SUMMARY
The complement system, which functions by lysing pathogens directly or by promoting their uptake by phagocytes, is critical for controlling many microbial infections. Here we show that in Streptococcus pneumoniae, increasing bacterial chain length sensitizes this pathogen to complement deposition and subsequent uptake by human neutrophils. Consistent with this, we show that minimizing chain length provides wild-type bacteria with a competitive advantage in vivo in a model of systemic infection. Investigating how the host overcomes this virulence strategy, we find that antibody promotes complement-dependent opsonophagocytic killing of Streptococcus pneumoniae and lysis of Haemophilus influenzae independent of Fc-mediated effector functions. Consistent with the agglutinating effect of antibody, F(ab′)2 but not Fab could promote this effect. Therefore, increasing pathogen size, whether by natural changes in cellular morphology or via antibody-mediated agglutination, promotes complement-dependent killing. These observations have broad implications for how cell size and morphology can affect virulence among pathogenic microbes.
INTRODUCTION
Streptococcus pneumoniae (the pneumococcus) is a leading cause of invasive bacterial infection (O’Brien et al., 2009). This pathogen was previously known as Diplococcus pneumoniae because it is typically seen clinically in the form of diplococci or short bacterial chains. This is in contrast to closely related streptococci, however, which typically appear as long bacterial chains. The underlying reason for this difference in bacterial chain length between pneumococci and other streptococci, however, has remained unclear.
Pneumococcal infections are controlled by host neutrophils, which kill this pathogen via opsonophagocytosis (OPH), a process that requires opsonization of bacteria by the complement system (Dalia et al., 2010; Lysenko et al., 2007; Matthias et al., 2008). Activation of this system results in the covalent deposition of complement component 3 (C3) onto bacterial surfaces (Lambris et al., 2008). On gram-negative bacteria, this can lead to direct complement-mediated lysis of cells, while gram-positives are resistant to lysis due to their thick peptidoglycan layer. However, C3 can also interact with complement receptors on neutrophils to promote phagocytosis. Deposition of C3 onto pneumococci can result from activation of either the classical or alternative pathways (Brouwer et al., 2008). Activation of the classical pathway can be directed to bacterial surfaces with the aid of antibodies, while the alternative pathway stochastically activates complement on bacterial surfaces. Once opsonized, bacteria can be recognized by surface receptors on neutrophils and ingested by phagocytosis. Once internalized, S. pneumoniae is efficiently killed in the phagolysosome (Standish and Weiser, 2009).
Pneumococci resist opsonization by complement due to their surface capsular polysaccharide (Hyams et al., 2010), which masks underlying structures and activates complement poorly. In addition to capsule, the pneumococcus has surface proteins that directly interact with serum components to evade complement and subsequent phagocytosis (Dalia et al., 2010; Jarva et al., 2003). To identify additional factors that promote resistance to this mechanism of killing, we screened a genomic library for mutants that were more sensitive to OPH killing. A common phenotype among mutants identified by this screen was an increase in bacterial chain length (CL). This lead us to hypothesize that minimization of CL in S. pneumoniae enhances resistance to OPH killing.
RESULTS
Increased CL enhances susceptibility to OPH killing
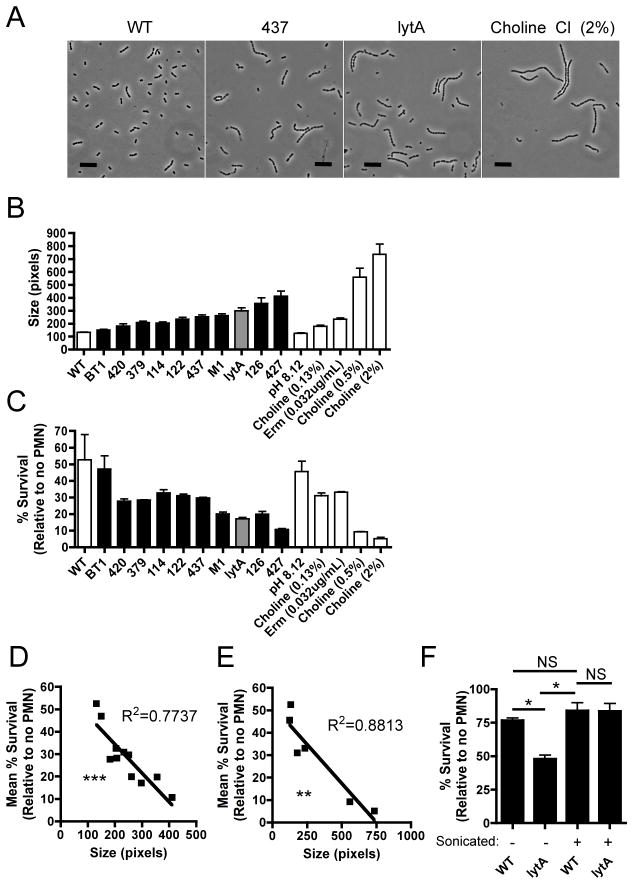
A library of pneumococcal mutants was created using the mariner transposon and screened for increased susceptibility to OPH killing by human neutrophils. In addition to genes affecting capsular polysaccharide expression, some of the genes identified by this screen encoded putative cell wall components or regulatory factors (Table S1). A common phenotype among many of these mutants was an increase in bacterial CL (Figure 1, A and B). The size of bacterial chains was defined as their two-dimensional area in phase contrast images and was used as a proxy for CL (Figure 1B). Mutant strains from the screen displayed varying degrees of chain formation, and as CL increased, resistance to OPH killing decreased (Figure 1, B and C) and this trend was highly significant (Figure 1D). A negative control was the BT1 mutant, which contains the mariner transposon, but does not display increased chain length and was not more susceptible to OPH killing (Figure 1, B and C). Since chain formation results from the incomplete cleavage of peptidoglycan between daughter cells following cell division, a positive control for increased CL was a mutant with an in-frame deletion in lytA, which encodes the major cell wall murein hydrolase in the pneumococcus (Figure 1, B and C) (Tomasz, 1968). Growth of pneumococci in varying concentrations of choline, which causes release of LytA and other choline binding proteins from the cell surface, and in sub-inhibitory concentrations of the bacteriostatic antibiotic erythromycin (Erm), also induced chains of increasing length, providing an independent way to confirm the effects of CL (Figure 1, A and C) (Tomasz, 1968). OPH killing assays performed with the WT grown under these conditions or with the lytA mutant (Figure 1, B and C) confirmed that as CL increased, resistance to OPH killing decreased (Figure 1E).
