Summary
In yeast, worms and flies, an extra copy of the gene encoding the Sirtuin Sir2 increases metabolic efficiency, as does administration of polyphenols like resveratrol, thought to act through Sirtuins. But evidence that Sirtuin gain-of-function results in increased metabolic efficiency in mammals is limited. We generated transgenic mice with moderate overexpression of SirT1, designed to mimic the Sirtuin gain-of-function that improves metabolism in C.elegans. These mice exhibit normal insulin sensitivity, but decreased food intake and locomotor activity, resulting in decreased energy expenditure. However, in various models of insulin resistance and diabetes, SirT1 transgenics display improved glucose tolerance due to decreased hepatic glucose production and increased adiponectin levels, without changes in body weight or composition. We conclude that SirT1 gain-of-function primes the organism for metabolic adaptation to insulin resistance, increasing hepatic insulin sensitivity and decreasing whole-body energy requirements. These findings have important implications for Sirtuin-based therapies in humans.
Introduction
The increased prevalence of obesity and diabetes, with the attendant increase in morbidity and mortality, pose a substantial therapeutic challenge (Narayan et al., 2003). Genetic screens in lower organisms provide evidence that gain-of-function of the deacetylase Sir2 results in beneficial metabolic effects and lifespan extension (Haigis and Guarente, 2006). Sirtuin agonists increase metabolic efficiency in rodents through a mechanism bearing similarity with calorie restriction (Baur et al., 2006; Lagouge et al., 2006; Milne et al., 2007). However, the specificity of these compounds remains undefined.
The function of mammalian Sir2α (also known as SirT1) in metabolism is controversial. Two models of SirT1 gain-of-function in either pancreatic β cells or adipocytes and brain result in improved insulin secretion and sensitivity, respectively (Bordone et al., 2007; Moynihan et al., 2005). But increasing SirT1 expression in liver impairs glucose tolerance (Rodgers et al., 2005), and resveratrol increases glucose production by hepatoma cells (Frescas et al., 2005). Thus, it remains unclear whether chronic, ubiquitous SirT1 activation ultimately benefits or impairs metabolic control.
