Abstract
This communication describes the development of a room temperature ligand-directed C–H arylation reaction using aryldiazonium salts. This was achieved by the successful merger of palladium-catalyzed C–H functionalization and visible light photoredox catalysis. The new method is general for a variety of directing groups and tolerates many common functional groups.
The biaryl motif is an important structural component of numerous natural products, pharmaceutical agents, and organic materials.1 As a result, the design of mild, general, and efficient methods for aryl-aryl bond construction continues to be an area of tremendous research effort.2 Over the past decade, transition metal-catalyzed C–H arylation reactions have been particularly well studied, and there have been numerous advances in the substrate scope, functional group tolerance, and range of different catalysts for promoting these transformations.2 However, despite this progress, the vast majority of C–H arylation methods still require elevated temperatures (>80 °C).3 The development of general room temperature C–H arylation reactions (particularly in non-acidic solvents) remains an important challenge for the field.3,4
In 2005, our group reported the Pd-catalyzed ligand-directed C–H arylation of amide and arylpyridine substrates with diaryliodonium salts.5 These transformations proceed in high yield at 100 °C; however, as shown in Scheme 1, very little product was obtained at room temperature. A detailed mechanistic study revealed that the rate-determining step of the catalytic cycle involves oxidation of dimeric intermediate 1 by [Mes–I–Ph]BF4 (Scheme 1).6 This result led us to hypothesize that the rate of the reaction could be accelerated (and therefore the temperature lowered) by substituting diaryliodonium salts with more kinetically reactive arylating reagents. We report herein the use of this strategy for the rational design of a new room temperature C–H arylation reaction. This achievement was made possible by merging Pd-catalyzed C–H functionalization with visible light photoredox catalysis.
Scheme 1.
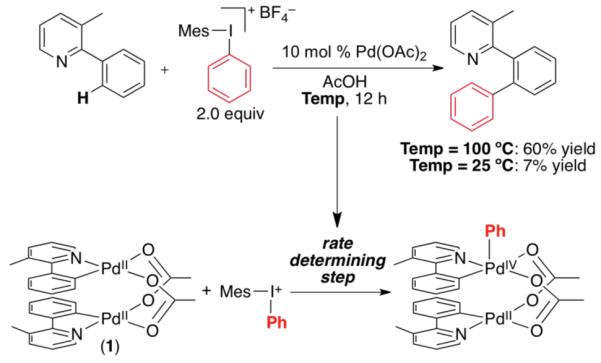
Pd-Catalyzed C–H Arylation with Diaryliodonium Salts
We reasoned that room temperature C–H arylation could be achieved by using Ph• as a highly kinetically reactive alternative to the diaryliodonium oxidant in Scheme 1. A recent report by Yu has suggested that phenyl radicals can participate in Pd-catalyzed C–H arylation reactions.7 However, high temperatures were required in this system in order to generate Ph• from dibenzoylperoxide via decarboxylation (eq. 1b).7 We sought to identify reagents that could form aryl radicals at room temperature, and a literature survey uncovered Deronzier’s Ru(bpy)3Cl2-photocatalyzed generation of aryl radicals at 25 °C from aryl diazonium salts.8a-e Inspired by this transformation, we envisioned that C–H activation intermediates could potentially be intercepted by aryl radicals formed at room temperature via visible light photocatalysis (eq. 1a).9
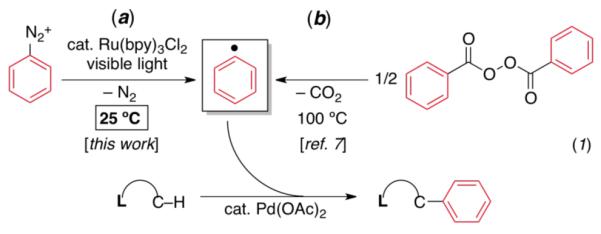 |
