Abstract
Background
Chronic pain is associated with depression. In rodents, pain is often assessed by sensory hypersensitivity, which does not sufficiently measure affective responses. Low-dose ketamine has been used to treat both pain and depression, but it is not clear whether ketamine can relieve depression associated with chronic pain and whether this antidepressant effect depends on its anti-nociceptive properties.
Methods
We examined whether the spared nerve injury (SNI) model of neuropathic pain induces depressive behavior in rats, using sucrose preference test and forced swim test, and tested whether a subanesthetic dose of ketamine treats SNI-induced depression.
Results
SNI-treated rats, compared with control, showed decreased sucrose preference (0.719 ± 0.068 (mean ± SEM) vs. 0.946 ± 0.010) and enhanced immobility in the forced swim test (107.3 ± 14.6s vs. 56.2 ± 12.5s). Further, sham-operated rats demonstrated depressive behaviors in the acute postoperative period (0.790 ± 0.062 on postoperative day 2). A single subanesthetic dose of ketamine (10mg/kg) did not alter SNI-induced hypersensitivity; however, it treated SNI-associated depression-like behaviors (0.896 ± 0.020 for ketamine vs. 0.663 ± 0.080 for control 1 day after administration; 0.858 ± 0.017 for ketamine vs. 0.683 ± 0.077 for control 5 days after administration).
Conclusions
Chronic neuropathic pain leads to depression-like behaviors. The postoperative period also confers vulnerability to depression, possibly due to acute pain. Sucrose preference test and forced swim test may be used to compliment sensory tests for assessment of pain in animal studies. Low-dose ketamine can treat depression-like behaviors induced by chronic neuropathic pain.
Introduction
Depression affects 30 to 100% of chronic pain patients and is likely underestimated in postoperative patients.1-6 Depression leads to additional emotional and cognitive deficits,7 and understanding the relationship between pain and depression will help to tailor treatments.8 There is evidence that depression alters the threshold of pain, though few studies examined whether depression is an integral affective component of the pain experience.9-14
In rodents, pain is often assessed by sensory hypersensitivity, measured by stereotyped behaviors such as limb withdrawal.15-16 Hypersensitivity is an important sensory feature, but does not reveal cognitive and emotional responses to pain, which can be assessed by Morris water maze, elevated plus maze, and open field tests.13,17-19 In rodents, depression can be measured by forced swim test (FST) and sucrose preference test (SPT).20 FST, a test for behavioral despair, was used to examine depression in the spinal nerve ligation model of neuropathic pain. Whereas Kontinen et al. reported no changes in the FST performance in rats 14 days after surgery,21 Suzuki et al. found rats displayed depressive traits 15 days post-operatively.22 SPT measures anhedonia, a key feature of depression.20 SPT, however, was rarely used in pain studies.
Ketamine has both analgesic and antidepressant properties and is ideally suited to treat pain-induced depression.23-24 Ketamine antagonizes N-Methyl-D-aspartic acid receptors in spinal dorsal horn neurons to decrease central sensitization,25-26 provides descending monoaminergic inhibition,27 and blocks Na+ channels and μ-opioid receptors in peripheral fibers.28 Ketamine is metabolized within an hour and useful as a short-acting analgesic.29 Although ketamine may block central sensitization to mediate long-acting analgesia;30 this remains to be proven in clinical practices.31 In contrast, clinical studies have shown that ketamine provides enduring antidepressant effects.32-34,35-36 Several proposed mechanisms explain the antidepressant properties of ketamine: increased presynaptic glutamate release and upregulation of post-synaptic machinery in the prefrontal cortex,37-38 and increased brain derived neurotrophic factor expression in the hippocampus.39 Thus, whereas short-lived analgesic properties of ketamine are mediated at peripheral and spinal levels, its antidepressant activities involve frontal and limbic structures and are long-lasting. Clinically, depression can be treated at a lower dose than pain.24,33 Similarly, in rats <10mg/kg of ketamine provides antidepressant effects but >25mg/kg is needed for analgesia.27,38,40-42 Therefore, determining whether ketamine can treat depression-like behaviors at a dose (<10mg/kg) that does not treat sensory hypersensitivity in rodents may provide a distinction between sensory and depressive components of pain.
We examined the spared nerve injury (SNI) model in rats.43 Using a combination of SPT and FST, we found that rats exhibited depression-like behaviors immediately after surgery. Whereas depression-like behaviors induced by sham operation were reversible, depression induced by SNI surgery was chronic. These results suggest that depression may be a key feature of post-operative and chronic pain in rodents; hence we favor the routine use of SPT and FST in pain assessments. Moreover, a single subanesthetic dose of ketamine produced rapid and enduring antidepressant effects in rats with chronic pain, without decreasing sensory hypersensitivity, suggesting that depressive symptoms of pain can be modulated independently of sensory symptoms.
