in EARLY and CILIA treatments. Incorrect gene expression was significantly different between EARLY and CILIA treatments for nodal and lefty, but not pitx2 (Figure 4A). All non-left expression was significantly different between EARLY and CILIA treatments for all three genes examined (Figure 4B), with a higher penetrance for CILIA treatments compared to EARLY treatments. Because we found an influence of animal model on the penetrance of organ laterality defects, we next asked whether the penetrance of abnormalities in asymmetric gene expression was also a function of the animal model used in the experiment. Both incorrect gene expression (Figure 4C), and all non-left expression were influenced by animal for all three genes examined (Figure 4D), with higher penetrance observed in mice compared to other animal models. Mice were especially different from the other animal models for all non-left expression, which illustrates the importance of absent gene expression in this animal. Thus, it is concluded that, similar to the penetrance of LR organ defects, asymmetric gene expression is influenced in a timing- and animal-dependent manner.
The relationship between asymmetric gene expression and organ situs is influenced by timing of treatments and animal model
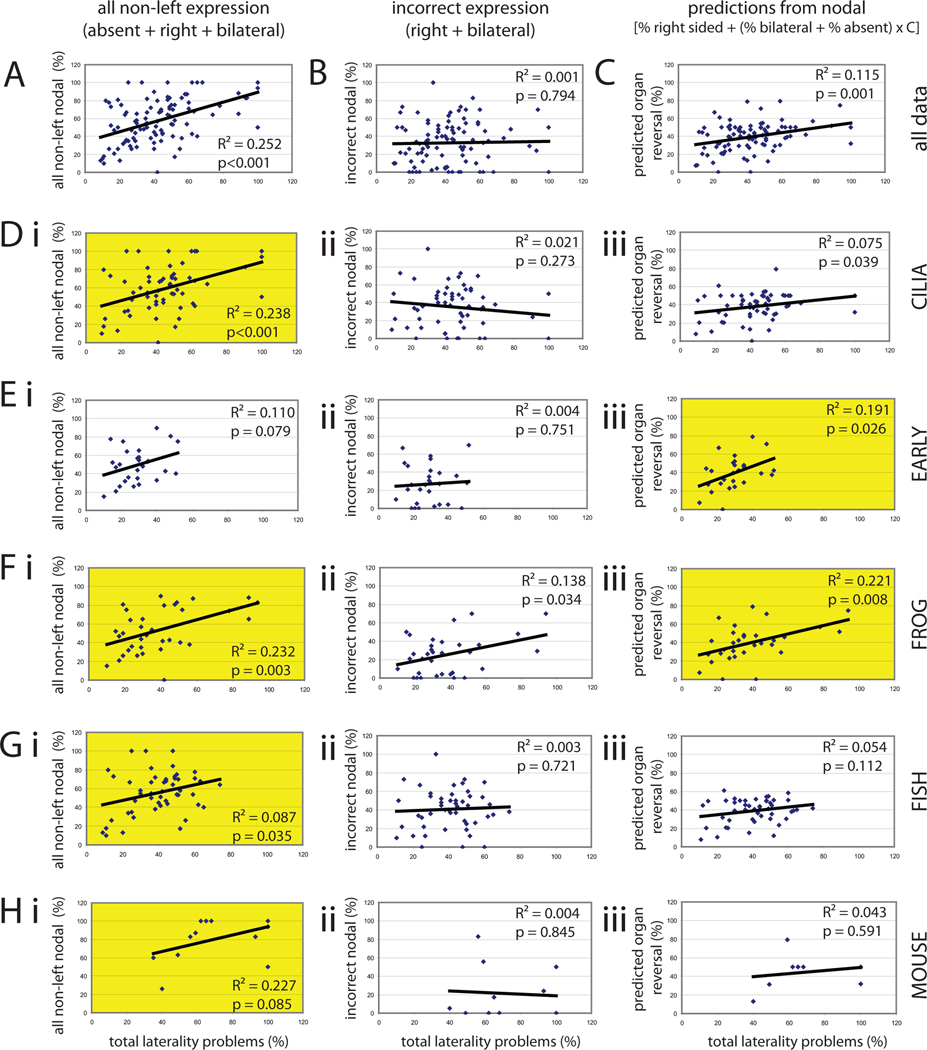
Many studies have examined asymmetric gene expression but have not verified whether these treatments were similarly effective at altering asymmetric organ placement (see Supplemental Table 1). In order to determine whether asymmetric expression of nodal is an adequate substitute for measurements of organ situs, I compared both incorrect nodal expression (which includes right and bilateral expression) and all non-left nodal expression (which includes right, bilateral and absent expression) with total laterality problems for any studies that assessed both organ situs and gene expression; these two gene expression parameters allow us to determine if “absent” nodal expression influences organ laterality. There was a significant correlation between all non-left nodal expression and total laterality problems (Figure 5A, R2=0.252, p<0.001) but no correlation between incorrect nodal expression and total laterality problems (Figure 5B, R2=0.001, p=0.794), confirming that absent gene expression cannot be ignored in further analyses.
Figure 5. Gene expression can be used to predict organ situs, but is dependent on several factors.
Regression analysis indicates that gene expression data can be used to predict organ situs, but only if the correct analysis is chosen based on timing and animal model. For all panels, total laterality problems are graphed along the X axis. For all graphs in the left column, the Y axis represents all non-left nodal expression (the sum of right, bilateral and absent gene expression). For all graphs in the middle column, the Y axis represents incorrect nodal expression (the sum of right and bilateral expression). For all graphs in the right column, the Y axis represents the predicted organ reversal rate derived from reported localization of Nodal. Yellow panels indicate the best fit regressions for each pathway (CILIA and EARLY) and each animal model. Panels A–C show regressions of all data using the three possible comparisons. Di–iii) The best regression for CILIA treatments is achieved by comparing all non-left nodal expression and total laterality problems. Ei–iii) The best regression for EARLY treatments is achieved by comparing predicted organ reversal rate and total laterality problems, although this relationship did not reach statistical significance. Fi–iii) Data from Xenopus fit two models equally well: regressions comparing all non-left nodal expression and total laterality problems and predicted organ reversal rate and total laterality problems. Gi–iii). Data from fish fit best to the regression between all non-left nodal expression and total laterality problems, but this relationship was not statistically significant. Hi–iii) Unexpectedly, data collected in mouse do not fit any of the regressions well, suggesting poor associations between asymmetric gene expression and organ laterality in this animal model. The best fit was observed between all non-left nodal expression and total laterality problems.
