Abstract
The formation and progression of mudulloblastoma (MB) is poorly understood. However, somatic inactivation of pRb/p105, in combination with a somatic or a germ-line TP53 inactivation, leads to MB in a mouse model. Presently, there is no specific evidence of pathway/s alterations for the other two members of the retinoblastoma family, pRb2/p130 and/or p107 in MB. JC virus (JCV) is a human polyomavirus. Although there is no firm evidence that this virus plays a causal role in human neoplasia, it has been clearly proven that JCV is highly oncogenic when injected into the brain of experimental animals. The mechanism of JCV-induced tumorigenesis is not entirely clear. However, several studies relate the oncogenic properties of JCV mainly to its early protein large T-antigen (T-Ag), which is able to bind and inactivate both TP53 and Rb family proteins. Here, we compared the protein expression profiles of p53, p73, pRb family proteins, and PCNA, as main regulators of cell proliferation and death, in different cell lines of mouse primitive neuroectodermal tumors (PNET), either T-Ag-positive or -negative, and in human MB cell lines. Our goal was to determine if changes in the relative expression of these regulators could trigger molecular perturbations underlying MB pathogenesis in mouse and human cells. Our results support that the presence of JCV T-Ag may interfere with the expression of pRb family proteins, specific p73 isoforms, and p53. In turn, this “perturbation” may trigger a network of signals strictly connected with survival and apoptosis.
Keywords: MEDULLOBLASTOMA, LARGE T-ANTIGEN, JC VIRUS, SV40, pRb FAMILY, p53/p73
Embryonal tumors of the central nervous system (CNS) include medulloblastomas (MB), primitive neuroectodermal tumors of the CNS (PNET), and atypical teratoid/rabdoid tumors (ATRT) [Louis et al., 2007]. The mechanism of MB tumorigenesis is poorly understood. Genetic alterations associated with this disease include loss of chromosome p17 and gain of 17q, losses on chromosomes 10q and 11, gains on chromosome 7, and rearrangements of chromosome 1 [Trojanek et al., 2006]. c-Myc and n-Myc amplification, constitutive Sonic hedgehog (SHH) signaling activation, and TP53 pathway alteration have also been reported [Rossi et al., 2008]. TP73 is a member of the p53 family and it has been indicated that overexpression of TP73 isoforms could have a role in the growth and treatment response in MB [Castellino et al., 2007]. pRb/p105, p107, and pRb2/p130 form the retinoblastoma family [Caracciolo et al., 2006; Macaluso et al., 2006]. pRb/p105 mutations are common in several cancers but not in human MB [Giacinti and Giordano, 2006]. However, somatic inactivation of pRb/p105, in combination with a somatic or a germ-line TP53 inactivation, leads to MB in a mouse model. Presently, there is no specific evidence of pRb2/p130 and/or p107 pathway/s alterations in MB, but a role for pRb2/p130 in glioblastoma has been suggested [Pucci et al., 2002].
JC virus (JCV) is a human polyomavirus of the polyomaviridae family, which also includes BK virus and Simian Virus 40 (SV40) [Maginnis and Atwood, 2009].
Several studies suggest an association between the occurrence of MB and JCV [Khalili et al., 1999; Krynska et al., 1999a; Del Valle et al., 2001a]. Although there is no firm evidence that this virus plays a causal role in human neoplasia, it has been clearly proven that JCV is highly oncogenic when injected into the brain of experimental animals including hamsters and nonhuman primates [Maginnis and Atwood, 2009]. The mechanism of JCV-induced tumorigenesis is not entirely clear. However, several studies relate the oncogenic properties of JCV mainly to its early protein large T-antigen (T-Ag), which is able to bind and inactivate both TP53 and Rb family proteins [Del Valle et al., 2001b; Caracciolo et al., 2006]. Del Valle et al. [2001] found a correlation between T-Ag, TP53, and pRb/p105 expression in human MB specimens by immunohistochemical analysis. They also observed a significant immunoreactivity to p107 and pRb2/p130 in most of the T-Ag-positive cases, suggesting that an association between T-Ag and/or pRb occurs in these tumors [Del Valle et al., 2001b].
Although mice models represent a powerful tool for the studies of mechanisms of cancers, there are fundamental differences in how the process can occur in mice and humans. Here, we compared the protein expression profiles of p53, p73, pRb family proteins, and PCNA, as main regulators of cell proliferation and death, in different cell lines of mouse PNET, T-Ag-positive or T-Ag-negative, and in human MB cell lines.
The mouse cell lines used for the experiments were obtained from cerebellar mouse PNET histologically resembling human MB in appearance, expression of neuronal marker proteins, and anatomical location [Krynska et al. 1999b].
Our goal was to determine if changes in the relative expression patterns of these regulators reflect a distinctive role of these proteins in the pathogenesis of MB in mouse and human cells.
