The night eating syndrome (NES) was first described in 1955 as a disorder defined by morning anorexia, evening hyperphagia (consuming 25% of the daily food intake after the evening meal), and insomnia.1 NES was originally thought to be a maladaptive response to stress in obese persons who were unsuccessful in weight loss treatment.1 Attention to NES was neglected until the late 1990’s, when the focus of eating-related research shifted in response to the growing prevalence of obesity in the United States.2 The increased attention to NES within the past decade has prompted several modifications to its core diagnostic criteria, aided in a better understanding its etiology, and promoted interest in developing effective treatments. This article outlines the basic features of the syndrome and summarizes the treatment approaches that have been developed for NES.
Conceptualization and clinical characteristics
NES has been attributed to a delay in the circadian rhythm of eating, characterized by appetite suppression during morning hours and appetite increase during evening hours.3 The diagnostic criteria of NES have been varied and disputed in the literature. NES is not listed in the DSM-IV-TR,4 although a consensus regarding its core diagnostic criteria was recently reached by a panel of NES experts (First International Night Eating Symposium, April 28, 2008).5 According to proposed criteria, an individual must first endorse evening hyperphagia (consumption of at least 25% of daily intake after the evening meal) and/or ≥ 2 nocturnal ingestions (defined as waking up at night to eat) per week. In addition, an individual must experience at least three of the five following features: morning anorexia (defined as absence of morning appetite); a strong urge to eat between dinner and sleep onset and/or during nocturnal awakenings; insomnia at least four to five times per week; a belief that eating is necessary to initiate or return to sleep; and depressed mood that worsens during evening hours. Finally, an awareness and ability to recall evening or nocturnal ingestions must be present; this criterion is necessary to differentiate NES and Sleep Related Eating Disorder (SRED), a disorder in which nocturnal ingestions occur without awareness and are unable to be recalled.6 The host of symptoms associated with NES makes the syndrome best conceptualized as a combination of eating, sleeping, mood, and stress disorder features. Using the proposed criteria can help standardize NES assessment and diagnosis, and can create a framework for future research of NES characteristics and treatments.
Treatment of night eating syndrome
Research on effective treatments specific to NES has been minimal, with just one randomized, controlled trial published to date. Case reports and open-label trials have suggested benefit from a variety of strategies including pharmacological treatment,7 cognitive behavior therapy8 (CBT) and several other treatment alternatives such as progressive muscle relaxation,9 phototherapy,10 and behavior therapy.11 We present the information currently available, with the recommendation that further research be conducted within each of the treatment modalities to build upon the nascent literature base of effective treatments for NES.
Pharmacological interventions
Pharmacotherapy has received the most research attention of any NES treatment. To date, one case series,12 two open label trials,13,14 and one randomized placebo-controlled trial7 have examined the effectiveness of pharmacotherapy in the reduction of NES symptoms.
In the first pharmacotherapy study of NES, Miyaoka et al.12 followed four NES patients throughout a treatment course of either paroxetine or fluvoxamine (selective serotonin reuptake inhibitors; SSRIs) and found that each patient reported a significant decrease in several core night eating symptoms. O’Reardon et al.13 conducted a 12-week open label trial of sertraline for 17 night eaters and found a significant reduction in the number of awakenings per week, nocturnal ingestions per week, and in the percentage of caloric intake consumed after the evening meal (Table 1). Patients whose NES symptoms remitted also averaged a 5 kg weight reduction at the end of active treatment, while patients whose symptoms did not remit averaged a 0.6 kg weight gain.
Table 1.
Treatment response (% reduction) across treatment trials for NES.
| Study | Treatment delivered |
N | Dur- ation (wks) |
Mean dose |
NESS | % intake after dinner |
# total awakenings |
# nocturnal ingest. |
weight | BDI- II |
QLES- Q |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pawlow et al. 9 | APMR | 10 active, 10 control |
1 | n/a | -- | -- | -- | 0.8 vs. 2.2 in 1 wk |
−0.8 kg 0.3 kg |
50% −5% |
-- |
| O’Reardon et al.13 | Sertraline | 17 | 12 | 188 mg |
24% | 50% | 60% | 67% | − 4.8 kg vs. + 0.6 kga |
33% | −6% |
| Stunkard et al.14 | Sertraline | 50 | 8 | 122 mg |
42% | 66% | 64% | 70% | −3 kgb | 58% | 19% |
| O’Reardon et al.7 | Sertraline or placebo | 17 active, 17 placebo |
8 | 127 mg |
57% 16% |
68% 29% |
74% 14% |
81% 14% |
−2.9 kgb −0.3 kg |
55% 46% |
15% |
| Allison et al.6 | Cognitive behavior therapy | 25 | 10 sessions/ 12 wks |
n/a | 43% | 29% | 37% | 70% | −3.1 kg | 28% | 5% |
Note: NESS – Night Eating Symptom Scale, BDI-II – Beck Depression Inventory-II, QLES-Q – Quality of Life, Satisfaction, and Enjoyment Questionnaire, APMR – abbreviated progressive muscle relaxation.