Materials and Methods
Animals
All procedures in this study were approved by the New York University School of Medicine Institutional Animal Care and Use Committee as consistent with the National Institute of Health Guide for the Care and Use of Laboratory Animals (publication number 85-23) to ensure minimal animal use and discomfort. Male Sprague-Dawley rats were purchased from Taconic Farms, Albany, NY and kept in the New York University Langone Medical Center’s Central Animal Facility, with controlled humidity, room temperature, and 12-h (6:30 AM to 6:30 PM) light-dark cycle. Food and water were available ad libitum. Animals arrived to the animal facility at 250 to 300 grams and were given approximately14 days to adjust to the new environment prior to the onset of any experiments.
Spared Nerve Injury (SNI) surgery
The SNI surgery has been previously described in detail.43 Briefly, under Isoflurane anesthesia (1.5 to 2%), the skin on the lateral surface of the right thigh of rat was incised and a section made through the biceps femoris muscle to expose three branches of the sciatic nerve: sural, common peroneal and tibial nerves. The common peroneal and tibial nerves were tied with non-absorbent 5.0 silk sutures at the point of trifurcation. The nerves were then cut distal to the knot, and about 3 to 5 mm of the distal ends were removed. In sham surgeries (control), above nerves were dissected but not cut. Muscle and skin layers were then sutured close in distinct layers. Naïve rats underwent no surgery.
Drugs
Ketamine Hydrochloride (Ketaset) was purchased from Fort Dodge Animal Health, Fort Dodge, IA. Ketamine was diluted in saline to a concentration of 1 to 50 mg/ml (depending on the final dose of ketamine). For the ketamine experiments, rats that underwent SNI surgery were assigned to two sub-groups, one receiving ketamine injections and the other saline (control) injections. 1, 3, 10, 20 or 50 mg/kg of ketamine was injected intraperitoneally in the ketamine group (0.3 to 0.5cc), while similar volume of saline was injected intraperitoneally to the control group. Injections were given at least fourteen days after SNI surgeries and were followed by behavioral tests.
Animal Behavioral Tests
Mechanical allodynia testing
A traditional Dixon up-down method with von Frey filaments was used to measure mechanical allodynia.16,44 In brief, rats were individually placed into plexiglass chambers over a mesh table and acclimated for 20 min before the onset of examination. Beginning with 2.55g, von Frey filaments in a set with logarithmically incremental stiffness (0.45, 0.75, 1.20, 2.55, 4.40, 6.10, 10.50, 15.10 g) were applied to the lateral 1/3 of right paws (in the distribution of the sural nerve) of animals prior to and up to 56 days after SNI or sham surgery and to rats that underwent no surgery but similar handling (naïve group). In addition, the tests were done on the left (uninjured) paws of the SNI animals as well. 50% withdrawal threshold was calculated as described previously.16 For ketamine experiments, mechanical allodynia tests were done 1 hour or 1 day after ketamine (or saline) injection, and observers were blinded to the test conditions (ketamine vs. saline treatments).
Cold allodynia testing
Animals were individually placed into plexiglass chambers as above and acclimated for 20 min. A drop of acetone was applied to the lateral plantar surface of the paws (in the distribution of the sural nerve). As previously described,15,25 the following scoring system was applied. 0: no visible response or startle response lasting <0.5 second; 1: paw withdrawal lasting <5 seconds; 2: withdrawal lasting 5 to 10 seconds, +/- licking of the paws; 3: prolonged repetitive withdrawal lasting > 10s. Acetone was applied 5 times to each paw, and an average score was calculated. Cold allodynia tests were typically done after mechanical allodynia tests on the same day, and observers were blinded to the test conditions (ketamine vs. saline treatments).
Sucrose preference test
Animals were trained approximately 2 hr each day for 7 to 10 days to drink from two identical bottles, one bottle containing 1% sucrose solution and the other water. In order to avoid side preference, the bottles were placed on alternating sides every day. Before the SNI surgery, baseline preference for sucrose was established. During each test, two bottles (1% sucrose solution vs. water) were presented to each animal for 30 min, and then the bottles were switched to the opposite side, and the test continued for an additional 30 min. At the end of each test, sucrose preference was calculated as volume of sucrose consumed divided by total liquid consumption for each rat. Based on their baseline preferences, animals were equitably assigned to naïve, sham surgery, or SNI surgery group. After undergoing surgery, sucrose preference was then tested on postoperative day 2, 7, 14 and 56 for the SNI and sham surgery groups and 2, 7, 14 for the naïve group. For ketamine experiments, all animals underwent SNI surgery, and animals were assigned to either the ketamine or saline group. Due to the variability of baseline pre-surgical sucrose preference, animals were assigned to treatment and control groups with the goal of ensuring that the average baseline preference for each group was approximately the same before each experiment. 14 days after the SNI surgery, ketamine or saline was injected into each animal, and sucrose preference was then tested 1 and 5 days after the injection, and observers were blinded to the test conditions (ketamine vs. saline treatments).
