Abstract
Background
Src activation is associated with cell migration, proliferation and metastasis. Saracatinib is an oral, tyrosine kinase inhibitor (TKI) selective for Src. We performed this trial to evaluate the efficacy and safety of saracatinib monotherapy in patients with estrogen receptor (ER)- and progesterone receptor (PR)-negative, metastatic breast cancer.
Patients and Methods
Patients with ≤1 prior chemotherapy regimen for measurable, ER- and PR-negative metastatic breast cancer received saracatinib 175 mg orally daily. The primary endpoint was disease control defined as complete response (CR) + partial response (PR) + stable disease (SD) >6 months. Secondary endpoints included toxicity and progression-free survival. Levels of circulating tumor cells in response to therapy were measured over time.
Results
Nine patients were treated on study. After a median of 2 cycles (range 1–3), no patients achieved CR, PR, or SD >6 months. The median time to treatment failure was 82 days (12–109).The majority (89%) of patients discontinued saracatinib because of disease progression. One patient developed potentially treatment-related grade 4 hypoxia with interstitial infiltrates and was removed from study. Common adverse events included fatigue, elevated liver chemistries, nausea, hyponatremia, dyspnea, cough, and adrenal insufficiency.
Conclusions
These efficacy results were not sufficiently promising to justify continued accrual to this study. Based on this series, saracatinib does not appear to have significant single-agent activity for the treatment of patients with ER(−)/PR(−) metastatic breast cancer.
INTRODUCTION
Src is a non-receptor tyrosine kinase that plays a role in cellular proliferation, motility, invasion, and differentiation through its interaction with growth factor receptors, cell adhesion receptors, integrins, and steroid hormone receptors. Src overexpression and deregulation occur in many solid tumors, and its increased activity correlates with metastasis and poor prognosis.(1–5)
Src activation has been demonstrated in more than 70% of invasive breast cancers.(6) Preclinical studies have identified a molecular signature that predicts for sensitivity to Src inhibition, which resembles that of the basal-like subtype of breast cancer: low expression of ER, PR, and HER2 and high expression of cytokeratins 5 and 17 .(7) Other groups have shown a significant correlation between basal-like breast cancer cell-lines and response to Src- inhibition with the oral agent, dasatinib.(8) Furthermore, hormone receptor (HR)-negative breast cancer cell- lines have shown increased sensitivity to Src- inhibition using dasatinib with the addition of chemotherapy.(9) The results from these preclinical trials suggest that Src may be an effective therapeutic target in patients with ER(−)/PR(−) breast cancer.
Saracatinib (AZD0530, AstraZeneca), is an oral aniline-quinazoline that has demonstrated activity in the regulation of Src-associated signaling.(10, 11) This small molecule is highly selective for non-receptor tyrosine kinases with nanomolar IC50 values for c-Src, c-Yes, Lck and Bcr-Abl. Saracatinib inactivates Src by competitive, reversible binding to the active conformation of the ATP binding-site.
The feasibility and safety of saracatinib in patients with advanced solid tumors was established in a phase I trial. Possible treatment- or disease-related respiratory failure occurred in 2 of 81 patients (grade 5 [1] at 200 mg; grade 4[1] at 175 mg). Intensive assessment and monitoring of pulmonary function was implemented in additional patients on phase I trials, with no further severe pulmonary toxicities reported, and no clinically relevant findings from assessments of pulmonary function.(12)
Saracatinib has shown modest antiproliferative effects in preclinical models yet significant activity in models of invasion and migration, supporting the hypothesis that Src may serve as an anti-invasive target rather than a direct antiproliferative target.(11, 13–16) Preclinical data in MDA-MB-231, an ER(−)/PR(−) breast cancer cell line and xenograft models, demonstrated decreased growth and cell migration in the presence of saracatinib.(11) In MCF-7 human breast cancer cell lines with wild-type and mutant ERα, saracatinib inhibited Src phosphorylation and cell growth.(15–17)
We designed this phase II trial to test the efficacy of saracatinib monotherapy in patients with ER(−)/PR(−) metastatic breast cancer (MBC) based on the preclinical data for Src inhibition in basal-like, HR-negative breast cancer. The primary endpoint of disease control rate, which included stabilization of disease >6 mo, was chosen considering the anti-invasive mechanism of action for the drug and the possibility that typical response assessment by RECIST alone may not have been an appropriate measure of saracatinib activity. Correlative studies included measurement of circulating tumor cells (CTCs) in response to saracatinib to explore their potential as biomarkers of Src inhibition.
