Abstract
OBJECTIVE
To estimate response of diagnosis and symptom-based subtypes to sertraline treatment.
METHODS
This was a secondary data analysis for women who were diagnosed with premenstrual syndrome (PMS) or premenstrual dysphoric disorder and treated in three National Institutes of Health-supported clinical trials (N=447). Three PMS subtypes were identified based on predominance of psychological, physical, or both symptom types. Scores for each symptom and a total premenstrual score at baseline and endpoint were calculated from daily symptom diaries. Change from baseline after three treated menstrual cycles (or endpoint if sooner) was estimated using linear regression models adjusted for baseline severity.
RESULTS
The PMS and premenstrual dysphoric disorder diagnoses improved similarly with sertraline relative to placebo, while symptom-based subtypes had differential responses to treatment. The mixed symptom subtype had the strongest response to sertraline relative to placebo (Daily Symptom Rating [DSR] difference 33.80, 95% CI: 17.16, 50.44, P<0.001), and the physical symptom subtype had the poorest response to sertraline (DSR difference 9.50, 95% CI: −16.29, 35.28, P=0.470). Results based on clinical improvement (50% decrease from baseline) indicated that 8.3 participants in the mixed symptom subtype, 3.9 in the psychological subtype, and 7.1 in the physical subtype are needed to observe one woman in the subtype who would achieve clinical improvement.
CONCLUSION
The PMS and premenstrual dysphoric disorder diagnoses have similar response to sertraline treatment, but symptom-based subtypes have significantly different responses to this treatment. Mixed and psychological symptom subtypes improved while the physical symptom subtype did not improve significantly. Identifying the patient’s predominant symptoms, and their severity is important for individualized treatment and possible response to a selective serotonin reuptake inhibitor.
Introduction
Premenstrual disorders that are diagnosed as premenstrual syndrome (PMS) or premenstrual dysphoric disorder (PMDD) (Box 1) are among the most common clinical complaints of reproductive-age women. The syndrome is associated with considerable disability, which results from the severity of the symptoms, impairment of work, personal relationships and activities, and the chronic occurrence of the disorder over many years of menstrual cycling (1). Although the distress caused by menstrually-related symptoms has long been recognized, the pathophysiology of the phenomena is complex and no treatment has been identified that is effective for all women with PMS or PMDD.
Box 1. Diagnostic Criteria.
A. Diagnosis of Premenstrual Syndrome (PMS) *
At least one affective (depression, angry outbursts, irritability, anxiety, confusion, social withdrawal) or somatic (breast tenderness, abdominal bloating, headache, swelling of extremities) symptom in the 5 days before menses.
Remission of the symptoms(s) from cycle days 4 to 13.
Symptoms impair functioning.
Symptoms confirmed in 2 cycles of prospective symptom ratings.
Symptoms are not due to another disorder.
B. Diagnosis of Premenstrual Dysphoric Disorder (PMDD) †
At least 5 of 11 specified symptoms at a severe level in the week before menses for the past year.
Remission for 1-week postmenses.
Symptoms impair functioning or relationships.
Symptoms confirmed in two consecutive cycles of prospective symptom ratings.
Symptoms are not due to another disorder.
PMDD symptoms: depression, anxiety, mood swings, irritability, decreased interest, difficulty concentrating, fatigue, appetite changes, sleep disturbance, feeling overwhelmed, physical symptoms. Diagnosis must include at least one of the first four symptoms and only one physical symptom.
Selective serotonin reuptake inhibitors (SSRIs) are a first-line treatment for PMS and PMDD, based on their reported efficacy in numerous clinical trials and several meta-analyses (2, 3). However, approximately 40% of women with PMS/PMDD do not respond well to SSRIs, for reasons that are not well-identified (4).
A possible factor in non-response to an SSRI may be the role of physical symptoms, which are predominant for many women who seek treatment but are less studied than the psychological symptoms of the syndrome. It has also been suggested that symptom-based subtypes in the heterogeneous PMS population may respond differently to treatments such as SSRIs or oral contraceptives (5). A recent consensus of international experts in premenstrual disorders proposed three symptom-based subtypes B predominantly psychological, predominantly physical and mixed psychological and physical symptom subtypes B but noted the absence of data that defined these subtypes (6). Apart from PMDD, which has considerable study in clinical trials, symptom-based subtypes and their responses to treatment have not been systematically examined.