Forced swim test
On the first session of the test, each animal was placed for fifteen minutes into a standard clear Porsolt chamber with water at 25° C filled to 25 cm. Afterwards, the animal was taken out of the chamber, dried and put back in its home cage. 24 hours later, the animal was placed into the Porsolt chamber again under the same conditions for 5 min. Both sessions were videotaped, but only the second session was analyzed. Immobility was defined as a lack of movement of the hind paws lasting greater than 1 second. Two independent observers, examined and graded the total time of immobility for each rat, and the average grade was presented for each animal. FST was conducted 14 days after surgeries. For the ketamine experiments, animals were randomly assigned to either treatment (ketamine) or control group (saline). FST was conducted one day after ketamine/saline injection, and observers were blinded to the test conditions (ketamine vs. saline treatments).
Corticosterone Measurements
Blood samples were procured from the jugular vein of each rat following decapitation. Blood samples then were spun down at X1000 g for 10 minutes, and serum was then extracted from the sample and immediately frozen at -80°C. A Corticosterone rat/mouse ELISA kit and the manufacturers recommended protocol (Immuno-Biological Laboratories, Inc., Minneapolis, MN) was used to determine corticosterone absorbance levels within each serum sample using plate reader detection. Corresponding corticosterone concentrations were determined using a standard curve, where the standards were provided by the manufacturer. EnVision plate reader software (PerkinElmer, Inc., Waltham, MA) was used to determine the standard curve. Absorbance levels of each sample was plugged into the standard curves equation generated by the EnVision software and the corresponding corticosterone concentrations was generated.
Statistics
The results of behavioral experiments were given as mean ± SEM. For mechanical allodynia, a two-way ANOVA with post hoc multiple pair-wise comparison Bonferroni tests was used to compare the 50% withdrawal threshold of the right and left legs of the SNI animals, and right legs of sham and naïve animals. Cold allodynia was analyzed using the Kruskal-Wallis test with post hoc Dunn’s multiple pair-wise comparison tests at each time point. A one-way ANOVA with post hoc multiple pair-wise comparison Tukey tests was used to analyze mechanical allodynia of SNI-treated animals which received higher doses of ketamine (20 or 50 mg/kg) or saline, and a Kruskal-Wallis test with post hoc Dunn’s multiple pair-wise comparison tests was used to analyze the cold allodynia data. Weight gain was analyzed using a two-way ANOVA with post hoc multiple pair-wise comparison Bonferroni tests. For the sucrose preference test, a two-way ANOVA with post hoc Bonferroni tests was used to compare the preference at different time points of the SNI, sham and naïve groups, and to compare the preference at different time points of ketamine or saline injected rats. In the dose response experiment, a two-way ANOVA with post hoc Bonferroni tests was used to compare sucrose preference for different doses of ketamine (1, 3, 10, and 20 mg/kg) vs. saline control. For the forced swim test, a unpaired two-tailed Student’s t test was used to compare the performances of sham and SNI groups as well as to compare the performances of ketamine and saline groups. For corticosterone levels and total fluid consumption during the SPT, a one way ANOVA was conducted. For all tests, a p value <0.05 was considered statistically significant. All data were analyzed using GraphPad Prism Version 4 software (GraphPad, La Jolla, CA). We did not perform a power analysis a priori for our experiments. In general, the numbers of subjects used in our experiments were based on our experience with these behavioral tests and in agreement with standard literature. Same or similar numbers of subjects were in control and test conditions. Thus, if the test condition provided statistical significance but the control did not, we would conclude that the control condition resulted in non-meaningful findings.