MATERIALS AND METHODS
CELL CULTURES
The cell lines used in the experiments included: (i) human MB DAOY cell line (ATCC # HTB-186; American Type Culture Collection, Manassas, VA), isolated from a posterior fossa tumor of a 4-year-old boy; and D283 Med cell line (ATCC # HTB-187; American Type Culture Collection), originated from peritoneal metastases from a boy with MB; (ii) WS-1 human normal skin fibroblasts (ATCC # CRL-1502; American Type Culture Collection); (iii) eight mouse cell lines (Bs1a, Bs1b, Bs1c, Bs1f, BsB7, BsB8, BsB13, and BsB14) derived from cerebellar MB that developed spontaneously in transgenic mice expressing the early genome of the archetype form of the JCV [Krynska et al., 2000]. All mouse cell lines were positive for the major neuronal markers. In addition, Bs1a, Bs1b, Bs1c, Bs1f cells were T-Ag-negative, whereas BsB7, BsB8, and BsB14 were T-Ag-positive [Krynska et al., 2000]; (iv) murine fibroblasts R503 and R503/T [Reiss et al., 1998]. R503/T cells were obtained by stable transfection of R503 cells with pcDNA/JCVTzeo expression vector [Lassak et al., 2002]. Parental R503 cells transfected with empty vectors were utilized as reference cell line.
All mouse cell lines were kindly provided by Drs Kamel Khalili and Krzysztof Reiss (Department of Neuroscience, Center for Neurovirology, Temple University School of Medicine, Philadelphia, PA).
All cells were grown in DMEM (CellGro, Mediatech Inc., Herndon, VA) containing 10% FBS serum (Atlanta Biological, Norcross, GA) at 37°C and 5% CO2 atmosphere. In particular, R503 cells were grown in DMEM containing 10% FBS and 150 μg/ml of selectant agent Hygromycin (Sigma-Aldrich, St. Louis, MO). The same medium with the addition of 400 μg/ml of zeocin was used for R503/T cells.
NUCLEAR/CYTOPLASMIC FRACTIONATION
Monolayer cultures that were 70–80% confluent were washed with PBS and dry pellets were collected and kept at −80°C until use. A cell fractionation procedure was applied to separate cytoplasmic and nuclear proteins using the NE-PER Nuclear and Cytoplasmic Extraction Reagents Kit (Pierce Biotechnology; Rockford, IL), according to the manufacturer’s instructions.
WESTERN BLOT ANALYSIS
Protein samples (40 μg/lane) were run on polyacrylamide gels according to the Laemmli procedure [Laemmli, 1970]. Protein bands were transferred on nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany) by wet electrophoretic transfer according to Towbin et al. [1979]. Nonspecific binding sites were blocked for 1 h at room temperature with 5% nonfat dry milk in TBS containing 0.05% Tween-20 (TBS-T). The blots were probed with the following primary antibodies at the appropriate dilutions: mouse monoclonal anti-PCNA (PC10; sc-56) 1:500, rabbit polyclonal anti-p73 (H79; sc-7957) 1:300, rabbit polyclonal anti-p53 (sc-6243 X) 1:400, and rabbit polyclonal anti-p107 (C-18; sc-318) 1:500 (Santa Cruz Biotechnology, Santa Cruz, CA); mouse monoclonal anti-Rb2 1:300 (BD 610261), mouse monoclonal anti-Rb (BD 554136) 1:400 (BD Biosciences, San Jose, CA); mouse monoclonal anti-T-Ag PAB416 1:10 produced in house [Howard et al., 1998]. Rabbit polyclonal antilamin A/C 1:1,000 (Cell Signaling Technology, Danvers, MA) and mouse monoclonal anti-α-tubulin 1:10,000 (Sigma) were used as internal controls for nuclear and cytoplasmic extracts, respectively. Mouse monoclonal anti-α-tubulin 1:10,000 (Sigma) or goat polyclonal anti-GAPDH (V-18) 1:400 (sc-20357; Santa Cruz Biotechnology) were used as internal controls for total extracts. All primary antibodies were diluted in 5% nonfat dry milk in TBS-T and incubated overnight at 4°C. After extensive washings, the blots were incubated with secondary antimouse or antirabbit peroxidase-conjugated antibodies (Amersham, Buckinghamshire, UK) diluted 1:10,000 in TBS-T containing 5% nonfat dry milk, for 1 h at room temperature. After rinsing, immunoreactive bands were visualized by ECL (Amersham). All experiments were performed in triplicates.
IMMUNOFLUORESCENCE
Immunofluorescence staining was performed on DAOY and D283 cells. The cells were fixed with 4% paraformaldehyde in PBS for 20 min at room temperature and permeabilized with 0.1% TritonX-100 (Fisher Scientific, Pittsburgh, PA) in PBS for 5 min at room temperature. Nonspecific binding sites were blocked with PBS containing 6% bovine serum albumin (BSA; Sigma-Aldrich) for 1 h at room temperature. The cells were then incubated overnight at 4°C with mouse monoclonal anti-PCNA 1:50 (Santa Cruz Biotechnology), diluted in PBS with 1% BSA. Secondary antibody, Alexa Fluor 488 goat antimouse IgG (Invitrogen, Eugene, OR) was diluted 1:600 in PBS containing 1% BSA. Negative controls were performed by omitting the primary antibody. Nuclear staining was performed with 100 ng/ml of DAPI (4′,6′-diamino-2-phenylindole; Molecular Probes, Carlsbad, CA), 1 min at room temperature. After washing, glasses were mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA) and visualized with an Olympus IX81 fluorescence microscope (Olympus Microscopes, Center Valley, PA).