It has been previously proposed that all non-left nodal expression can be used to calculate an expected organ reversal rate (Lohr et al., 1997; Mogi et al., 2003) using the formula:
where 0.5 is used to account for randomization of a single organ. If two organs are examined, the formula must be adjusted, because the chance of having at least one of two organs placed inappropriately raises to 75%; if three organs are examined, the formula is adjusted to 87.5%, and so on. To deal with the variability in the available data (i.e. studies examining anywhere from 1 to 5 organs), I used this modified equation:
I compared the predicted organ reversal rate with total laterality problems and also found a statistically significant correlation (Figure 5C, R2=0.115, p=0.001).
I next asked whether CILIA and EARLY treatments fit one or more of these regression models. I found that CILIA treatments best fit a regression model comparing all non-left nodal expression with total laterality problems (Figure 5Di–iii). When the same regressions were repeated for EARLY treatments, the strongest statistical relationship was observed for comparisons of predicted organ reversal rate and total laterality problems (Figure 5Ei–iii), although this regression did not reach the level of statistical significance. From these data, I conclude that gene expression can be used to predict organ situs, but that different regression models are needed to make the best predictions depending on the timing of treatments.
To address the question of whether animal model influences the relationship between gene expression and organ situs, I performed each of these regression analyses separately for studies that used frog, fish or mouse as an animal model; the sample size for chick was too small for such analysis. Organ laterality in frogs fit well with all three regression models, although all non-left nodal expression and predicted organ reversal rate provided the best fits with the organ situs data (Figure 5F i–iii). In fish (Figure 5G i–iii) and mouse (Figure 5H i–iii), organ situs best correlated with all non-left nodal expression, although neither reached statistical significance. There were no significant correlations between organ situs and incorrect nodal expression or predicted organ reversal rate for either animal.
One major concern with the comparison between gene expression and organ situs is that treatments that affect survival could bias the relationship between these two factors. For example, it is plausible that a treatment could lead to higher mortality in animals with altered laterality, but that mortality would manifest between the stages where asymmetric gene expression and organ position were examined. To address this concern, I performed the same regression analyses described above, but limited to those treatments that were not reported to induce embryonic/perinatal lethality. The removal of animals with reported lethality had little effect on the relationships between gene expression and organ situs for the CILIA and EARLY groups (Table 2). Similarly, the strong relationships observed in frog were maintained, and the relationship between organ situs and all non-left nodal expression in fish became even stronger. In contrast, there were no correlations even approaching significance in the mouse model.
Table 2.
Results of regression analysis limited to animals without embryonic/perinatal lethality
| Group | Comparison of situs & all non-left nodal |
Comparison of situs & incorrect nodal |
Comparison of situs & predicted values |
|---|---|---|---|
| all data | R2 = 0.157 p < 0.001 |
R2 = 0.017 p = 0.230 |
R2 = 0.097 p = 0.004 |
| CILIA | R2 = 0.102 p = 0.017 |
R2 < 0.001 p = 0.983 |
R2 = 0.042 p = 0.143 |
| EARLY | R2 = 0.122 p = 0.069 |
R2 = 0.015 p = 0.545 |
R2 = 0.175 p = 0.037 |
| frog | R2 = 0.239 p = 0.002 |
R2 = 0.187 p = 0.014 |
R2 = 0.210 p = 0.011 |
| fish | R2 = 0.123 p = 0.013 |
R2 < 0.001 p = 0.948 |
R2 = 0.066 p = 0.081 |
| mouse | R2 = 0.028 p = 0.787 |
R2 = 0.704 p = 0.076 |
R2 = 0.125 p = 0.559 |
Because several studies have suggested that absent gene expression correlates with the incidence of isomerisms (Yan et al., 1999; Meno et al., 1998; Ibanes and Izpisua Belmonte, 2009), and because both absent gene expression and isomerisms are common in the mouse model (Figure 4 and data not shown), I also addressed whether there was a direct relationship between the incidence of absent nodal expression and the frequency of isomerisms. When all animals with these two measures were included in this regression analysis, there was no statistically significant relationship observed (R2=0.004, p=0.750). Even when regression analyses comparing isomerisms and absent nodal were limited to the mouse model, no statistically significant relationship was revealed (R2=0.084, p=0.417).
From these results, I conclude that gene expression data can be used to predict organ situs only in some animal models, and the best use of gene expression data is different in frog, fish and mouse studies. Gene expression data collected in frogs could be used to accurately predict organ situs directly, and calculations similar to those proposed previously were also found to be useful (Lohr et al., 1997; Mogi et al., 2003). Yet, mathematical manipulations of gene expression data were not predictive of mouse organ situs data, an unexpected finding that contradicts previous attempts to model small datasets (Ibanes and Izpisua Belmonte, 2009). Even fish, which have a large number of treatments to include in this type of analysis, have relatively weak relationships between gene expression and organ situs.
Discussion
A meta-analysis approach identifies previously unknown and important trends in the LR asymmetry field
Meta-analysis approaches have been employed in many fields of biology and medicine, allowing large datasets to be utilized to make conclusions that would be difficult or impossible to make from smaller, hypothesis-driven studies. Here, I present the results of an analysis of more than 120 studies examining the effects of mutations, molecular and genetic alterations, and pharmaceutical treatments on the orientation of the LR axis. This analysis is the first of its kind, and has produced robust findings that should allow researchers in the field to address how the timing of treatments influences their effectiveness at randomizing LR asymmetry and whether the various laboratory animals utilized in controlled experiments are appropriate to make broad conclusions about the evolutionary conservation of LR asymmetry (Vandenberg and Levin, 2010b).
This analysis used two separate but related techniques to make statistical comparisons about the orientation in the LR axis. In the first, comparisons were made across groups based on treatment timing (EARLY versus CILIA), as well as across animal models. One flaw in this approach is that these data were not originally collected for this purpose, and therefore caution should be taken when making conclusions from the statistical comparisons across groups. Another source of error that should be recognized is that the treatments utilized to generate the LR defects in the original studies included LR-relevant mutants, pharmacological treatments, and molecular reagents with a range of penetrance. While acknowledging the inherent variability in producing this data, striking differences are still apparent between groups, with the strongest trend being the high incidence of LR patterning defects in the mouse model. Even when analyses were limited to mutants, these striking differences remained.