To address this question, we developed transgenic mice designed to mimic the chromosomal duplication leading to moderate gain-of-function that improves metabolism in C.elegans. In the latter organism, Sir2 function requires the FoxO1 ortholog daf-16 (Tissenbaum and Guarente, 2001). Thus, in our studies we sought to address whether SirT1 gain-of-function mediates its effects through FoxO1.
Results
Generation and analysis of SirT1 Transgenic Mice
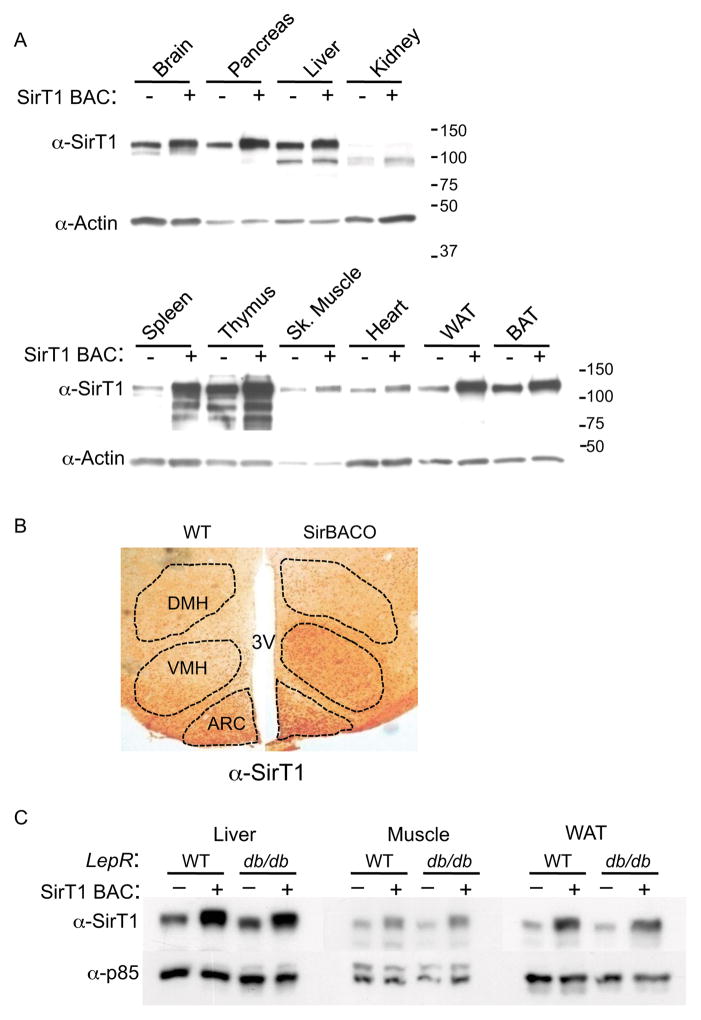
To test the hypothesis that SirT1 participates in the regulation of metabolism, we generated BAC transgenic mice overexpressing SirT1 (SirT1 Bacterial Artificial Chromosome Overexpressor, SirBACO). SirBACO mice were born in Mendelian ratios and displayed no gross anatomical or reproductive defects. SirT1 levels were two- to threefold higher than control littermates, except in the spleen (~seven-fold higher), and distribution patterns were indistinguishable from those of the endogenous protein in all tissues examined (Figure 1A–1B). SirT1 overexpression was preserved when SirBACO mice were backcrossed onto the db/db background (Figure 1C). To probe the transgene’s function, we intercrossed SirBACO with Sirt1−/− mice (McBurney et al., 2003). The transgene readily rescued developmental defects and postnatal lethality of Sirt1−/− mice (data not shown). These findings are consistent the generation of a mouse model of SirT1 gain-of-function.
Figure 1. Expression Levels and Tissue Distribution of Sirt1 in SirBacO Mice.
(A) Western blot of SirT1 in mouse tissues. (B) Immunohistochemistry of SirT1 in mouse brain; DMH: dorsomedial hypothalamus; VMH: ventromedial hypothalamus; ARC: arcuate nucleus. (C) SirT1 expression in SirBACO::db/db mice and controls.
Altered energy balance in SirBACO Mice
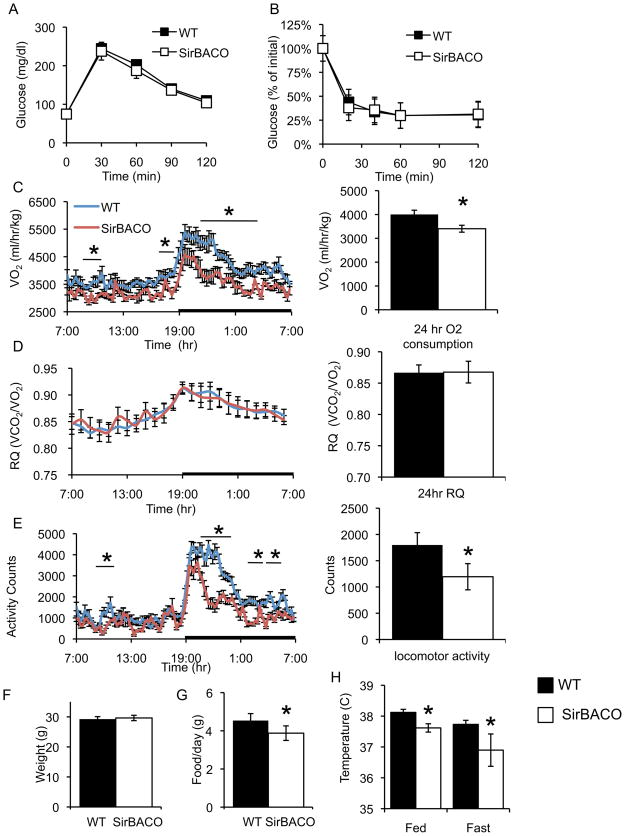
We carried out a metabolic characterization of SirBACO mice on a standard diet. Serum metabolite levels–including insulin and glucose–were normal (Table S1), as was the response to intraperitoneal glucose or insulin challenge tests (Figure 2A–2B). However, we detected a ~30% increase in plasma adiponectin levels (Table S1).