RESULTS
PROTEIN EXPRESSION PROFILE OF pRb FAMILY IN MOUSE T-Ag-POSITIVE AND -NEGATIVE PNET CELLS
We detected that T-Ag-positive and -negative PNET cells express both the hypophosphorylated and hyperphosphorylated forms of pRb2/p130 (Fig. 1A,B). However, in the T-Ag-negative PNET cells, the hyperphosphorylated form of pRb2/p130 appeared to be prevalent (Fig. 1A). Interestingly, T-Ag-positive fibroblast cells exhibited only the hyperphosphorylated form of pRb2/p130 (Fig. 1B), whereas the T-Ag-negative fibroblast cells expressed both the hypophosphorylated and the hyperphosphorylated forms of pRb2/p130 (Fig. 1A). Several studies have reported that pRb2/p130 in its hypophosphorylated form acts as a key regulator of the G1–S phase transition mediating G1 growth arrest. On the contrary, the hyperphosphorylated form of pRb2/p130, which appears to be essentially cytoplasmic, is typical of cells progressing into G1 [Sun et al., 2007]. Moreover, we previously reported that JCV T-Ag targets the hypophosphorylated form of pRb2/p130 in JCV-induced hamster brain tumor HJC12 and HJCΔ5 cells [Howard et al., 1998]. These observations along with our results may suggest that the presence of T-Ag could interfere with the regulatory function of pRb2/p130 by interacting with the active hypophosphorylated form of pRb2/p130 and/or altering its nuclear sublocalization, thus accelerating the progression of the cell cycle.
Fig. 1.
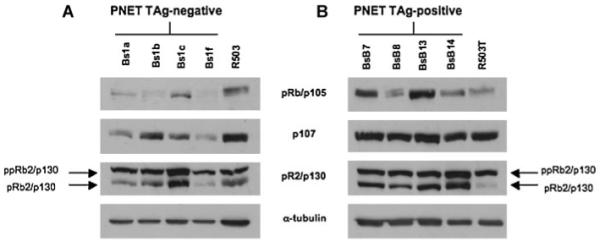
pRb family expression in mouse T-antigen-negative (A) and -positive (B) PNET cells and in mouse T-antigen-negative (R503; A) and -positive (R503/T; B) fibroblasts. The relative protein levels of pRb/p105, p107, and pRb2/p130 were detected by Western blotting using 40 μg of whole lysate. The expression of α-tubulin protein was assessed to normalize protein loading (ppRb2/p130, phosphorylated protein; pRb2/p130, hypophosphorylated protein).
T-Ag-positive PNET cells express significant levels of p107 compared with T-Ag-negative PNET cells. On the contrary, we did not observe significant changes in p107 expression between the T-Ag-positive and -negative fibroblasts (Fig. 1A,B).
We detected an increased expression of pRb/p105 in the T-Ag-positive PNET cells compared with the T-Ag-negative PNET cells. Interestingly, we found that the Bs1f T-Ag-negative PNET cell line does not express pRb/p105. Furthermore, we did not detect significant changes in the protein expression pattern of pRb/p105 between T-Ag-positive and -negative fibroblasts (Fig. 1A,B).
p53, p73, AND PCNA EXPRESSION
In accordance with what was previously reported by Krynska et al. [2000], we detected expression of p53 protein exclusively in T-Ag-positive PNET cells (Fig. 2B). Interestingly, in the same study, it was reported that T-Ag-negative PNET cell lines express only a smaller p53 transcript [Krynska et al., 2000]. In our study, we observed that the T-Ag-positive BsB14 PNET cell line exhibited a p53 protein expression pattern that is different from the one observed in the other T-Ag-positive PNET cells (Fig. 2B). In fact, we found that the BsB14 PNET cell line does not express p53, similar to the T-Ag-negative PNET cells (Fig. 2A,B). In an attempt to understand the atypical behavior of BSB14 cells, we tested the latter for the presence of the T-Ag and found no evidence of T-Ag expression (Fig. 2C). This result may suggest that in this cell line, which was originally T-Ag-positive, the loss of T-Ag expression could be directly or indirectly correlated with the concomitant loss of p53 expression. Furthermore, as both T-Ag-positive and -negative fibroblast cells exhibit similar levels of p53, it is reasonable to hypothesize that the event/s encompassing the concomitant loss of both p53 and T-Ag, observed in the BsB14 PNET cell line, are triggered only if a specific tumor microenviroment is established.
Fig. 2.
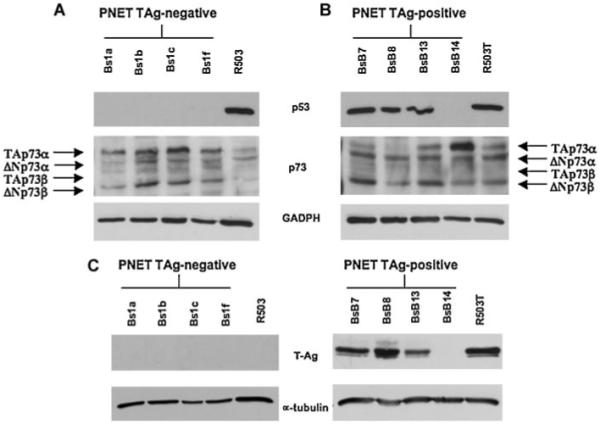
p53 and p73 expression in mouse T-antigen-negative (A) and -positive (B) mouse PNET cells and in mouse T-antigen-negative (R503; A) and -positive (R503/T; B) fibroblasts. The relative protein levels of p53 and p73 were detected by Western blotting using 40 μg of whole lysate. As shown in (A), the T-Ag-negative PNET cells do not express p53 but exhibit significant levels of TAp73α and ΔNp73α p73 isoforms. The T-Ag-positive PNET cells (B) express a prevalence of ΔNp73α and ΔNp73β isoforms. The presence of T-Ag in PNET cells was tested by Western blotting (C). The expression of GAPDH protein was assessed to normalize protein loading.