Patients and Methods
Patient eligibility
Patients with histologically confirmed breast cancer were eligible if they had unresectable, locally advanced or metastatic disease that was ER(−) and PR(−), defined as <10% expression by immunohistochemistry (IHC). Eligibility requirements included measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) and no more than 1 prior chemotherapy regimen for MBC. Patients with locally advanced disease must have had one first-line chemotherapy regimen prior to study consideration. Patients with HER2-positive breast cancer (IHC 3+ or FISH >2.1) must have previously received trastuzumab.
Additional eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2, absolute neutrophil count ≥1,500/μL, platelets ≥100,000/μL, hemoglobin ≥ 9 g/dL, total bilirubin and creatinine within normal institutional limits and AST/ALT ≤2.5 times the institutional upper limit of normal. Patients with severe restrictive or obstructive lung disease were excluded from participation if any baseline pulmonary function test (PFT) measured: total lung capacity <60%, forced vital capacity <50%, forced expiratory volume 1 <50%, diffusing capacity <50%, room air oxygen saturation at rest <92%, or decline in oxygen saturation of >4% with exercise. Baseline high resolution CT of the chest was required. Chemotherapy, radiotherapy, or investigational therapy ≤ 3 weeks before initiating study treatment, presence of brain metastases, baseline proteinuria (urine protein:creatinine [UPC] ratio >1), QTc prolongation >500msecs and concurrent use of CYP3A4-active agents were also exclusion criteria. Patients with impaired ability to comply with oral therapy were not permitted on study.
The MSKCC and Montefiore Medical Center institutional review boards approved this protocol. All patients gave written informed consent.
Study design and treatment
This was a nonrandomized, open-label, bi-institutional, phase II trial (NCT00559507). The primary objective was to estimate the efficacy of saracatinib in terms of disease control rate (CR+PR+SD >6 mo). Secondary endpoints included toxicity, progression free survival (PFS), and overall response rate (CR+PR). Correlative endpoints were to prospectively describe changes in CTCs from pretreatment levels in response to saracatinib.
Saracatinib 175 mg was administered orally as one 125 mg tablet and one 50 mg tablet daily. Patients kept a medication diary to document compliance. Twenty-eight days of continuous dosing constituted a treatment cycle.
Patients were treated until disease progression or unacceptable adverse events. Saracatinib dose reductions were required for grade (gr) ≥ 3, treatment-related, nonhematologic toxicity. A maximum of 2 dose reductions were permitted per patient, and were stipulated according to grade and number of appearances. Use of hematopoietic growth factors was permitted on study.
Special management guidelines were included for dyspnea, proteinuria, and adrenal insufficiency. New onset or worsening dyspnea or cough with associated abnormal PFTs or new pulmonary radiographic findings that could not be definitively attributed to a cause other than saracatinib required delay of study drug, pulmonary medicine consultation, and repeat high resolution CT of the chest to evaluate for interstitial lung disease.
New proteinuria (UPC ratio >1) prompted additional evaluation and gr ≥ 2 proteinuria required that saracatinib be held. If saracatinib was delayed >4 weeks, the patient was removed from study. If patients developed symptoms consistent with adrenal insufficiency (ie, hypotension, fatigue, skin pigmentation, weight loss), adrenal function testing was recommended. Patients were removed from study for symptomatic adrenal insufficiency.