Figure 1. Increased chain length enhances susceptibility to killing by human neutrophils ex vivo.
Chain formation was assessed among mutant strains and growth conditions by phase contrast microscopy (Scale bar = 10 μm) (A). Details of bacterial strains and mutants are described in Table S1. Chain formation was induced in the WT by growth in varying concentrations of choline chloride or in the presence of sub-inhibitory concentrations of erythromycin (Erm). The size of bacterial chains was calculated from phase contrast images and is shown for mutant strains (black bars), a defined lytA mutant (gray bar), and culture conditions used to induce chain formation in the WT (white bars) (B). Data are the result of at least 100 chains analyzed per sample ±SEM. The same bacterial cultures analyzed in B were used in opsonophagocytic (OPH) killing assays where bacteria were opsonized in a final concentration of 66% baby rabbit serum, which lacks specific anti-pneumococcal antibodies, followed by incubation with human neutrophils (PMNs) (C). Data are from one of two representative experiments performed in duplicate ±Range. Linear regressions showing the trend between size (from B) and percent survival (from C) in OPH assays for mutant strains (D) and conditions that induce chain formation in the WT (E). Each data point in D and E represents the mean size and mean percent survival for one strain or condition tested. OPH killing assays were performed where WT and lytA mutant bacteria were mildly sonicated to mechanically disrupt bacterial chains towards diplococcal morphology prior to opsonization in BRS at a final concentration of 66% (F). Mechanical disruption of chains towards a diplococcal morphology by sonication was confirmed by analysis of phase contrast images. Data from unsonicated samples are from one of two representative experiments performed in duplicate ±Range and data for sonicated samples are from one of two representative experiments and the result of 4 independently sonicated biological replicates performed in duplicate ±Range. See also Figure S1. NS = not significant, *=P<0.05, **=P< 0.01, and ***=P<0.001.
Since some of the genes identified by our screen have putative roles in peptidoglycan synthesis and modification, it is formally possible that our mutant strains are more susceptible to OPH killing due to an altered peptidoglycan structure and not due to their increased chain length (Table S1). To distinguish between these possibilities, we performed OPH killing assays where WT and lytA mutant bacteria were mechanically disrupted towards a diplococcal morphology. Under these conditions, the lytA mutant was rendered as resistant to OPH killing as the WT strain (Figure 1F). As mechanical disruption should not alter the chemical structure of the cell wall in these chains, we attributed the increased sensitivity of a lytA mutant to its increased size (chain length). In addition to this phenotypic complementation, we generated a lytA revertant strain (lytA::pE916), and found that it chained significantly less than the lytA mutant and was significantly more resistant to OPH killing (Figure S1).
Capsular polysaccharide expression is one of the primary mechanisms the pneumococcus uses to resist complement deposition and subsequent phagocytic killing. To determine if mutant strains were more sensitive to opsonophagocytic killing due to decreased expression of capsular polysaccharide, we performed quantitative capture ELISA assays and have determined that our mutant strains expressed WT levels of capsule (data not shown). Thus, differences in capsular polysaccharide expression cannot account for the increased sensitivity of mutant strains in our OPH killing assays.
Increased CL enhances susceptibility to uptake by human neutrophils
To determine the mechanism for how CL affects resistance to OPH killing, we first assessed uptake by human neutrophils by performing competitive neutrophil association assays. When the lytA mutant was competed against the WT, it was significantly more cell-associated, suggesting that longer chains were more effectively taken up by host neutrophils (Figure 2A). Similar results were also obtained when analyzed by microscopy (Figure 2B). Additionally, phagocytosis was likely required for this effect since cell association was almost completely abrogated and the competitive differences lost in reactions where neutrophils were pre-incubated with cytochalasin D, an inhibitor of actin polymerization (data not shown). Complement activity in serum is heat-labile, and when strains of increasing CL were opsonized in heat-inactivated serum, OPH killing was completely abrogated, indicating that complement was required for phagocytosis in the context of CL (Figure 2C).
Figure 2. Increased chain length enhances association of bacteria with neutrophils and is dependent on complement activity.
To determine the effect of chain length on neutrophil association, competitive assays were performed between bacterial cultures of the WT and lytA mutant labeled with either FITC or Cy5 (A). To detect cell association, samples were examined by flow cytometry and the percentage of neutrophils with a positive fluorescent signal (FITC or CY5) was determined. The output ratio of FITC:CY5 labeled PMNs was then normalized to the input ratio of FITC:CY5 labeled bacteria to calculate a competitive index value (CI). In these assays, a CI of less than 1 indicates that the Cy5 labeled strain is more cell associated than the FITC labeled strain, while CI values of greater than 1 indicate that the FITC labeled strain is more cell associated than the Cy5 labeled strain. The dotted line indicates a CI of 1 – the value expected if strains compete equally well. Similar experiments were performed where the ratio of bacteria:PMNs was changed from ~2:1 to ~50:1 and then analyzed by phase contrast and fluorescence microscopy (Scale bar = 10 μm)(B). FITC labeled WT (green) and Cy5 labeled lytA mutant bacteria (red) are distinguished from the auto fluorescence of neutrophils by the size and shape of stained particles. The importance of complement in OPH killing of pneumococci was determined by performing OPH killing assays where bacteria were opsonized in either active (BRS) or heat-inactivated (HI BRS) serum (66%) (C). The triangle above columns indicates the trend of chain length among the strains or conditions tested. All data are from at least two independent experiments performed in duplicate ±SEM. NS=not significant, **=P<0.01, ***=P<0.001.
Increased CL promotes complement deposition on pneumococci
Since active complement was required for OPH killing, we assessed the impact of CL on complement deposition. Within a population of pneumococci, complement deposition is bimodal, where chains either have C3 bound or do not (Figure 3A) (Brown et al., 2002). Therefore, we hypothesized that longer chains of the pneumococcus would be more likely to have complement bound compared to shorter chains and diplococci. Studying either the mutant strains or by inducing chain formation in the WT, increased CL correlated with an increased likelihood of having C3 bound, and this trend was highly significant (Figure 3B). When grown in tryptic soy broth, the WT strain produced chains of varying length, allowing us to examine of the effect of CL on complement deposition within a clonal population. Even within a WT population, we found that longer chains of pneumococci were more likely to have C3 deposited on their surface (Figure 3D). This trend was also observed when human serum was used as a source of complement to opsonize bacteria (Figure 3E). Chain formation in the WT is not only evident when pneumococci are grown in tryptic soy broth, but was also observed when pneumococci were grown in serum, a physiologically relevant growth medium. Growth of the WT strain in serum induced a small proportion (~1%) of the population to form long bacterial chains (Figure 3A). This phenomenon of long chain formation in the WT was also observed when human serum was used as a growth medium (data not shown). As observed for long chain mutant strains, the long chains produced by growth of the WT in serum were more likely to have C3 bound compared to shorter chains in the population (Figure 3F).