We observed that both T-Ag-positive and -negative PNET cells express significant levels of p73 isoforms (Fig. 2A,B).
Specifically, we observed that T-Ag-negative PNET cells express significant levels of TAp73α and ΔNp73β isoforms as well as low levels of ΔNp73α and TAp73β isoforms (Fig. 2A). Indeed, T-Ag-positive PNET cells express a prevalence of the ΔNp73α and ΔNp73β isoforms and very low levels of the other isoforms (Fig. 2B). Interestingly, we observed that the BsB14 PNET cell line, which lost T-Ag expression, exhibits very high levels of TAp73α (Fig. 2B).
Moreover, the T-Ag-negative fibroblasts expressed very low levels of all p73 isoforms (Fig. 2A) whereas the T-Ag-positive fibroblasts expressed all p73 isoforms similar to the T-Ag-positive PNET cells (Fig. 2B), thereby supporting a role for T-Ag in the expression of specific p73 isoforms.
Expression of PCNA protein was detected in all cell lines analyzed (Fig. 3A,B). However, we found that in T-Ag-negative PNET cells, PCNA was accumulated exclusively in the cytoplasm (Fig. 3C). On the contrary, in T-Ag-positive PNET cells we detected both nuclear and cytoplamic PCNA distribution (Fig. 3D). T-Ag-positive and -negative fibroblast cells exhibited only a cytoplasmic PCNA distribution (Fig. 3C,D). Again, the BsB14 PNET cell line exhibited only a cytoplasmic PCNA accumulation, similar to the T-Ag-negative PNET cells (Fig. 3D).
Fig. 3.
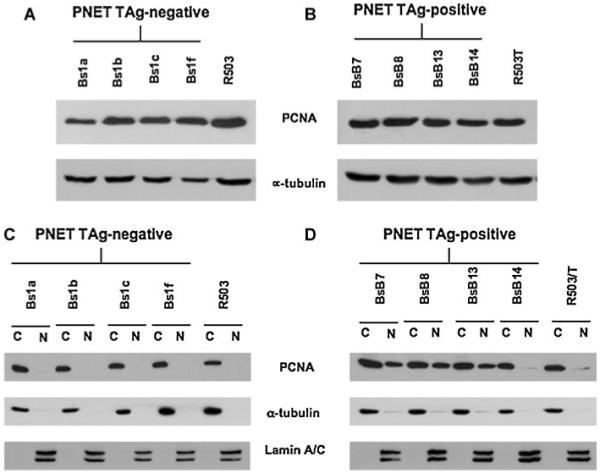
PCNA expression and distribution in mouse PNET cells and in mouse fibroblasts. Western blot analysis from total lysates shows similar expression levels of PCNA in all PNET cells (A, B). In the T-Ag-negative PNET cells, PCNA accumulates in the cytoplasm, whereas in the T-Ag-positive PNET cells PCNA is detected in both the nuclear and cytoplasmic fractions (C, D). Efficient cytoplasmic and nuclear fractionation was confirmed by Western blotting analysis using anti-α-tubulin antibody for the cytoplasmic fraction and antilamin A/C antibody for the nuclear fraction.
PCNA may function in DNA replication when located in the nucleus. Cytoplasmic accumulation of PCNA has also been reported but the precise function of cytoplasmic PCNA is still unclear, although a role for PCNA in the translation in postmitotic cells, such as neurons, has been suggested [White et al., 2004].
Interestingly, our results may suggest that the presence of T-Ag may interfere with PCNA cytoplasmic/nuclear trafficking, promoting the shuttling of PCNA from the cytoplasm to the nucleus.
PROTEIN EXPRESSION PROFILE OF pRb FAMILY IN HUMAN MB CELLS
D283 and DAOY cell lines represent the two phenotypic profiles of MB, neuronal and glial, respectively. We tested these two cell lines for the presence of JCV T-Ag and both resulted negative (data not shown). Although several studies have reported that JCV infection is associated with various types of human cancers including MB, glioblastoma, colorectal cancer, and lung cancer, the association of JCV with human cancer remains controversial [Del Valle et al., 2001a; Shiramizu et al., 2007; Zheng et al., 2007; Lin et al., 2008; Maginnis and Atwood, 2009]. However, it has been reported that D283 cells express SV40 large T-Ag and that T-Ag may contribute to the immortalized phenotype of D283 [Kim et al., 2002]. Moreover, several studies have reported the presence of SV40 T-Ag in a variety of human cancers and suggested a viral role in their tumorigenesis [Cheng et al., 2009]. Several studies also indicated that both SV40 T-Ag and JCV T-Ag bind the retinoblastoma family proteins and interfere with their oncosuppressor function [DeCaprio et al., 1988; Dyson et al., 1989; Ewen et al., 1989; Sheng et al., 1997; Stubdal et al., 1997; Zalvide et al., 1998; Maginnis and Atwood, 2009]. As SV40 T-Ag and JCV T-Ag share close similarity in both mechanism of action and cellular targets, D283 cells represent a good model to explore the interplay between T-Ag presence and pRb family and p53/p73 signaling in human MB.
