Abstract
The contribution of the metabolic state of human erythrocytes to maintenance of cellular deformability was studied during and after in vitro incubation in serum for periods up to 28 hr. An initial loss of membrane deformability became apparent between 4 and 6 hr when cellular adenosine triphosphate (ATP) levels were approximately 70% of initial values. Membrane deformability then remained stable between 6 and 10 hr.
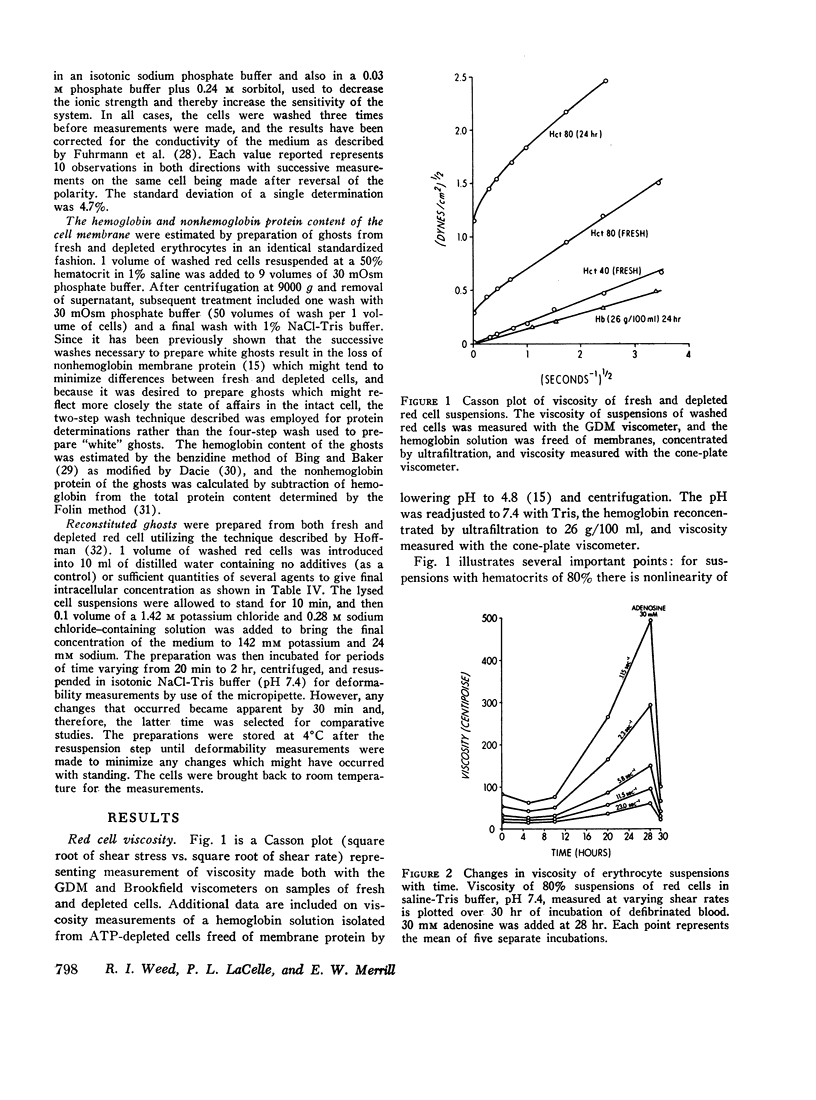
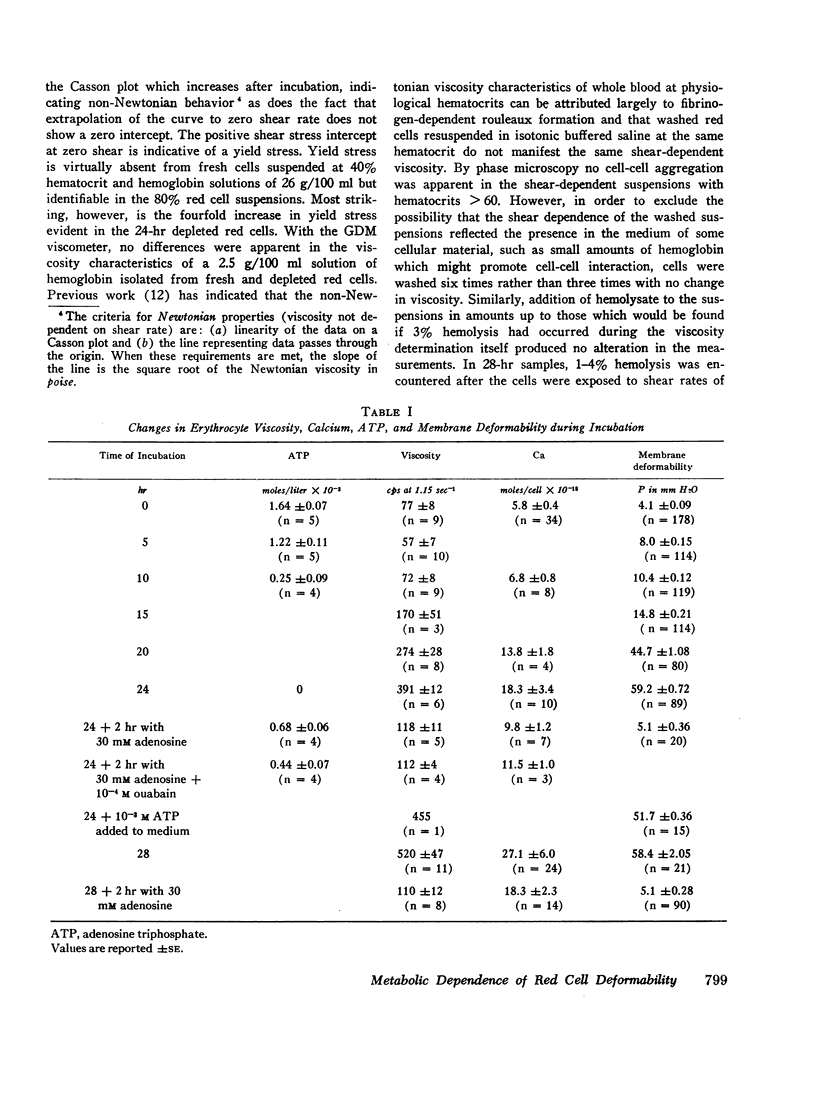
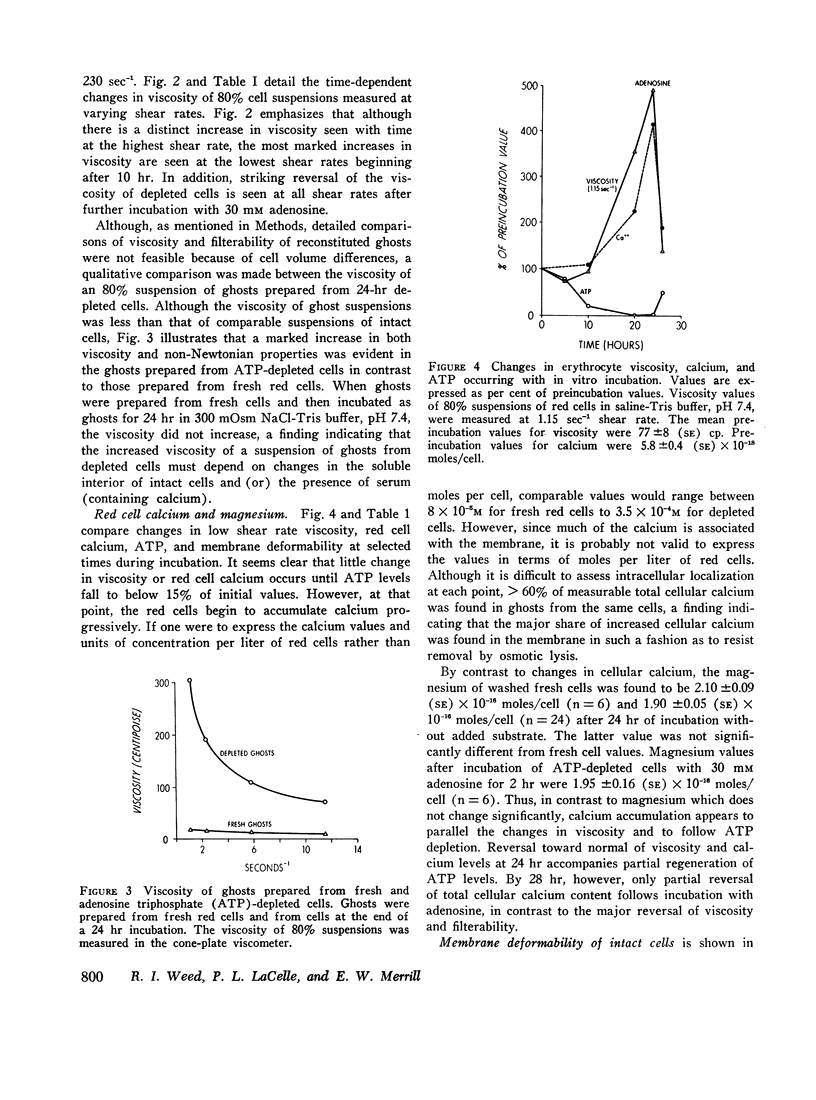
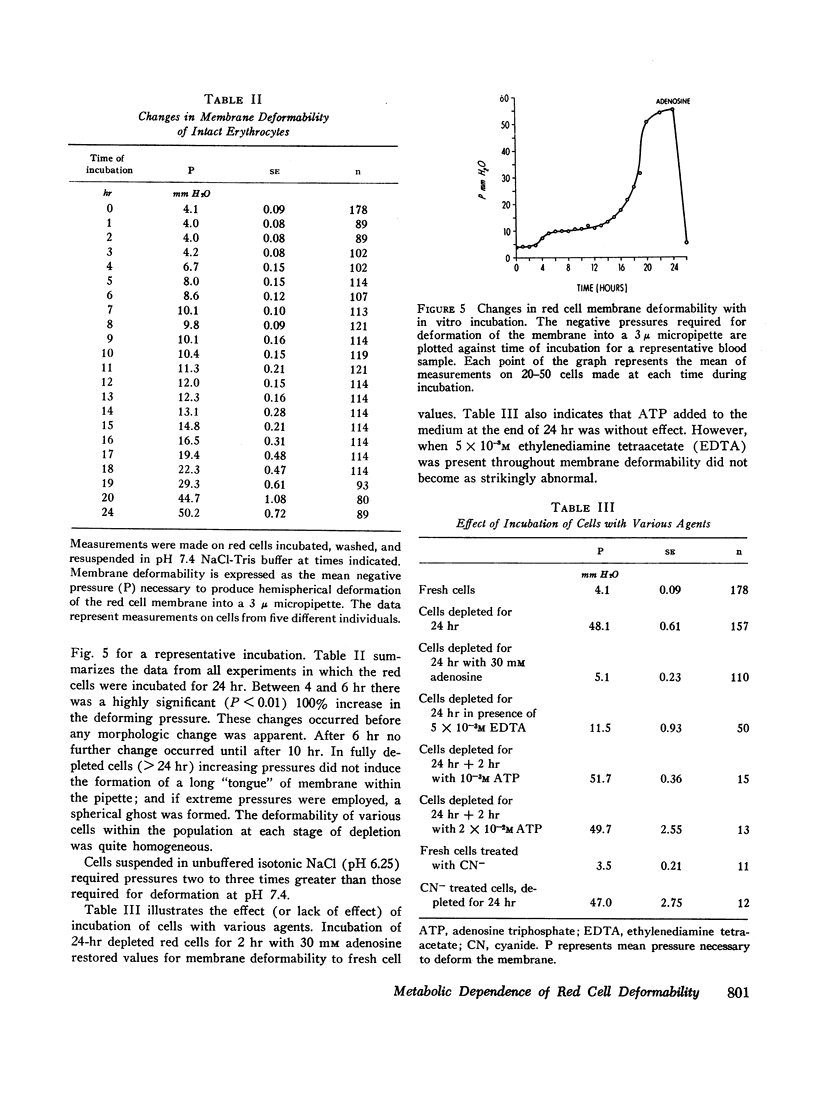
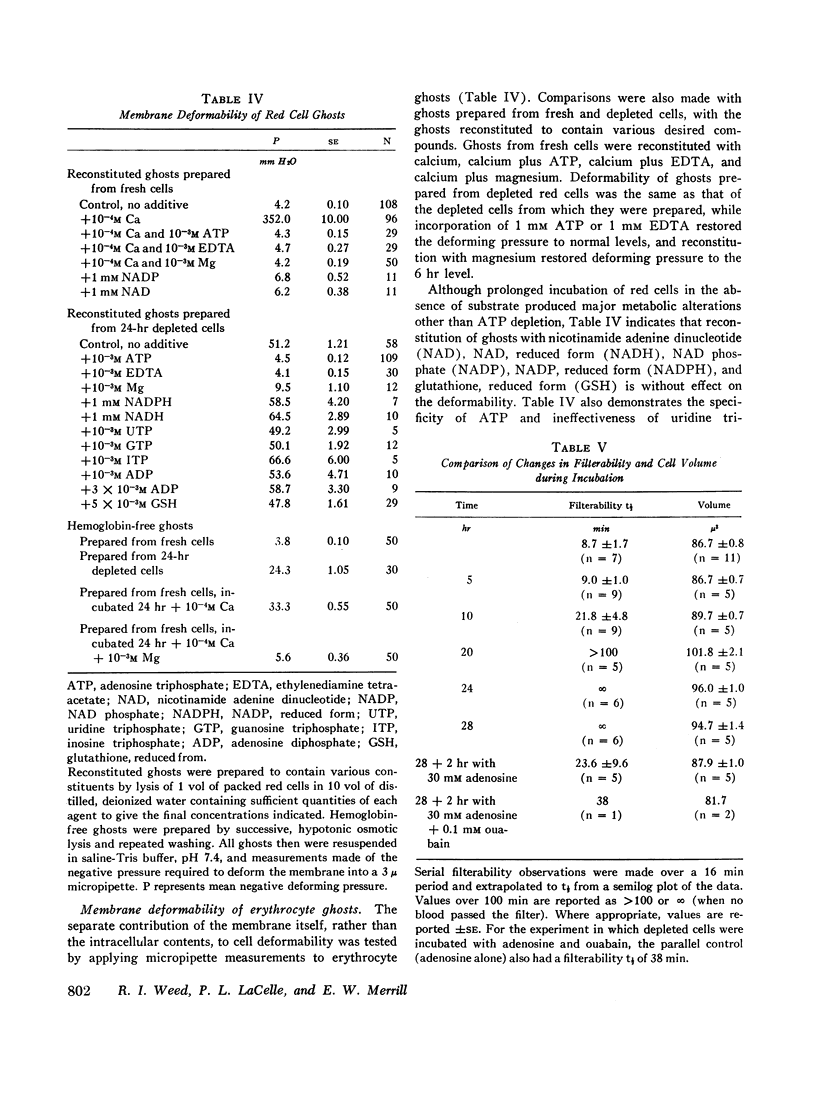