Mean weight loss for those whose NES remitted on sertraline vs. those who did not respond
Mean weight loss among overweight and obese participants
These results were supported by Stunkard et al.’s14 long-distance treatment trial of 50 NES patients. Patients who contacted the authors’ program through their website consented to participate in an innovative trial in which they would receive treatment with sertraline prescribed by their own physicians. Over the course of the 8-week study, they participated in phone or email check-ins with study staff and completed questionnaires every 2 weeks to assess treatment effect. Significant improvements were noted on all core aspects of NES, including the Night Eating Symptom Scale (NESS),13 nocturnal ingestions, percent of intake after the evening meal, and weight (among overweight and obese participants) (Table 1).
O’Reardon and colleagues7 conducted the first randomized, placebo-controlled trial of sertraline for the treatment of NES. Thirty-four participants diagnosed with NES were randomized to either an 8-week course of sertraline (N = 17) or to a placebo condition (N=17). Sertraline dosages were administered flexibly and ranged from 50mg/day to 200mg/day across participants. The sertraline group endorsed significantly greater reductions than the placebo group in the number of nocturnal ingestions, the number of total awakenings, and scores on the NESS. The percentage of calories consumed after the evening meal was reduced by 68% in the sertraline group and 29% in the control group, although this difference was not significant after statistical correction for multiple comparisons. Overweight patients in the sertraline group lost significantly more weight than overweight patients in the control group (2.9 kg vs. 0.3 kg). Overall, 71% of patients on sertraline were classified as responders on the Clinical Global Impression of Improvement Scale as compared to 18% in the placebo group.
Interestingly, improvements on the primary outcome measures were not significantly correlated with improvements on the Beck Depression Inventory-II (BDI),15 suggesting that the participants did not decrease their night eating symptoms due to an improvement in mood. Instead, it seems that the sertraline had an independent effect on the disordered eating behaviors. The authors suggested that this research demonstrates the use of sertraline as a promising treatment for NES and its associated weight gain.
Sertraline case example
Julie is a high functioning professional. She is married and is the mother of two young adult children. Her daughter had been born prematurely and required 24-hour care for over a year. During this time, Julie began eating while she was up caring for her daughter. Even after her daughter required less and less nighttime attention, Julie’s nightly nocturnal ingestions continued.
Julie sought treatment 18 years after the onset of her nocturnal ingestions. She recalled that her father had also risen from bed to eat. Julie also described herself as a night owl; she would often stay up late at night and would graze on snacks during this time. Coupled with frequent breakfast skipping, she was consuming at least a quarter of her intake after dinner. Her nocturnal ingestions became more frequent, and at her initial evaluation, she was eating three times per night. Typically she would have peanut butter and jelly sandwiches, cookies, fruit, or other snacks or leftovers. As she reached menopause, she found it increasing difficult to lose weight, even though she exercised regularly. At the time she sought treatment her body mass index (BMI) was 31.5 kg/m2, with her weight loss attempts through various, structured programs never yielding more than a 5 lb weight loss.
Julie also reported a history of restless legs syndrome and generalized anxiety disorder. She had been prescribed venlafaxine (75 mg) for her anxiety, gabapentin (600 mg) for her restless legs symptoms, and zolpidem (10 mg) for insomnia and restless legs symptoms. Julie stated that she “had not thought to mention” her night eating symptoms to her sleep doctor when she sought help for her restless legs syndrome and insomnia. After a comprehensive assessment by a psychiatrist with knowledge of NES, it was evident that the venlafaxine was increasing her restless legs symptoms, making it necessary to increase her dose of gabapentin over time, neither of which was helping her nocturnal ingestions. She was tapered from her venlafaxine and simultaneously began taking sertraline. Within the first two weeks, Julie’s nocturnal ingestions decreased to two episodes per week. She currently takes 100 mg of sertraline at dinnertime, and her dose of gabapentin has been decreased to 300 mg. She continues taking zolpidem. Most weeks she has reported no nocturnal ingestions, although break-through ingestions occur occasionally, typically related to pain due to an injury. She is looking forward to having surgery to alleviate this discomfort so that she can continue to experience nights free of eating. She is amazed that after all of these years she can sleep more soundly and start to lose weight more consistently. She still struggles to some extent with after-dinner snacking, but, overall, she also perceives a significant improvement in her control over her eating during this time.