This study evaluated three symptom-based subtypes in a clinical population of women who met stated criteria for PMS or PMDD. We estimated response of the three subtypes to treatment with sertraline and tested the hypothesis that psychological symptoms respond to sertraline better than physical symptoms. We also estimated treatment response in the PMS and PMDD groups and hypothesized that the PMDD response to sertraline is stronger due to the predominance of mood symptoms that are required for the diagnosis.
Material and Methods
The study included all women who met stated criteria for PMS or PMDD and reported at least one response to treatment with sertraline or placebo in three previous clinical trials (N=447) (7–9). The trials were supported by the National Institutes of Health (NIH) and conducted between 1998 and 2007 to determine efficacy of sertraline compared to alprazolam and placebo (9), continuous or intermittent dosing with sertraline and placebo (8), and efficacy of short-term versus long-term treatment with luteal phase sertraline, an 18-month survival study with a randomized, double-blind switch to placebo after 4 or 12 months of sertraline treatment (7). The studies were approved by the Institutional Review Board of the University of Pennsylvania and written consent was obtained from all participants.
In the original trials, all women rated symptoms daily throughout the studies on the validated Daily Symptom Rating form (DSR) (10). Eligibility required a total premenstrual DSR score >=80, a total postmenstrual score <40, a difference >=50% between the postmenstrual and premenstrual scores, and a moderate to severe rating of impairment on one or more impairment items of work, family life, social activity and overall interference. A PMDD diagnosis was identified by applying these same criteria with the additional requirement that 5 or more of the 11 symptoms specified for PMDD be severe premenstrually. At least one mood symptom and no more than one physical symptom was included in this diagnostic count (11). The PMS and PMDD diagnoses were confirmed by prospective daily symptom ratings for 2–3 menstrual cycles in the screen period.
Each trial had a three-month screen period with single-blind placebo administered in the third screen cycle followed by double-blind randomization of eligible participants to treatment assignment. All participants received sertraline hydrochloride at 50 mg/d or a matching placebo capsule in the first treatment cycle. The dose could be increased to 100 mg or two placebo capsules per day (double blind) in the second or third cycles, in the absence of clear improvement or dose-limiting adverse events.
Participant ages were 18–45 years, all had regular menstrual cycles of 22–35 days and persistent premenstrual symptoms for 6 months or more. Exclusions included major axis 1 psychiatric diagnosis, alcohol or substance abuse within the past year, history of psychosis or bipolar disorder as identified in the Structured Clinical Interview for DSM IV Diagnoses (SCID) (12); current use of psychotropic medications or any current prescription, over-the-counter, herbal, or nonmedical therapies for PMS, pregnancy, breast feeding, hysterectomy, symptomatic endometriosis, irregular menstrual cycles, and any serious or unstable medical illness.
Symptom scores were calculated from the DSR (10), which lists 17 symptoms that include the 11 PMDD symptoms: fatigue, poor coordination, feeling out of control, feeling worthless/guilty, headache, anxiety/tension, aches, irritability, mood swings, weight gain, food cravings, no interest in usual activities, cramps, sad/depressed/blue, breast tenderness, sleep problems, difficulty concentrating. Each symptom was rated daily on a 5-point scale from 0 (none) to 4 (severe). Premenstrual and postmenstrual scores were computed for each menstrual cycle in the same manner as reported elsewhere (7–9, 13, 14). For the premenstrual scores, each daily symptom rating was summed for the 6 days before menses, and 6-day symptom scores were summed for a total premenstrual score (7–9, 14). Other study variables were obtained from the participants in the screen period: age, self-reported race, employment, and history of depression as determined in SCID interview.
PMS subtypes were defined by classifying each participant based on the severity of psychological and physical symptoms at baseline. First we established a severity threshold for each symptom (the top quartile of the mean baseline scores) and identified the most prevalent psychological and physical symptoms of the disorder. The symptoms and DSR score cutpoints for classification into the psychological subtype were irritability (>=16), anxiety (>=16), sad/depressed (>=14) or hopelessness (>=12). The symptoms and DSR score cutpoints for classification into the physical subtype were swelling/bloating (>=16), breast tenderness (>=12), aches/joint pains (>=12) or cramps (>=6). Each participant was then classified by her levels of psychological symptoms (threshold yes or no) and her level of physical symptoms (threshold yes or no). This 2 × 2 classification produced 4 cells (subtypes) as follows: threshold for psychological symptoms only, threshold for physical symptoms only, threshold for both types, and threshold for neither type. The 3rd and 4th cells were combined as the mixed subtype after further evaluation and statistical tests. Treatment response was the change from baseline (mean of 3 screen cycles) after three treatment cycles (or at the participant’s endpoint if sooner) for the total premenstrual DSR score and the premenstrual score for each of 17 symptoms. A secondary outcome of clinical response was defined as >=50% decrease from baseline for the total premenstrual DSR score at the participant’s endpoint. Analyses were based on intent-to-treat and included all participants with DSR treatment data.