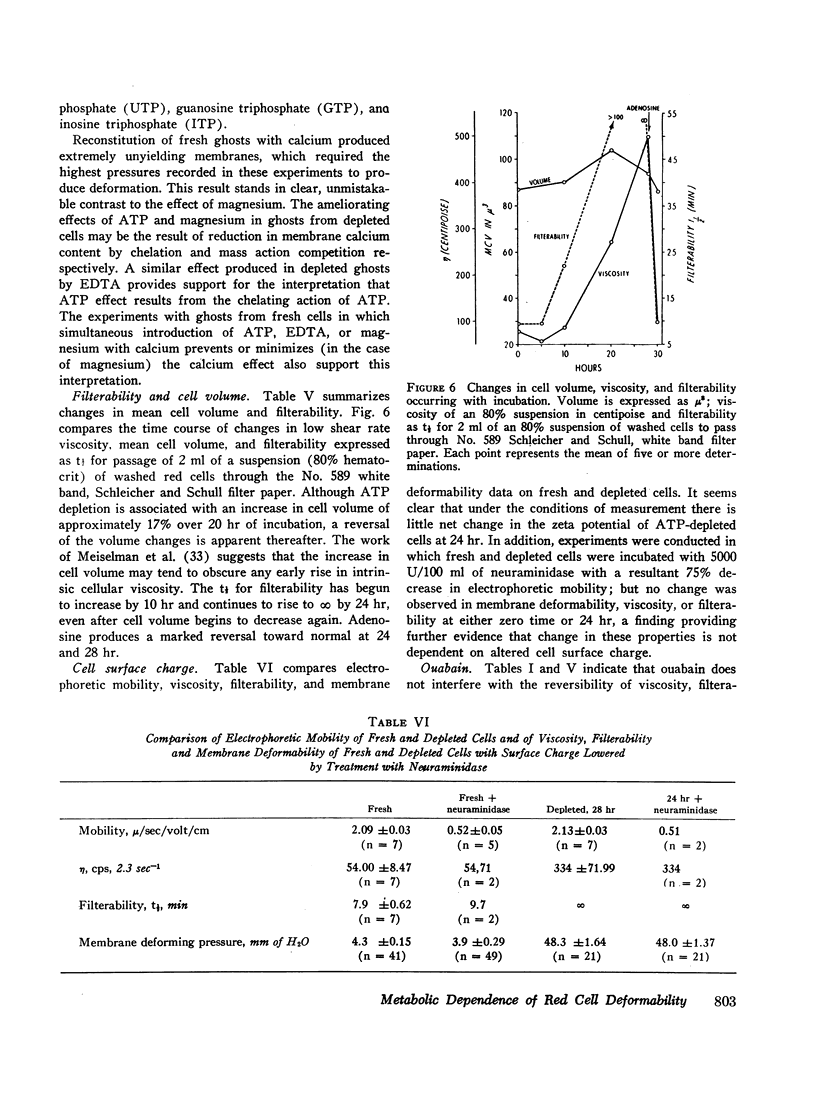
After 10 hr, when cellular ATP had decreased to < 15% of initial values, progressive parallel changes occurred in red cell calcium which increased 400% by 24 hr and in the viscosity of red cell suspensions which had risen 500-750% at 24 hr. A further progressive decrease in membrane deformability also occurred and was reflected by a 1000% increase in negative pressure required to deform the membrane. Red cell filterability decreased to zero as the disc-sphere shape transformation ensued. These changes were accompanied by an increase in ghost residual hemoglobin and nonhemoglobin protein.