Patient evaluation
Patients were evaluated for toxicity according to the National Cancer Institute Common Toxicity Criteria Adverse Events v3.0 (NCI CTCAE v3). During the first cycle, electrolytes and creatinine were monitored weekly, and PFTs were repeated during week 3. In subsequent cycles, patients were evaluated for toxicity once per cycle, including history and physical examination, complete blood count and serum chemistries, UPC ratio, PFTs, and electrocardiogram (ECG). Tumor assessment was performed every 3 cycles (12 weeks), with scans reviewed by a designated study radiologist according to RECIST. Imaging included high-resolution CT scans of the chest to evaluate lung parenchyma. For patients enrolled at Memorial Sloan-Kettering Cancer Center (MSKCC), quantitative CTC analysis was performed using CellSearch® (Veridex, LLC, Raritan, NJ) at baseline and after cycles 1 and 3.
Statistical considerations
The primary endpoint of this trial was the disease control rate (DCR) defined as the proportion of patients achieving a CR + PR + SD >6 months using RECIST. A Simon 2-stage optimal design was used to test the null hypothesis of 5% DCR against the alternative of a 20% DCR for saracatinib monotherapy. The probabilities of type I and type II errors were 0.1 and 0.05, respectively. By design, 21 patients would be accrued to the first stage. If >1 patient demonstrated CR, PR, or SD >6 mo, enrollment would be expanded to 41 patients. If >4 of 41 patients achieved response or SD >6 months, saracatinib would be considered worthy of further study. Investigator flexibility allowed for the early termination of the study in response to an unfavorable risk-benefit ratio.
There were several secondary endpoints. Toxicities were summarized using the NCI CTCAE v3, and the maximum grade per patient was used as the summary measure. Response rate was calculated with exact 95% confidence intervals (CI). PFS was defined from start of therapy to the time of progression or death, and analyzed using Kaplan-Meier methodology. The statistical analysis of CTC data was descriptive. CTC levels were to be plotted over time for individual patients and as a summary of all. Paired t tests were used to examine whether response was related to change from baseline.
RESULTS
Nine patients were enrolled and treated on study before the trial was closed to accrual at the investigators' request, based on the observed risk:benefit ratio. All patients were evaluable for response and toxicity. Patient characteristics are shown in Table 1.
Table 1.
Patient characteristics at baseline (N=9)
| Characteristic | Number (%) |
|---|---|
| Median age (range) | 52 (31–85) |
| ER and PR <10% by IHC | 9 (100%) |
| HER2 positive (IHC 3+, FISH >2.1) | 1 (11%) |
| Median ECOG performance status | 1 (0–1) |
| Median number of prior chemotherapy regimens for MBC | 1 (0–1) |
| No. patients with visceral metastases | 8 (89%) |
Efficacy
After a median of 2 cycles (range 1–3), no patients achieved CR, PR, or SD >6 months. Three patients had SD <6 months. Six patients experienced progression of disease as best response. The median time to treatment failure was 82 days (12– 109).
Adverse Events
The most common, possibly treatment-related nonhematologic adverse events were: fatigue (78%—gr 1, 66%; gr 3, 11%), AST (44%—gr 1, 22%; gr 3, 22%), nausea (44%—gr 1, 44%), hyponatremia (44%—gr 1, 33%, gr 3, 11%), ALT (33%—gr 1, 22%, gr 2, 11%), and alkaline phosphatase (33%—gr 1, 33%). Dyspnea and cough occurred in 22% of patients. Adrenal insufficiency was observed in 2 patients (gr 3, 1 and gr 2, 1). Table 2 summarizes gr ≥ 2 treatment-related adverse events. There were no gr 5 events.
Table 2.