Our analysis regarding pRb family protein expression revealed that DAOY MB cells, which are SV40 T-Ag-negative, exhibited very low levels of pRb2/p130 compared with D283 MB cells, which are SV40 T-Ag-positive (Fig. 4A). Moreover, in DAOY cells the only form of pRb2/p130 that appears to be expressed is the hypophosphorylated form, which was detected in both nuclear and cytoplasmic fractions (Fig. 4B). D283 cells exhibited high levels of the hyperphosphorylated form of pRb2/p130, which appears to be accumulated in the cytoplasm (Fig. 4B). WS1 fibroblast cells expressed both the hypophosphorylated and hyperphosphorylated forms of pRb2/p130, in both the nucleus and cytoplasm; however, nuclear accumulation of the hypophosphorylated form of pRb2/p130 was predominant in both the nuclear and cytoplasmic compartments (Fig. 4A,B).
Fig. 4.
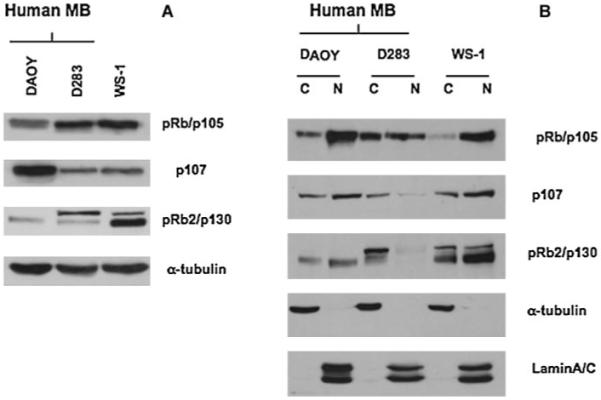
pRb family expression in human medulloblastoma cells (DAOY, D283) and human fibroblast cells (WS-1). The relative protein levels of pRb/p105, p107, and pRb2/p130 were detected by Western blotting using total (A) or nuclear or cytoplamic (B) extract. The expression of α-tubulin protein was assessed to normalize protein loading (A). Efficient cytoplasmic and nuclear fractionation was confirmed by Western blotting analysis using anti-α-tubulin antibody for the cytoplasmic fraction and antilamin A/C antibody for the nuclear fraction (B).
Furthermore, we found an increased level of pRb/p105 in the nucleus of DAOY cells compared with the D283 cells where pRb/p105 was equally distributed between the nucleus and cytoplasm (Fig. 4B). On the contrary, WS1 fibroblast cells exhibited only a nuclear pRb/p105 (Fig. 4B).
Increased levels of p107 were detected in DAOY cells compared with D283 cells. Moreover, D283 cells expressed very low levels of nuclear p107 (Fig. 4A,B).
p53, p73, AND PCNA EXPRESSION
p53 was expressed at very high levels in DAOY cells in both nuclear and cytoplasmic fractions (Fig. 5A,B). On the contrary, D283 cells expressed very low p53 levels and p53 was accumulated exclusively in the cytoplasm (Fig. 5A,B). Also WS1 fibroblast cells expressed very low levels of p53 but it was accumulated in the nucleus (Fig. 5A,B).
Fig. 5.
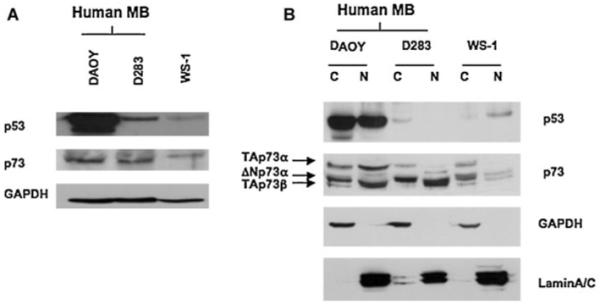
p53 and p73 expression in human medulloblastoma cells (DAOY, D283) and human fibroblast cells (WS-1). Western blot analysis reveals a high expression of p53 in DAOY cells, whereas p53 is expressed at very low levels and accumulates in the cytoplasm in D283 cells (A, B). p73 expression is similar in both DAOY and D283 cells (A).Very low expression of TAp73α isoform is observed in D283 cells compared with the DAOY cells (B). The expression of GAPDH protein was assessed to normalize protein loading (A). Efficient cytoplasmic and nuclear fractionation was confirmed by Western blotting analysis using anti-GAPDH antibody for the cytoplasmic fraction and antilamin A/C antibody for the nuclear fraction (B).
Similar levels of p73 were detected in both DAOY and D283 cells (Fig. 5A). Interestingly, the analysis of cytoplasmic and nuclear fractions from both cell lines revealed the presence of different p73 isoforms (Fig. 5B). Specifically, we observed that DAOY cells exhibited high levels of the TAp73α isoform in both the cytoplasm and nucleus, compared with the D283 cells (Fig. 5B). Moreover, both DAOY and D283 cells exhibited similar and significant levels of ΔNp73α and TAp73β isoforms accumulated in the cytoplasm and nucleus, respectively (Fig. 5B).
EXPRESSION OF PCNA WAS DETECTED IN ALL CELL LINES ANALYZED
Interestingly, PCNA was detected only in the cytoplasm (Fig. 6A,B). Cytoplasmic distribution of PCNA was also confirmed in both DAOY and D283 by immunofluorescence (Fig. 6C,D).
Fig. 6.