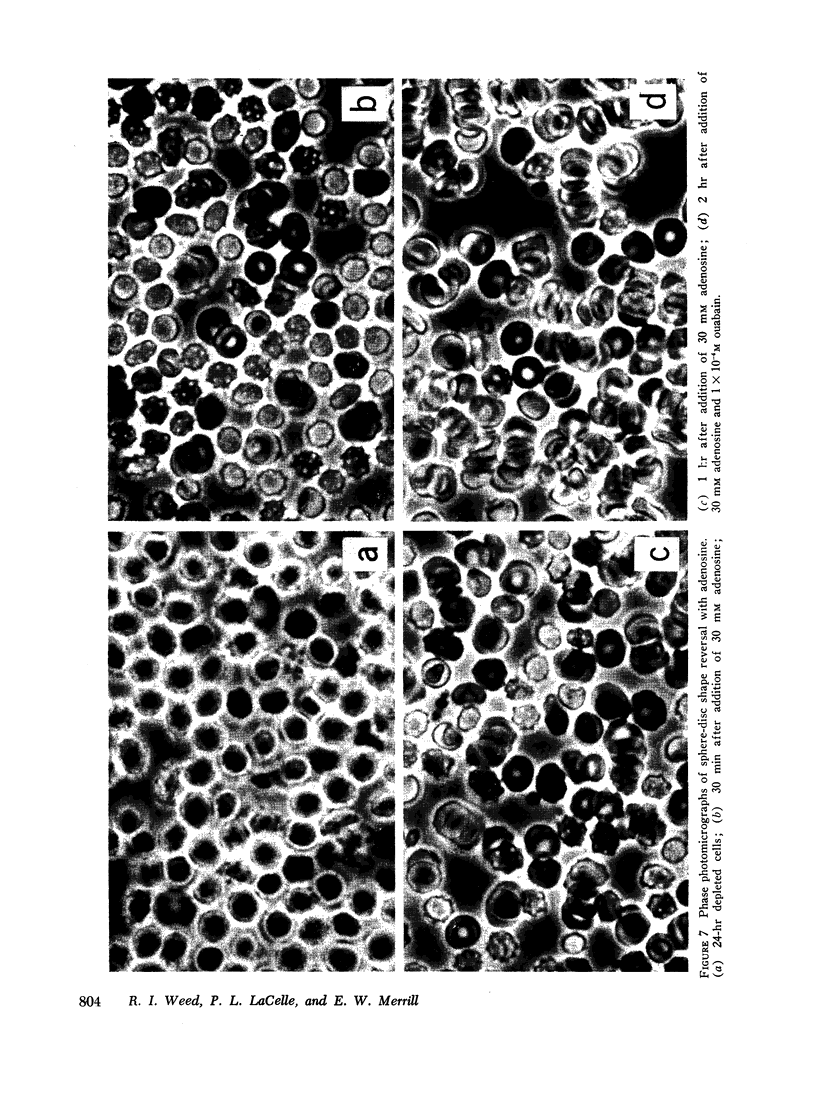
Regeneration of ATP in depleted cells by incubation with adenosine produced significant reversal of these changes, even in the presence of ouabain. Introduction of calcium into reconstituted ghosts prepared from fresh red cells mimicked the depleted state, and introduction of ATP, ethylenediamine tetraacetate (EDTA), and magnesium into depleted cells mimicked the adenosine effects in intact depleted cells. ATP added externally to 24-hr depleted cells was without effect. Simultaneous introduction of EDTA, ATP, or magnesium along with calcium into reconstituted ghosts prevented the marked decrease in deformability produced by calcium alone. Incorporation of adenosine diphosphate (ADP), nicotinamide adenine dinucleotide (NAD), NAD phosphate (NADP), NADP, reduced form (NADPH), glutatione, reduced form (GSH), inosine triphosphate (ITP), guanosine triphosphate (GTP), and uridine triphosphate (UTP) was without effect.