Adverse events considered by the investigators to be related to saracatinib by grade according to National Cancer Institute Common Toxicity Criteria v3 (N=9)
| Grade, No. (%) |
|||
|---|---|---|---|
| Toxicity | 2 | 3 | 4 |
| Hypoxia | - | - | 1 (11) |
| AST | - | 2 (22) | - |
| Fatigue | - | 1 (11) | - |
| Lymphopenia | - | 1 (11) | - |
| Sodium, serum low | - | 1 (11) | - |
| Potassium, serum low | - | 1 (11) | - |
| Adrenal Insufficiency | 1 (11) | 1 (11) | - |
| Phosphate, serum low | 1 (11) | 1 (11) | - |
| Dyspnea | 1 (11) | - | - |
| Bilirubin | 1 (11) | - | - |
| ALT | 1 (11) | - | - |
| Glucose, serum high | 1 (11) | - | - |
| Hemoglobin | 1 (11) | - | - |
| Neutrophils/granulocytes | 1 (11) | - | - |
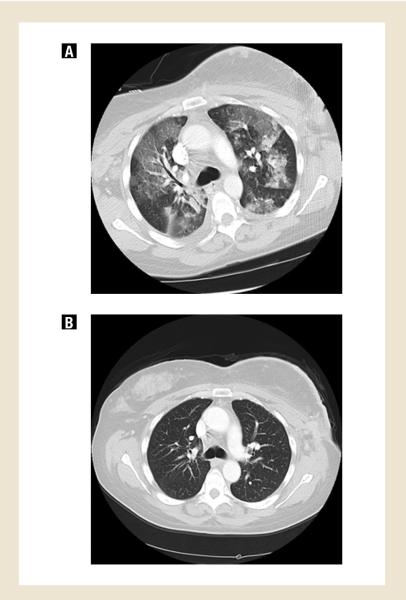
Three patients experienced possibly treatment-related serious adverse events. One patient experienced grade 4 hypoxia leading to discontinuation of protocol treatment. She presented with fever and cough 11 days after initiating saracatinib. Study drug was held, and broad-spectrum, empiric antibiotics were initiated. She quickly became hypoxic requiring intubation and vasopressor support. High-resolution CT scan of the chest demonstrated bilateral interstitial ground glass opacities and an ARDS-like picture; no pulmonary emboli were seen (Fig. 1a). A comprehensive infectious workup included negative bacterial, fungal, and viral cultures of blood and sputum. Legionella urinary antigens were negative. Bronchoscopy was negative for PCP and AFB, and cultures were negative; cytology was negative for malignant cells. An echocardiogram confirmed a normal left ventricular ejection fraction. Gr 2 adrenal insufficiency and hypotension were managed with hydrocortisone. With maximal supportive care and discontinuation of saracatinib, the patient gradually improved. Chest CT performed 18 days later showed resolution of the pulmonary infiltrates and confirmed stable disease (Fig. 1b). This patient was removed from study due to this possible drug-related pneumonitis.
Fig 1.
High resolution, chest CT scan of patient treated with saracatinib who experienced grade 4 hypoxia, possibly treatment-related. Figure 1A shows bilateral interstitial ground glass opacities 13 days after beginning study treatment. Figure 1B shows resolution of these findings 18 days after discontinuation of saracatinib, which corresponded to clinical improvement.
One patient was hospitalized with gr 3 fatigue, hyponatremia, adrenal insufficiency, and dyspnea. PFTs showed stable lung function, and a CT of the chest demonstrated SD but increased interstitial lung markings. Saracatinib was held for 14 days; hydrocortisone was initiated. Symptoms resolved, and she was permitted to restart saracatinib at a reduced dose without recurrence of her symptoms.
One patient experienced gr 3 elevated liver enzymes and was hospitalized for evaluation. Saracatinib was held for 6 days, and her laboratory values improved. A CT of the abdomen revealed mild fatty liver infiltration and no evidence for biliary obstruction. Gastroenterology consultation deemed hyperbilirubinemia and transaminitis were drug-related. The patient resumed treatment at a reduced dose without recurrence of elevated liver enzymes.