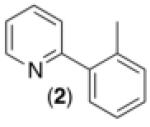
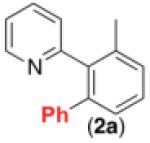
Initial investigations focused on the Pd-catalyzed C–H arylation of 2 with phenyldiazonium tetrafluoroborate. Ru(bpy)3Cl2•6H2O was employed as the photocatalyst, and a 26 W household fluorescent compact bulb was utilized as the source of visible light. We were pleased to find that the desired C–H arylation product 2a was formed in 31% yield at room temperature with 10 mol % of Pd(OAc)2, 2.5 mol % of Ru(bpy)3Cl2•6H2O, and 2 equiv of PhN2BF4 in MeOH (Table 1, entry 1). The yield was improved to 84% by using 4 equiv of PhN2BF4 in conjunction with 10 mol % of Ag2CO3 as an additive (entry 3). Importantly, [Pd], [Ru], and visible light were all critical for this transformation. In the absence of any of these components significantly reduced yield of 2a (≤8%) was observed (Table 1, entries 4-6). This transformation has several attractive features compared to most current C–H arylation methods,2 including a low operating temperature (25 °C),10 a non-acidic solvent (MeOH), and the generation of innocuous nitrogen and easily removable HBF4 as the only stoichiometric byproducts. In addition, this is the first example of palladium-catalyzed C–H functionalization using visible light photoredox catalysis.
Table 1.
Optimization of Reaction Between 2 and [PhN2]BF4[a]
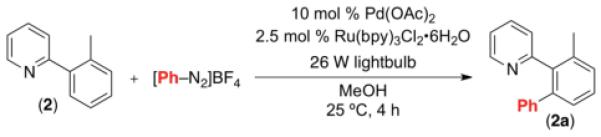
| Entry | [PhN2]BF4 (equiv) |
Additive (equiv) |
Yield[b] |
|---|---|---|---|
| 1 | 2 | None | 31% |
| 2 | 4 | None | 49% |
| 3 | 4 | Ag2CO3 (0.10) | 84% |
| 4[c] | 4 | Ag2CO3 (0.10) | 0% |
| 5[d] | 4 | Ag2CO3 (0.10) | 8% |
| 6[e] | 4 | Ag2CO3 (0.10) | 5% |
General conditions: Pd(OAc)2 (0.1 equiv), Ru(bpy)3Cl2•6H2O (0.025 equiv), MeOH (0.1 M in substrate), rt, 4 h, 26 W compact fluorescent light bulb.
Calibrated yields determined by gas chromatographic analysis of the crude reaction mixtures.
General conditions, but with no Pd(OAc)2.
General conditions, but with no Ru(bpy)3Cl2•6H2O.
General conditions, but with no light.
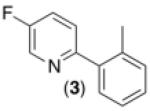
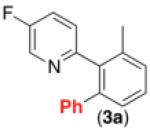
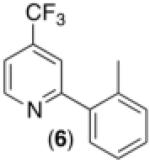
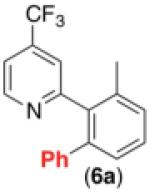
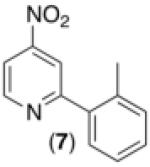
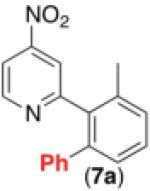
With optimal conditions in hand, we next explored the functional group compatibility of this transformation with a series of 2-arylpyridine derivatives. As shown in Table 2, C–H phenylation proceeded at room temperature in modest to good yield with a variety of electronically diverse pyridine substrates. In all cases, Pd(OAc)2, Ru(bpy)3Cl2•6H2O, and visible light were required to achieve >9% yield (see Supporting Information for control reactions). Electron donating and electron withdrawing substituents were well-tolerated on both the pyridine (entries 1-6) and aryl ring undergoing functionalization (entries 7 and 8). Additionally, aryl chloride and bromide substituents on the pyridine ring were compatible with the reaction conditions, suggesting against the intermediacy of Pd0 in this transformation (entries 3 and 4).