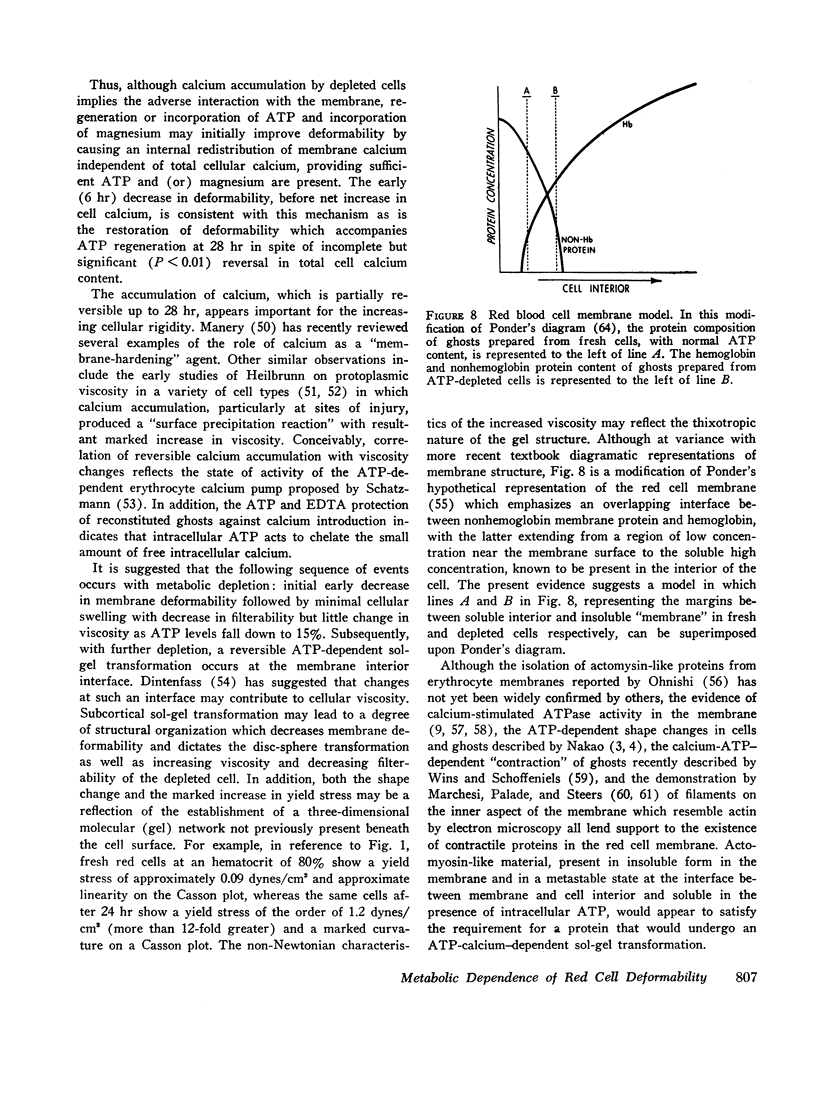
These data suggest that a major role of ATP in maintenance of red cell viability relates to preservation of red cell membrane deformability. It is proposed that the changes seen in the physical properties of ATP-depleted erythrocytes represent ATP-calcium-dependent sol-gel changes occurring at the interface between the membrane and the cell interior, and that the sol-gel balance determines membrane deformability.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN D. W., JANDL J. H. Oxidative hemolysis and precipitation of hemoglobin. II. Role of thiols in oxidant drug action. J Clin Invest. 1961 Mar;40:454–475. doi: 10.1172/JCI104273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aledort L. M., Weed R. I., Troup S. B. Ionic effects on firefly bioluminescence assay of red blood cell ATP. Anal Biochem. 1966 Nov;17(2):268–277. doi: 10.1016/0003-2697(66)90205-3. [DOI] [PubMed] [Google Scholar]
- Burton A. C. Role of geometry, of size and shape, in the microcirculation. Fed Proc. 1966 Nov-Dec;25(6):1753–1760. [PubMed] [Google Scholar]
- CROSBY W. H., MUNN J. I., FURTH F. W. Standardizing a method for clinical hemoglobinometry. U S Armed Forces Med J. 1954 May;5(5):693–703. [PubMed] [Google Scholar]
- Cahn R. D. Detergents in membrane filters. Science. 1967 Jan 13;155(3759):195–196. doi: 10.1126/science.155.3759.195. [DOI] [PubMed] [Google Scholar]
- Charache S., Conley C. L., Waugh D. F., Ugoretz R. J., Spurrell J. R. Pathogenesis of hemolytic anemia in homozygous hemoglobin C disease. J Clin Invest. 1967 Nov;46(11):1795–1811. doi: 10.1172/JCI105670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien S., Usami S., Dellenback R. J., Gregersen M. I. Blood viscosity: influence of erythrocyte deformation. Science. 1967 Aug 18;157(3790):827–829. doi: 10.1126/science.157.3790.827. [DOI] [PubMed] [Google Scholar]
- Chien S., Usami S., Dellenback R. J., Gregersen M. I., Nanninga L. B., Guest M. M. Blood viscosity: influence of erythrocyte aggregation. Science. 1967 Aug 18;157(3790):829–831. doi: 10.1126/science.157.3790.829. [DOI] [PubMed] [Google Scholar]
- DINTENFASS L. MOLECULAR AND RHEOLOGICAL CONSIDERATIONS OF THE RED CELL MEMBRANE IN VIEW OF THE INTERNAL FLUIDITY OF THE RED CELL. Acta Haematol. 1964 Nov;32:299–313. doi: 10.1159/000209575. [DOI] [PubMed] [Google Scholar]
- DINTENFASS L. RHEOLOGY OF PACKED RED BLOOD CELLS CONTAINING HEMOGLOBINS A-A, S-A, AND S-S. J Lab Clin Med. 1964 Oct;64:594–600. [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERSLEV A. J., ATWATER J. EFFECT OF MEAN CORPUSCULAR HEMOGLOBIN CONCENTRATION ON VISCOSITY. J Lab Clin Med. 1963 Sep;62:401–406. [PubMed] [Google Scholar]
- Gregersen M. I., Bryant C. A., Hammerle W. E., Usami S., Chien S. Flow Characteristics of Human Erythrocytes through Polycarbonate Sieves. Science. 1967 Aug 18;157(3790):825–827. doi: 10.1126/science.157.3790.825. [DOI] [PubMed] [Google Scholar]
- HARLEY J. D., MAUER A. M. Studies on the formation of Heinz bodies. II. The nature and significance of Heinz bodies. Blood. 1961 Apr;17:418–433. [PubMed] [Google Scholar]