Correlative studies
Only 3 of 6 patients had measurable CTCs at baseline. One of those 3 patients had stable CTC levels over time. The other 2 patients did not have cycle 1 and cycle 3 CTCs tested because of disease progression prior to time of subsequent CTC ascertainment.
DISCUSSION
Notwithstanding encouraging preclinical and phase I data, our phase II trial of saracatinib failed to show clinical benefit for patients with hormone receptor-negative metastatic breast cancer. Due to the short time to treatment failure observed in the first nine patients on study and the severe pulmonary adverse event that occurred, we terminated this study early as this suggested an unacceptable risk:benefit ratio. In fact, 33% of patients experienced at least one serious adverse event while on treatment with sarcatinib. We recognize that without having accrued the expected number of patients, it is difficult to make definitive statements regarding efficacy. Nevertheless, clinical benefit against overt disease was clearly not demonstrated. This result is consistent with a recent study that found that a gene expression signature of src-activation, while critical for the survival of latent breast cancer cells in bone, and hence associated with late-onset bone metastases in hormone receptor-negative as well as positive cases, was not critical for the survival of these cells once they exited latency and grew as overt metastases.(18) It is of note in this regard that these clinical results and our emerging understanding of the biology of src in breast cancer do not preclude the possibility that src inhibition might be effective in treating latent disease.
In a previous phase I trial, pulmonary toxicity was reported in 2 of 81 patients with metastatic solid tumors treated with saracatinib. One patient, treated at the 200 mg dose level, presented with symptoms of dyspnea and hypoxia. Diffuse alveolar damage consistent with acute respiratory distress syndrome developed, ultimately resulting in death. An additional subject treated at the 175 mg dose level developed grade 4 pleural effusions and interstitial infiltrates while on therapy, which resolved upon discontinuation of saracatinib. Both patients had multiple confounding factors that may have been implicated in the causality of these events, but causality of saracatinib could not definitively be excluded and the events were considered by the investigators as possibly related to the study drug.(12) These findings prompted inclusion of routine pulmonary monitoring with PFTs and high resolution chest CT scans in future trials. No consistent changes in PFTs were noted in this trial or in a randomized, placebo-controlled study of saracatinib conducted in healthy volunteers (data on file, AstraZeneca). Note however, that despite the inclusion of increased pulmonary monitoring during our study, grade 4 pulmonary toxicity occurred nonetheless.
Other published phase II studies of saracatinib to date have not reported pulmonary toxicity as a significant adverse event.(19, 20) A randomized, placebo-controlled trial of chemotherapy with or without saracatinib has been completed in 189 patients with metastatic ovarian cancer, and no difference in the frequency of respiratory events were noted between study arms.(21)
Src inhibitor-associated lung abnormalities have been reported for other compounds within this class. Dasatinib, a TKI with activity at Src as well as other targets is approved by the FDA for the treatment of chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia. Respiratory adverse events, particularly dyspnea and pleural effusions, have been reported with this drug. In one metaanalysis of 138 patients with CML, the incidence of dasatinib-related pleural effusions was 35% (17% gr 3 or 4).(22) Across phase I and II studies, treatment-related pulmonary toxicity has been noted in 7%–35% of patients receiving dasatinib.(23–27) In one case series, 9 of 40 patients treated with dasatinib experienced pulmonary toxicity while on therapy. The majority of these patients (7 of 9) had parenchymal findings on high resolution chest CT, including nodular consolidations, ground-glass opacities, and septal thickening. Complete resolution of drug-related lung manifestations was seen in 7 of 9 patients, with partial improvement in the remaining 2 patients. Interruption of dasatinib was the most successful intervention in this group, with only one patient requiring corticosteroid therapy and 2 patients receiving empiric antibiotic treatment. (28) Pulmonary events have been described with other src-inhibitors under development, but definitive attribution to study therapy has been difficult.(29)
In our study, 2 of the 3 patients with documented serious adverse events had SD at first response assessment. For example, despite discontinuing study drug secondary to toxicity, one patient with grade 4 hypoxia was reported to have clinical improvement in her chest wall nodules and stable disease by RECIST on CT scans 10 weeks after discontinuing saracatinib, in the absence of new anticancer therapy. Given the small number of patients enrolled on this study and the limited response rate, it is difficult to draw definitive conclusions from the above narratives. Nevertheless, the hypothesis that toxicity may correlate with drug activity deserves attention in other ongoing studies with saracatinib. Such a link could imply either off-target effects with similar pharmacology or on-target effects in sensitive host tissues.