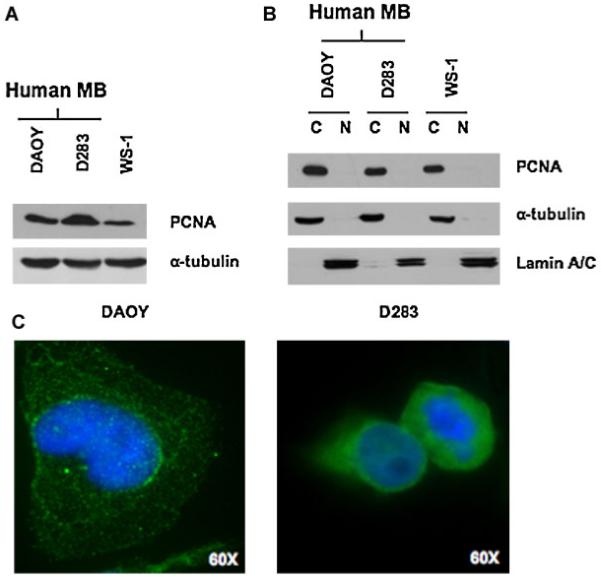
PCNA is expressed in the cytoplasm of human medulloblastoma cells and in human fibroblast cells. Western blot results from total lysates (A) and from cytoplasmic and nuclear lysates (B) from DAOY, D283, and WS-1 cells. The expression of α-tubulin protein was assessed to normalize protein loading (A). Efficient cytoplasmic and nuclear fractionation was confirmed by Western blotting analysis using anti-α-tubulin antibody for the cytoplasmic fraction and antilamin A/C antibody for the nuclear fraction (B). C: Fluorescence immunostaining of DAOY and D283 medulloblastoma cells, showing a cytoplasmic localization of PCNA; 60 × oil objective.
DISCUSSION
Several authors have indicated a compensation role, but not a complete redundancy, among the pRb family proteins in regulating the cell cycle and cellular growth. Interestingly, it has been proposed that intrinsic genetic compensation between pRb/p105 and p107 prevents retinoblastoma in pRb/p105- or p107-deficient mice, but this compensation does not occur in humans [Donovan et al., 2006]. In our study, we found that the T-Ag-positive mouse PNET cells express higher levels of p107 compared with the T-Ag-negative PNET cells. Interestingly, we observed a different p107 expression pattern in human MB cell lines with the T-Ag-positive D283 cells expressing low levels of p107 compared with the T-Ag-negative DAOY cells. This may support the existence of different functions and expression pattern of p107 between mouse and human cells, which may also rely on a different effect of the T-Ag on p107 and pRb2/p130 in these two different cellular systems.
Both in mouse PNET cells and human MB cells the protein expression pattern and/or cellular distribution of pRb family, p53, p73, and PCNA may be altered by the presence of T-Ag. Our idea is that alterations of these tumor suppressor pathways cooperate in tumorigenesis in a cell type-specific manner, suggesting that cell specificity of tumor suppression mechanisms depend on driving signals. For instance, a mutant p53 may interact with p73 and p63, thus inactivating their functions in MB. Furthermore, concomitant presence of T-Ag could stabilize the mutant p53 through pRb signaling alteration. In support of this hypothesis, it has been reported that inhibition of p73 and p63 functions is dependent on p53 alteration, and that many p53 mutants, but not wild-type p53, interact with p73 and p63 [Lozano, 2007]. In addition, it has been reported that during the course of tumor evolution of JCV T-Ag mouse MB, a mutation occurs that inactivates p53, allowing tumor progression even in the absence of continued T-Ag expression [Krynska et al., 2000; White et al., 2006]. As tumor progression is allowed even in the absence of T-Ag, then it will be important to determine whether the T-Ag represents the initial “hit event” necessary to establish specific p53 mutants. In addition, does the presence of T-Ag affect the equilibrium between pRb and p53/p73 pathways? Does the affected equilibrium lead to aberrant epigenetic events dictating a tissue-specific altered microenvironment?
Importantly, several studies have suggested a potential role for p73 isoforms in tumor growth and in the response to chemotherapeutic treatment. In particular, it has been reported that MB expresses significant levels of p73 isoforms and overexpression of TAp73β and ΔNp73β induces apoptosis in MB cells transfected with wild-type p53 [Castellino et al., 2007]. Moreover, it has been shown that the ΔNp73α isoform exhibits oncogenic properties, inhibits apoptosis and may contribute to drug resistance in vitro and in vivo [Stiewe and Putzer, 2002; Concin et al., 2005; Vilgelm et al., 2008]. The mechanism/s by which p73 isoforms exert these activities as well as the signals governing the presence of specific isoforms still remains unclear. However, our results may support the idea that the presence of JCV T-Ag interferes with the expression of specific p73 isoforms and p53, thus altering the delicate balance between p53 and p73 isoforms activity. In turn, this “perturbation” may trigger a network of signals strictly connected with survival and apoptosis. For instance, we observed significant levels of TAp73α isoform in both T-Ag-negative PNET cells and DAOY cells, whereas this isoform was expressed at very low levels in T-Ag-positive PNET cells and D283 cells, suggesting a correlation between the expression of specific p73 isoforms and the presence of T-Ag.
Disclosing the interplay among pRb family, p53 and p73 signaling, and the role played by the presence of T-Ag in their molecular network, will be critical in understanding the biology of MB.
Acknowledgments
Grant sponsor: National Institute of Health; Grant sponsor: Department of Defense; Grant sponsor: Human Health Foundation, Spoleto, Italy.