Figure 2. Metabolic effects of SirT1 overexpression.
(A) Intraperitoneal glucose tolerance tests and (B) Insulin tolerance tests in four-month-old mice on standard diet (n= 9–11 each). (C) Oxygen consumption (VO2), (D) respiratory quotient (RQ), and (E) locomotor activity in eight-week-old mice over 24-hr (line chart) and mean 24-hr values (bar graphs) in WT (full bars) and SirBACO mice (empty bars) (n= 7–8 each). (F) Body weight of chow-fed, four-month-old mice (n= 10–11 each). (G) 24-hr food intake (n= 9–11). (H) Body temperature in fed and 24-hr-fasted mice (n=9–12 each). *= P<0.05.
In C.elegans and D.melanogaster, increased Sir2 mimics the effects of calorie restriction to extend lifespan (Haigis and Guarente, 2006). In mammals, prolonged calorie restriction decreases energy expenditure and increases metabolic efficiency by decreasing O2 consumption (Fontana and Klein, 2007). To determine whether SirT1 overexpression reproduces these sub-phenotypes, we carried out indirect calorimetry experiments. SirBACO mice demonstrated a coordinate ~15% decrease in O2 consumption and CO2 production (Figure 2C and data not shown). Due to the combined fall in VO2 and VCO2, the respiratory quotient (RQ = VCO2/VO2) was unchanged (Figure 2D). In addition, spontaneous locomotor activity decreased by 33.6 ± 1.4% (Figure 2E), more markedly during the dark phase.
To investigate differences in basal metabolic rate, we compared the energy expenditure required for locomotor activity (Ravussin et al., 1986). SirBACO mice had decreased resting energy expenditure, but no differences at higher activity levels (Figure S1). Body weight and composition were similar in SirBACO mice and non-transgenic littermates (Figure 2F). Food intake decreased by 15 ± 1.3% in SirBACO mice (Figure 2G), consistent with the preservation of normal body weight, in the face of decreased metabolic rate and activity.
In response to 24-hr food deprivation SirBACO mice showed a normal drop in RQ, reflecting increased fat oxidation (not shown). These data indicate that, although hypometabolic, SirBACO mice have normal rates of fatty acid oxidation. They also exhibited lower body temperature than control littermates under fed and fasted conditions (Figure 2H), but did not display changes in blood pressure or heart rate (Figure S2), suggesting that the effect on body temperature is secondary to decreased locomotion (Weinert and Waterhouse, 1998).
SirBACO mice are protected from insulin-resistant diabetes
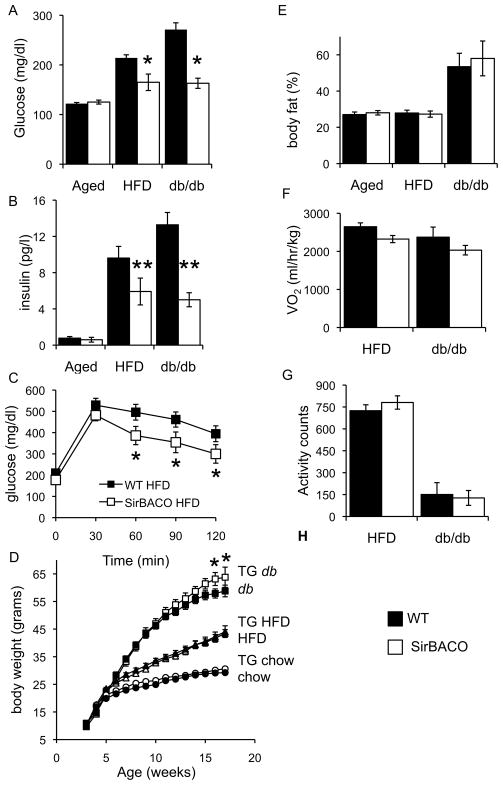
We next asked whether SirT1 overexpression affects the susceptibility to insulin resistance and diabetes in environmental and genetic models of these conditions. When SirBACO mice on an inbred C57BL/6J background were placed on a HFD or backcrossed onto db/db mice, they showed lower blood glucose and plasma insulin levels (Figures 3A–3B and Table S1) and better glucose tolerance (Figure 3C) than non-transgenic controls, despite similar body weight (Figure S3A), weight gain during the diet (Figure 3D), and body fat content (Figure 3E). We provisionally conclude that SirT1 overexpression does not improve glucose tolerance per se, but prevents the adverse effects of obesity on glucose metabolism.