Possible future therapeutic medication targets
The trials with sertraline are promising, but more treatment trials with other SSRIs and other agents are warranted. Some successful case reports of NES treatment with topiramate have been published, but no open label or randomized trials have been conducted. These initial reports have described treatment with topiramate for persons with sleep-related eating disorder, as well as those with NES. In these case descriptions, patients experience significant decreases in nocturnal ingestions, weight, and the percentage of calories consumed after dinner.16,17 Controlled trials of topiramate and/or other similar antiepileptic agents may be warranted. Particular attention should be given to the neurological side-effects that may accompany topiramate, such as confusion, memory difficulties, and difficulty concentrating. If these occur, the medication must be discontinued.
Contraindications
O’Reardon et al.13 assessed previous medication trials for each of the participants presenting in their NES study. Not many of these trials had an effect, but of note, none of the participants described success with zolpidem or over the counter hypnotics. In fact, the participants reported that these agents promoted SRED. These medications helped them to fall asleep initially, but they still rose from bed during the night seeking food. The participants described little to no recollection of their eating during these occasions, and they found evidence of consuming larger amounts of foods that they typically did not eat when not taking the hypnotics. Others have since described this phenomenon in case studies. e.g., 18, 10 While the patient in our case study, Julia, above did not experience SRED while taking zolpidem, if night eating is the presenting concern, hypnotics should not be the first line treatment. If patients are already taking a hypnotic, an assessment of the onset of nocturnal eating and level awareness should be determined.
Cognitive Behavior Therapy
The first study to investigate cognitive behavioral therapy (CBT) for NES was conducted by Allison and colleagues8 in response to the observed cognitive component of night eating behavior. A central feature of this disorder is, “If I don’t eat, I won’t be able to fall asleep.” Vinai and colleagues have found that this thought distinguishes persons with NES from those with binge eating disorder.20 Allison et al.8 designed a cognitive behavioral treatment to target NES-specific symptoms by adapting features from CBT protocols for binge eating disorder21 and behavioral weight loss.22 The primary goal of CBT for NES is to correct the delay in circadian eating rhythms by shifting food intake to earlier in the day, while simultaneously interrupting the overlearned relationship between night time eating, faulty cognitions, and sleep onset.23 The strategies used to achieve these treatment goals include a combination of behavioral weight management components (monitoring food consumption, regulating meals and snacks, restricting daily caloric intake) and cognitive therapy components (identifying, evaluating, and restructuring maladaptive thoughts).
Allison et al.8 studied 25 participants with NES who were enrolled in a 10-session, uncontrolled CBT intervention that lasted 12 weeks; 14 participants completed the treatment. It is difficult to know with certainty why the attrition rate was so high, but clinical observations suggested factors that included: 1) feeling overwhelmed with keeping a food and sleep log each day; and 2) not losing more weight than desired in the early weeks. Future studies should stress the importance of daily logging and should set realistic weight management goals to help maximize treatment adherence.
Similar to observations from the sertraline treatment studies, significant reductions in all primary and secondary treatment outcomes were noted following completion of the 10-week CBT program, including reductions in the proportion of calories consumed after dinner, falling from 35% to 25%, and the number of nocturnal ingestions, falling from 8.7 per week to 2.6 ingestions per week (see Table 1). Mixed modeling regression models were used to account for participant drop-out. Closer examination of night time eating showed a significant reduction in the proportion of calories consumed between sleep onset and morning awakening (15% to 5%), but not in the proportion of calories consumed between the evening meal and sleep onset (21.8% to 21.0%). Results from secondary outcome measures indicated significant decreases in daily caloric intake (2365 kcal/d to 1759 kcal/d), total number of awakenings (13.5/wk to 8.5/wk), body weight (−3.1kg), and NESS scores (29 to 16). Finally, BDI-II scores significantly decreased (9 to 6.5) and QLES-Q scores significantly increased (47.2 to 49.6), suggesting improved mood and quality of life, respectively. However, these latter improvements are small in magnitude when examining the clinical relevance of these changes. Study limitations, including the high attrition rate and the absence of a control group, require a tentative interpretation of the findings until these results are replicated under more controlled settings.