Figure 3. Increased CL promotes complement deposition on pneumococci.
Complement deposition on WT pneumococci grown in heat-inactivated baby rabbit serum (HI BRS) was analyzed by immunofluorescence microscopy using a FITC-labeled antibody to C3 (Scale bar = 10 μm)(A). Open arrows indicate pneumococci that have no complement bound. C3 deposition on pneumococci was compared among the same mutant strains and growth conditions defined in Figure 1B by immunofluorescence microscopy after pneumococci were incubated in baby rabbit serum (BRS) at a final concentration of 66%. Data is presented as a linear regression to show the trend between the percentages of chains positive for C3 and chain length (size) (B). Also shown, is a linear regression to analyze the trend between the C3 deposited per chain and chain length (C). Each data point in this plot represents the mean size and mean C3 deposited per chain for each strain or condition tested, where each data set was normalized relative to the WT. The slope is indicated on the plot ±SE. Complement deposition was also analyzed within a clonal population of the WT. Bacteria were grown in tryptic soy broth (TS) and opsonized in BRS at a final concentration of 66% (D) or an equivalent concentration of normal human serum (E). WT bacteria were also grown in 100% HI BRS followed by opsonization in BRS at a final concentration of 66%. For quantitative analysis of C3 deposition in D, E, and F, data were binned by size (diplococci 50–100 pixels). All data are from one of at least two representative experiments and the result of at least 100 chains analyzed per sample ±SEM. ***=P<0.001.
Once C3 is deposited on a surface, it can spread via the alternative pathway of complement activation. This has been observed in the fungal pathogen Cryptococcus neoformans, as well as on artificial surfaces (Hed et al., 1984; Kozel et al., 1991; Rosas et al., 2002). To determine if complement can spread on pneumococci, we assessed the trend between the C3 deposited per chain and chain length between our mutant strains and conditions where chain formation was induced in the WT. To simplify this analysis, both data sets were normalized to the WT (Figure 3C). If C3 deposition cannot spread from a focus of complement activation, we would expect this trend to have a slope of 1, indicating that longer chains bind complement proportionately to their increased CL. If C3 deposition can spread from a focus of complement activation on pneumococci, however, we would expect this trend to have a slope of >1, which would indicate that as CL increased there was a disproportionate increase in the amount C3 deposited per chain. When we perform this analysis, we find that the slope is significantly >1, consistent with the hypothesis that C3 deposition can spread on pneumococci from a focus of complement activation (Figure 3C). Additionally, when assessing C3 deposition on pneumococci by immunofluorescence microscopy, it is clear that long bacterial chains within the population are almost fully opsonized by C3, while some diplococci in the population remain free of complement, which is also consistent with the hypothesis that C3 deposition can spread from a focus of complement activation (Figure 3A).
Together, these data indicate that longer chains of the pneumococcus have an increased likelihood of attaining a focus of complement activation, which can then deposit and spread C3 throughout the bacterial chain.
Decreased CL provides a competitive advantage in vivo
Next, we addressed if CL affected susceptibility to complement-dependent clearance in vivo. WT and mutant strains were competed in a mouse model of systemic infection. Mutants that displayed increased CL were outcompeted by the WT, and the magnitude of the effect correlated with the degree of CL observed in mutant strains (Figure 4). When the WT was competed against a mutant strain (BT1) that did not display increased CL, we found that both strains competed equally (Figure 4). To confirm that the attenuation of mutants with increased CL in vivo was due to complement-dependent clearance, animals were depleted of complement activity using cobra venom factor prior to infection (Van den Berg et al., 1991). Under these conditions, all mutants competed significantly better with the WT (Figure 4). In most cases, mutants competed equally with the WT in the absence of active complement. As mutants could compete equally with the WT, these data indicate that mutant strains were not attenuated due to an artificial reduction in colony forming units as a result of chain formation or to other growth defects in vivo. Thus, increased chain length promoted complement-dependent clearance during a systemic infection in vivo, consistent with what was observed in OPH killing assays ex vivo.
Figure 4. Decreased chain length provides a competitive advantage in vivo.
Competition between the indicated mutant and the WT was determined in a model of systemic infection. Mice were inoculated with pneumococci by intraperitoneal injection and bacteremia was assessed 16 hours post-infection by quantitative culture of blood. To assess the role of complement, assays were repeated using animals that were depleted of complement activity by treatment with cobra venom factor (CoVF)(25 μg/animal) 24 hours prior to inoculation of bacteria. In this assay CI values of less than one indicate that the WT outcompeted the mutant, while CI values of greater than one indicate that the mutant outcompeted the WT. The dotted line indicates a CI of 1 – the value expected if strains compete equally well. The triangle above the graph indicates the trend of chain length among the mutant strains. Comparisons were made by Mann-Whitney test, NS=not significant, *=P<0.05, **=P<0.01.
Agglutination by antibody subverts minimization of size as a complement evasion strategy
Antibodies are canonically thought to promote complement deposition directly via interaction of their Fc domain with complement component C1q to activate the classical pathway of complement. Growth of S. pneumoniae in serum containing specific anti-pneumococcal antibodies has previously been shown to induce the formation of long bacterial chains; a phenomenon originally termed the thread reaction (Stryker, 1916). This reaction is seen when bacteria are grown in sera from animals immunized with a strain of homologous serotype as well as in sera from patients convalescent from pneumococcal infection (Stryker, 1916; White et al., 1938). Threading results from the agglutination of cells during growth as pneumococci divide in a single plane. During a systemic infection, clearance correlates with agglutination, although the mechanism for this effect of antibody has not been defined (Bull, 1915a, b). Similar to increased CL, agglutination increases the size of bacterial targets by amassing them together. We, therefore, examined the hypothesis that antibodies promote complement-dependent OPH killing by increasing the size of bacterial targets via agglutination.