Figure 3. SirT1 increases insulin sensitivity in obese mice.
(A) Fasting glucose and (B) insulin levels in SirBACO and WT mice in three conditions: 11-month-old on standard diet (aged), five-month-old on HFD, or eight-week-old on db/db background. (C) Glucose tolerance in four-month-old SirBACO mice on HFD. (D) Body weight in SirBACO (empty symbols) and WT mice (filled symbols) fed regular chow (n=13–14 each), HFD (n=12–16 each) or bred with db/db (n= 9–14 each). (E) Fat mass, (F) indirect calorimetry, and (G) locomotor activity in db/db (full bars), high fat-fed WT (full bars) and SirBACO::db/db or HFD-fed SirBACO mice (empty bars) (n= 5–8 each). *= P<0.05, **= P<0.01.
Catalytically inactive SirT1 fails to protect aging mice from glucose intolerance
To assess the specificity of these findings, we carried out glucose tolerance tests in aging transgenic mice expressing a catalytically inactive SirT1 transgene (H355Y) (Luo et al., 2001). Unlike SirBACO mice, SirT1H355Y transgenics showed no differences in glucose tolerance from control littermates, demonstrating that SirT1 catalytic activity is required for its insulin-sensitizing effects (Figure S3B).
Normal energy expenditure in diabetes-resistant SirBACO mice
Based on the decrease of food intake and energy expenditure in SirBACO mice, we wanted to determine whether the transgenes ’ ability to improve glucose homeostasis in diabetes models could be explained by changes in physical activity or metabolic rate. In both the HFD and db/db model of insulin-resistant diabetes, body weight and composition were similar between SirT1 transgenic and non-transgenic mice (Figure 3D–3E). In contrast to the findings in SirBACO mice on a normal diet (Figure 2), there were no differences in metabolic rate, spontaneous locomotor activity, or food intake in SirBACO mice on a HFD or crossed onto db/db compared to littermate controls (Figure 3F-3G and data not shown). We conclude that SirT1 promotes a state of increased energy efficiency that protects against insulin resistance and hyperglycemia.
Hyperadiponectinemia and increased insulin sensitivity in SirBACO mice on HFD
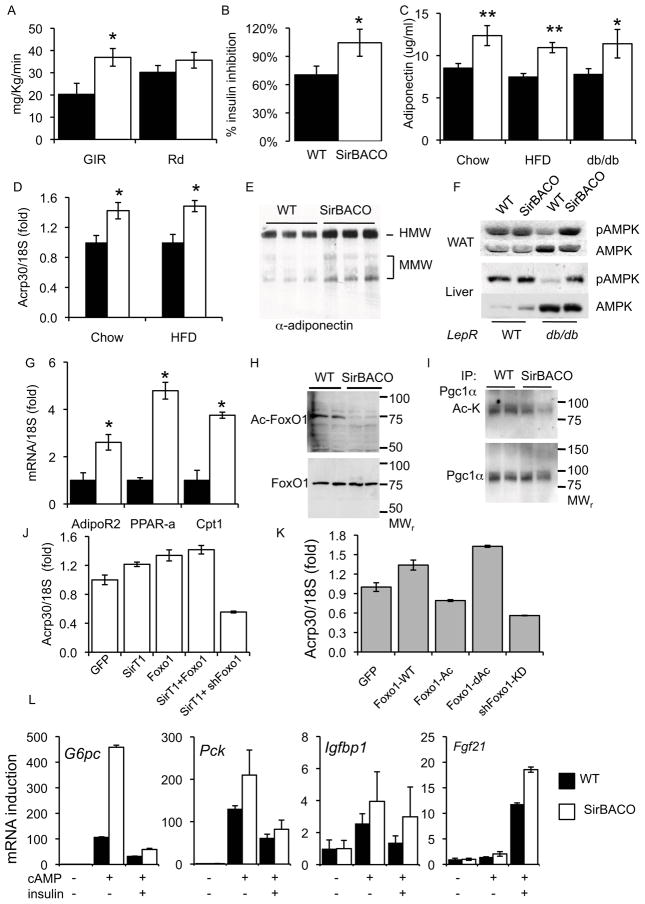
To determine the mechanism of improved glucose tolerance in SirBACO mice in a diabetes-predisposing background, we performed hyperinsulinemic euglycemic clamps in high fat-fed and in aging mice (twelve-month-old). In both models, we detected increased insulin-stimulated glucose disposal–as reflected by a higher glucose infusion rate (GIR)–without changes in glucose disappearance rates (Rd), when compared to controls (Figure 4A and S4). Moreover, insulin’s ability to suppress hepatic glucose production rose by >30% in high fat-fed SirBACO mice, relative to wild-type littermates (Figure 4B and S4). These findings indicate that the improvement of glucose homeostasis is due to increased hepatic insulin sensitivity (Accili, 2004).