Src integrates numerous signaling pathways in the development of breast cancer and, as such, blocking Src activity alone may not be sufficient to prevent tumor progression. Similar to our study, other trials of saracatinib monotherapy in patients with prostate or pancreatic cancer did not demonstrate objective durable responses or improvement in survival.(19, 20) It has been suggested that Src inhibitors may have a role in cancer therapy if added to other agents for dual inhibition of redundant signal transduction pathways or in combination with known endocrine or chemotherapies.(16, 30) Drug resistance to saracatinib is associated with upregulation of MEK, phosphatidylinositol 3-kinase (PI3K), and mTOR, suggesting that the use of other targeted therapies in combination with saracatinib may be an effective intervention for the treatment of breast cancer.(31) Based on these preclinical findings, a phase I/II trial of neoadjuvant saracatinib and anastrozole is ongoing (NCT01216176).
The identification of predictors of response and biomarkers of Src inhibition would allow for strategic selection of patients most likely to benefit from this targeted therapy. Multiple studies have shown that detection of disseminated tumor cells in bone marrow by immunocytochemistry is strongly prognostic of recurrence and death.(32) Additionally, the presence of CTCs in patients with metastatic disease is associated with poorer survival, and in multivariate analysis, was a significant predictor of survival. Unfortunately, only 3 of 6 patients evaluated in our trial had detectable CTCs prior to treatment with saracatinib, 2 of whom did not have serial measurable values.
Saracatinib, as a monotherapy, did not induce meaningful responses in this small set of patients with hormone receptor-negative metastatic breast cancer. These data were not considered sufficiently promising to continue accrual to this study. Furthermore, the toxicity profile of this agent reminds us of the potential for significant adverse events, even with presumably targeted therapies.
Acknowledgments
Supported in part by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute. Drug provided by AstraZeneca through CTEP.
Supported in part by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute and AstraZeneca.
Memorial Sloan-Kettering Cancer Center Grant: N01-CM-62206; PI David P. Kelsen, MD Montefiore Medical Center Grant: N01-CA-62240; PI Joseph A. Sparano, MD
The authors would like to thank Carol Pearce for her assistance with formatting this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest All authors have no conflict of interest.
Presented in part at the 46th Annual Meeting of the American Society of Clinical Oncology, June 4–8, 2010, Chicago, IL.