To study the effects of agglutination, IgG was purified from immune rabbit serum generated to a homologous pneumococcal serotype. We dissected the role of agglutination in promoting killing of pneumococci by comparing the IgG digestion products F(ab′)2, which can agglutinate bacteria, and Fab, which cannot (Figure 5A). Both of these antibody fragments lack Fc, so they cannot directly activate the classical pathway or interact with Fc-receptors (Fig 5B). IgG and F(ab′)2 both promoted OPH killing of pneumococci, while Fab did not, demonstrating that agglutination can promote complement-dependent killing independent of Fc-effector function (Figure 5C). Additionally, when bacteria were grown with decreasing concentrations of F(ab′)2, killing correlated with the degree of agglutination observed (Figure S2). As expected, promotion of OPH killing by F(ab′)2 required serotype-specific antibody, as antibody generated to a heterologous serotype did not show this effect (Figure S2). Complement was necessary for killing by IgG or F(ab′)2 since opsonization of bacteria in heat-inactivated serum or pre-incubation of neutrophils with a blocking antibody to complement receptor 3 (CR3) abrogated killing (Figure 5, C and D). Since the effect of IgG was similar to that of F(ab′)2, this indicated that Fc receptors on neutrophils did not dramatically enhance IgG function under these conditions (Figure 5C). Additionally, OPH killing was still observed when bacteria were opsonized in MgEGTA buffer, which blocks the classical pathway of complement, suggesting that the alternative pathway was sufficient to promote killing in the presence of IgG and F(ab′)2 (Figure 5E). Importantly, Fab was able to bind pneumococci as well as IgG and F(ab′)2, indicating that differences in binding affinity cannot account for the different phenotype observed using Fab (Figure 5F).
Figure 5. Agglutination subverts minimization of bacterial size as a complement evasion strategy and promotes opsonophagocytic killing of a gram-positive pathogen.
Effects of agglutination on OPH killing and complement deposition were studied by growing bacteria in the presence of serotype-specific IgG (500 μg/mL) or equimolar concentrations of F(ab′)2 or Fab. Phase contrast images of bacteria grown in media alone, or supplemented with F(ab′)2 or Fab (Scale bar = 10 μm)(A). To confirm absence of Fc, antibody-binding assays were performed on pneumococci using an Fc-specific secondary antibody (GMFI = geometric mean fluorescence intensity) (B). OPH killing assays were performed where bacteria were opsonized in BRS (25%) (black bars) or HI BRS (25%) (white bars) (C). OPH killing assay performed in the presence of 21 μg/106 PMNs of either a blocking antibody to complement receptor 3 (CBRM1/5) or an isotype control antibody (MOPC21) (D). OPH killing assay performed in the presence of MgEGTA to block activation of the classical pathway (E). To confirm equivalent binding between IgG, F(ab′)2, and Fab, antibody-binding assays were performed using a polyclonal secondary antibody to rabbit F(ab′)2, which recognizes IgG as well as its digestion products (F). The percentage of bacterial particles positive for C3 was determined by flow cytometry after bacteria were opsonized in BRS (25%) (G). OPH killing assay performed where bacteria were pre-opsonized in BRS (66%) (black bars) or HI BRS (66%) (white bars) followed by agglutination with IgG (100ug/mL) or equimolar concentrations of F(ab′)2 or Fab (H). C3 deposition on F(ab′)2 agglutinated cells was analyzed by immunofluorescence microscopy at the time indicated after the addition of 25% BRS (Scale bar = 10 μm) (I). All data are the result of at least 2 independent experiments performed in duplicate ±SEM. See also Figure S2. *=P<0.05, ***=P<0.001.
Next, we wanted to determine if increasing bacterial size via agglutination promoted OPH killing by increasing the likelihood that larger bacterial particles would have C3 bound. Indeed, in the presence of agglutinating concentrations of IgG or F(ab′)2, the percentage of bacterial particles positive for complement increased, similar to what was observed in the context of increased CL (Figure 5G and Figure 3, F, G, and H). Additionally, we observed that C3 deposition on F(ab′)2 agglutinated pneumococci occurs stochastically over time and appears to spread from a focus (or few foci) of complement activation, consistent with what was observed on long bacterial chains (Figure 5I). Thus, our findings demonstrate that agglutinated bacteria, by virtue of their increased size, are indirectly more susceptible to complement deposition due to an increased likelihood of attaining a focus of complement activation that can then spread throughout the agglutinated mass.
Agglutination may also enhance OPH killing independent of its ability to alter the dynamics of complement activation. By amassing bacteria that are pre-opsonized in complement, agglutination could increase the total C3 present on an opsonized target to promote its binding and uptake by phagocytic cells. To address this possibility, we performed OPH killing assays where bacteria were pre-opsonized in serum and then incubated with IgG, F(ab′)2, or Fab after the complement source was removed. Under these conditions, we found that IgG and F(ab′)2 at agglutinating concentrations enhanced killing of pneumococci, while Fab did not (Figure 5H). The density of C3 on individual cells is equal in these experiments; however, agglutination increased the total C3 on a bacterial particle by amassing these cells together. Thus, agglutination either before or after opsonization by complement can promote opsonophagocytic killing. Pre-opsonization also confirmed that the interaction between complement and antibody components does not need to be direct to promote phagocytosis.
Agglutination by antibody promotes complement-dependent lysis
Since the effect of agglutination on killing pneumococci was complement-dependent, we predicted that it would also promote killing of a gram-negative pathogen by lysis (i.e. without phagocytes). To study the effect of agglutination on killing of Haemophilus influenzae, we purified IgG from serotype-specific immune serum (type b) and generated the digestion products F(ab′)2 and Fab. At agglutinating concentrations, F(ab′)2 was comparable to IgG, and superior to Fab at promoting complement-dependent lysis (Figure 6, A and B). Lack of Fc on Fab and F(ab′)2 was confirmed and binding of Fab to H. influenzae was at least equal to that of F(ab′)2 and IgG (Figure 6, C and D). Therefore, in addition to promoting complement-dependent opsonophagocytosis, antibody also enhanced complement-mediated lysis that was Fc-independent via agglutination.
Figure 6. Agglutination promotes complement-dependent lysis of a gram-negative pathogen.
Haemophilus influenzae was incubated with serotype specific IgG (600 μg/mL) or equimolar concentrations of F(ab′)2 or Fab and analyzed by phase contrast microscopy (Scale bar = 10 μm)(A). Serum killing assays performed using the same bacterial cultures analyzed in A where bacteria were incubated in active (BRS) or heat-inactivated (HI BRS) serum (33%) (B). To confirm equivalent binding between IgG, F(ab′)2, and Fab on H. influenzae, antibody-binding assays were performed as in Figure 5F (C), and to confirm absence of Fc, assays were performed as in Figure 5B (D). All data are from 2 independent experiments performed in duplicate ±SEM. *=P<0.05, **=P<0.01.