Associations between change in DSR scores and random treatment assignment were estimated using linear regression models adjusted for baseline severity. Baseline scores were median-split based on previous findings that baseline severity significantly modified treatment response (8). We report the P values for main effects and interaction terms where significant using Wald tests (the standard test of statistical significance generated via linear regression) (15). Hypothesized modifiers of treatment were evaluated in the multivariable models.
A priori power calculations for the indicator of treatment response with three symptom subtypes indicated that a sample size of 328 subjects was sufficient to detect statistically significant results (with alpha=0.008) for a risk ratio >=1.6 for treatment response comparing one subtype versus any other subtype with 80% power. We assumed equal group sizes for the subtypes and a response rate of 40% in the lowest responding subtype. These pre-study calculations assumed a dichotomous outcome variable, while the analyses as presented used continuous outcome variables with greater power.
Randomization to treatment was balanced and baseline DSR scores were similar in the original trials (7–9). Because this study defined subtypes by baseline symptoms without respect to treatment assignment, all analyses were adjusted for baseline symptom levels. Study was considered as an additional covariate but was not included in the final multivariable models as it was not significant. Baseline characteristics were compared among study groups using chi-square or Student t tests. Analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC) with a 2-sided P value <=0.05 considered statistically significant. With the Bonferroni adjustment for multiple comparisons of the 17 individual DSR symptoms, P values <=0.003 were required for significance.
RESULTS
Table 1 shows clinical characteristics at baseline for the total sample of 447 women. There were no significant differences between the sertraline and placebo groups.
Table 1.
Baseline Characteristics
| Sertraline (n=328) | Placebo (n=119) | P* | |
|---|---|---|---|
| Age, mean (SD), y | 33.2 (6.8) | 34.1 (5.8) | 0.213 |
| Race | 0.110 | ||
| White | 233 (71.0) | 96 (80.7) | |
| African American | 71 (21.6) | 12 (10.1) | |
| Other | 24 (7.3) | 11 (9.2) | |
| Employed | 250 (77.2) | 98 (83.1) | 0.181 |
| History of depression | 114 (35.2) | 40 (33.6) | 0.758 |
| Diagnosis | |||
| PMS | 286 (87.2) | 104 (87.4) | 0.955 |
| PMDD (2 or more cycles) | 42 (12.8) | 15 (12.6) | |
| Study | |||
| 1 | 62 (18.9) | 62 (52.1) | |
| 2 | 109 (33.2) | 57 (47.9) | |
| 3 | 157 (47.9) | 0 † | |
SD, standard deviation; PMS, premenstrual syndrome; PMDD, premenstrual dysphoric disorder.
P-value for chi-square test or t test
This was an 18-month double-blind, placebo-controlled discontinuation study. All patients had sertraline for 3 months and were then switched to placebo at 4 months or 12 months.
Sertraline significantly reduced premenstrual symptoms, consistent with the reports from the primary clinical trials (7–9). The adjusted mean difference in symptom reduction with sertraline relative to placebo was 24.48 (95% CI, 6.35, 42.62, P=0.008) (Table 2). The secondary outcome of clinical improvement (decrease of 50% or more from baseline) was significantly greater with sertraline compared to placebo treatment (61% vs 40%, P=0.001).
Table 2.
Subtype Responses to Sertraline Treatment
| DSR Mean* | Total Sample | Mixed Subtype 1 | Psychological Subtype 2 | Physical Subtype 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | (95% CI) | n | Mean | (95% CI) | n | Mean | (95% CI) | n | Mean | (95% CI) | |
| Baseline | ||||||||||||
| Sertraline | 328 | 152.15 | (145.51, 158.80) | 196 | 170.47 | (161.56, 179.38) | 51 | 154.24 | (142.57, 165.90) | 81 | 131.75 | (125.86, 137.65) |
| Placebo | 119 | 140.84 | (127.24, 154.43) | 78 | 155.06 | (141.23, 168.90) | 9 | 138.44 | (115.79, 161.09) | 32 | 129.00 | (118.38, 139.62) |
| Change from baseline | ||||||||||||
| Sertraline | 78.27 | (70.35, 86.20) | 87.37 | (78.44, 96.30) | 83.68 | (66.40, 100.98) | 63.76 | (49.88, 77.65) | ||||
| Placebo | 53.79 | (37.46, 70.12) | 53.57 | (39.58, 67.56) | 53.53 | (12.23, 94.83) | 54.27 | (32.36, 76.18) | ||||
| Change vs placebo | 24.48 | (6.35, 42.62) † | 33.80 | (17.16, 50.44) ‡ | 30.15 | (−14.65, 74.95) § | 9.50|| | (−16.29, 35.28) | ||||
DSR, Daily Symptom Rating; CI, confidence interval.