Figure 4. SirT1 decreases HGP and regulates adiponectin.
(A) Glucose infusion (GIR) and disappearance rates (Rd) and (B) Insulin suppression of hepatic glucose production (HGP) in high fat-fed mice (n= 5–11 each). (C) Plasma adiponectin (n=11–13) and (D) WAT mRNA levels (Acrp30) in 16-week-old mice (eight-week-old in the SirBACO::db/db cross). (E) Plasma adiponectin isoform distribution. (F) AMPK phosphorylation in WAT and liver. (G) mRNA levels of adiponectin target genes (n= 4–5 each). (H) FoxO1 acetylation in hepatocytes and (I) Pgc1α acetylation in gastrocnemius muscle. (J–K) Regulation ofAcrp30 mRNA levels in 3T3-L1. *= P<0.05, **= P<0.01. (L) Gene expression in primary mouse hepatocytes (n=4 each).
The decrease in hepatic glucose production observed in clamp studies could be due to increased insulin production, improved insulin sensitivity, or both. However, plasma insulin levels were lower in high fat-fed SirBACO and SirBACO::db/db mice (Figure 3B), consistent with improved insulin sensitivity. Islet β cell mass, β cell insulin content and insulin secretion from isolated pancreatic islets were nearly identical in SirBACO and control mice (Figure S5). Based on prior evidence that adiponectin increases the hepatic insulin sensitivity (Combs et al., 2001), we analyzed the role of adiponectin in our transgenic model. Whereas high fat feeding was associated with a modest decrease in plasma adiponectin levels in wild-type mice, in SirBACO mice we observed a ~30–40% rise (Figure 4C), suggesting that the ability to prevent hyperglycemia is due to increased adiponectin synthesis/secretion, independent of differences in body fat mass. In contrast, we found no differences in resistin, PAI-1, TNFα, or leptin levels (Table S1). To identify the source of increased adiponectin levels, we measured adiponectin mRNA in white adipose tissue (WAT) from mice fed either a standard or HFD and observed a similar increase (Figure 4D). Plasma adiponectin isoform distribution was unchanged, with increases in both the high and medium molecular weight components (Figure 4E).
Adiponectin has been proposed to signal through at least two receptors, AdipoR1 and AdipoR2, which activate AMPK and PPARα, respectively (Yamauchi et al., 2003). Consistent with the observed hyperadiponectinemia, high fat-fed and SirBACO::db/db mice showed elevated phospho-AMPK levels in WAT, liver, and skeletal muscle (Figure 4F and Figure S6). Notably, SirT1 appears to prevent the obesity-driven decrease in phospho-AMPK levels (Martin et al., 2006). Furthermore, in high fat-fed SirBACO mice we also found higher mRNA levels of Pparα and its target genes, Cpt1 and Adipor2 in liver (Figure 4G). These results are consistent with increased adiponectin signaling through AdipoR1 and AdipoR2 and provide a potential mechanism for the increase in hepatic insulin sensitivity.