DISCUSSION
The complement system is critical for controlling many microbial infections. As a result, resistance to complement is common among pathogenic microbes. Previously described mechanisms to resist complement deposition include the expression of a surface capsular polysaccharide to mask the microbial surface, the expression of surface proteins to recruit complement regulatory factors and serum proteases, and the secretion of inhibitors to specifically block complement activation (Lambris et al., 2008). In this study, we demonstrate that minimization of bacterial size represents a complement evasion strategy that is critical for resistance to opsonophagocytic killing in S. pneumoniae both ex vivo and in vivo. In our studies, the effect of bacterial size on resistance to OPH killing was determined with bacterial chains that can be successfully ingested even at the maximum size tested. Within this range, which included natural variants in CL, we found that smaller chains were more resistant to OPH killing. There has recently been an appreciation for how the cellular morphology of pathogens affects their virulence (Justice et al., 2004; Okagaki et al., 2010; Sycuro et al., 2010; Zaragoza et al., 2010). Our study demonstrates that minimization of pathogen size can promote virulence. If the size of a pathogen, however, exceeds the limit for what a phagocyte is able to successfully ingest, this may also be an effective strategy to evade OPH killing. This has been demonstrated in recent in vivo studies on “titan” forms of Cryptococcus neoformans and long filamentous forms of uropathogenic Escherichia coli (Justice et al., 2004; Okagaki et al., 2010; Zaragoza et al., 2010).
It has long been appreciated that in clinical samples pneumococci exhibit a diplococcal morphology. In this study, however, we show that growth of pneumococci in serum, a physiologically relevant medium, causes a small proportion of the population (~1%) to form long bacterial chains. These long chain variants were more susceptible to complement deposition by a mechanism identical to that observed for long chain mutant strains. Thus, while the pneumococcus may grow in a long chain form in vivo, these chains may be rapidly cleared by opsonophagocytosis, resulting in their absence in clinical samples.
While a small population of bacteria grew as long bacterial chains in serum, pneumococci predominantly grew as diplococci. Among the genus Streptococcus, the growth of S. pneumoniae as diplococci or short chains is a distinguishing characteristic. The underlying cause of this difference in bacterial chain length between pneumococci and other streptococci, however, is unclear. As for traditional studies of bacterial “virulence factors”, we sought to determine the role that diplococcal morphology had on pneumococcal virulence using mutant strains and growth conditions that “knockout” this phenotype. Using these mutants and growth conditions, we have determined that the diplcoccal morphology of the pneumococcus is critical for evasion of complement-mediated phagocytic clearance. As this morphology is critical for the virulence of S. pneumoniae, it is interesting to speculate that evolutionarily, resistance to opsonophagocytic clearance was a selective pressure that resulted in the diplococcal morphology of this species. Minimization of chain length may have been specific to the pneumococcus as a complement evasion strategy because other potentially virulent streptococci may have evolved other mechanisms to evade complement, which resulted in less selective pressure on the bacterial chain length of those species. Conversely, pneumococci may have a greater potential to cause invasive disease compared to other virulent streptococci. Indeed, the incidence of invasive pneumococcal disease prior to introduction of the pneumococcal conjugate vaccine were at least 20-fold higher than those observed for other virulent streptococci (CDC, 2008; Davies et al., 1996). While many factors may govern the incidence rates observed for invasive streptococcal infections, it is interesting to speculate that decreased bacterial chain length in the pneumococcus may be one factor that promotes the enhanced invasiveness of this streptococcal species. Moreover, a general characteristic of bacterial pathogens that commonly cause invasive infections, such as Haemophilus influenzae and Neisseria meningitidis, is their small size.
The positive control for increased pneumococcal chain length in this study was a defined lytA mutant. This gene encodes the major lytic murein hydrolase in the pneumococcus and has previously been implicated in the separation of daughter cells following cell division (Sanchez-Puelles et al., 1986). LytA is also a well-characterized virulence factor in the pneumococcus, which has been shown to play a role during models of pneumonia and bacteremia (Berry et al., 1989a; Orihuela et al., 2004). LytA is thought to promote virulence by enhancing the release of pneumolysin, an intracellular toxin that is critical for for the pathogenesis of this organism (Berry et al., 1989b; Sanchez-Puelles et al., 1986). In this study, we show that a lytA mutant had increased sensitivity to complement-dependent clearance both ex vivo and in vivo, and have attributed this attenuation to its increased bacterial chain length. Thus, in addition to its role in pneumolysin release, LytA activity may be critical in vivo to promote the efficient separation of daughter cells, which allows for evasion of complement-dependent opsonophagocytic clearance.
We show that increased cell size attenuated the pneumococcus during a systemic infection. However, in the absence of complement and phagocytes, increasing pathogen size may be beneficial at distinct sites of infection in vivo. As larger microbes will likely have more adhesins as a result of their enlarged surface area, increasing pathogen size may aid in the establishment of a mucosal infection, where binding to the epithelium is critical. As we observed long chain forms of the pneumococcus when it was grown in a physiologically relevant medium (serum), we are interested in determining whether these long chain variants, and whether chain formation in general, is important for pneumococcal infections at mucosal sites (i.e. during the carrier state).
We also addressed how the host may subvert minimization of size as a virulence strategy via the agglutinating effect of antibody. Agglutination of pathogens in vivo is thought to be an important function of antibodies, although the mechanism for this effect has not been defined. During a systemic infection, it has been shown that agglutinating antibodies are more effective at promoting clearance compared to non-agglutinating antibodies (Margni et al., 1983). Specifically for pneumococci, it was demonstrated almost 100 years ago that intravenous challenge of immunized animals resulted in rapid bacterial agglutination (within 30 s), which correlated with clearance, although the mechanism for this effect was unclear (Bull, 1915a, b).
In this study, we have dissected the role of agglutination in promoting killing of bacteria by comparing IgG to its digestion products F(ab′)2 and Fab. We found that F(ab′)2 ≫ Fab at promoting complement-dependent opsonophagocytic killing of S. pneumoniae and lysis of H. influenzae, and have attributed this difference to the ability of F(ab′)2 to agglutinate and, therefore, increase the size of bacterial targets. This effect is likely not specific to these two microbes, since opsonophagocytic killing of Pseudomonas aeruginosa is also enhanced by F(ab′)2 but not Fab, and importantly, this required the presence of active complement (Trautmann et al., 1985). Additionally, others have shown that F(ab′)2 could promote clearance of invasive bacterial infection while Fab could not, suggesting that agglutination may play a role independent of Fc-effector function in vivo as well (Ax et al., 1981).