Results
Spared nerve injury (SNI) produced long lasting sensory hypersensitivity without changes in the stress level or overall well being of the animals
We studied the SNI model for neuropathic pain. Compatible with previous reports,43 we found that SNI surgery produced significant mechanical and cold hypersensitivity in the (spared) sural nerve distribution acutely, on postoperative day 1, and these effects persisted for up to two months (fig. 1A, 1B). In contrast, rats that underwent sham operation or no surgical manipulation (naïve rats) did not demonstrate any sensory hypersensitivity in the spared nerve distribution. In addition, we observed no hypersensitivity in the uninjured paw of an animal after the SNI surgery. SNI results in neuropathy, which may produce chronic stress to complicate behavioral tests. To rule out this possibility, we used weight gain as a measure for the overall health and well being of rats and found no difference among SNI-treated rats, naïve (non-operated) rats or sham-operated rats (fig. 1C). Furthermore, we measured levels of the stress hormone corticosterone of rats 14 days after surgeries and found no differences among rats in the SNI, sham, and naïve groups (fig. 1D).
Fig. 1.
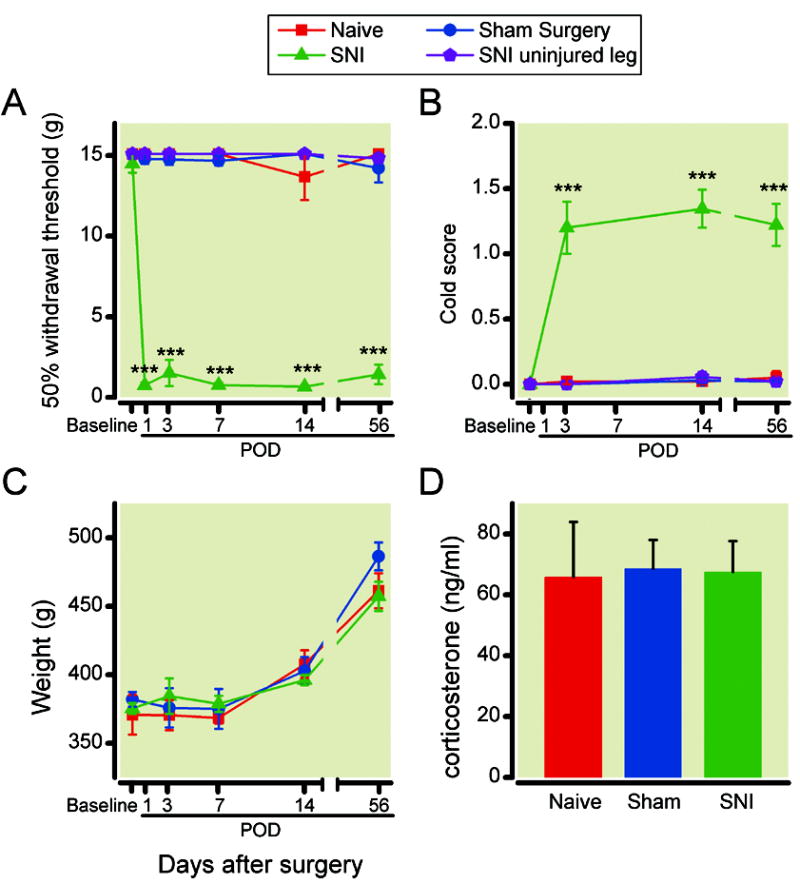
Spared nerve injury (SNI) surgery induced long lasting mechanical and cold hypersensitivity without causing changes in weight and stress hormone levels. (A) Animals after SNI surgery developed mechanical hypersensitivity starting on postoperative day (POD) 1, and this hypersensitivity lasted until POD56. The mechanical hypersensitivity only manifested in the injured leg. Mechanical hypersensitivity was tested using von Frey filaments. 50% withdrawal threshold (WT) was calculated (see Methods for details). *** p<0.001, WT for injured legs in SNI-operated animals (n= 24) versus WT of naïve (n= 14), sham-operated animals (n= 22), or contralateral legs of SNI-operated animals (n= 12), two-way ANOVA, with post-hoc Bonferroni tests. (B) Animals after SNI surgery developed cold hypersensitivity from POD3 to POD56. *** p<0.001, cold score (see Methods for details) for injured legs in SNI-operated animals (n=11) versus naïve (n=9), sham-operated animals (n=11), or contralateral legs of SNI-operated animals (n=14), Kruskal-Wallis test with post hoc Dunn’s multiple pair-wise comparison tests. (C) Weight gain was not altered by SNI surgery. Naïve: n= 16; sham: n=14; SNI: n=15. (D) SNI surgery did not cause changes in corticosterone levels. Naïve: n= 10; sham: n=10; SNI: n=12.