FoxO1 deacetylation phenocopies the effects of SirT1 overexpression
Finally, we sought to delineate a mechanism linking SirT1 overexpression to hyperadiponectinemia. Among SirT1 substrates, the transcription factor FoxO1 promotes adiponectin expression (Qiao and Shao, 2006) and FoxO1 haploinsufficiency decreases adiponectin levels (Nakae et al., 2003). SirT1 deacetylates FoxO1 at multiple sites, increasing FoxO1 transcriptional activity (Kitamura et al., 2005). Indeed, acetyl-FoxO1 levels were decreased in hepatocytes from SirBACO mice compared to controls (Figure 4H), as were acetyl-Pgc1α levels in muscle (Figure 4I).
To test the hypothesis that SirT1 and FoxO1 coordinately regulate adiponectin expression, we examined Acrp30 (the gene encoding adiponectin) expression in adipocytes. As constitutive activation of either FoxO1 or SirT1 inhibits adipocyte differentiation (Nakae et al., 2003; Picard et al., 2004), we used 3T3-L1 CARΔ, overexpressing the adenoviral receptor, to introduce adenoviruses encoding FoxO1 and SirT1 into differentiated cells (Orlicky et al., 2001). Wild-type FoxO1 increased Acrp30 by ~40%. Similarly, SirT1 increased Acrp30 transcription in a FoxO1-dependent manner (Figure 4J). Conversely, FoxO1 knock-down (Matsumoto et al., 2007) resulted in decreased Acrp30 levels (Figure 4K). Expression of a mutant FoxO1 designed to mimic SirT1-mediated deacetylation (FoxO1-dAc) increased Acrp30 expression, while a mutant FoxO1 mimicking “constitutive acetylation” (FoxO1-Ac) (Kitamura et al., 2005) failed to activate Acrp30 transcription (Figure 4K). These results indicate that FoxO1-dependent Acrp30 transcription can be regulated by SirT1-mediated deacetylation.
To assess the cell nonautonomous effects of SirT1 on liver gene expression, we isolated primary hepatocytes from WT or SirBACO mice and measured Foxo1/Pgc1α target genes. Consistent with prior observations (Frescas et al., 2005; Rodgers et al., 2005), we observed increased levels of G6pc, Pck1 and Igfbp1 in response to cAMP (Figure 4L). The Pparα target Fgf21 was also elevated in SirBACO hepatocytes, but was not regulated by cAMP (Figure 4L). These results indicate that the cell-autonomous and nonautonomous actions of SirT1 on hepatic glucose production are opposite. We speculate that circulating factors account for the latter.
Discussion
The ability of Sirtuin gain-of-function to extend lifespan and improve metabolism in simple organisms, together with the insulin-sensitizing properties of Sirtuin agonists in rodents, have led to the exploration of this pathway for therapeutic ends in metabolic disease. But a rigorous genetic test of the hypothesis that Sirtuin gain-of-function results in positive metabolic effects has not been carried out.
Two models of SirT1 gain-of-function had previously been reported. SirT1 overexpression in β cells increased insulin secretion (Moynihan et al., 2005). SirBACO mice did not show this phenotype, possibly due to differences in the levels of SirT1 expression. In SirT1 knock-in mice (Bordone et al., 2007), the transgene is expressed off the β-actin locus, leading potentially to developmental effects that impair adipocyte differentiation (Picard et al., 2004), and increase insulin sensitivity. Thus, the SirT1 knock-in model cannot distinguish between a direct effect of SirT1 and an indirect one due to reduced body fat.
Complex metabolic effects of SirT1 overexpression
Our results demonstrate complex, and not easily generalizable effects of SirT1 over-expression. In chow-fed mice, SirT1 gain-of-function has no discernible effects on insulin sensitivity and glucose utilization, but engenders a behavioral response that partly mimics calorie restriction. While further studies will be required to dissect the site(s) of these Sirtuin effects, they are likely to reflect an involvement of the central nervous system, and thus point to the need for rigorous behavioral/psychometric testing in early-phase clinical trials of Sirtuin agonists. In light of the current prevalence of overweight, the effects of SirT1 to reduce food intake are of some interest. In the present study we cannot identify whether these effects are mediated directly in the central nervous system, or by circulating factors.