The second technique that was used here was a regression of gene expression and organ situs data collected from the same studies, allowing for a direct comparison between these two endpoints. Many published studies report only gene expression data which are often used to infer about the detrimental effects of treatments and mutations on organ placement (see Supplemental Table 1 for examples). Some researchers have proposed that gene expression can be used to predict altered organ laterality, but the mathematical models put forward to date have been applied to extremely limited datasets (Lohr et al., 1997; Mogi et al., 2003; Ibanes and Izpisua Belmonte, 2009). This meta-analysis offered the opportunity to examine mathematically the best ways to use gene expression to predict effects on organ laterality, producing new challenges to the molecular models that have been proposed for how gene expression influences organ placement.
The strength of a meta-approach is that it allows conclusions to be made by examining studies performed by specialists in subfields with expertise working with different animal models. Very few labs are examining LR mechanisms across species, and yet this is a fundamental piece of the LR asymmetry puzzle. Without this type of analysis, scientists in this field are handicapped in their ability to address differences across animal models. This analysis is bolstered by the similarities in the types of data collected in these studies (see Supplemental Table 1), and because of the size of the dataset utilized, the results reported here are unlikely to be significantly altered by the inadvertent exclusion of a few studies.
Treatments affecting cilia produce highly penetrant laterality defects
The results presented here indicate that there are differences between the penetrance of LR phenotypes in CILIA versus EARLY treatments. While this could be interpreted to mean that treatments and mutations affecting cilia have a more potent effect on LR patterning, it should be noted that only some of the studies that implicate cilia as a mechanism for symmetry breaking actually examined cilia, and only two studies functionally tested ciliary motion itself in the absence of genetic perturbations that also affect intracellular functions (Schweickert et al., 2007; Nonaka et al., 2002). Those studies suggest that altering cilia motion has very different effects on mice and frogs, as predicted from our meta-analysis: while altering nodal flow causes almost complete reversal of heart situs in mouse [88%, see (Nonaka et al., 2002)], altering flow in Xenopus embryos has a much more tempered effect on organ situs [33% heterotaxia of 3 organs, see (Schweickert et al., 2007)].
Several studies clearly show little concordance between ciliary defects and LR randomization [reviewed in (Vandenberg and Levin, 2010b)]. For example, some animals where genes implicated in LR patterning have been targeted still have normal appearing cilia (Serluca et al., 2009; Kishimoto et al., 2008), while some ciliary mutants have normal laterality (Zhao and Malicki, 2007; Zeng et al., 2010). This finding is unexpected if cilia are necessary and sufficient for symmetry breaking, and calls into question some of the major tenets of the cilia model (Vandenberg and Levin, 2010b).
The results from this meta-analysis suggest that there are differences between CILIA and EARLY treatments, not just in the penetrance of LR phenotypes (organ situs and gene expression), but also in the incidence of developmental defects and isomerisms (Table 1 & Supplemental Table 1) and the distribution of non-left gene expression (Figure 4 and Supplemental Table 1). Here, mutants and treatments were classified as CILIA or EARLY based on information provided by the original authors. Yet, it is important to note that some gene products that have been defined as “ciliary” and classified as CILIA in this meta-analysis have other roles. For example, LRD is expressed in the limbs and headfolds of the developing mouse (Supp et al., 1997) and has non-ciliary roles in chromatid segregation (Armakolas and Klar, 2007) and intracellular transport, and is asymmetrically expressed in early cleavage stage embryos (Qiu et al., 2005). This suggests that the classification of the timing of some of these mutants may not be completely appropriate; additionally, the lack of information on early roles of some proteins and the presence of altered cilia is not strong enough evidence to indicate causation (Vandenberg and Levin, 2010b). Information about the early roles of all of the treatments previously examined is needed, and should be collected in future studies.
The mouse model: high penetrance of LR phenotypes compared to other animal models
While it is often assumed that ciliary mechanisms are more fundamental because the penetrance of loss-of-function treatments are higher than those targeting other mechanisms (Blum et al., 2009), examination of each animal model separately shows that the mouse model has a propensity to have high levels of randomization, and this may contribute to the trends seen in the CILIA group; mice had higher levels of organ laterality problems (Figure 2B), altered gene expression (Figure 4C–D), developmental problems (Table 1), and absent expression of nodal, lefty, and pitx2 (Supplemental Table 1). In fact, the data clearly indicate that studies of mice drew conclusions that were quantifiably different in almost every way from studies using other animals. This striking finding suggests that the mechanisms used by the mouse to orient the LR axis, which involve cilia, may be divergent from the mechanisms used by other animals, which may include cilia-independent, early mechanisms of orienting the LR axis; other mammals appear to establish the LR axis without developing a structure that resembles the mouse node (Blum et al., 2007) or without cilia altogether (Gros et al., 2009).
Previous discussions about the appropriateness of the mouse model to extrapolate understanding of LR mechanisms to other animals have focused on the differences between LR mutations in mouse and human (Vandenberg and Levin, 2010b; Levin, 2005), the impact of embryonic architecture (Blum et al., 2009; Gros et al., 2009), and the difficulties inherent in working with mouse embryos, which are randomized when cultured (Levin and Palmer, 2007; Fujinaga and Baden, 1991). The general assumption is that there is conservation of mechanisms across species (Levin and Palmer, 2007; Palmer, 2004; Tabin, 2006); thus, the question is whether the central importance of cilia to mouse LR asymmetry makes this animal an outlier, or whether the many examples of animals that orient the LR axis without the benefit of, or prior to the appearance of cilia, are the outliers (Vandenberg and Levin, 2010b). The results of this study suggest that mice are particularly vulnerable to LR randomization, to a degree that sets them apart from other animal models.
Developmental defects: frequently observed, but with variable effects on LR patterning
This meta-analysis indicated that CILIA treatments were more likely to have every developmental malformation examined compared to EARLY treatments. It also indicated that fish and mouse studies often utilized animals with other developmental abnormalities for LR endpoints, whereas these were infrequently reported in studies using frogs or chick (Table 1).