Spared nerve injury (SNI) produced depressive behaviors
Next, we applied standard tests of depression to rats in chronic pain. The first test we used was the forced swim test. Increased time of immobility (instead of swimming) in a water tank is considered a measurement of behavioral despair. We performed our tests 14 days after the surgery – a standard time for the development of chronic neuropathic pain – and found that compared with sham-operated rats, rats after SNI developed significantly increased time of immobility (fig. 2A). We confirmed this expression of depressive behavior with the sucrose preference test, which assesses anhedonia, measured by the preference of sucrose solution to water. At baseline, sham and SNI-operated rats demonstrated approximately > 90% preference for sucrose (1%) solution over water. However, 14 days after surgery, SNI-operated rats demonstrated significantly decreased preference for sucrose compared with sham-operated rats, and this difference lasted up to two months (fig. 2B), concurrent with the time course of chronic sensory hypersensitivity demonstrated by SNI animals (fig. 1A, 1B). Next, we turned our attention to the early postoperative period (postoperative days 1 to 14). Because we were concerned about post-operative incisional pain associated with sham operations, we added an additional control group – naïve rats which underwent no surgeries. Rats in both SNI and sham surgery groups developed significantly decreased preference for sucrose in the immediate postoperative period (fig. 2C, postoperative day 2), compared with the naïve group. This decrease in sucrose preference was transient in the sham surgery group, lasting less than 7 days. In contrast, the decrease in the SNI group was stable. To ensure that neuropathy resulting from SNI did not interfere with drinking behaviors, we measured the total fluid consumption and did not find any differences among three groups of animals (fig. 2D).
Fig. 2.
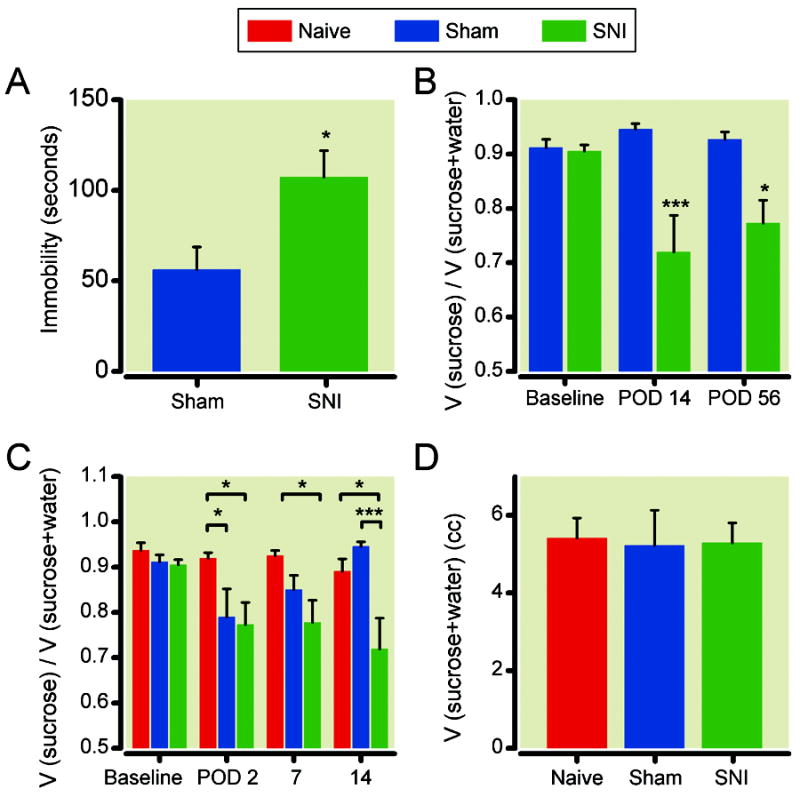
Spared nerve injury (SNI) surgery induced immediate and long lasting depressive changes. (A) Animals after SNI demonstrated increased immobility during the forced swim test, compared with sham-operated rats. * p<0.05, student t test. n= 6. (B) Rats after SNI demonstrated chronic decreases in preference for 1% sucrose solution compared with sham-operated rats. Sucrose preference = volume (sucrose solution) / volume (sucrose solution + water). *** p<0.001, * P<0.05, two-way ANOVA with post-hoc Bonferroni tests. sham: n=13; SNI: n=17.C) Both SNI-operated and sham-operated rats demonstrated an initial loss of sucrose preference post-operatively (on postoperative day 2- POD2), but this loss of preference only persisted in SNI-operated rats. *** p<0.001, * P<0.05, two-way ANOVA with post-hoc Bonferroni tests. Naïve: n= 17; sham: n=17; SNI: n=19. (D) Total fluid consumption was not altered with either sham or SNI surgeries. Naïve: n= 4; sham: n=7; SNI: n=9.