Resveratrol-treated mice fed a HFD display increased O2 consumption (Lagouge et al., 2006) and decreased body weight, (Baur et al., 2006; Lagouge et al., 2006). SirBACO mice on a HFD do not display these phenotypes, but–similar to resveratrol-treated mice–show decreased locomotor activity (Lagouge et al., 2006). The data suggest that resveratrol acts on additional Sirtuins or on different targets. While both exogenous resveratrol and SirT1 overexpression improve glucose tolerance in high fat–fed and db/db mice, the mechanisms appears to differ: our studies point to a mechanistic link with hyperadiponectinemia, while improved ATP utilization seems to protect resveratrol-treated mice from diet-induced diabetes (Baur et al., 2006; Lagouge et al., 2006). The hyperadiponectinemia model allows us to reconcile the decreased hepatic glucose production in SirBACO mice with SirT1’s ability to increase it (Rodgers et al., 2005). We propose that, in SirBACO mice, the insulin-sensitizing effect of adiponectin trumps SirT1’s cell-nonautonomous activation of the gluconeogenic program.
In vivo SirT1 substrates and metabolic control
In C.elegans, the FoxO1 ortholog daf-16 is required for lifespan extension in response to SirT1 gain-of-function (Tissenbaum and Guarente, 2001). The widespread role of FoxO1 in mammalian metabolism provides a testable hypothesis on SirT1’s mechanism of action. In this regard we found that, unlike common forms of obesity and type 2 diabetes (Hu et al., 1996), SirBACO mice in diabetic and obese backgrounds display increased adiponectin. We propose that SirT1 acts through FoxO1 to increase adiponectin production. Given the conflicting results on the effect of SirT1 on adiponectin expression (Qiang et al., 2007; Qiao and Shao, 2006), the hyperadiponectinemia of SirBACO mice may have heterogeneous causes, including changes in β-adrenergic signaling or redox potential (Fasshauer et al., 2001; Furukawa et al., 2004). Nonetheless, this phenotype bears similarities with the effect of adiponectin overexpression in ob/ob mice (Kim et al., 2007). Moreover, knock-in mice bearing constitutively deacetylated FoxO1 alleles mimic the energy balance phenotype of SirBACO mice, supporting the contention that the effects of SirT1 are mediated by FoxO1 (A.B. and D.A., unpublished observation).
Conclusions
The ability of SirBACO mice to adapt their basal phenotype and thus prevent insulin-resistant diabetes will rekindle the debate on the evolutionary function of Sirtuins. SirT1 appears to function as a “reverse thrifty gene” that protects against metabolic diseases by instructing the organism to limit energy consumption and expenditure in the physiologic state. The likely behavioral aspects of SirT1 activation deserve closer scrutiny, if Sirtuin-based approaches are to be introduced in the clinic.
Experimental Procedures
Mice
We used BAC RP23-390D8 to generate SirBACO and SirT H355Y mice. Two lines of WT SirT1 transgenics with similar expression levels (Figure S7) had similar metabolic features (not shown). db/+ m/+ were from Jackson Labs. HFD (60% of calories from fat) (Research Diets) was administered from 4- to 20-weeks of age. All data are from male mice backcrossed at least 10 generations to C57BL/6J.
Antibodies and cell culture
Antibodies were from: Affinity BioReagents (adiponectin); Cell Signaling Technologies (Acetyl-lysine, Phospho and total AMPK, and PGC-1α); Santa Cruz Biotechnology (acetyl- and total-FoxO1); Upstate Biotechnology (Sir2, p85); and Calbiochem (actin antibody JLA20). Culture and infection of 3T3-L1 CARΔ cells have been described (Ross et al., 2003).
Metabolic and glucose clamp studies
We performed all assays as described (Lin et al., 2007; Matsumoto et al., 2007).
Energy balance
Indirect calorimetry was performed using LabMaster (TSE Systems). All mice were acclimatized for 24 hr before measurements every 14 min for 96 hr. Resting metabolic rate was determined by generating average energy expenditure and locomotor activity at each 14-min time point. Energy efficiency plots were obtained as described (Ravussin et al., 1986). Food intake was measured daily for two weeks with feeding hoppers. Core body temperature was measured with a probe (YSI Incorporated), blood pressure and heart rate with Hatteras Instrument MC4000, and body composition with either Piximus DEXA scanner (GE Healthcare) or NMR (Bruker Optics).