Data are least squares means from the general linear regression model for DSR change from baseline with treatment assignment, subtypes, baseline severity, and interaction of treatment by subtypes.
P=0.008
P=<0.001
P=0.187
P=0.470
Treatment effects were not modified by history of depression, P=0.948; daily vs luteal phase dosing, P=0.837; race, P=0.304, or study, P=0.215. A significant interaction between treatment and age as a continuous variable (P=0.018) indicated that older age women responded better to sertraline and were less likely to respond to placebo.
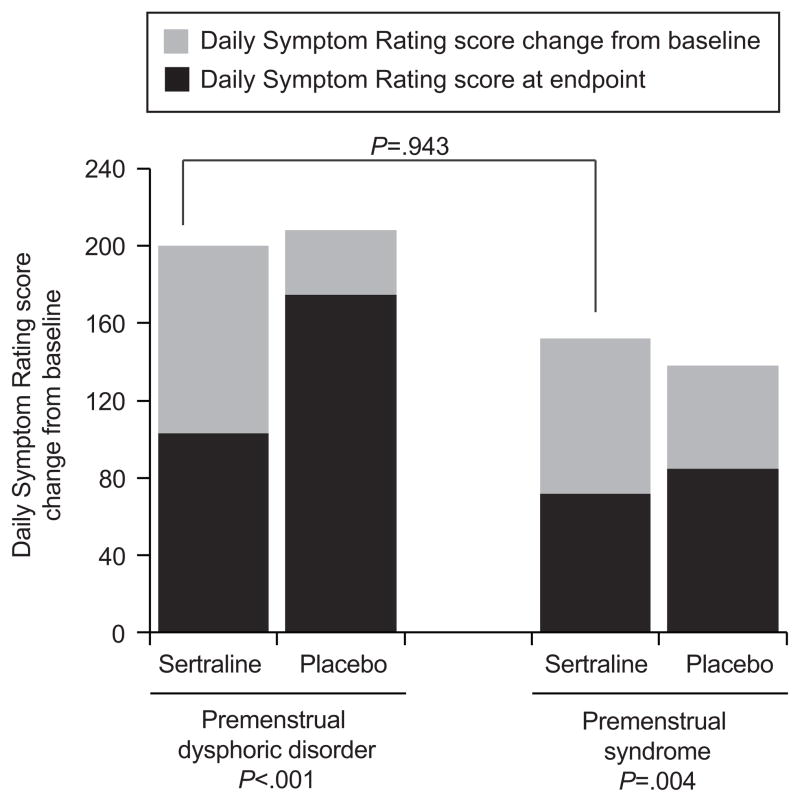
Both PMS and PMDD significantly improved with sertraline relative to placebo (Figure 1). In the PMDD group, DSR difference relative to placebo was 63.19; 95% CI: 26.97, 100.41, P=<0.001); and in the PMS group was 21.15; 95% CI: 6.89, 35.42, P=0.004. Figure 1 shows that although the PMDD group had a higher symptom baseline, the magnitude of change was nearly identical in the PMDD and PMS groups (79.89; 95% CI: 60.23, 99.55 and 80.90; 95% CI: 73.58, 88.22, respectively, P=0.943).
Figure 1.
Mean Daily Symptom Rating change (gray bars) from baseline (total bars) by treatment and diagnosis. The number of women taking sertraline was 42 women with premenstrual dysphoric disorder, and 266 with premenstrual syndrome; the number of women taking the placebo was 15 (premenstrual dysphoric disorder) and 104 (premenstrual syndrome). All P values are from the linear regression model of change from baseline with treatment, diagnosis, baseline severity and interaction of treatment by diagnosis. P=.119 for interaction of treatment by diagnosis.