REFERENCES
- Aligayer H, Boyd DD, Heiss MM, Abdalla EK, Curley SA, Gallick GE. Activation of Src kinase in primary colorectal carcinoma: an indicator of poor clinical prognosis. Cancer. 2002;94:344–51. doi: 10.1002/cncr.10221. [DOI] [PubMed] [Google Scholar]
- 2.Ito Y, Kawakatsu H, Takeda T, Tani N, Kawaguchi N, Noguchi S, et al. Activation of c-Src is inversely correlated with biological aggressiveness of breast carcinoma. Breast Cancer Res Treat. 2002;76:261–7. doi: 10.1023/a:1020860221099. [DOI] [PubMed] [Google Scholar]
- 3.Masaki T, Igarashi K, Tokuda M, Yukimasa S, Han F, Jin YJ, et al. pp60c-src activation in lung adenocarcinoma. Eur J Cancer. 2003;39:1447–55. doi: 10.1016/s0959-8049(03)00276-4. [DOI] [PubMed] [Google Scholar]
- 4.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–58. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 5.Wiener JR, Windham TC, Estrella VC, Parikh NU, Thall PF, Deavers MT, et al. Activated SRC protein tyrosine kinase is overexpressed in late-stage human ovarian cancers. Gynecol Oncol. 2003;88:73–9. doi: 10.1006/gyno.2002.6851. [DOI] [PubMed] [Google Scholar]
- 6.Chu I, Sun J, Arnaout A, Kahn H, Hanna W, Narod S, et al. p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell. 2007;128:281–94. doi: 10.1016/j.cell.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang F, Reeves K, Han X, Fairchild C, Platero S, Wong TW, et al. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67:2226–38. doi: 10.1158/0008-5472.CAN-06-3633. [DOI] [PubMed] [Google Scholar]
- 8.Finn RS, Dering J, Ginther C, Wilson CA, Glaspy P, Tchekmedyian N, et al. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/“triple-negative” breast cancer cell lines growing in vitro. Breast Cancer Res Treat. 2007;105:319–26. doi: 10.1007/s10549-006-9463-x. [DOI] [PubMed] [Google Scholar]
- 9.Tryfonopoulos D, O'Donovan N, Corkery B, Clynes M, Crown J. Activity of dasatinib with chemotherapy in triple-negative breast cancer cells. ASCO Meeting Abstracts. 2009;27:e14605. [Google Scholar]
- 10.Chang YM, Bai L, Liu S, Yang JC, Kung HJ, Evans CP. Src family kinase oncogenic potential and pathways in prostate cancer as revealed by AZD0530. Oncogene. 2008;27:6365–75. doi: 10.1038/onc.2008.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green TP, Fennell M, Whittaker R, Curwen J, Jacobs V, Allen J, et al. Preclinical anticancer activity of the potent oral Src inhibitor AZD0530. Mol Oncol. 2009;3:248–261. doi: 10.1016/j.molonc.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baselga J, Cervantes A, Martinelli E, Chirivella I, Hoekman K, Hurwitz HI, et al. Phase I safety, pharmacokinetics, and inhibition of SRC activity study of saracatinib in patients with solid tumors. Clin Cancer Res. 2010;16:4876–83. doi: 10.1158/1078-0432.CCR-10-0748. [DOI] [PubMed] [Google Scholar]
- 13.AstraZeneca . Investigators Brochure for AZD0530. Macclesfield, Chesire; England: 2005. [Google Scholar]
- 14.Hennequin LF, Allen J, Costello GF, Fennell M, Green TP, Jacobs V, Morgentin R, Olivier A, Ple PA. The discovery of AZD0530: a novel, oral, highly selective and dual-specific inhibitor of the Src and Abl family kinases. Proceedings of the American Association for Cancer Research.2005. p. 46. [Google Scholar]
- 15.Hiscox S, Jordan NJ, Smith C, James M, Morgan L, Taylor KM, et al. Dual targeting of Src and ER prevents acquired antihormone resistance in breast cancer cells. Breast Cancer Res Treat. 2009;115:57–67. doi: 10.1007/s10549-008-0058-6. [DOI] [PubMed] [Google Scholar]
- 16.Hiscox S, Morgan L, Green TP, Barrow D, Gee J, Nicholson RI. Elevated Src activity promotes cellular invasion and motility in tamoxifen resistant breast cancer cells. Breast Cancer Res Treat. 2006;97:263–74. doi: 10.1007/s10549-005-9120-9. [DOI] [PubMed] [Google Scholar]