Table 2.
Substrate Scope of Room Temperature Pd/Ru-Catalyzed C–H Arylation of 2-Arylpyridine Derivatives
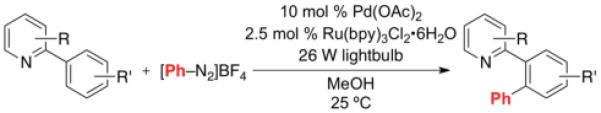
| Entry | Substrate | Product | Isolated Yielda |
|---|---|---|---|
| 1 b |
|
|
76% |
| 2c,d |
|
|
66% |
| 3b,d,e,f |
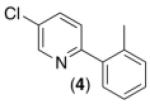
|
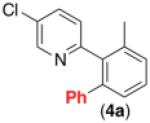
|
62% |
| 4b,c,d,e,f |
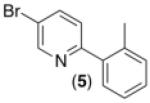
|
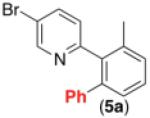
|
63% |
| 5d,g |
|
|
59% |
| 6c,d,e,h |
|
|
47% |
| 7d,f |
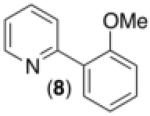
|
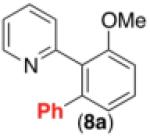
|
69% |
| 8c,d,e,g |
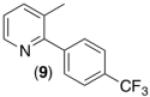
|
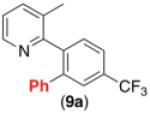
|
60% |
General procedure: Substrate (1.0 equiv), Pd(OAc)2 (0.1 equiv), Ru(bpy)3Cl2•6H2O (0.025 equiv), PhN2BF4 (4.0 equiv), MeOH (0.1 M in substrate), rt, 4 h, 26 W compact fluorescent light bulb.
Ag2CO3 (0.1 equiv) used as an additive.
General conditions but with 0.15 equiv of Pd(OAc)2.
MeOH (0.05 M in substrate).
5.0 equiv of PhN2BF4
Reaction run for 10 h.
Reaction run for 8 h.
Reaction run for 9.5 h.
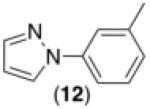
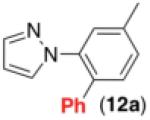
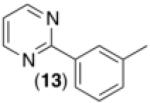
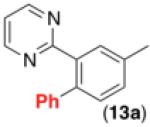
This room temperature C–H arylation reaction was also effective for substrates containing different directing groups, including amides, pyrazoles, pyrimidines, and oxime ethers (Table 3, entries 1-5). In addition, the mild reaction conditions enabled the first example of Pd-catalyzed C–H functionalization directed by a free oxime (Table 3, entry 6). This functional group typically undergoes rapid hydrolysis in the acidic solvents and at the elevated temperatures required for most related reactions.11 The oxime serves as a versatile functional handle for elaboration of the products, as it can be readily transformed into a ketone, alcohol, amine, or amide.11b Again, for the examples in Table 3, Pd(OAc)2, Ru, and visible light were all critical for achieving good yields of the products (see Supporting Information for details).
Table 3.
Scope of Directing Groups for Pd/Ru-Catalyzed C–H Arylation
General procedure: Substrate (1.0 equiv), Pd(OAc)2 (0.1 equiv), Ru(bpy)3Cl2•6H2O (0.025 equiv), PhN2BF4 (4.0 equiv), MeOH (0.1 M in substrate), rt, 4 h, 26 W compact fluorescent light bulb.
General conditions but with 0.05 equiv of Ru(bpy)3Cl2•6H2O.
Ag2CO3 (1.0 equiv) used as an additive.
Reaction run for 5 h.
3.5 equiv of PhN2BF4
General conditions but with 0.20 equiv of Pd(OAc)2.