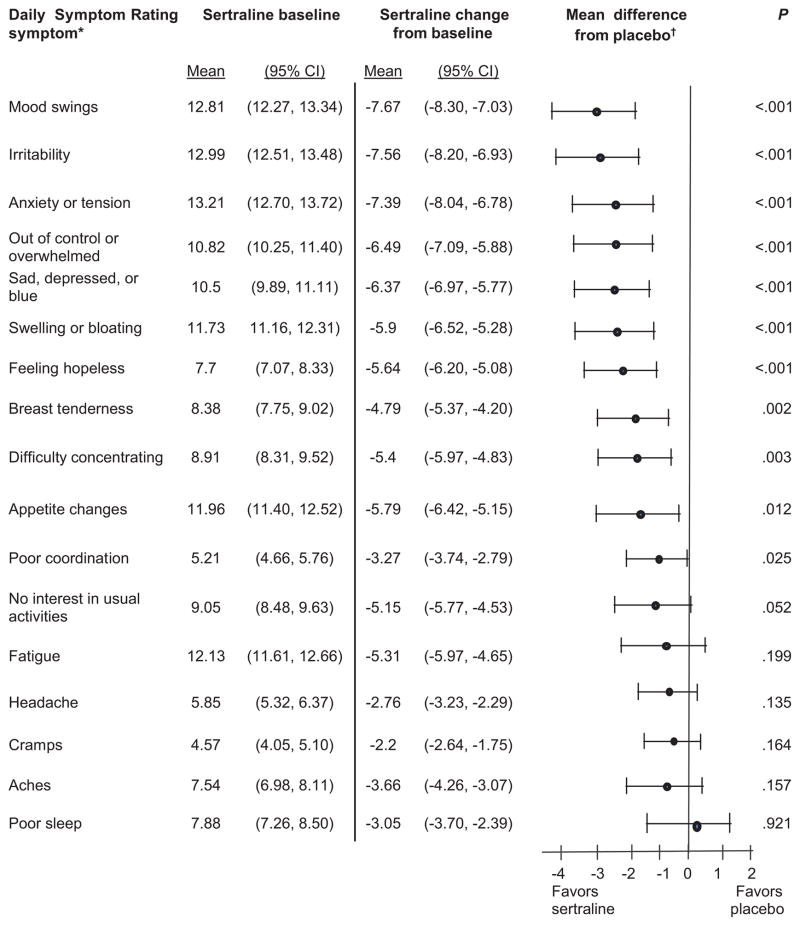
All psychological symptoms significantly improved with sertraline relative to placebo in the total sample: mood swings, irritability, anxiety/tension, out of control/overwhelmed, sad/depressed, hopelessness (each at P<0. 001), and difficulty concentrating (P=0.003) (Figure 2). Two of the most common physical symptoms of PMS significantly improved with sertraline relative to placebo: swelling, bloating (P<0.001) and breast tenderness (P=0.002). Improvement in appetite, poor coordination and decreased interest did not reach the threshold for statistical significance after adjustment for multiple comparisons. Symptoms that clearly did not respond to sertraline relative to placebo were fatigue, headache, cramps, aches, and poor sleep (P>0.20).
Figure 2.
Symptom response to sertraline treatment. Sertraline n=328; placebo n=119. Significance level is P≤.003 with Bonferroni adjustment. *Raw score range: 0–24; higher scores are more severe. †General linear regression model adjusted for baseline severity.
Evaluation of the 3 symptom-based subtypes showed no significant differences in demographic characteristics of age, race, education or history of depression. The three most severe symptoms in the mixed and psychological subtypes were irritability, anxiety/tension and mood swings. The most severe symptoms in the physical subtype were swelling/bloating, appetite changes, fatigue, mood swings and breast tenderness. Of the participants with a PMS diagnosis, 58% were in the mixed subtype, 29% were in the physical subtype, and 13% were in the psychological subtype. Of participants with a PMDD diagnosis, 82% were in the mixed subtype, 16% were in the psychological subtype, and only 1 woman was in the physical subtype.