The literature on activation of the alternative pathway by F(ab′)2 and Fab fragments is controversial. Some studies show that both fragments promote alternative pathway-dependent effects equally, while others show that show that F(ab′)2 is better than Fab at activating the complement system (Ax et al., 1981; Ehrnst, 1978; Moore et al., 1982; Trautmann et al., 1985; Winkelstein and Shin, 1974). Without Fc, it is unlikely that either molecule directly activates the complement cascade by binding C1q. F(ab′)2 and Fab, however, can be bound by C3, so they may promote complement deposition by coating a surface that is poor at binding complement directly (like capsule) and providing a substrate for C3 deposition. We have found that F(ab′)2 ≫ Fab at promoting complement-dependent effects, and importantly, these effects correlated with the degree of agglutination observed. Therefore, instead of a difference in the inherent ability of F(ab′)2 to promote complement dependent effects over Fab fragments (for which a mechanism remains unclear), the discrepancy in the literature between the effects of F(ab′)2 and Fab may be attributed to whether antibody concentrations used were agglutinating or not. As agglutination was not examined in these studies it is difficult to conclude that it accounts for the different phenotypes observed. Based on this hypothesis, however, one would expect that at sub-agglutinating concentrations the effect of F(ab′)2 = Fab, while at agglutinating concentrations the effect of F(ab′)2 ≫ Fab, and indeed, our data supports this claim (Figure S2, Figure 5C, and Figure 6B).
IgG was used in this study to examine the effects of agglutination. The mechanism described here, however, may play a larger role for immunoglobulins with a higher valency, like polymeric IgA (4 antigen-binding sites) and IgM (10 antigen-binding sites) due to their enhanced ability to agglutinate antigen. It is difficult to determine the role that agglutination plays in the function of these antibodies, since their ability to agglutinate is not readily separated from their Fc-effector functions.
In summary, we have shown that minimization of bacterial size allows for complement evasion and have characterized a mechanism for how the host may subvert this virulence strategy via agglutinating antibody. Since complement is critical for the clearance of many pathogenic microbes, this study has broad implications for how pathogen size and morphology can affect virulence.
EXPERIMENTAL PROCEDURES
Bacterial strains and culture conditions
Bacterial strains (Table S1) were grown as previously described (Dalia et al., 2010; Gould and Weiser, 2002). When appropriate, media was supplemented with streptomycin (Str, 100 μg/mL), spectinomycin (Spec, 200 μg/mL), or erythromycin (Erm, 1 μg/mL).
Mariner mutants of TIGR4 were generated exactly as previously described (Hava and Camilli, 2002). The M1 and LytA revertant strains were also generated in this study. For details please refer to the Supplementary Methods.
Complement Sources
Three-four week baby rabbit serum (BRS) was aliquoted and frozen at −80°C until use (Pel-Freez Biologicals). Normal human serum (NHS) was isolated from human whole blood as previously described (Dalia et al., 2010).
Opsonophagocytic Killing Assays
OPH killing assays were performed exactly as previously described (Dalia et al., 2010). Briefly, 103 PBS-washed late log-phase bacteria (in 10 μL) were pre-opsonized by addition of 20 μL of 100% BRS (unless otherwise indicated) for 30 minutes at 37°C, followed by the addition of 105 PMNs (40 μL) in +++ buffer (130 μL), which were isolated from human whole blood as previously described (Dalia et al., 2010). Reactions were then incubated at 37°C for 45 minutes with rotation. Reactions were then stopped by placing on ice and viable counts of bacteria were determined by dilution plating. Percent survival was determined relative to control reactions where no PMNs were added. Thus, for mutants with increasing chain formation, an artificial reduction in CFUs due to growth of mutant strains as chains during the duration of the experiment did not alter percent survival data since data were normalized to control reactions where an equal amount of growth should have occurred.
When specified, OPH killing assays were modified. To determine the role of complement, reactions were performed where bacteria were opsonized in HI BRS. To determine the role of the alternative pathway on OPH killing, bacteria were opsonized in gelatin veronal buffer containing MgEGTA to block the classical pathway of complement activation (Boston Bioproducts). Where indicated, PMNs were pre-incubated with 20 μM cytochalasin D, to inhibit actin polymerization (block phagocytosis), or a vector control (DMSO) (Sigma Aldrich). To determine the role of complement receptor 3 (CR3) on OPH killing, neutrophils were pre-treated with 21 μg/106 neutrophils of a blocking antibody (CBRM1/5) or an isotype control antibody (MOPC21) at 37°C for 30 minutes with rotation (Biolegend) prior to use in OPH killing assays. Where indicated, bacterial cultures at an OD620 of ~0.5 were mildly sonicated for 3 s using a 40TL probe (Ultra Sonic Power Corp.).
For data when percent survival or C3 deposition is related to the size of bacterial chains, samples for microscopy were prepared from the same cultures used in OPH killing assays. The degree of chain formation observed between mutant strains and growth conditions where chain formation was induced in the WT was variable from day to day. Therefore, chain length (size) was determined for each experiment independently when correlating chain length with percent survival in OPH killing assays or the amount of C3 deposited in complement deposition assays.
For additional details about control experiments please refer to the Supplementary Methods.
Serum Bactericidal Killing Assays
Bactericidal killing assays were performed as previously described (Nakamura et al., 2011). Briefly, 105 mid-log phase bacteria (OD620 = 0.3−0.4) were diluted into +++ buffer (70 μL), and then added to 120 μL of active BRS (50% in +++ buffer) or 140 μL of heat-inactivated BRS (50% in +++ buffer) for 45 minutes at 37°C with rotation. Assays were stopped by placing reactions on ice, and dilutions were made for quantitative culture. Killing in BRS was determined relative to reactions where bacteria were incubated in heat-inactivated BRS.
Microscopy
Samples (8 μl) were prepared for microscopy on agarose pads (1% in PBS) as previously described (Batchelor and Goulian, 2006).
Samples were analyzed on an Olympus IX81 microscope with a 100X phase contrast objective (Olympus UPLFLN). Fluorescence microscopy was performed using a 100W halogen bulb and Chroma filter cubes 41017 and 41043. Images were taken using an Andor IXON+ 887 camera. For fluorescence microscopy, separate images were taken using each filter set as well as a phase contrast image.
All images were analyzed using image J. The size (in pixels) of bacterial chains is defined as their two-dimensional area based on the “Auto Threshold” function, which defined the outline of chains. Shapes that were not chains and chains on the border of images were manually excluded from subsequent analysis.