We showed previously that the penetrance of LR defects increased from 25% to 40% when Xenopus tadpoles with slight axial defects were included for analysis (Vandenberg and Levin, 2010a) and similar results have been reported by others as well (Danos and Yost, 1996; 1995; Lohr et al., 1997). Here, I found that many LR phenotypes occur concurrently with malformations of midline structures (i.e. neural tube / notochord problems and bent axes, see Table 1) and 35% of the studies using fish report bent axes as a secondary phenotype, including some studies that selected mutants specifically because of their curly tails (Schottenfeld et al., 2007; Zhao and Malicki, 2007). The results presented here indicate that defects in the dorsal-ventral and anterior-posterior axes, manifesting as bent axes, do not increase penetrance of laterality problems in other animals as they do in Xenopus.
Gene expression: predictive power is dependent on how it is measured, timing of treatment, and animal model
Previously, researchers have proposed that gene expression can be used to calculate expected organ reversals (Lohr et al., 1997; Mogi et al., 2003; Ibanes and Izpisua Belmonte, 2009); these authors demonstrated a high correlation between gene expression and organ placement in their own small datasets. I applied three regression models to determine whether incorrect (right and bilateral expression), all non-left (right, bilateral and absent expression) or calculated predictions from gene expression would best fit organ situs data (Figure 5). When all studies were considered, all non-left gene expression was slightly better than calculated predicted values (compare Figures 5A and 5C). This result suggests that any non-left gene expression causes randomization of organ laterality, but that no specific relationships can be predicted between inappropriate gene expression and specific organ placement (i.e. bilateral Nodal cannot necessarily be calculated as a 50:50 chance of correct/incorrect heart orientation). Furthermore, it should be noted that a 1:1 ratio between organ situs and predictions from asymmetric gene expression was expected, but this ratio was not generated by any of our regressions; if a 1:1 ratio were observed, the regression lines should intercept the y-axis at 0, but most intercept between 20 and 40 (Figure 5). This indicates that the data predict a low (~20%) amount of non-left gene expression (right, bilateral and absent) would have little to no effect on organ situs, an unexpected finding.
Another surprising finding from this meta-analysis was the frequency and importance of absent gene expression. A considerable number of studies have reported complete absence of nodal, lefty, or pitx2 expression (Field et al., 2011; Oki et al., 2010; Sakano et al., 2010; Gaio et al., 1999; Takeuchi et al., 2007; Zhang et al., 2001; Yan et al., 1999; Tsukui et al., 1999; Collignon et al., 1996; Kramer-Zucker et al., 2005; Krebs et al., 2003; Pennekamp et al., 2002), with some reporting 100% absent expression for all three genes. What does complete absence of gene expression mean? Several studies report that some fraction of untreated animals do not express an asymmetric gene, yet these controls have no reported defects in organ laterality [for example, (Schweickert et al., 2007) reports absent pitx2 expression in 6% of controls and (Adams et al., 2006) and (Bunney et al., 2003b) both report absent Xnr-1 expression in 6% of controls]. Absent gene expression was common in treated animals, especially in mice (Figure 4 and Supplemental Table 1), and had a significant role when using gene expression to predict organ situs (Figure 5, compare all left panels with middle panels). Yet the findings presented here suggest that the use of absent gene expression to predict incidence of isomerisms is unfounded; there was no statistical relationship between absent nodal and isomerisms. Understanding what absent gene expression means and how it is translated to organ situs is an important area for future research.
Another interesting result was that data from CILIA studies best fit the regression generated with all non-left expression (Figure 5Di) and data from EARLY studies best fit the regression using predicted values (Figure 5Eiii). This is more evidence that there are fundamental differences between EARLY and CILIA treatments. Even more surprising was that data collected in different animal models fit to different regression models: the best regressions for data collected in Xenopus were for all non-left gene expression and predicted values, which fit equally well (Figure 5Fi and 5Fiii); the best regression for data collected in fish and mice was for all non-left gene expression (Figure 5Gi,Hi), but surprisingly, these relationships were not statistically robust.
Finally, the regression analyses highlight that there are exceptions to the relationship between gene expression and organ situs data. For example, some groups report high levels of asymmetric gene expression and lower than expected levels of randomized organ situs (Kim et al., 2011; Field et al., 2011; Meyers and Martin, 1999; Schottenfeld et al., 2007; Yasuhiko et al., 2001; May-Simera et al., 2010; Carneiro et al., 2011); others report the opposite phenomenon (Bajoghli et al., 2007; Kishimoto et al., 2008; Song et al., 2010). These mutations and treatments may be of interest to researchers that are studying the amplification and transmission of asymmetric gene expression signals to multiple organ systems (Hackett, 2002).
Conclusions
I have presented here results from an analysis of more than 120 studies with almost 400 mutants, molecular/genetic and pharmaceutical treatments affecting the orientation of the LR axis. My analysis indicates that the timing of when treatments are implicated and the animal model used for study both significantly affect penetrance of LR phenotypes. Most important is that the mouse model is quantifiably different from the other animals examined, and this is likely to skew the conclusions made about the importance of cilia to LR patterning events. To allow the field of LR asymmetry to move forward, increased collaboration is needed among labs to study LR-relevant pathways in a wider variety of taxa. In particular, studies of EARLY pathways are needed in fish (and mice), and more information is needed about the role of CILIA pathways in frogs. Additionally, while others have compared gene expression and organ situs in select datasets, this meta-analysis showed that gene expression can be used to predict randomization of organs, but the relationship between these two factors is also influenced by timing and animal model. In both the fish and mouse models, there was relatively poor concordance between gene expression and organ position, suggesting that there may be missing pieces in the molecular pathway of how genetic information becomes organ positional information in these animals. This analysis reveals the need for scientists to approach research questions in a theory-neutral manner, remaining open to the idea that the origin of LR asymmetry is not yet solved (Vandenberg and Levin, 2009; 2010b).
Highlights.
Treatments affecting cilia produce the most penetrant left-right phenotypes.
The mouse has higher penetrance of left-right defects compared to other animals.
Gene expression can predict organ placement in frog, but less so in fish or mouse.