The implications from this research are promising and highlight the need for future randomized controlled trials. In particular, the CBT treatment seemed to be more effective in reducing eating during the night, than in reducing the proportion of eating before bedtime. This suggests that more time during sessions may be dedicated to addressing eating during the evening. It may be that more distress is associated with eating during the night, such that more effort is expended trying to eliminate that behavior. Future CBT studies for this population should improve intervention efforts in shifting this evening eating to earlier in the day.
Although food log data are imperfect, Allison et al.’s8 study suggested that patients can engage in behavioral weight loss while decreasing their nocturnal ingestions. The data revealed that participants had reduced their total daily intake by about 600 kcal/day, starting after week 3 when calorie counting was introduced. Given this information, the lack of change in the proportion of intake after the evening meal suggests that the circadian delay in energy intake remained present at the end of treatment, although not as pronounced when nocturnal ingestions were more frequent. It therefore appears that these individuals may benefit from maintaining their current level of energy intake but distributing it more evenly across breakfast, lunch, and daytime snacks. A recent study suggests that a positive and independent relationship exists between intake after 8:00 pm and BMI,24 although larger studies are needed to confirm this finding.
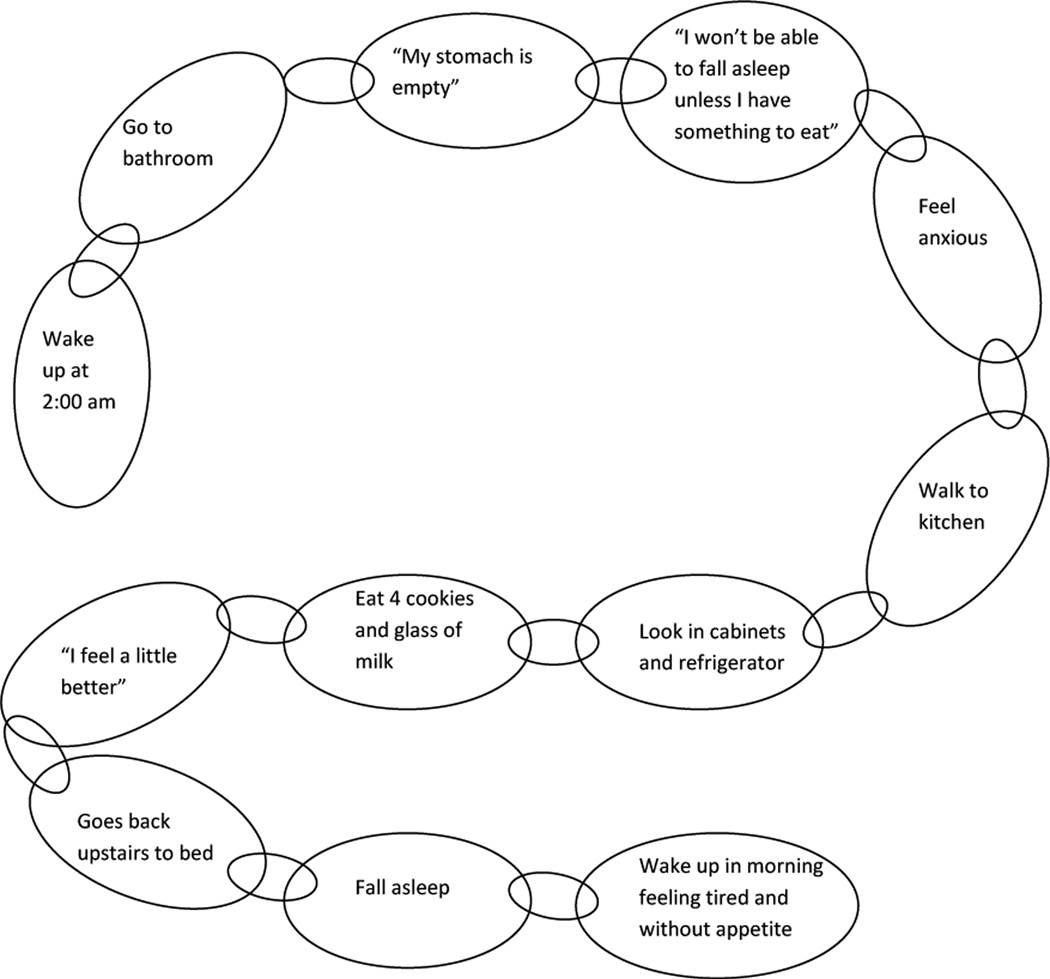
CBT case example (Fig. 1)
Figure 1.