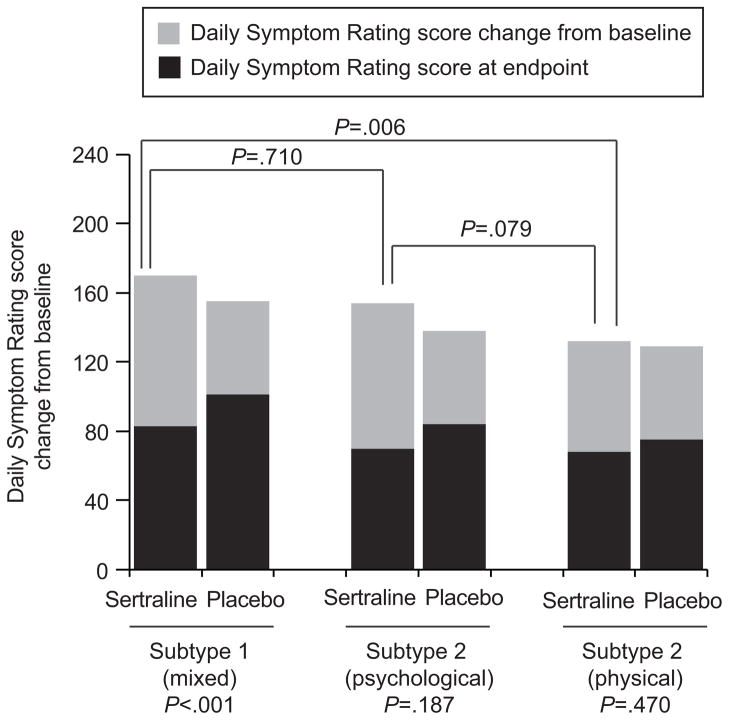
In the multivariable model, only the mixed symptom subtype significantly improved with sertraline relative to placebo adjusted for baseline severity (mean difference from placebo: 33.80, 95% CI: 17.16, 50.44, P<0.001) (Table 2, Figure 3). Although the psychological subtype had a similar change from baseline with sertraline treatment, the small number of participants in the placebo group reduced statistical power within this subtype. The physical symptom subtype did not improve with sertraline relative to placebo. A further comparison of improvement of only the physical symptoms in the mixed and physical symptom subtypes showed no significant difference (P=0.713), indicating that the strong improvement in the mixed subtype was driven by the psychological symptoms.
Figure 3.
Adjusted mean Daily Symptom Rating change (gray bars) from baseline (total bars) by treatment and subtype. The number of women in the Subtype 1 (mixed psychological and physical symptoms) group taking sertraline was 196, and the number taking the placebo was 78; for women in the Subtype 2 (predominant psychological symptoms)group, 51 women took sertraline, and nine took the placebo; for women in the Subtype 3 (predominant physical symptoms)group, 81 took sertraline, and 32 took the placebo. All P values are from the linear regression model of change from baseline with treatment, subtype, baseline severity and interaction of treatment by subtype.
Improvement of individual symptoms differed in the subtypes. In the mixed subtype, all psychological symptoms and physical symptoms of swelling/bloating and breast tenderness significantly improved, similar to the symptom improvement shown in Table 3 for the total sample. In the psychological subtype, all psychological but no physical symptoms improved relative to placebo. In the physical subtype, only swelling/bloating and mood swings significantly improved relative to placebo. It is noteworthy that in each subtype, symptoms that improved were those that were most severe, indicating that severity was an important factor in improvement relative to placebo. Within subtypes, the less severe symptoms – psychological as well as physical -- did not improve relative to placebo
The secondary outcome of clinical improvement was significant in the mixed symptom subtype (64% vs 42%, P<0.001). Clinical improvement was not significant in the physical subtype (52% vs 38%, P=0.169) or the psychological subtype (59% vs 33%, P=0.109). Based on clinical improvement (50% decrease from baseline), the results indicated that 8.3 participants in the mixed symptom subtype, 3.9 in the psychological subtype and 7.1 in the physical subtype are needed to observe one woman in the subtype who would achieve clinical improvement.
DISCUSSION
Premenstrual symptoms improved significantly with sertraline relative to placebo in the sample overall, regardless of whether the diagnosis was PMS or PMDD. These findings are consistent with other clinical trials and a previous meta-analysis of SSRI treatment for this disorder (3). In contrast, symptom-based subtypes differed significantly in response to sertraline treatment, with significant improvement in the mixed subtype and no significant improvement relative to placebo in the physical symptom subtype.
Symptom severity was an important factor in observed improvement. The two physical symptoms that significantly improved with sertraline (swelling/bloating and breast tenderness) were reported as severe in the total sample and in the physical subtype, while the mean ratings of unimproved physical symptoms were below the severity threshold. Moreover, although women in the physical subtype had multiple psychological symptoms, their severity was below the threshold and did not improve relative to placebo. Even irritability, which has been reported as the most responsive symptom to SSRI treatment (5, 16), did not improve in the context of the physical subtype, where its mean level was below the severity threshold. These findings indicate that symptom improvement varies with both severity and context and support the diagnostic principle that symptom severity is a primary determinant in treatment response.