These results challenge prior ideas about conservation of left-right mechanisms.
Supplementary Material
Acknowledgments
Acknowledgements & Funding
The author would like to thank Michael Levin, Douglas Blackiston, Cheryl Schaeberle and Dany Adams for suggestions with statistical analyses and comments on the manuscript, and members of the Levin lab for helpful discussions. I apologize to any authors whose work was inadvertently overlooked in the compilation of this dataset. This study was supported by NRSA grant 1F32GM087107. The funders had no role in the design of the experiments or the analysis of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams DS, Robinson KR, Fukumoto T, Yuan S, Albertson RC, Yelick P, Kuo L, McSweeney M, Levin M. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development (Cambridge, England) 2006;133:1657–1671. doi: 10.1242/dev.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amack JD, Wang X, Yost HJ. Two T-box genes play independent and cooperative roles to regulate morphogenesis of ciliated Kupffer's vesicle in zebrafish. Dev Biol. 2007;310:196–210. doi: 10.1016/j.ydbio.2007.05.039. [DOI] [PubMed] [Google Scholar]
- Antic D, Stubbs JL, Suyama K, Kintner C, Scott MP, Axelrod JD. Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis. PLoS ONE. 2010;5:e8999. doi: 10.1371/journal.pone.0008999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armakolas A, Klar AJ. Left-right dynein motor implicated in selective chromatid segregation in mouse cells. Science. 2007;315:100–101. doi: 10.1126/science.1129429. [DOI] [PubMed] [Google Scholar]
- Aw S, Adams DS, Qiu D, Levin M. H,K-ATPase protein localization and Kir4.1 function reveal concordance of three axes during early determination of left-right asymmetry. Mech Dev. 2008;125:353–372. doi: 10.1016/j.mod.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajoghli B, Aghaallaei N, Soroldoni D, Czerny T. The roles of Groucho/Tle in left-right asymmetry and Kupffer's vesicle organogenesis. Dev Biol. 2007;303:347–361. doi: 10.1016/j.ydbio.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Basu B, Brueckner M. Cilia: multifunctional organelles at the center of vertebrate left-right asymmetry. Curr Top Dev Biol. 2008;85:151–174. doi: 10.1016/S0070-2153(08)00806-5. [DOI] [PubMed] [Google Scholar]
- Blum M, Andre P, Muders K, Schweickert A, Fischer A, Bitzer E, Bogusch S, Beyer T, van Straaten HW, Viebahn C. Ciliation and gene expression distinguish between node and posterior notochord in the mammalian embryo. Differentiation. 2007;75:133–146. doi: 10.1111/j.1432-0436.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- Blum M, Beyer T, Weber T, Vick P, Andre P, Bitzer E, Schweickert A. Xenopus, an ideal model system to study vertebrate left-right asymmetry. Dev Dyn. 2009;238:1215–1225. doi: 10.1002/dvdy.21855. [DOI] [PubMed] [Google Scholar]
- Brown NA, Wolpert L. The development of handedness in left/right asymmetry. Development. 1990;109:1–9. doi: 10.1242/dev.109.1.1. [DOI] [PubMed] [Google Scholar]
- Brueckner M. Cilia propel the embryo in the right direction. Am J Med Genet. 2001;101:339–344. doi: 10.1002/1096-8628(20010715)101:4<339::aid-ajmg1442>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Bunney TD, De Boer AH, Levin M. Fusicoccin signaling reveals 14-3-3 protein function as a novel step in left-right patterning during amphibian embryogenesis. Development (Cambridge, England) 2003a;130:4847–4858. doi: 10.1242/dev.00698. [DOI] [PubMed] [Google Scholar]
- Bunney TD, De Boer AH, Levin M. Fusicoccin signaling reveals 14-3-3 protein function as a novel step in left-right patterning during amphibian embryogenesis. Development. 2003b;130:4847–4858. doi: 10.1242/dev.00698. [DOI] [PubMed] [Google Scholar]
- Burdine R, Schier A. Conserved and divergent mechanisms in left-right axis formation. Genes & Development. 2000;14:763–776. [PubMed] [Google Scholar]
- Carneiro K, Donnet C, Rejtar T, Karger BL, Barisone GA, Diaz E, Kortagere S, Lemire JM, Levin M. Histone deacetylase activity is necessary for left-right patterning during vertebrate development. BMC Dev Biol. 2011;11:29. doi: 10.1186/1471-213X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B. Two rights make a wrong: human left-right malformations. Hum Mol Genet. 1998;7:1565–1571. doi: 10.1093/hmg/7.10.1565. [DOI] [PubMed] [Google Scholar]
- Casey B, Hackett BP. Left-right axis malformations in man and mouse. Curr Opin Genet Dev. 2000;10:257–261. doi: 10.1016/s0959-437x(00)00085-x. [DOI] [PubMed] [Google Scholar]
- Collignon J, Varlet I, Robertson EJ. Relationship between asymmetric nodal expression and the direction of embryonic turning. Nature. 1996;381:155–158. doi: 10.1038/381155a0. [DOI] [PubMed] [Google Scholar]
- Danilchik MV, Brown EE, Riegert K. Intrinsic chiral properties of the Xenopus egg cortex: an early indicator of left-right asymmetry? Development. 2006;133:4517–4526. doi: 10.1242/dev.02642. [DOI] [PubMed] [Google Scholar]
- Danos MC, Yost HJ. Linkage of cardiac left-right asymmetry and dorsal-anterior development in Xenopus. Development. 1995;121:1467–1474. doi: 10.1242/dev.121.5.1467. [DOI] [PubMed] [Google Scholar]
- Danos MC, Yost HJ. Role of notochord in specification of cardiac left-right orientation in zebrafish and Xenopus. Dev Biol. 1996;177:96–103. doi: 10.1006/dbio.1996.0148. [DOI] [PubMed] [Google Scholar]
- Duboc V, Lepage T. A conserved role for the nodal signaling pathway in the establishment of dorso-ventral and left-right axes in deuterostomes. J Exp Zool B Mol Dev Evol. 2008;310:41–53. doi: 10.1002/jez.b.21121. [DOI] [PubMed] [Google Scholar]