Behavioral chain for Carol’s typical night eating episode.
Carol presented for treatment of her NES, stating that her night eating began in her teen years. She offered that both of her parents had NES, and that her husband also now gets up to eat with her. When she married her husband and first lived independently at the age of 20, she did not think her NES was significant, because her parents did it too. Now in her 40s, the behavior had become particularly distressing because she was having trouble controlling her weight, which had risen to a BMI of 26 kg/m2 from 21 kg/m2 that she had maintained since the birth of her children about 20 years prior.
Carol woke most days before 7 am. She drank coffee, but did not eat until the afternoon, sometime between 12 – 2 pm; a couple days per week she did not eat anything until dinnertime. She prepared a home-cooked meal for dinner at 6 pm. Carol enjoyed coffee afterwards, but generally did not snack. Her bedtime has varied over the years, but at treatment intake she was going to bed at 9 or 10 pm without any initial insomnia. Then, every night at 2 am, she awoke and felt compelled to eat. She would occasionally use the bathroom first, but then headed to the kitchen where she would eat milk and cookies, pasta, peanut butter and jelly, and/or raisins. She reported that before she goes to bed, she knows she will wake up to eat. She makes it a priority to have food on hand, even when traveling. She stated that she would surely be up all night if she did not have something to eat. She described her experience as feeling the need for her stomach to be full before she can go back to sleep.
At treatment baseline, she was consuming 25.1% of her intake after dinner, and she reported 6 awakenings with 6 nocturnal ingestions in the previous week. Her average daily caloric intake from her food and sleep journal was 2078 kcals, and her baseline NESS score was a 29. She reported no significantly depressed mood, with a BDI score of 5.
Carol successfully started using stimulus control techniques by ridding her kitchen of the foods she most often ate during the night. Living with her husband and son prevented complete control of these foods, but her nocturnal ingestions decreased slowly and consistently across the weeks. She also worked to eat earlier in the day and to monitor her caloric intake while aiming for a goal of 1200–1500 kcals per day. She also engaged in cognitive restructuring regarding her beliefs about her need to eat during the night. We framed our approach with the idea that she needed to re-train her body not to expect food during the night.
At the end of the 10 sessions, Carol reported her first week without any nocturnal ingestions. Her proportion of calories consumed after dinner had decreased to 16.3%, based on her food journal, and her NESS score decreased to 13. Her BDI score stayed low throughout treatment, ranging between a score of 0–3. Carol moved out of the country after treatment. At an 8-month follow-up, her NESS score remained at a 14, and she was consuming 14% of her intake after dinner. However, she reported four awakenings and two nocturnal ingestions in that previous week, suggesting that she could have benefitted from a longer duration of treatment or occasional booster sessions.
Behavioral and light therapies
Behavior therapy
Therapies traditionally used for the treatment of mood and anxiety disorders, such as behavior therapy, may also be adapted to successfully treat NES. To date, two case study reports have examined the effectiveness of behavioral therapy for NES. Coates25 reported partial success for one NES client through behavioral techniques like leaving notes, restricting access to food, and chaining the refrigerator closed. After several modifications to his behavioral prevention plan, the client’s night eating symptoms remitted and his morning anorexia improved. This behavioral plan remained effective in controlling the client’s night eating symptoms at an 18-month follow up, but his symptoms reportedly returned if his plan was not in place.
Williamson and colleagues26 designed a behavior contingency plan to target night eating symptoms in a woman with bulimia nervosa and rumination. This contingency plan was designed to extinguish the client’s night eating by rewarding increasingly difficult treatment goals (e.g., one night/week without a binge episode, two nights/week without a binge episode, etc.). Although the client experienced several lapses in the first phase of treatment, her symptoms remitted completely by treatment end and she remained symptom-free at 3-month and 2-year follow-up assessments. Because the client was also undergoing cognitive behavioral group therapy for her bulimia symptoms, the authors cautioned against drawing firm conclusions about the contingency plan’s unique impact on her night eating symptoms.