RNA and protein analysis
See Supplemental Methods.
Adenovirus generation
Adenovirus encoding constitutively acetylated (Foxo1-Ac), and deacetylated mutants (Foxo1-dAc), or Foxo1-GFP were generated using previously described cDNAs (Frescas et al., 2005; Kitamura et al., 2005), cloned into AdEasy pShuttle-CMV (Stratagene). Other adenoviruses have been described (Matsumoto et al., 2006).
Statistical analysis
P values were calculated by unpaired Student’s t –tests.
Supplementary Material
Acknowledgments
Supported by NIH grants (DK07328, DK079496, DK64819 and HL87123), by a pilot grant from the Columbia Diabetes & Endocrinology Research Center (DK63608), and by the Albert Einstein Diabetes Research & Training Center (DK20541). We thank members of the Gu and Accili laboratories, Jeff Flier, Rudy Leibel, and Eric Ravussin for discussions and critical reading of the manuscript, and Yitian Liu and Kumiko Aizawa for technical assistance.
Footnotes
Author Contributions
A.B. designed and performed experiments and wrote the manuscript. N.K. generated transgenic mice. M.M. generated FoxO1 adenovirus. C.K. and R.G-J. performed clamps. L.R., W.G., and D.A. designed experiments and wrote the manuscript.
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accili D. Lilly lecture 2003: the struggle for mastery in insulin action: from triumvirate to republic. Diabetes. 2004;53:1633–1642. doi: 10.2337/diabetes.53.7.1633. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, Guarente L. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Adiponectin gene expression is inhibited by beta-adrenergic stimulation via protein kinase A in 3T3-L1 adipocytes. FEBS Lett. 2001;507:142–146. doi: 10.1016/s0014-5793(01)02960-x. [DOI] [PubMed] [Google Scholar]
- Fontana L, Klein S. Aging, adiposity, and calorie restriction. Jama. 2007;297:986–994. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280:20589–20595. doi: 10.1074/jbc.M412357200. [DOI] [PubMed] [Google Scholar]
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, Accili D. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lin HV, Kim JY, Pocai A, Rossetti L, Shapiro L, Scherer PE, Accili D. Adiponectin resistance exacerbates insulin resistance in insulin receptor transgenic knockout mice. Diabetes. 2007 doi: 10.2337/db07-0127. [DOI] [PubMed] [Google Scholar]
- Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- Martin TL, Alquier T, Asakura K, Furukawa N, Preitner F, Kahn BB. Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J Biol Chem. 2006;281:18933–18941. doi: 10.1074/jbc.M512831200. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006;116:2464–2472. doi: 10.1172/JCI27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor foxo1 in liver. Cell Metab. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Meneur C, Permutt MA, Imai S. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Nakae J, Kitamura T, Kitamura Y, Biggs WH, Arden KC, Accili D. The forkhead transcription factor foxo1 regulates adipocyte differentiation. Dev Cell. 2003;4:119–129. doi: 10.1016/s1534-5807(02)00401-x. [DOI] [PubMed] [Google Scholar]
- Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. Jama. 2003;290:1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- Orlicky DJ, DeGregori J, Schaack J. Construction of stable coxsackievirus and adenovirus receptor-expressing 3T3-L1 cells. J Lipid Res. 2001;42:910–915. [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Wang H, Farmer SR. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-L alpha. Mol Cell Biol. 2007;27:4698–4707. doi: 10.1128/MCB.02279-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem. 2006;281:39915–39924. doi: 10.1074/jbc.M607215200. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78:1568–1578. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Ross SA, Song X, Burney MW, Kasai Y, Orlicky DJ. Efficient adenovirus transduction of 3T3-L1 adipocytes stably expressing coxsackie-adenovirus receptor. Biochem Biophys Res Commun. 2003;302:354–358. doi: 10.1016/s0006-291x(03)00180-3. [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- Weinert D, Waterhouse J. Diurnally changing effects of locomotor activity on body temperature in laboratory mice. Physiol Behav. 1998;63:837–843. doi: 10.1016/s0031-9384(97)00546-5. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.