Low-dose (10mg/kg) ketamine provided long lasting antidepressant effects
If SNI causes depressive behaviors, then an antidepressant should be able to treat these behaviors. We applied a single dose of ketamine (10mg/kg) known to treat depression38 vs. saline (control) intraperitoneally to animals that had undergone the SNI surgery 14 days earlier. One day after drug administration, we tested the sucrose preference for these animals. While animals that received saline injection remained anhedonic, animals that received ketamine injections demonstrated a return to baseline pre-surgical sucrose preference (fig. 3A). Although ketamine improved sucrose preference, it did not affect total volume of fluid consumption (fig. 3B). We repeated these tests five days after the injection to see if the antidepressant effect of ketamine was enduring. We found animals that received ketamine 5 days earlier continued to demonstrate improvements in anhedonia (fig. 3A). We next used the forced swim test to confirm the antidepressant effects of ketamine in animals after SNI surgeries. One day after ketamine administration, animals with neuropathy showed a significant improvement in their immobility score, as compared with those animals that received saline injections (fig. 4). In fact, the immobility score for SNI-operated animals after ketamine treatment was comparable to that of sham-operated animals.
Fig. 3.
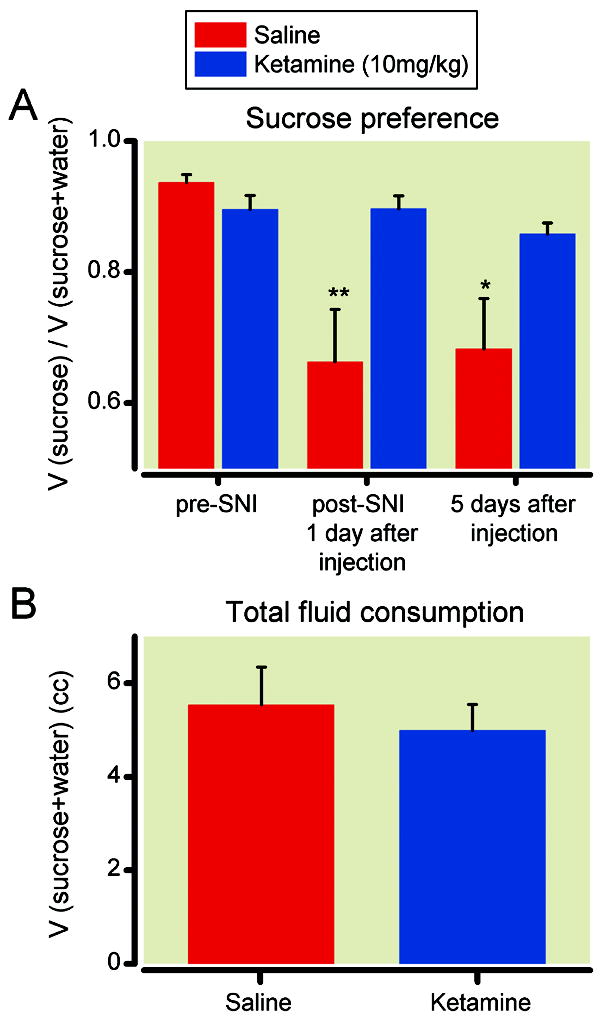
Ketamine (10mg/kg) restored sucrose preference in animals after spared nerve injury (SNI). (A) All rats had spared nerve injury 14 days prior to testing. Ketamine, compared with saline, increased sucrose preference 1 day and 5 days after injection. ** p<0.01, * p<0.05, two-way ANOVA with post-hoc Bonferroni tests. Ketamine group: n= 11; saline group: n=12. (B) Ketamine did not affect total fluid consumption. n= 10.
Fig. 4.