As NES has been associated with obesity, behavioral weight management may also represent a possible treatment approach. Gluck and colleagues27 reported that patients with NES did not lose as much weight in a behavioral weight loss group as patients without NES. More recently, however, Dalle Grave and colleagues11 compared obese night eaters pursuing weight loss treatment and matched controls following intensive inpatient weight loss treatment. Treatment included psychoeducation, a low calorie diet, and regular exercise. At program completion, patients with NES and matched controls did not differ in weight loss at program completion or at the 6-month follow-up assessment. However, only 8 of the 32 patients with NES continued to endorse clinically significant night eating symptoms at the 6-month follow up. Although night eating symptoms were not a specific treatment target, the authors posited that the intensity of the dietary and behavioral weight loss strategies effectively treated NES by reducing night time hunger and resetting circadian eating rhythms. Dalle Grave et al.’s11 results suggest a promising future for behavioral weight management in NES treatment, although future studies should explore the efficacy of less intensive programs.
Progressive muscle relaxation
Progressive muscle relaxation (PMR) is a therapeutic technique designed to achieve muscular relaxation through the tension and release of various muscle groups.28 Additionally, PMR has been shown to alleviate a variety of cognitive and physical conditions including anxiety29 and, psychological and physiological stress.30 Pawlow, O’Neil, and Malcolm9 conducted the first controlled treatment trial of PMR for NES. Ten participants who met criteria for NES and received one week of abbreviated progressive muscle relaxation (APMR) were compared to a control group of NES participants who did not receive the APMR intervention. The intervention group demonstrated a significant reduction in evening hyperphagia and increase in morning appetite. The treatment group also evidenced reductions in stress, anxiety, fatigue, anger, depression and cortisol levels. The authors concluded that APMR represents a valid treatment choice for NES patients.
A major limitation of this trial was its brevity. Future studies should follow adherence and outcome over a longer period of time. This criticism applies to all of the trials described thus far. None has demonstrated efficacy longer than 12 weeks, which is a clear weakness of this literature.
Phototherapy
Phototherapy was first introduced as a treatment for seasonal affective disorder in 1984 by Rosenthal and colleagues.31 After witnessing changes in mood that followed seasonal patterns, Rosenthal et al.31 proposed that melatonin played a role in the onset of seasonal depression, and that adjusting the levels of melatonin in the body would lead to symptom improvement. Because NES has been conceptualized as a disorder of circadian rhythm, and because melatonin is involved in the regulation of circadian rhythms, phototherapy has been proposed as a logical treatment choice for NES.
Two case studies of phototherapy for NES have been published. In one, a 51-year-old obese woman presented with comorbid depression and night eating; she was already receiving pharmacotherapy (paroxetine) for her symptoms, but it was not effective in significantly reducing them. She was exposed to 30 minute sessions of 10,000 lux white light therapy each morning for a period of 2 weeks.32 After completing her phototherapy program, the client no longer met clinical criteria for major depression or night eating. Although the woman’s night eating symptoms returned at the one month follow-up assessment, they fully remitted following 12 additional days of phototherapy.
A second case study involved a 46-year-old non-obese man presenting to treatment with non-seasonal recurrent major depression and NES.10 This client was prescribed 30 minutes of phototherapy (10,000 lux) every morning for 14 days. Upon completing the 2 week phototherapy program, both depressive and NES symptoms significantly improved, and the client no longer met diagnostic criteria for either disorder. The results of these case studies suggest that phototherapy may have a role in treating comorbid depression and NES. However, future research is needed to confirm the validity of these initial findings, as well as the effect on NES for patients presenting without co-morbid depression.
Summary
Although treatment research for NES remains quite limited, several options are available for patients whose symptoms require clinical attention. Pharmacotherapy has received the most empirical support of the proposed treatments. Controlled trials are needed to confirm the initial results from pilot studies with CBT,, behavioral therapy, and phototherapy, and an extended controlled trial of progressive muscle relaxation would be useful.
In their comprehensive review of the field, Striegel-Moore and colleagues have questioned the clinical utility of NES as a diagnostic entity and stress the very limited nature of treatment studies to date.33 Research in this field has to provide a systematic examination of the approaches described here, as well as others yet to be identified. This pursuit seems warranted given that persons suffering with the cluster of symptoms identified as NES are approaching healthcare providers for relief and are often frustrated by the lack of recognition of this syndrome.34
Future studies should test a wider variety of medications that would target serotonin or the circadian timing of eating. Additionally, trials comparing and combining medication treatments and CBT (or progressive muscle relaxation alone) would also be useful in addressing which treatment should be used as a first line treatment. With NES being considered for inclusion as a Feeding and Eating Condition Not Elsewhere Classified (NEC-FEC) in the DSM 5 (www.dsm5.org), it is likely that more clinical attention and studies will address these important issues in the coming years.