- Field S, Riley KL, Grimes DT, Hilton H, Simon M, Powles-Glover N, Siggers P, Bogani D, Greenfield A, Norris DP. Pkd1l1 establishes left-right asymmetry and physically interacts with Pkd2. Development. 2011;138:1131–1142. doi: 10.1242/dev.058149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelgren B, Lin SY, Zuo X, Jaffe KM, Park KM, Reichert RJ, Bell PD, Burdine RD, Lipschutz JH. The exocyst protein Sec10 interacts with Polycystin-2 and knockdown causes PKD-phenotypes. PLoS Genet. 2011;7:e1001361. doi: 10.1371/journal.pgen.1001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga M, Baden JM. Evidence for an adrenergic mechanism in the control of body asymmetry. Dev Biol. 1991;143:203–205. doi: 10.1016/0012-1606(91)90067-d. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Blakely R, Levin M. Serotonin transporter function is an early step in left-right patterning in chick and frog embryos. Dev Neurosci. 2005a;27:349–363. doi: 10.1159/000088451. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Kema IP, Levin M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr Biol. 2005b;15:794–803. doi: 10.1016/j.cub.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Gaio U, Schweickert A, Fischer A, Garratt AN, Muller T, Ozcelik C, Lankes W, Strehle M, Britsch S, Blum M, Birchmeier C. A role of the cryptic gene in the correct establishment of the left-right axis. Curr Biol. 1999;9:1339–1342. doi: 10.1016/s0960-9822(00)80059-7. [DOI] [PubMed] [Google Scholar]
- Gilbert SF. Developmental Biology. Sinauer Associates, Massachusetts. 2006 [Google Scholar]
- Gros J, Feistel K, Viebahn C, Blum M, Tabin CJ. Cell movements at Hensen's node establish left/right asymmetric gene expression in the chick. Science. 2009;324:941–944. doi: 10.1126/science.1172478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett BP. Formation and malformation of the vertebrate left-right axis. Curr Mol Med. 2002;2:39–66. doi: 10.2174/1566524023363031. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Tanaka Y, Okada Y. Left-right determination: involvement of molecular motor KIF3, cilia, and nodal flow. In: Li R, Bowerman B, editors. Symmetry breaking in biology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2010. pp. 277–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Tanaka Y, Okada Y, Takeda S. Nodal flow and the generation of left-right asymmetry. Cell. 2006;125:33–45. doi: 10.1016/j.cell.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Houde C, Dickinson RJ, Houtzager VM, Cullum R, Montpetit R, Metzler M, Simpson EM, Roy S, Hayden MR, Hoodless PA, Nicholson DW. Hippi is essential for node cilia assembly and Sonic hedgehod signaling. Dev Biol. 2006;300:523–533. doi: 10.1016/j.ydbio.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanes M, Izpisua Belmonte JC. Left-right axis determination. Wiley Interdiscip Rev Syst Biol Med. 2009;1:210–219. doi: 10.1002/wsbm.31. [DOI] [PubMed] [Google Scholar]
- Kim S, Zaghloul NA, Bubenshchikova E, Oh EC, Rankin S, Katsanis N, Obara T, Tsiokas L. Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nat Cell Biol. 2011;13:351–360. doi: 10.1038/ncb2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto N, Cao Y, Park A, Sun Z. Cystic kidney gene seahorse regulates cilia-mediated processes and Wnt pathways. Dev Cell. 2008;14:954–961. doi: 10.1016/j.devcel.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Kosaki K, Casey B. Genetics of human left-right axis malformations. Semin Cell Dev Biol. 1998;9:89–99. doi: 10.1006/scdb.1997.0187. [DOI] [PubMed] [Google Scholar]
- Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development. 2005;132:1907–1921. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]
- Krebs LT, Iwai N, Nonaka S, Welsh IC, Lan Y, Jiang R, Saijoh Y, O'Brien TP, Hamada H, Gridley T. Notch signaling regulates left-right asymmetry determination by inducing Nodal expression. Genes Dev. 2003;17:1207–1212. doi: 10.1101/gad.1084703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander A, King T, Brown NA. Left-right development: mammalian phenotypes and conceptual models. Semin Cell Dev Biol. 1998;9:35–41. doi: 10.1006/scdb.1997.0185. [DOI] [PubMed] [Google Scholar]
- Levin M. Hypothesis: motor proteins and ion pumps, not ciliary motion, initiate LR asymmetry. BioEssays. 2003;25:1002–1010. doi: 10.1002/bies.10339. [DOI] [PubMed] [Google Scholar]
- Levin M. Left-right asymmetry in embryonic development: a comprehensive review. Mech Dev. 2005;122:3–25. doi: 10.1016/j.mod.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Levin M. Is the early left-right axis like a plant, a kidney, or a neuron? The integration of physiological signals in embryonic asymmetry. Birth Defects Res C Embryo Today. 2006;78:191–223. doi: 10.1002/bdrc.20078. [DOI] [PubMed] [Google Scholar]
- Levin M. Large-scale biophysics: ion flows and regeneration. Trends Cell Biol. 2007;17:262–271. doi: 10.1016/j.tcb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Levin M, Johnson R, Stern C, Kuehn M, Tabin C. A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell. 1995;82:803–814. doi: 10.1016/0092-8674(95)90477-8. [DOI] [PubMed] [Google Scholar]
- Levin M, Palmer AR. Left-right patterning from the inside out: widespread evidence for intracellular control. Bioessays. 2007;29:271–287. doi: 10.1002/bies.20545. [DOI] [PubMed] [Google Scholar]
- Levin M, Thorlin T, Robinson KR, Nogi T, Mercola M. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell. 2002;111:77–89. doi: 10.1016/s0092-8674(02)00939-x. [DOI] [PubMed] [Google Scholar]
- Lohr JL, Danos MC, Groth TW, Yost HJ. Maintenance of asymmetric nodal expression in Xenopus laevis. Developmental Genetics. 1998;23:194–202. doi: 10.1002/(SICI)1520-6408(1998)23:3<194::AID-DVG5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Lohr JL, Danos MC, Yost HJ. Left-right asymmetry of a nodal-related gene is regulated by dorsoanterior midline structures during Xenopus development. Development. 1997;124:1465–1472. doi: 10.1242/dev.124.8.1465. [DOI] [PubMed] [Google Scholar]
- Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci U S A. 1999;96:5043–5048. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May-Simera HL, Kai M, Hernandez V, Osborn DP, Tada M, Beales PL. Bbs8, together with the planar cell polarity protein Vangl2, is required to establish left-right asymmetry in zebrafish. Dev Biol. 2010;345:215–225. doi: 10.1016/j.ydbio.2010.07.013. [DOI] [PubMed] [Google Scholar]
- McGrath J, Brueckner M. Cilia are at the heart of vertebrate left-right asymmetry. Curr Opin Genet Dev. 2003;13:385–392. doi: 10.1016/s0959-437x(03)00091-1. [DOI] [PubMed] [Google Scholar]
- McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- Meno C, Shimono A, Saijoh Y, Yashiro K, Mochida K, Ohishi S, Noji S, Kondoh H, Hamada H. Lefty-1 is required for left-right determination as a regulator of lefty-2 and nodal. Cell. 1998;94:287–297. doi: 10.1016/s0092-8674(00)81472-5. [DOI] [PubMed] [Google Scholar]
- Meyers EN, Martin GR. Differences in left-right axis pathways in mouse and chick: functions of FGF8 and SHH. Science. 1999;285:403–406. doi: 10.1126/science.285.5426.403. [DOI] [PubMed] [Google Scholar]
- Mogi K, Goto M, Ohno E, Azumi Y, Takeuchi S, Toyoizumi R. Xenopus neurula left-right asymmetry is respeficied by microinjecting TGF-beta5 protein. Int J Dev Biol. 2003;47:15–29. doi: 10.1387/13. [DOI] [PubMed] [Google Scholar]
- Morokuma J, Blackiston D, Adams DS, Seebohm G, Trimmer B, Levin M. Modulation of potassium channel function confers a hyperproliferative invasive phenotype on embryonic stem cells. Proc Natl Acad Sci U S A. 2008a;105:16608–16613. doi: 10.1073/pnas.0808328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morokuma J, Blackiston D, Levin M. KCNQ1 and KCNE1 K+ channel components are involved in early left-right patterning in Xenopus laevis embryos. Cell Physiol Biochem. 2008b;21:357–372. doi: 10.1159/000129628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Asaoka Y, Namae M, Saito K, Momose H, Mitani H, Furutani-Seiki M, Katada T, Nishina H. The LIM protein Ajuba is required for ciliogenesis and left-right axis determination in medaka. Biochem Biophys Res Commun. 2010;396:887–893. doi: 10.1016/j.bbrc.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Shiratori H, Saijoh H, Hamada H. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature. 2002;418:96–99. doi: 10.1038/nature00849. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Yoshiba S, Watanabe D, Ikeuchi S, Goto T, Marshall WF, Hamada H. De novo formation of left-right asymmetry by posterior tilt of nodal cilia. PLoS Biol. 2005;3:e268. doi: 10.1371/journal.pbio.0030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki S, Kitajima K, Meno C. Dissecting the role of Fgf signaling during gastrulation and left-right axis formation in mouse embryos using chemical inhibitors. Dev Dyn. 2010;239:1768–1778. doi: 10.1002/dvdy.22282. [DOI] [PubMed] [Google Scholar]
- Palmer AR. Symmetry breaking and the evolution of development. Science. 2004;306:828–833. doi: 10.1126/science.1103707. [DOI] [PubMed] [Google Scholar]
- Pathak N, Obara T, Mangos S, Liu Y, Drummond IA. The zebrafish fleer gene encodes an essential regulator of cilia tubulin polyglutamylation. Mol Biol Cell. 2007;18:4353–4364. doi: 10.1091/mbc.E07-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennekamp P, Karcher C, Fischer A, Schweickert A, Skryabin B, Horst J, Blum M, Dworniczak B. The ion channel polycystin-2 is required for left-right axis determination in mice. Curr Biol. 2002;12:938–943. doi: 10.1016/s0960-9822(02)00869-2. [DOI] [PubMed] [Google Scholar]
- Qiu D, Cheng SM, Wozniak L, McSweeney M, Perrone E, Levin M. Localization and loss-of-function implicates ciliary proteins in early, cytoplasmic roles in left-right asymmetry. Dev Dyn. 2005;234:176–189. doi: 10.1002/dvdy.20509. [DOI] [PubMed] [Google Scholar]
- Ramsdell AF. Left-right asymmetry and congenital cardiac defects: Getting to the heart of the matter in vertebrate left-right axis determination. Dev Biol. 2005 doi: 10.1016/j.ydbio.2005.07.038. [DOI] [PubMed] [Google Scholar]
- Raya A, Kawakami Y, Rodriguez-Esteban C, Ibanes M, Rasskin-Gutman D, Rodriguez-Leon J, Buscher D, Feijo JA, Izpisua Belmonte JC. Notch activity acts as a sensor for extracellular calcium during vertebrate left-right determination. Nature. 2004;427:121–128. doi: 10.1038/nature02190. [DOI] [PubMed] [Google Scholar]
- Sakano D, Kato A, Parikh N, McKnight K, Terry D, Stefanovic B, Kato Y. BCL6 canalizes Notch-dependent transcription, excluding Mastermind-like1 from selected target genes during left-right patterning. Dev Cell. 2010;18:450–462. doi: 10.1016/j.devcel.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath K, Cheng AM, Frisch A, Wright CV. Functional differences among Xenopus nodal-related genes in left-right axis determination. Development. 1997;124:3293–3302. doi: 10.1242/dev.124.17.3293. [DOI] [PubMed] [Google Scholar]
- Schlueter J, Brand T. Left-right axis development: examples of similar and divergent strategies to generate asymmetric morphogenesis in chick and mouse embryos. Cytogent Genome Res. 2007;117:256–267. doi: 10.1159/000103187. [DOI] [PubMed] [Google Scholar]
- Schottenfeld J, Sullivan-Brown J, Burdine RD. Zebrafish curly up encodes a Pkd2 ortholog that restricts left-side-specific expression of southpaw. Development. 2007;134:1605–1615. doi: 10.1242/dev.02827. [DOI] [PubMed] [Google Scholar]
- Schweickert A, Weber T, Beyer T, Vick P, Bogusch S, Feistel K, Blum M. Cilia-driven leftward flow determines laterality in Xenopus. Curr Biol. 2007;17:60–66. doi: 10.1016/j.cub.2006.10.067. [DOI] [PubMed] [Google Scholar]