REFERENCES
- Caracciolo V, Reiss K, Khalili K, De Falco G, Giordano A. Role of the interaction between large T antigen and Rb family members in the oncogenicity of JC virus. Oncogene. 2006;25:5294–5301. doi: 10.1038/sj.onc.1209681. [DOI] [PubMed] [Google Scholar]
- Castellino RC, De Bortoli M, Lin LL, Skapura DG, Rajan JA, Adesina AM, Perlaky L, Irwin MS, Kim JY. Overexpressed TP73 induces apoptosis in medulloblastoma. BMC Cancer. 2007;7:127. doi: 10.1186/1471-2407-7-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, DeCaprio JA, Fluck MM, Schaffhausen BS. Cellular transformation by Simian virus 40 and murine polyoma virus T antigens. Semin Cancer Biol. 2009;19:218–228. doi: 10.1016/j.semcancer.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concin N, Hofstetter G, Berger A, Gehmacher A, Reimer D, Watrowski R, Tong D, Schuster E, Hefler L, Heim K, Mueller-Holzner E, Marth C, Moll UM, Zeimet AG, Zeillinger R. Clinical relevance of dominant-negative p73 isoforms for responsiveness to chemotherapy and survival in ovarian cancer: Evidence for a crucial p53-p73 cross-talk in vivo. Clin Cancer Res. 2005;11:8372–8383. doi: 10.1158/1078-0432.CCR-05-0899. [DOI] [PubMed] [Google Scholar]
- DeCaprio JA, Ludlow JW, Figge J, Shew JY, Huang CM, Lee WH, Marsilio E, Paucha E, Livingston DM. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988;54:275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Del Valle L, Baehring J, Lorenzana C, Giordano A, Khalili K, Croul S. Expression of a human polyomavirus oncoprotein and tumour suppressor proteins in medulloblastomas. Mol Pathol. 2001a;54:331–337. doi: 10.1136/mp.54.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle L, Gordon J, Assimakopoulou M, Enam S, Geddes JF, Varakis JN, Katsetos CD, Croul S, Khalili K. Detection of JC virus DNA sequences and expression of the viral regulatory protein T-antigen in tumors of the central nervous system. Cancer Res. 2001b;61:4287–4293. [PubMed] [Google Scholar]
- Donovan SL, Schweers B, Martins R, Johnson D, Dyer MA. Compensation by tumor suppressor genes during retinal development in mice and humans. BMC Biol. 2006;3:4–14. doi: 10.1186/1741-7007-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N, Buchkovich K, Whyte P, Harlow E. The cellular 107K protein that binds to adenovirus E1A also associates with the large T antigens of SV40 and JC virus. Cell. 1989;58:249–255. doi: 10.1016/0092-8674(89)90839-8. [DOI] [PubMed] [Google Scholar]
- Ewen ME, Ludlow JW, Marsilio E, DeCaprio JA, Millikan RC, Cheng SH, Paucha E, Livingston DM. An N-terminal transformation-governing sequence of SV40 large T antigen contributes to the binding of both p110Rb and a second cellular protein, p120. Cell. 1989;58:257–267. doi: 10.1016/0092-8674(89)90840-4. [DOI] [PubMed] [Google Scholar]
- Giacinti C, Giordano A. RB and cell cycle progression. Oncogene. 2006;25:5220–5227. doi: 10.1038/sj.onc.1209615. [DOI] [PubMed] [Google Scholar]
- Howard CM, Claudio PP, Gallia GL, Gordon J, Giordano GG, Hauck WW, Khalili K, Giordano A. Retinoblastoma-related protein pRb2/p130 and suppression of tumor growth in vivo. J Natl Cancer Inst. 1998;90:1451–1460. doi: 10.1093/jnci/90.19.1451. [DOI] [PubMed] [Google Scholar]
- Khalili K, Krynska B, Del Valle L, Katsetos CD, Croul S. Medulloblastomas and the human neurotropic polyomavirus JC virus. Lancet. 1999;353:1152–1153. doi: 10.1016/s0140-6736(99)00357-8. [DOI] [PubMed] [Google Scholar]
- Kim JY, Koralnik IJ, LeFave M, Segal RA, Pfister LA, Pomeroy SL. Medulloblastomas and primitive neuroectodermal tumors rarely contain polyomavirus DNA sequences. Neuro Oncol. 2002;4:165–170. doi: 10.1093/neuonc/4.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krynska B, Del Valle L, Croul S, Gordon J, Katsetos CD, Carbone M, Giordano A, Khalili K. Detection of human neurotropic JC virus DNA sequence and expression of the viral oncogenic protein in pediatric medulloblastomas. Proc Natl Acad Sci. 1999a;96:11519–11524. doi: 10.1073/pnas.96.20.11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krynska B, Otte J, Franks R, Khalili K, Croul S. Human ubiquitous JCV·CY T-antigen gene induces brain tumors in experimental animals. Oncogene. 1999b;18:39–46. doi: 10.1038/sj.onc.1202278. [DOI] [PubMed] [Google Scholar]
- Krynska B, Del Valle L, Gordon J, Otte J, Croul S, Khalili K. Identification of a novel p53 mutation in JCV-induced mouse medulloblastoma. Virology. 2000;274:65–74. doi: 10.1006/viro.2000.0450. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lassak A, Del Valle L, Peruzzi F, Wang JY, Enam S, Croul S, Khalili K, Reiss K. Insulin receptor substrate 1 translocation to the nucleus by the human JC virus T-antigen. J Biol Chem. 2002;277:17231–17238. doi: 10.1074/jbc.M110885200. [DOI] [PubMed] [Google Scholar]