Cell Association Assays
Neutrophil cell association assays were performed essentially as previously described (Dalia et al., 2010). Bacteria were labeled with fluorescein isothiocyanate (FITC, 0.2 mg/mL) or Cy5 (2pmol/mL). For competitive assays strains were mixed 1:1 and 4 × 105 bacteria/mix were opsonized in baby rabbit serum (66%), followed by incubation with 2 × 105 neutrophils. By flow cytometry, the proportion of FITC:Cy5 positive bacteria in the inocula is defined as the input ratio, while the proportion of FITC:Cy5 positive neutrophils is defined as the output ratio.
Complement Deposition Assays
C3 deposition assays were performed as previously described (Dalia et al., 2010). Briefly, 106 late-log phase bacteria were incubated in 100 μl of 66% BRS or 66% NHS (diluted in +++) for 30 minutes at 37°C. Bacteria were then washed and resuspended in --+ buffer containing 5% fetal calf serum. C3 binding was then detected by incubating bacteria in 100 μl of a 1:100 dilution of a FITC-conjugated anti-rabbit C3 or anti-human C3 antibody as appropriate (MP Biomedical). Control reactions included bacteria that were opsonized in heat-inactivated serum, as well as reactions where bacteria were incubated in buffer alone.
For analysis by microscopy, the size and outline of cells was determined from the phase contrast image using Auto Threshold. Using the fluorescence image, the Integrated Density (IntDen) (defined as the sum of the values of the pixels in the image or selection) was determined within the outline of each chain as defined by the phase contrast image. Reactions where bacteria were opsonized in heat-inactivated BRS or NHS were used to define the background fluorescence. The IntDen of chains from these reactions was divided by their size (in pixels) to define the “background intensity per pixel”. For experimental samples:
The percent of cells positive for complement (%C3 positive) was determined by first analyzing a histogram of C3/chain data from a given experiment to determine a threshold value above which cells were scored as positive for C3. The proportion of cells that scored positive for complement was also analyzed by flow cytometry as previously described (Dalia et al., 2010).
Mouse model
Six-eight week C57BL/6 mice were inoculated intraperitoneally with 2×106 CFU of a 1:1 mixture of mutant and WT bacteria. The proportion of mutant and WT bacteria was determined by plating on both selective (using appropriate antibiotics – supports growth of mutant) and non-selective (supports growth of mutant and WT) media. The input ratio was defined by the inocula and the output ratio was defined by quantitative culture of blood, and the CI was calculated as in cell association assays. Animals that cleared the infection were excluded from subsequent analysis since a CI could not be calculated for these animals.
Cleavage of IgG
Rabbit serum generated to pneumococcal serotypes 4 and 23 was provided by Robert Austrian. Rabbit serum to Haemophilus influenzae type b was commercially available (Becton, Dickinson, and Co.). IgG was purified from sera using a Hi-Trap protein G columns (GE Healthcare, Uppsala, Sweden), and cleaved using F(ab′)2 and Fab cleavage kits according to manufacturers instructions (Thermo Scientific).
Antibody binding assays
Antibody binding experiments were performed as previously described (Dalia et al., 2010). Briefly, 106 bacteria were incubated with IgG (50 μg/mL) or equimolar concentrations of F(ab′)2 or Fab. Binding was determined using a goat anti-rabbit secondary antibody as specified (1:4000) (Jackson ImmunoResearch). Binding was detected using a FITC-conjugated anti-goat IgG (1:100) and analyzed by flow cytometry.
Statistical Analysis
Comparisons were made by two-tailed Student’s t-test unless otherwise specified.
Supplementary Material
HIGHLIGHTS.
Increasing pneumococcal chain length promotes complement deposition and phagocytosis
Minimizing chain length confers a competitive advantage during systemic infection
Agglutination by F(ab′)2 promotes opsonophagocytosis of a gram-positive pathogen
Agglutination by F(ab′)2 promotes lysis of a gram-negative pathogen
Acknowledgments
We thank Mark Goulian from the University of Pennsylvania for technical assistance with performing and analyzing microscopy experiments and for helpful discussions. This work was supported by US Public Health Service Grant numbers AI44231 and AI38446 to J.N.W..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ax W, Kanzy EJ, Seiler FR. In-vivo phagocytosis: enhancement of bacterial clearance by native and enzyme-treated immunoglobulins. Immunobiology. 1981;159:349–365. doi: 10.1016/S0171-2985(81)80092-7. [DOI] [PubMed] [Google Scholar]
- Batchelor E, Goulian M. Imaging OmpR localization in Escherichia coli. Mol Microbiol. 2006;59:1767–1778. doi: 10.1111/j.1365-2958.2006.05048.x. [DOI] [PubMed] [Google Scholar]
- Berry AM, Lock RA, Hansman D, Paton JC. Contribution of autolysin to virulence of Streptococcus pneumoniae. Infect Immun. 1989a;57:2324–2330. doi: 10.1128/iai.57.8.2324-2330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AM, Yother J, Briles DE, Hansman D, Paton JC. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989b;57:2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer N, Dolman KM, van Houdt M, Sta M, Roos D, Kuijpers TW. Mannose-binding lectin (MBL) facilitates opsonophagocytosis of yeasts but not of bacteria despite MBL binding. J Immunol. 2008;180:4124–4132. doi: 10.4049/jimmunol.180.6.4124. [DOI] [PubMed] [Google Scholar]
- Brown JS, Hussell T, Gilliland SM, Holden DW, Paton JC, Ehrenstein MR, Walport MJ, Botto M. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc Natl Acad Sci U S A. 2002;99:16969–16974. doi: 10.1073/pnas.012669199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull CG. The agglutination of bacteria in vivo. J Exp Med. 1915a;22:484–491. doi: 10.1084/jem.22.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull CG. The mechanism of the curative action of antipneumococcus serum. J Exp Med. 1915b;22:457–464. doi: 10.1084/jem.22.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction--eight states, 1998–2005. MMWR Morb Mortal Wkly Rep. 2008;57:144–148. [PubMed] [Google Scholar]
- Dalia AB, Standish AJ, Weiser JN. Three surface exoglycosidases from Streptococcus pneumoniae, NanA, BgaA, and StrH, promote resistance to opsonophagocytic killing by human neutrophils. Infect Immun. 2010;78:2108–2116. doi: 10.1128/IAI.01125-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HD, McGeer A, Schwartz B, Green K, Cann D, Simor AE, Low DE. Invasive group A streptococcal infections in Ontario, Canada. Ontario Group A Streptococcal Study Group. N Engl J Med. 1996;335:547–554. doi: 10.1056/NEJM199608223350803. [DOI] [PubMed] [Google Scholar]