- HARRIS J. W., BREWSTER H. H., HAM T. H., CASTLE W. B. Studies on the destruction of red blood cells. X. The biophysics and biology of sickle-cell disease. AMA Arch Intern Med. 1956 Feb;97(2):145–168. doi: 10.1001/archinte.1956.00250200021002. [DOI] [PubMed] [Google Scholar]
- HOFFMAN J. F. The active transport of sodium by ghosts of human red blood cells. J Gen Physiol. 1962 May;45:837–859. doi: 10.1085/jgp.45.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huishman T. H., Dozy A. M., Horton B. F., Nechtman C. M. Studies on the heterogeneity of hemoglobin. X. The nature of various minor hemoglobin components produced in human red blood cell hemolysates on aging. J Lab Clin Med. 1966 Mar;67(3):355–373. [PubMed] [Google Scholar]
- JANDL J. H., ENGLE L. K., ALLEN D. W. Oxidative hemolysis and precipitation of hemoglobin. I. Heinz body anemias as an acceleration of red cell aging. J Clin Invest. 1960 Dec;39:1818–1836. doi: 10.1172/JCI104206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H., SIMMONS R. L., CASTLE W. B. Red cell filtration and the pathogenesis of certain hemolytic anemias. Blood. 1961 Aug;18:133–148. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Manery J. F. Effects of Ca ions on membranes. Fed Proc. 1966 Nov-Dec;25(6):1804–1810. [PubMed] [Google Scholar]
- Marchesi V. T., Palade G. E. The localization of Mg-Na-K-activated adenosine triphosphatase on red cell ghost membranes. J Cell Biol. 1967 Nov;35(2):385–404. doi: 10.1083/jcb.35.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T., Steers E., Jr Selective solubilization of a protein component of the red cell membrane. Science. 1968 Jan 12;159(3811):203–204. doi: 10.1126/science.159.3811.203. [DOI] [PubMed] [Google Scholar]
- Meiselman H. J., Merrill E. W., Gilliland E. R., Pelletier G. A., Salzman E. W. Influence of plasma osmolarity on the rheology of human blood. J Appl Physiol. 1967 Apr;22(4):772–781. doi: 10.1152/jappl.1967.22.4.772. [DOI] [PubMed] [Google Scholar]
- Murphy J. R. The influence of pH and temperature on some physical properties of normal erythrocytes and erythrocytes from patients with hereditary spherocytosis. J Lab Clin Med. 1967 May;69(5):758–775. [PubMed] [Google Scholar]
- NAKAO K., WADA T., KAMIYAMA T., NAKAO M., NAGANO K. A direct relationship between adenosine triphosphate-level and in vivo viability of erythrocytes. Nature. 1962 Jun 2;194:877–878. doi: 10.1038/194877a0. [DOI] [PubMed] [Google Scholar]
- NAKAO M., NAKAO T., YAMAZOE S. Adenosine triphosphate and maintenance of shape of the human red cells. Nature. 1960 Sep 10;187:945–946. doi: 10.1038/187945a0. [DOI] [PubMed] [Google Scholar]
- NAKAO M., NAKAO T., YAMAZOE S., YOSHIKAWA H. Adenosine triphosphate and shape of erythrocytes. J Biochem. 1961 Jun;49:487–492. doi: 10.1093/oxfordjournals.jbchem.a127333. [DOI] [PubMed] [Google Scholar]
- NICOLAU C. T., TEITEL P., FOTINO M. Loss of plasticity of erythrocytes coated with incomplete antibodies. Nature. 1959 Dec 5;184(Suppl 23):1808–1809. doi: 10.1038/1841808b0. [DOI] [PubMed] [Google Scholar]
- OHNISHI T. Extraction of actin- and myosin-like proteins from erythrocyte membrane. J Biochem. 1962 Oct;52:307–308. doi: 10.1093/oxfordjournals.jbchem.a127620. [DOI] [PubMed] [Google Scholar]