Although recent surveys indicate that physical symptoms are the most prevalent symptoms of the syndrome (e.g., swelling/bloating, breast tenderness and abdominal pain, followed by irritability and mood swings (17, 18)), there has been little study of the response of physical symptoms to treatment. Other clinical trials of SSRIs reported improvement of swelling/bloating and breast tenderness (16, 19, 20) but did not evaluate other physical symptoms. We also found that no physical symptoms improved relative to placebo in the PMDD diagnosis group, consistent with one previous SSRI trial for PMDD (21), possibly because the diagnosis gives little emphasis to physical symptoms. These findings suggest that the limited response of physical symptoms may be a factor in a poor response to sertraline. The greater drug-placebo difference in women with more severe symptoms was similar to other but not all studies of mood disorders (22–24), where placebo response ranged from 12% to over 55% (22). Our findings indicate the importance of placebo control for assessing the benefit of medication (25) but also suggest that women who improved with placebo treatment, particularly those with less severe symptoms, might benefit from other non-drug therapies.
A limitation of the study is that only one treatment was examined. It would be important to evaluate the treatment response of symptom-based subtypes to other classes of medication, particularly oral contraceptives, which are frequently prescribed for PMS. The study participants did not have other major physical or mood disorders, and comorbidity remains an important and unstudied factor in the clinical responses to treatments for PMS and PMDD. The study participants were in general good health, primarily in their mid-reproductive years, city dwellers and primarily white, and results may not be generalizable to all women with premenstrual symptoms.
The strengths of the study include investigation of treatment response in randomized clinical trials, prospective symptom ratings in daily diaries throughout the clinical trials, appropriate statistical power, investigation of both psychological and physical symptoms, and new information on subtypes in the PMS population.
The findings indicate that the majority of women were distressed by both psychological and physical symptoms and were in the mixed symptom subtype, regardless of a PMS or PMDD diagnosis. While both diagnoses had a similar and significant response to sertraline, symptom-based subtypes responded differentially to SSRI treatment. The mixed subtype significantly improved, while the physical subtype did not significantly improve relative to placebo. The prevalent PMS symptoms of breast tenderness and swelling/bloating improved, but most physical symptoms did not respond to sertraline. This suggests that clinicians carefully evaluate the individual symptoms of each patient to determine which symptoms are predominant and whether they are severe. Symptoms that are not severe, particularly physical symptoms, may not respond to sertraline treatment. Further studies of PMS subtypes and their responses to other medications are needed, as are comparisons of SSRIs with oral contraceptives or other hormone treatments for premenstrual disorders.
Acknowledgments
Supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development: RO1 HD018633.
Footnotes
Data from the American College of Obstetricians and Gynecologists. Premenstrual syndrome. ACOG Practice Bulletin 15. Washington, DC: ACOG; 2000.
Data from the American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed.; 2000. p. 774.
Financial Disclosure: Dr. Freeman has received research support from Forest Laboratories Inc., Wyeth, and Xanodyne Pharmaceuticals. Dr. Rickels received research grants (issued to the University of Pennsylvania) from Euthymics Bioscience, Inc., NIMH, Pamlab, and Pfizer, Inc. The other authors did not report any potential conflicts of interest.
References
- 1.Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD) Psychoneuroendocrinology. 2003;28:1–23. doi: 10.1016/s0306-4530(03)00098-2. [DOI] [PubMed] [Google Scholar]
- 2.Dimmock PW, Wyatt KM, Jones PW, O’Brien PM. Efficacy of selective serotonin reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;30(356):1131–6. doi: 10.1016/s0140-6736(00)02754-9. [DOI] [PubMed] [Google Scholar]
- 3.Brown J, O’Brien PM, Marjoribanks J, Wyatt K. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2009;2:CD001396. doi: 10.1002/14651858.CD001396.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Mitwally MF, Kahn LS, Halbreich U. Pharmacotherapy of premenstrual syndromes and premenstrual dysphoric disorder: current practices. Expert Opin Pharmacother. 2002;3:1577–90. doi: 10.1517/14656566.3.11.1577. [DOI] [PubMed] [Google Scholar]