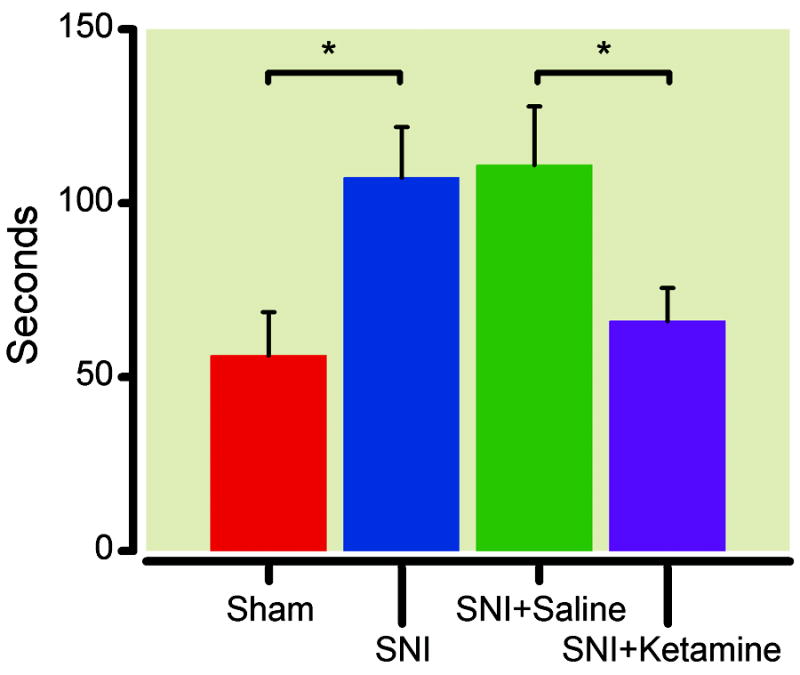
Ketamine improved immobility during the forced swim test. Rats after spared nerve injury (SNI) demonstrated increased immobility during the forced swim test, compared with sham-operated rats. * p<0.05, student t test. n= 6. For rats that had spared nerve injury for 14 days, ketamine (10mg/kg) treatment improved immobility compared with saline treatment. * p<0.05, student t test. Sham: n=9; SNI: n=11.
Low-dose (<50 mg/kg) ketamine did not modify sensory hypersensitivity in animals after SNI surgery
Because ketamine also carries anti-nociceptive properties, we next asked whether the antidepressant effect of ketamine that we found at this dose (10mg/kg) caused improvements in sensory hypersensitivity. We administered a single dose of ketamine (10mg/kg) intraperitoneally to animals that had undergone SNI surgery 14 days earlier. Because the half-life of ketamine is less than 1 hour and its analgesic effects are short acting, we tested its analgesic effects one hour and one day after injection. At this dose, we did not observe any changes in mechanical or cold hypersensitivity compared with saline (control) either one hour after the injection, or one day after injection (fig. 5A-D). To further verify the lack of long-acting (>1 hr) anti-nociceptive effects of ketamine, we also tested sensory hypersensitivities at two higher doses, 20 mg/kg and 50 mg/kg, one day after administration (we did not perform these tests 1 hr after drug administration because sedative side effects of ketamine interfered with behavior tests). At these doses, we still did not observe any changes in mechanical or cold hypersensitivity compared with saline (fig. 5E-F). This lack of long-lasting analgesia contrasted with the enduring antidepressant effects that we observed up to 5 days after ketamine injection (fig. 3A). These results suggested that the antidepressant effects of ketamine we observed are independent of the anti-nociceptive effects of ketamine at low subanesthetic doses.
Fig. 5.
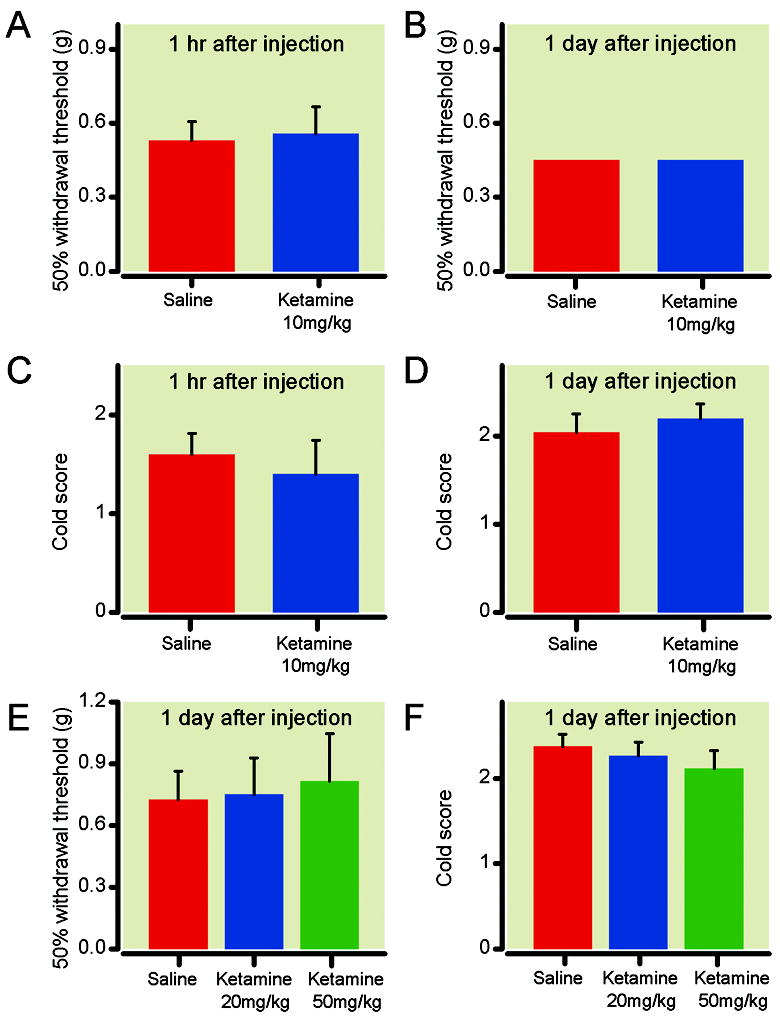