Reaction run for 5.5 h.
MeOH (0.2 M in substrate).
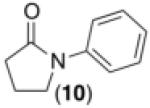
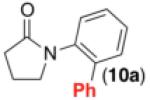
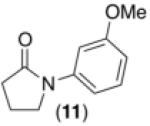
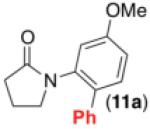
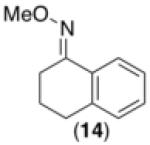
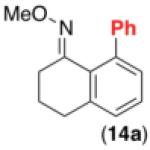
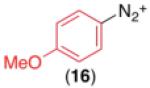
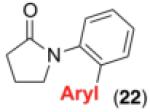
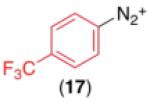
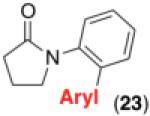
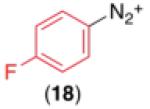
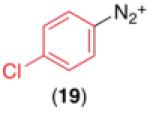
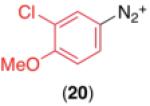
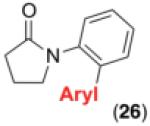
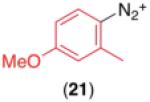
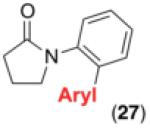
We next examined the use of diverse aryldiazonium salts in these transformations. As depicted in Table 4, C–H arylation proceeded in good to excellent yield with electron deficient, electron rich, and relatively sterically hindered arylating reagents. Interestingly, the electron deficient derivatives 17-19 showed modest to high photoreactivity with 1-phenylpyrrolidin-2-one (10) in the absence of Ru(bpy)3Cl2•6H2O. In one of the most pronounced cases, 10 underwent Pd-catalyzed photoreaction with [4-CF3C6H4N2]BF4 to afford 23 in 97% calibrated GC yield without [Ru] (Scheme 2). This yield was comparable to that obtained using 2.5 mol % of Ru(bpy)3Cl2•6H2O under otherwise analogous conditions (92% by GC). Interestingly, this uncatalyzed photoreaction was highly substrate dependent; for example, relatively little reaction was observed between [4-CF3C6H4N2]BF4 and oxime ether 14 in the absence of [Ru] (Scheme 2).12
Table 4.
Scope for Aryldiazonium Salts for Pd/Ru-Catalyzed C–H Arylation
General procedure: Substrate (1.0 equiv), Pd(OAc)2 (0.1 equiv), Ru(bpy)3Cl2•6H2O (0.025 equiv), ArylN2BF4 (4.0 equiv), MeOH (0.1 M in substrate), rt, 4 h, 26 W compact fluorescent light bulb.
Reaction run for 6 h.
General conditions but with 3.4 equiv of ArylN2BF4.
General conditions but with 0.05 equiv of Ru(bpy)3Cl2•6H2O.
Reaction run for 10 h.
MeOH (0.2 M in substrate).
Scheme 2.