- Serluca FC, Xu B, Okabe N, Baker K, Lin SY, Sullivan-Brown J, Konieczkowski DJ, Jaffe KM, Bradner JM, Fishman MC, Burdine RD. Mutations in zebrafish leucine-rich repeat-containing six-like affect cilia motility and result in pronephric cysts, but have variable effects on left-right patterning. Development. 2009;136:1621–1631. doi: 10.1242/dev.020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimeld SM. Calcium turns sinister in left-right asymmetry. Trends Genet. 2004;20:277–280. doi: 10.1016/j.tig.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Shiratori H, Hamada H. The left-right axis in the mouse: from origin to morphology. Development. 2006;133:2095–2104. doi: 10.1242/dev.02384. [DOI] [PubMed] [Google Scholar]
- Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, Yang Y. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466:378–382. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speder P, Petzoldt AG, Suzanne M, Noselli S. Strategies to establish left/right asymmetry in vertebrates and invertebrates. Curr Opin Genet Dev. 2007;17:351–358. doi: 10.1016/j.gde.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Supp DM, Brueckner M, Kuehn MR, Witte DP, Lowe LA, McGrath J, Corrales J, Potter SS. Targeted deletion of the ATP binding domain of left-right dynein confirms its role in specifying development of left-right asymmetries. Development - supp. 1999;126:5495–5504. doi: 10.1242/dev.126.23.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supp DM, Witte DP, Potter SS, Brueckner M. Mutation of an axonemal dynein affects left-right asymmetry in inversus viscerum mice. Nature. 1997;389:963–966. doi: 10.1038/40140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin CJ. The key to left-right asymmetry. Cell. 2006;127:27–32. doi: 10.1016/j.cell.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Tabin CJ, Vogan KJ. A two-cilia model for vertebrate left-right axis specification. Genes Dev. 2003;17:1–6. doi: 10.1101/gad.1053803. [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Lickert H, Bisgrove BW, Sun X, Yamamoto M, Chawengsaksophak K, Hamada H, Yost HJ, Rossant J, Bruneau BG. Baf60c is a nuclear Notch signaling component required for the establishment of left-right asymmetry. Proc Natl Acad Sci U S A. 2007;104:846–851. doi: 10.1073/pnas.0608118104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SY, Rosenthal J, Zhao XQ, Francis RJ, Chatterjee B, Sabol SL, Linask KL, Bracero L, Connelly PS, Daniels MP, Yu Q, Omran H, Leatherbury L, Lo CW. Heterotaxy and complex structural heart defects in a mutant mouse model of primary ciliary dyskinesia. J Clin Invest. 2007;117:3742–3752. doi: 10.1172/JCI33284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukui T, Capdevila J, Tamura K, Ruiz-Lozano P, Rodriguez-Esteban C, Yonei-Tamura S, Magallon J, Chandraratna RA, Chien K, Blumberg B, Evans R, Belmonte JC. Multiple left-right asymmetry defects in Shh(−/−) mutant mice unveil a convergence of the shh and retinoic acid pathways in the control of Lefty-1. Proc Natl Acad Sci U S A. 1999;96:11376–11381. doi: 10.1073/pnas.96.20.11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Levin M. Perspectives and open problems in the early phases of left-right patterning. Semin Cell Dev Biol. 2009;20:456–463. doi: 10.1016/j.semcdb.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Levin M. Consistent left-right asymmetry cannot be established by late organizers in Xenopus unless the late organizer is a conjoined twin. Development. 2010a;137:1095–1105. doi: 10.1242/dev.041798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Levin M. Far from solved: a perspective on what we know about early mechanisms of left-right asymmetry. Dev Dyn. 2010b;239:3131–3146. doi: 10.1002/dvdy.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick P, Schweickert A, Weber T, Eberhardt M, Mencl S, Shcherbakov D, Beyer T, Blum M. Flow on the right side of the gastrocoel roof plate is dispensable for symmetry breakage in the frog Xenopus laevis. Dev Biol. 2009;331:281–291. doi: 10.1016/j.ydbio.2009.05.547. [DOI] [PubMed] [Google Scholar]
- Yan YT, Gritsman K, Ding J, Burdine RD, Corrales JD, Price SM, Talbot WS, Schier AF, Shen MM. Conserved requirement for EGF-CFC genes in vertebrate left-right axis formation. Genes Dev. 1999;13:2527–2537. doi: 10.1101/gad.13.19.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhiko Y, Imai F, Ookubo K, Takakuwa Y, Shiokawa K, Yokoyama T. Calmodulin binds to inv protein: implication for the regulation of inv function. Dev Growth Differ. 2001;43:671–681. doi: 10.1046/j.1440-169x.2001.00604.x. [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Meno C, Koshiba K, Sugihara M, Itoh H, Ishimaru Y, Inoue T, Ohuchi H, Semina EV, Murray JC, Hamada H, Noji S. Pitx2, a bicoid-type homeobox gene, is involved in a lefty-signaling pathway in determination of left-right asymmetry. Cell. 1998;94:299–305. doi: 10.1016/s0092-8674(00)81473-7. [DOI] [PubMed] [Google Scholar]
- Yost HJ. Development of the left-right axis in amphibians. CIBA Foundation Symposium. 1991;162:165–176. doi: 10.1002/9780470514160.ch10. [DOI] [PubMed] [Google Scholar]
- Yost HJ. Regulation of vertebrate left-right asymmetries by extracellular matrix. Nature. 1992;357:158–161. doi: 10.1038/357158a0. [DOI] [PubMed] [Google Scholar]
- Zeng H, Hoover AN, Liu A. PCP effector gene Inturned is an important regulator of cilia formation and embryonic development in mammals. Dev Biol. 2010;339:418–428. doi: 10.1016/j.ydbio.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Ramalho-Santos M, McMahon AP. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R symmetry by the mouse node. Cell. 2001;106:781–792. [PubMed] [Google Scholar]
- Zhao C, Malicki J. Genetic defects of pronephric cilia in zebrafish. Mech Dev. 2007;124:605–616. doi: 10.1016/j.mod.2007.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.