Synopsis.
This article reviews the research published to date for night eating syndrome. These include trials of selective serotonin reuptake inhibitors, cognitive behavior therapy, behavior therapy, abbreviated progressive muscle relaxation, and light therapy. Case examples are included to illustrate the clinical features and response patterns of those who present for treatment of night eating syndrome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kelly C. Allison, Department of Psychiatry, Perelman School of Medicine at the University of Pennsylvania, 3535 Market St., Philadelphia, PA 19104-3309; kca@mail.med.upenn.edu; 215-898-2823.
Ellen Tarves, Department of Psychology, LaSalle University, Philadelphia, PA; tarvese1@student.lasalle.edu.
References
- 1.Stunkard AJ, Grace WJ, Wolff HG. The night-eating syndrome: a pattern of food intake among certain obese patients. Am J Med. 1955;19:78–86. doi: 10.1016/0002-9343(55)90276-x. [DOI] [PubMed] [Google Scholar]
- 2.Birketvedt GS, Florholmen J, Sundsfjord J, et al. Behavioral and neuroendocrine characteristics of the night-eating syndrome. JAMA. 1999;282:657–663. doi: 10.1001/jama.282.7.657. [DOI] [PubMed] [Google Scholar]
- 3.Stunkard AJ, Allison KC. Two forms of disordered eating in obesity: binge eating and night eating. Int J Obesity. 2003;27:1–12. doi: 10.1038/sj.ijo.0802186. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Text Revision. 4th edition. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 5.Allison KC, Lundgren JD, O’Reardon J, et al. Proposed diagnostic criteria for night eating syndrome. Int J Eat Disord. 2010;43:241–247. doi: 10.1002/eat.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howell MJ, Schenck CH, Crow SJ. A review of nighttime eating disorders. Sleep Med. 2009;13:23–34. doi: 10.1016/j.smrv.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 7.O’Reardon JP, Allison KC, Martino NS, et al. A randomized placebo controlled trial of sertraline in the treatment of the night eating syndrome. Am J Psychiatry. 2006;163:893–898. doi: 10.1176/ajp.2006.163.5.893. [DOI] [PubMed] [Google Scholar]
- 8.Allison KC, Lundgren J, Moore RH, et al. Cognitive behavior therapy for night eating syndrome: a pilot study. Amer J Psychotherapy. 2010;64:91–106. doi: 10.1176/appi.psychotherapy.2010.64.1.91. [DOI] [PubMed] [Google Scholar]
- 9.Pawlow LA, Jones GE. The impact of abbreviated progressive muscle relaxation on salivary cortisol. Biol Psychol. 2002;60:1–16. doi: 10.1016/s0301-0511(02)00010-8. [DOI] [PubMed] [Google Scholar]
- 10.Friedman S, Even C, Dardennes R, Guelfi JD. Light therapy, non-seasonal depression, and night eating syndrome. Can J Psychiatry. 2004;49:790. [PubMed] [Google Scholar]
- 11.Dalle Grave R, Calugi S, Ruocco A, Marchesini G. Night eating syndrome and weight loss outcome in obese patients. Int J Eat Disord. 2011;44:150–156. doi: 10.1002/eat.20786. [DOI] [PubMed] [Google Scholar]
- 12.Miyaoka T, Yasukawa R, Tsubouchi K, et al. Successful treatment of nocturnal eating/drinking syndrome with selective serotonin reuptake inhibitors. Int J Clinical Psychopharm. 2003;18:175–177. doi: 10.1097/01.yic.0000068440.56680.8e. [DOI] [PubMed] [Google Scholar]
- 13.O’Reardon JP, Stunkard AJ, Allison KC. A clinical trial of sertraline in the treatment of the night eating syndrome. Int J Eat Disord. 2004;35:16–26. doi: 10.1002/eat.10224. [DOI] [PubMed] [Google Scholar]
- 14.Stunkard AJ, Allison KC, Lundgren JD, et al. A paradigm for facilitating pharmacotherapy at a distance: Sertraline treatment of the night eating syndrome. J Clin Psychiatry. 2006;67:1568–1572. doi: 10.4088/jcp.v67n1011. [DOI] [PubMed] [Google Scholar]
- 15.Beck AT, Steer RA, Brown GK. BDI-II Manual. San Antonio, TX: Harcourt Brace; 1996. [Google Scholar]