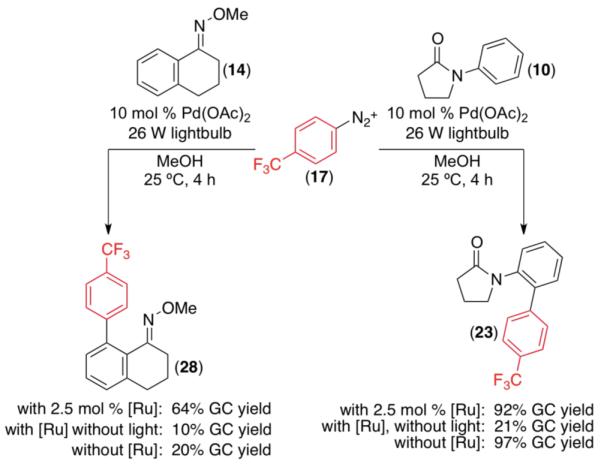
Background Reactions with Electron Deficient Aryldiazonium Salts
Although the mechanistic details of Pd/Ru photo-catalyzed C–H arylation remain to be elucidated, a possible catalytic cycle is shown in Figure 1. The key steps involve: (i) photo-excitation of the Ru catalyst to generate Ru(bpy)32+*, (ii) reduction of the diazonium salt to Ar• and concomitant oxidation of the Ru center to Ru(bpy)33+,8 (iii) reaction of Ar• with palladacycle 29 (generated by C–H activation of the substrate) to afford the PdIII intermediate 30, (iv) one electron oxidation of 30 by Ru(bpy)33+ to regenerate the photocatalyst and form PdIV intermediate 31, and finally (v) C–C bond-forming reductive elimination to release the arylated product and regenerate the PdII catalyst. Efforts to obtain a detailed mechanistic understanding of this transformation are currently underway.
Figure 1.
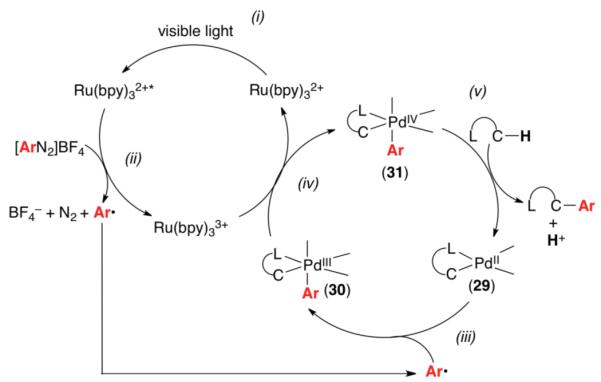
Possible Mechanism for Pd/Ru-Catalyzed C–H Arylation Reactions
In summary, this communication describes a mild approach for the palladium-catalyzed ligand-directed arylation of aromatic C–H bonds. These reactions proceed at room temperature and are compatible with a range of functional groups, directing ligands, and aryl diazonium salts. This system introduces the idea of merging visible light photoredox catalysis (which can be used to generate diverse reactive intermediates) with palladium-catalyzed C–H functionalization. We anticipate this new concept will find application in the development of many new synthetically useful transformations.
Supplementary Material
Acknowledgements
We gratefully acknowledge the NIH NIGMS (GM073836) as well as an ARRA supplement to this grant for financial support of this work.
Footnotes
Supporting Information Available: Experimental details and spectroscopic and analytical data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Hassan J, Sevignon M, Gozzi C, Schulz E, Lemaire M. Chem. Rev. 2002;102:1359. doi: 10.1021/cr000664r. [DOI] [PubMed] [Google Scholar]
- 2.For representative reviews on C–H arylation, see: Alberico D, Scott ME, Lautens M. Chem. Rev. 2007;107:174. doi: 10.1021/cr0509760. Kakiuchi F, Kochi T. Synthesis. 2008:3013. McGlacken GP, Bateman L. Chem. Soc. Rev. 2009;38:2447. doi: 10.1039/b805701j. Chen X, Engle KM, Wang D-H, Yu J-Q. Angew. Chem. Int. Ed. 2009;48:5094. doi: 10.1002/anie.200806273. Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147. doi: 10.1021/cr900184e. Daugulis O. Top. Curr. Chem. 2010;292:57. doi: 10.1007/128_2009_10. Chiusoli GP, Catellani M, Costa M, Motti E, Ca’ ND, Maestri G. Coord. Chem. Rev. 2010;254:456.