- 17.Herynk MH, Beyer AR, Cui Y, Weiss H, Anderson E, Green TP, et al. Cooperative action of tamoxifen and c-Src inhibition in preventing the growth of estrogen receptor-positive human breast cancer cells. Mol Cancer Ther. 2006;5:3023–31. doi: 10.1158/1535-7163.MCT-06-0394. [DOI] [PubMed] [Google Scholar]
- 18.Zhang XH, Wang Q, Gerald W, Hudis CA, Norton L, Smid M, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lara PN, Jr., Longmate J, Evans CP, Quinn DI, Twardowski P, Chatta G, et al. A phase II trial of the Src-kinase inhibitor AZD0530 in patients with advanced castration-resistant prostate cancer: a California Cancer Consortium study. Anticancer Drugs. 2009;20:179–84. doi: 10.1097/CAD.0b013e328325a867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nallapareddy SAJ, Touban B, et al. A phase II trial of saracatinib (AZD0530), an oral Src inhibitor, in previously treated metastatic pancreatic cancer. ASCO 2010 Gastrointestinal Cancers Symposium. 2010 [Google Scholar]
- 21.Poole CLA, Rodenhuis S, Kristensen G, Pujade Lauraine E, Cantarini M, Emeribe U, Stuart M, Ray Coquard I. A Randomized Phase II Clinical Trial of the Src Inhibitor Saracatinib (AZD0530) and Carboplatin and Paclitaxel (C +P) Versus C+P in patients (Pts) with Advanced Platinum-Sensitive Epithelial Ovarian Cancer (EOC) Abstract 9720. Annals of Oncology; Abstract Book of the 35th ESMO Congress Milan, Italy. 2010 October 8–12;21:viii304–viii13. [Google Scholar]
- 22.Quintas-Cardama A, Kantarjian H, O'Brien S, Borthakur G, Bruzzi J, Munden R, et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol. 2007;25:3908–14. doi: 10.1200/JCO.2007.12.0329. [DOI] [PubMed] [Google Scholar]
- 23.Cortes J, Kim DW, Raffoux E, Martinelli G, Ritchie E, Roy L, et al. Efficacy and safety of dasatinib in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blast phase. Leukemia. 2008;22:2176–83. doi: 10.1038/leu.2008.221. [DOI] [PubMed] [Google Scholar]
- 24.Hochhaus A, Baccarani M, Deininger M, Apperley JF, Lipton JH, Goldberg SL, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22:1200–6. doi: 10.1038/leu.2008.84. [DOI] [PubMed] [Google Scholar]
- 25.Kantarjian H, Pasquini R, Hamerschlak N, Rousselot P, Holowiecki J, Jootar S, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood. 2007;109:5143–50. doi: 10.1182/blood-2006-11-056028. [DOI] [PubMed] [Google Scholar]
- 26.Shah NP, Kantarjian HM, Kim DW, Rea D, Dorlhiac-Llacer PE, Milone JH, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26:3204–12. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 27.Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–41. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 28.Bergeron A, Rea D, Levy V, Picard C, Meignin V, Tamburini J, et al. Lung abnormalities after dasatinib treatment for chronic myeloid leukemia: a case series. Am J Respir Crit Care Med. 2007;176:814–8. doi: 10.1164/rccm.200705-715CR. [DOI] [PubMed] [Google Scholar]
- 29.Khoury HJ, Kim D, Zaritskey A, Apperley J, Besson N, Volkert A, et al. Safety and efficacy of third-line bosutinib in imatinib (IM) and dasatinib (DAS) resistant or intolerant chronic phase (CP) chronic myeloid leukemia (CML) ASCO Meeting Abstracts. 2010;28:6514. [Google Scholar]
- 30.Hiscox S, Morgan L, Barrow D, Dutkowskil C, Wakeling A, Nicholson RI. Tamoxifen resistance in breast cancer cells is accompanied by an enhanced motile and invasive phenotype: inhibition by gefitinib ('Iressa', ZD1839) Clin Exp Metastasis. 2004;21:201–12. doi: 10.1023/b:clin.0000037697.76011.1d. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Guggisberg N, Jorda M, Gonzalez-Angulo A, Hennessy B, Mills GB, et al. Combined Src and aromatase inhibition impairs human breast cancer growth in vivo and bypass pathways are activated in AZD0530-resistant tumors. Clin Cancer Res. 2009;15:3396–405. doi: 10.1158/1078-0432.CCR-08-3127. [DOI] [PubMed] [Google Scholar]
- 32.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]