- 16.Winkelman JW. Treatment of nocturnal eating syndrome and sleep-related eating disorder with topiramate. Sleep Med. 2003;4:243–246. doi: 10.1016/s1389-9457(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 17.Allison KC. Symposium talk presented at the annual meeting of the Eating Disorders Research Society. Toronto, Canada: 2005. Treatment of the night eating syndrome. [Google Scholar]
- 18.Morgenthaler TI, Silber MH. Amnestic sleep-related eating disorder associated with zolpidem. Sleep Med. 2002;3:323–327. doi: 10.1016/s1389-9457(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 19.Najjar M. Zolpidem and amnestic sleep related eating disorder. J Clin Sleep Med. 2007;3:637–638. [PMC free article] [PubMed] [Google Scholar]
- 20.Vinai P, Allison KC, Cardetti S, et al. Psychopathology and treatment of night eating syndrome: a review. Eat Weight Disord. 2008;13:54–63. doi: 10.1007/BF03327604. [DOI] [PubMed] [Google Scholar]
- 21.Fairburn CG, Marcus MD, Wilson GT. Cognitive-behavioral therapy for binge eating and bulimia nervosa: A comprehensive treatment manual. In: Fairburn CG, Wilson GT, editors. Binge Eating: Nature, Assessment, and Treatment (pp. 361–404) New York: Guilford Press; 1993. pp. 361–404. [Google Scholar]
- 22.Brownell KD. The LEARN Program for Weight Management. 10th edition. Dallas: LEARN – The Lifestyle Company; 2004. [Google Scholar]
- 23.Allison KC, Stunkard AJ. Treatment for night eating syndrome. In: Grilo CM, Mitchell JE, editors. The Treatment of Eating Disorders. New York: Guilford Press; 2010. pp. 458–470. [Google Scholar]
- 24.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity. doi: 10.1038/oby.2011.100. in press. [DOI] [PubMed] [Google Scholar]
- 25.Coates TJ. Successive self-management strategies towards coping with night eating. J Behav Therapy Exper Psychiatry. 1978;9:181–183. [Google Scholar]
- 26.Williamson DA, Lawson OD, Bennett SM, Hinz L. Behavioral treatment of night bingeing and rumination in an adult case of bulimia nervosa. J Behav Therapy Experiment Psychiatry. 1989;20:73–77. doi: 10.1016/0005-7916(89)90010-4. [DOI] [PubMed] [Google Scholar]
- 27.Gluck ME, Geliebter A, Satov T. Night eating syndrome is associated with depression, low self-esteem, reduced daytime hunger, and less weight loss in obese outpatients. Obes Res. 2001;9:264–267. doi: 10.1038/oby.2001.31. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson E. Progressive Relaxation. Second Edition. Oxford, England: University of Chicago Press; 1938. [Google Scholar]
- 29.Conrad A, Roth WT. Muscle relaxation therapy for anxiety disorders: It works but how? J Anxiety Disord. 2007;21:243–264. doi: 10.1016/j.janxdis.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Pawlow LA, O’Neil PM, Malcolm RJ. Night eating syndrome: Effects of brief relaxation training on stress, mood hunger and eating patterns. Int J Obesity Rel Metab Disord. 2003;27:970–978. doi: 10.1038/sj.ijo.0802320. [DOI] [PubMed] [Google Scholar]
- 31.Rosenthal NE, Sack DA, Gillin C, et al. Seasonal affect disorder: a description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- 32.Friedman S, Even C, Dardennes R, Guelfi JD. Light therapy, obesity, and night-eating syndrome. Am J Psychiatry. 2002;159:875–876. doi: 10.1176/appi.ajp.159.5.875. [DOI] [PubMed] [Google Scholar]
- 33.Striegel-Moore RH, Franko D, Garcia J. The validity and utility of night eating syndrome. Int J Eat Disord. 2009;42:720–738. doi: 10.1002/eat.20721. [DOI] [PubMed] [Google Scholar]
- 34.Goncalves MD, Moore RH, Stunkard AJ, Allison KC. The treatment of night eating: the patient’s perspective. Eur Eat Disord Rev. 2009;17:184–190. doi: 10.1002/erv.918. [DOI] [PubMed] [Google Scholar]