- Ehrnst A. Separate pathways of C activation by measles virus cytotoxic antibodies: subclass analysis and capacity of F(ab) molecules to activate C via the alternative pathway. J Immunol. 1978;121:1206–1212. [PubMed] [Google Scholar]
- Gould JM, Weiser JN. The inhibitory effect of C-reactive protein on bacterial phosphorylcholine platelet-activating factor receptor-mediated adherence is blocked by surfactant. J Infect Dis. 2002;186:361–371. doi: 10.1086/341658. [DOI] [PubMed] [Google Scholar]
- Hava DL, Camilli A. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol Microbiol. 2002;45:1389–1406. [PMC free article] [PubMed] [Google Scholar]
- Hed J, Johansson M, Lindroth M. Complement activation according to the alternate pathway by glass and plastic surfaces and its role in neutrophil adhesion. Immunol Lett. 1984;8:295–299. doi: 10.1016/0165-2478(84)90013-0. [DOI] [PubMed] [Google Scholar]
- Hyams C, Camberlein E, Cohen JM, Bax K, Brown JS. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect Immun. 2010;78:704–715. doi: 10.1128/IAI.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarva H, Jokiranta TS, Wurzner R, Meri S. Complement resistance mechanisms of streptococci. Mol Immunol. 2003;40:95–107. doi: 10.1016/s0161-5890(03)00108-1. [DOI] [PubMed] [Google Scholar]
- Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, Footer MJ, Hultgren SJ. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci U S A. 2004;101:1333–1338. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel TR, Wilson MA, Murphy JW. Early events in initiation of alternative complement pathway activation by the capsule of Cryptococcus neoformans. Infect Immun. 1991;59:3101–3110. doi: 10.1128/iai.59.9.3101-3110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat Rev Microbiol. 2008;6:132–142. doi: 10.1038/nrmicro1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysenko ES, Clarke TB, Shchepetov M, Ratner AJ, Roper DI, Dowson CG, Weiser JN. Nod1 signaling overcomes resistance of S. pneumoniae to opsonophagocytic killing. PLoS Pathog. 2007;3:e118. doi: 10.1371/journal.ppat.0030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margni RA, Parma EA, Cerone S, Erpelding A, Perdigon G. Agglutinating and non-agglutinating antibodies in rabbits inoculated with a particulate antigen (Salmonella typhimurium) Immunology. 1983;48:351–359. [PMC free article] [PubMed] [Google Scholar]
- Matthias KA, Roche AM, Standish AJ, Shchepetov M, Weiser JN. Neutrophil-toxin interactions promote antigen delivery and mucosal clearance of Streptococcus pneumoniae. J Immunol. 2008;180:6246–6254. doi: 10.4049/jimmunol.180.9.6246. [DOI] [PubMed] [Google Scholar]
- Moore FD, Jr, Austen KF, Fearon DT. Antibody restores human alternative complement pathway activation by mouse erythrocytes rendered functionally deficient by pretreatment with pronase. J Immunol. 1982;128:1302–1306. [PubMed] [Google Scholar]
- Nakamura S, Shchepetov M, Dalia AB, Clark SE, Murphy TF, Sethi S, Gilsdorf JR, Smith AL, Weiser JN. Molecular basis of increased serum resistance among pulmonary isolates of non-typeable Haemophilus influenzae. PLoS Pathog. 2011;7:e1001247. doi: 10.1371/journal.ppat.1001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- Okagaki LH, Strain AK, Nielsen JN, Charlier C, Baltes NJ, Chretien F, Heitman J, Dromer F, Nielsen K. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 2010;6:e1000953. doi: 10.1371/journal.ppat.1000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihuela CJ, Gao G, Francis KP, Yu J, Tuomanen EI. Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J Infect Dis. 2004;190:1661–1669. doi: 10.1086/424596. [DOI] [PubMed] [Google Scholar]
- Rosas AL, MacGill RS, Nosanchuk JD, Kozel TR, Casadevall A. Activation of the alternative complement pathway by fungal melanins. Clin Diagn Lab Immunol. 2002;9:144–148. doi: 10.1128/CDLI.9.1.144-148.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Puelles JM, Ronda C, Garcia JL, Garcia P, Lopez R, Garcia E. Searching for autolysin functions. Characterization of a pneumococcal mutant deleted in the lytA gene. Eur J Biochem. 1986;158:289–293. doi: 10.1111/j.1432-1033.1986.tb09749.x. [DOI] [PubMed] [Google Scholar]
- Standish AJ, Weiser JN. Human neutrophils kill Streptococcus pneumoniae via serine proteases. J Immunol. 2009;183:2602–2609. doi: 10.4049/jimmunol.0900688. [DOI] [PubMed] [Google Scholar]
- Stryker LM. Variations in the pneumococcus induced by growth in immune serum. J Exp Med. 1916;24:49–68. doi: 10.1084/jem.24.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sycuro LK, Pincus Z, Gutierrez KD, Biboy J, Stern CA, Vollmer W, Salama NR. Peptidoglycan crosslinking relaxation promotes Helicobacter pylori’s helical shape and stomach colonization. Cell. 2010;141:822–833. doi: 10.1016/j.cell.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chain formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968;59:86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann M, Triest K, Hofstaetter T, Seiler FR, Hahn H. Influence of different immunoglobulin G preparations on phagocytosis of Pseudomonas aeruginosa by polymorphonuclear granulocytes. Zentralbl Bakteriol Mikrobiol Hyg A. 1985;259:104–117. doi: 10.1016/s0176-6724(85)80012-2. [DOI] [PubMed] [Google Scholar]
- Van den Berg CW, Aerts PC, Van Dijk H. In vivo anti-complementary activities of the cobra venom factors from Naja naja and Naja haje. J Immunol Methods. 1991;136:287–294. doi: 10.1016/0022-1759(91)90015-8. [DOI] [PubMed] [Google Scholar]
- White B, Robinson ESA, Barnes LA. The biology of pneumococcus; the bacteriological, biochemical, and immunological characters and activities of Diplococcus pneumoniae. London: The Commonwealth fund; H. Milford: Oxford University press; 1938. [Google Scholar]
- Winkelstein JA, Shin HS. The role of immunoglobulin in the interaction of pneumococci and the properdin pathway: evidence for its specificity and lack of requirement for the Fc portion of the molecule. J Immunol. 1974;112:1635–1642. [PubMed] [Google Scholar]
- Zaragoza O, Garcia-Rodas R, Nosanchuk JD, Cuenca-Estrella M, Rodriguez-Tudela JL, Casadevall A. Fungal cell gigantism during mammalian infection. PLoS Pathog. 2010;6:e1000945. doi: 10.1371/journal.ppat.1000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.