- Lin PY, Fung CY, Chang FP, Huang WS, Chen WC, Wang JY, Chang D. Prevalence and genotype identification of human JC virus in colon cancer in Taiwan. J Med Virol. 2008;80:1828–1834. doi: 10.1002/jmv.21296. [DOI] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano G. The oncogenic roles of p53 mutants in mouse models. Curr Opin Genet Dev. 2007;17:66–70. doi: 10.1016/j.gde.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Macaluso M, Montanari M, Giordano A. Rb family proteins as modulators of gene expression and new aspects regarding the interaction with chromatin remodeling enzymes. Oncogene. 2006;25:5263–5267. doi: 10.1038/sj.onc.1209680. [DOI] [PubMed] [Google Scholar]
- Maginnis MS, Atwood WJ. JC virus: An oncogenic virus in animals and humans? Semin Cancer Biol. 2009;19:261–269. doi: 10.1016/j.semcancer.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci B, Claudio PP, Masciullo V, Bellincampi L, Terrinoni A, Khalili K, Melino G, Giordano A. pRb2/p130 promotes radiation-induced cell death in the glioblastoma cell line HJC12 by p73 upregulation and Bcl-2 downregulation. Oncogene. 2002;21:5897–5905. doi: 10.1038/sj.onc.1205750. [DOI] [PubMed] [Google Scholar]
- Reiss K, Valentinis B, Tu X, Xu SQ, Baserga R. Molecular markers of IGF-I-mediated mitogenesis. Exp Cell Res. 1998;242:361–372. doi: 10.1006/excr.1998.4113. [DOI] [PubMed] [Google Scholar]
- Rossi A, Caracciolo V, Russo G, Reiss K, Giordano A. Medulloblastoma: From molecular pathology to therapy. Clin Cancer Res. 2008;14:971–976. doi: 10.1158/1078-0432.CCR-07-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Q, Denis D, Ratnofsky M, Roberts TM, DeCaprio JA, Schaffhausen B. The DnaJ domain of polyomavirus large T antigen is required to regulate Rb family tumor suppressor function. J Virol. 1997;71:9410–9416. doi: 10.1128/jvi.71.12.9410-9416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiramizu B, Hu N, Frisque RJ, Nerurkar VR. High prevalence of human polyomavirus JC VP1 gene sequences in pediatric malignancies. Cell Mol Biol (Noisy-le-grand) 2007;53:4–12. [PMC free article] [PubMed] [Google Scholar]
- Stiewe T, Putzer BM. Role of p73 in malignancy: Tumor suppressor or oncogene? Cell Death Differ. 2002;9:237–245. doi: 10.1038/sj.cdd.4400995. [DOI] [PubMed] [Google Scholar]
- Stubdal H, Zalvide J, Campbell KS, Schweitzer C, Roberts TM, DeCaprio JA. Inactivation of pRB-related proteins p130 and p107 mediated by the J domain of simian virus 40 large T antigen. Mol Cell Biol. 1997;17:4979–4990. doi: 10.1128/mcb.17.9.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A, Bagella L, Tutton S, Romano G, Giordano A. From G0 to S phase: A view of the roles played by the retinoblastoma (Rb) family members in the Rb-E2F pathway. J Cell Biochem. 2007;102:1400–1404. doi: 10.1002/jcb.21609. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanek J, Ho T, Croul S, Wang JY, Chintapalli J, Koptyra M, Giordano A, Khalili K, Reiss K. IRS-1-Rad51 nuclear interaction sensitizes JCV T-antigen positive medulloblastoma cells to genotoxic treatment. Int J Cancer. 2006;119:539–548. doi: 10.1002/ijc.21828. [DOI] [PubMed] [Google Scholar]
- Vilgelm A, Wei JX, Piazuelo MB, Washington MK, Prassolov V, El-Rifai W, Zaika A. DeltaNp73alpha regulates MDR1 expression by inhibiting p53 function. Oncogene. 2008;27:2170–2176. doi: 10.1038/sj.onc.1210862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F, McCaig D, Brown SM, Graham DI, Harland J, Macrae IM. Up-regulation of a growth arrest and DNA damage protein (GADD34) in the ischaemic human brain: Implications for protein synthesis regulation and DNA repair. Neuropathol Appl Neurobiol. 2004;30:683–691. doi: 10.1111/j.1365-2990.2004.00584.x. [DOI] [PubMed] [Google Scholar]
- White MK, Skowronska A, Gordon J, Del Valle L, Deshmane SL, Giordano A, Khalili K. Analysis of a mutant p53 protein arising in a medulloblastoma from a mouse transgenic for the JC virus early region. Anticancer Res. 2006;26:4079–4092. [PubMed] [Google Scholar]
- Zalvide J, Stubdal H, DeCaprio JA. The J domain of simian virus 40 large T antigen is required to functionally inactivate RB family proteins. Mol Cell Biol. 1998;18:1408–1415. doi: 10.1128/mcb.18.3.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Abdel Aziz HO, Nakanishi Y, Masuda S, Saito H, Tsuneyama K, Takano Y. Oncogenic role of JC virus in lung cancer. J Pathol. 2007;212:306–315. doi: 10.1002/path.2188. [DOI] [PubMed] [Google Scholar]