- PONDER E. Present concepts of the structure of the mammalian red cell. Blood. 1954 Mar;9(3):227–235. [PubMed] [Google Scholar]
- RAND R. P., BURTON A. C. MECHANICAL PROPERTIES OF THE RED CELL MEMBRANE. I. MEMBRANE STIFFNESS AND INTRACELLULAR PRESSURE. Biophys J. 1964 Mar;4:115–135. doi: 10.1016/s0006-3495(64)86773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND R. P. MECHANICAL PROPERTIES OF THE RED CELL MEMBRANE. II. VISCOELASTIC BREAKDOWN OF THE MEMBRANE. Biophys J. 1964 Jul;4:303–316. doi: 10.1016/s0006-3495(64)86784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed C. F., Swisher S. N. Erythrocyte lipid loss in hereditary spherocytosis. J Clin Invest. 1966 May;45(5):777–781. doi: 10.1172/JCI105392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rega A. F., Weed R. I., Reed C. F., Berg G. G., Rothstein A. Changes in the properties of human erythrocyte membrane protein after solubilization by butanol extraction. Biochim Biophys Acta. 1967 Oct 23;147(2):297–312. doi: 10.1016/0005-2795(67)90408-4. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J. ATP-dependent Ca++-extrusion from human red cells. Experientia. 1966 Jun 15;22(6):364–365. doi: 10.1007/BF01901136. [DOI] [PubMed] [Google Scholar]
- Teitel P. Le test de la filtrabilité érythrocytaire (TFE). Une méthode simple d'étude de certaines propriétés microrhéologiques des globules rouges. Nouv Rev Fr Hematol. 1967 Mar-Apr;7(2):195–214. [PubMed] [Google Scholar]
- Teitel P., Marcu I., Xenakis A. Erythrocyte microrheology: its dependence on the reduced sulfhydryl groups and hemoglobin integrity. Folia Haematol Int Mag Klin Morphol Blutforsch. 1968;90(2):281–295. [PubMed] [Google Scholar]
- WEED R. I., REED C. F., BERG G. Is hemoglobin an essential structural component of human erythrocyte membranes? J Clin Invest. 1963 Apr;42:581–588. doi: 10.1172/JCI104747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS L. The structure of fine splenic arterial vessels in relation to hemoconcentration and red cell destruction. Am J Anat. 1962 Sep;111:131–179. doi: 10.1002/aja.1001110203. [DOI] [PubMed] [Google Scholar]
- Weed R. I., Bowdler A. J. Metabolic dependence of the critical hemolytic volume of human erythrocytes: relationship to osmotic fragility and autohemolysis in hereditary spherocytosis and normal red cells. J Clin Invest. 1966 Jul;45(7):1137–1149. doi: 10.1172/JCI105420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed R. I., Reed C. F. Membrane alterations leading to red cell destruction. Am J Med. 1966 Nov;41(5):681–698. doi: 10.1016/0002-9343(66)90030-1. [DOI] [PubMed] [Google Scholar]
- Wins P., Schoffeniels E. ATP+ca++-linked contraction of red cell ghosts. Arch Int Physiol Biochim. 1966 Nov;74(5):812–820. doi: 10.3109/13813456609059954. [DOI] [PubMed] [Google Scholar]
- Wins P., Schoffeniels E. Studies on red-cell ghost ATPase systems: properties of a (Mg2+ + Ca2+)-dependent ATPase. Biochim Biophys Acta. 1966 Jul 13;120(3):341–350. doi: 10.1016/0926-6585(66)90301-3. [DOI] [PubMed] [Google Scholar]