- 5.Halbreich U, O’Brien PM, Eriksson E, Bäckström T, Yonkers KA, Freeman EW. Are there differential symptom profiles that improve in response to different pharmacological treatments of premenstrual syndrome/premenstrual dysphoric disorder? CNS Drugs. 2006;20:523–47. doi: 10.2165/00023210-200620070-00001. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien PM, Bäckström T, Brown C, Dennerstein L, Endicott J, Epperson CN, et al. Towards a consensus on diagnostic criteria, measurement and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13–21. doi: 10.1007/s00737-010-0201-3. Epub 2011 Jan 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman EW, Rickels K, Sammel MD, Lin H, Sondheimer SJ. Time to relapse after short- or long-term treatment of severe premenstrual syndrome with sertraline. Arch Gen Psychiatry. 2009;66:537–44. doi: 10.1001/archgenpsychiatry.2008.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman EW, Rickels K, Sondheimer SJ, Polansky M, Xiao S. Continuous or intermittent dosing with sertraline for patients with severe premenstrual syndrome or premenstrual dysphoric disorder. Am J Psychiatry. 2004;161:343–51. doi: 10.1176/appi.ajp.161.2.343. [DOI] [PubMed] [Google Scholar]
- 9.Freeman EW, Rickels K, Sondheimer SJ, Polansky M. Differential response to antidepressants in women with premenstrual syndrome/premenstrual dysphoric disorder: a randomized controlled trial. Arch Gen Psychiatry. 1999;56:932–9. doi: 10.1001/archpsyc.56.10.932. [DOI] [PubMed] [Google Scholar]
- 10.Freeman EW, DeRubeis RJ, Rickels K. Reliability and validity of a daily diary for premenstrual syndrome. Psychiatry Res. 1996;65:97–106. doi: 10.1016/s0165-1781(96)02929-0. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 12.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis 1 Disorders - Patient Edition (SCID-1/P, version 2.0) New York, NY: New York State Psychiatric Institute; 1995. [Google Scholar]
- 13.Mortola JF, Girton L, Beck L, Yen SS. Diagnosis of premenstrual syndrome by a simple, prospective, and reliable instrument: the calendar of premenstrual experiences. Obstet Gynecol. 1990;76:302–7. [PubMed] [Google Scholar]
- 14.Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41–9. doi: 10.1007/s00737-005-0103-y. [DOI] [PubMed] [Google Scholar]
- 15.Frank E, Harrell . Regression modelling strategies. Vol. 9.2 Springer-Verlag; 2001. [Google Scholar]
- 16.Eriksson E, Hedberg MA, Andersch B, Sundblad C. The serotonin reuptake inhibitor paroxetin is superior to the noradrenaline reuptake inhibitor maprotiline in the treatment of premenstrual syndrome. Neuropsychopharmacology. 1995;12:167–76. doi: 10.1016/0893-133X(94)00076-C. [DOI] [PubMed] [Google Scholar]
- 17.Dennerstein L, Lehert P, Keung LS, Pal SA, Choi D. A population-based survey of Asian women’s experience of premenstrual symptoms. Menopause Int. 2010;16:139–45. doi: 10.1258/mi.2010.010034. [DOI] [PubMed] [Google Scholar]
- 18.Dennerstein L, Lehert P, Bäckström TC, Heinemann K. Premenstrual symptoms --severity, duration and typology. Menopause Int. 2009;15:120–6. doi: 10.1258/mi.2009.009030. [DOI] [PubMed] [Google Scholar]
- 19.Steiner M, Romano SJ, Babcock S, Dillon J, Shuler C, Berger C, et al. The efficacy of fluoxetine in improving physical symptoms associated with premenstrual dysphoric disorder. BJOG. 2001;108:462–8. doi: 10.1111/j.1471-0528.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 20.Cohen LS, Miner C, Brown EW, Freeman E, Halbreich U, Sundell K, et al. Premenstrual daily fluoxetine for premenstrual dysphoric disorder: a placebo-controlled, clinical trial using computerized diaries. Obstet Gynecol. 2002;100:435–44. doi: 10.1016/s0029-7844(02)02166-x. [DOI] [PubMed] [Google Scholar]
- 21.Halbreich U, Bergeron R, Yonkers KA, Freeman E, Stout AL, Cohen L. Efficacy of intermittent, luteal phase sertraline treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2002;100:1219–29. doi: 10.1016/s0029-7844(02)02326-8. [DOI] [PubMed] [Google Scholar]
- 22.Stein DJ, Baldwin DS, Dolberg OT, Despiegel N, Bandelow B. Which factors predict placebo response in anxiety disorders and major depression? An analysis of placebo-controlled studies of escitalopram. J Clin Psychiatry. 2006;67:1741–6. doi: 10.4088/jcp.v67n1111. [DOI] [PubMed] [Google Scholar]
- 23.Rickels K, Lipman R, Raab E. Previous medication, duration of illness and placebo response. J Nerv Ment Dis. 1966;142:548–54. doi: 10.1097/00005053-196606000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Khan A, Leventhal RM, Khan SR, Brown WA. Severity of depression and response to antidepressants and placebo: an analysis of the Food and Drug Administration database. J Clin Psychopharmacol. 2002;22:40–5. doi: 10.1097/00004714-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287:1840–7. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]