- 3.Wencel-Delord J, Droge T, Liu F, Glorius F. Chem. Soc. Rev. 2011;40:4740. doi: 10.1039/c1cs15083a. [DOI] [PubMed] [Google Scholar]
- 4.For isolated examples of room temperature Pd-catalyzed C–H arylation, see: Hull KL, Lanni EL, Sanford MS. J. Am. Chem. Soc. 2006;128:14047. doi: 10.1021/ja065718e. Xiao B, Fu Y, Xu J, Gong T-J, Dai J-J, Yi J, Liu L. J. Am. Chem. Soc. 2010;132:468. doi: 10.1021/ja909818n. Nishikata T, Abela AR, Huang S, Lipshutz BH. J. Am. Chem. Soc. 2010;132:4978. doi: 10.1021/ja910973a. Nishikata T, Abela AR, Lipshutz BH. Angew. Chem. Int. Ed. 2010;49:781. doi: 10.1002/anie.200905967. Haffemayer B, Gulias M, Gaunt MJ. Chem. Sci. 2011;2:312. Tredwell MJ, Gulias M, Bremeyer N, Johansson CCC, Collins BSL, Gaunt MJ. Angew. Chem. Int. Ed. 2011;50:1076. doi: 10.1002/anie.201005990.
- 5.Kalyani D, Deprez NR, Desai LV, Sanford MS. J. Am. Chem. Soc. 2005;127:7330. doi: 10.1021/ja051402f. [DOI] [PubMed] [Google Scholar]
- 6.(a) Deprez NR, Sanford MS. J. Am. Chem. Soc. 2009;131:11234. doi: 10.1021/ja904116k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ariafard A, Hyland CJT, Canty AJ, Sharma M, Yates BF. Inorg. Chem. 2011;50:6449. doi: 10.1021/ic102323s. [DOI] [PubMed] [Google Scholar]
- 7.Yu W-Y, Sit W, Zhou Z, Chan ASC. Org. Lett. 2009;11:3174. doi: 10.1021/ol900756g. [DOI] [PubMed] [Google Scholar]
- 8.(a) Cano-Yelo H, Deronzier A. J. Chem. Soc., Perkin Trans. 2. 1984:1093. [Google Scholar]; (b) Cano-Yelo H, Deronzier A. J. Chem. Soc., Faraday Trans. 1. 1984:3011. [Google Scholar]; (c) Cano-Yelo H, Deronzier A. Tetrahedron Lett. 1984;25:5517. [Google Scholar]; (d) Cano-Yelo H, Deronzier A. J. Photochemistry. 1987;37:315. [Google Scholar]; (e) Cano-Yelo H, Deronzier A. New J. Chem. 1987;11:479. [Google Scholar]; (f) Lalevee J, Blanchard N, Tehfe M-A, Peter M, Morlet-Savary F, Fouassier JP. Macromol. Rapid Commun. 2011;32:917. doi: 10.1002/marc.201100098. [DOI] [PubMed] [Google Scholar]
- 9.(a) Narayanam JMR, Stephenson CRJ. Chem. Soc. Rev. 2011;40:102. doi: 10.1039/b913880n. [DOI] [PubMed] [Google Scholar]; (b) Yoon TP, Ishchay MA, Du J. Nature Chem. 2010;2:527. doi: 10.1038/nchem.687. [DOI] [PubMed] [Google Scholar]; (c) Nicewicz D, MacMillan DWC. Science. 2008;322:77. doi: 10.1126/science.1161976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regulation of the reaction temperature at 25 °C was found to be important for obtaining reproducible yields in these reactions. See Supporting Information for details.
- 11.(a) Desai LV, Hull KL, Sanford MS. J. Am. Chem. Soc. 2004;126:9542. doi: 10.1021/ja046831c. [DOI] [PubMed] [Google Scholar]; (b) Neufeldt SR, Sanford MS. Org. Lett. 2010;12:532. doi: 10.1021/ol902720d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The origin of this photoreactivity in the absence of [Ru] is currently under investigation. We tentatively hypothesize that certain palladacyclic intermediates may participate in photoinduced electron transfer to electron deficient aryldiazonium salts to initiate this transformation.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.