Ketamine did not improve mechanical and cold hypersensitivity in rats after spared nerve injury (SNI). (A) All rats underwent spared nerve injury 14 days prior to testing. No changes in mechanical hypersensitivity were observed 1 hr after ketamine (10mg/kg) injection. (B) No changes in mechanical hypersensitivity 1 day after ketamine (10mg/kg) injection. (C) No changes in cold hypersensitivity 1 hr after ketamine (10mg/kg) injection. (D) No changes in cold hypersensitivity 1 day after ketamine (10mg/kg) injection. n= 5 for A-D. (E) No changes in mechanical hypersensitivity 1 day after administration of higher doses of ketamine (20 and 50mg/kg). (F) No changes in cold hypersensitivity 1 day after high dose ketamine (20 and 50mg/kg) treatment. E-F: Saline: n=8; 20 mg/kg: n=6; 50 mg/kg: n=5. p> 0.05, one-way ANOVA.
Dose response relationship for the antidepressant activity of ketamine
Next we sought to determine the dosage required to establish antidepressant effects for ketamine. We applied a series of doses – 1 mg/kg, 3 mg/kg, 10 mg/kg, and 20 mg/kg – and compared the antidepressant effects of these doses of ketamine with saline control using the SPT. As shown in fig 6, at 10 or 20 mg/kg, antidepressant effects were fully established statistically (p<0.01 for 10 mg/kg, p<0.05 for 20 mg/kg).
Fig. 6.
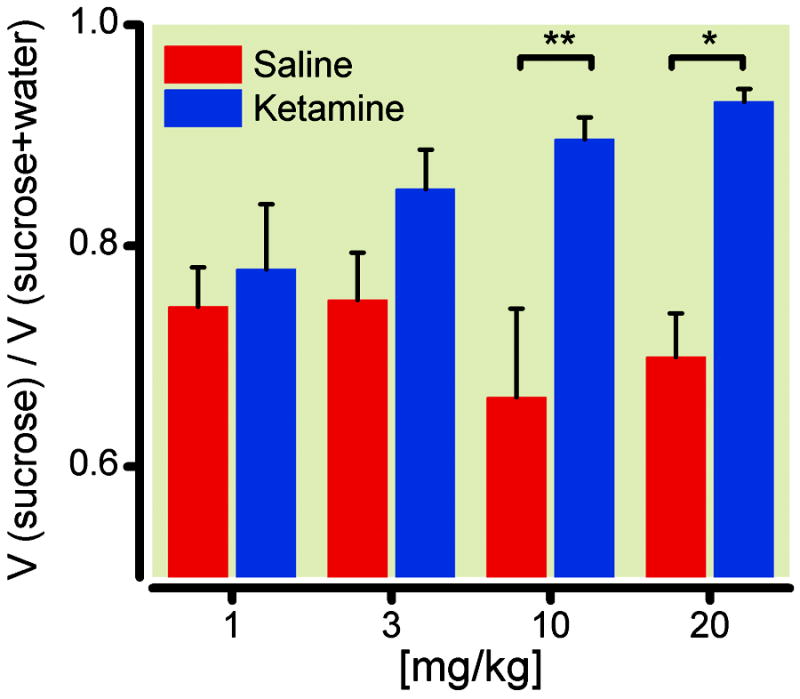
Dose response relationship for the antidepressant effects of ketamine. A series of escalating doses of ketamine (1 mg/kg, 3 mg/kg, 10 mg/kg, and 20 mg/kg) were applied to rats 14 days after spared nerve injury (SNI) and are compared with saline control. Sucrose preference test was performed one day after drug administration. Statistical significance was found at 10 and 20 mg/kg of ketamine. ** p<0.01 for 10 mg/kg, and * p<0.05 for 20 mg/kg, two-way ANOVA with post-hoc Bonferroni tests. 1 mg/kg group: n= 4; 3 mg/kg: n=10; 10 mg/kg: n=10; 20 mg/kg: n=6.
Discussion
Depression and pain are important comorbidities.1-4 In the current study, we used a rodent neuropathic pain (SNI) model to test if pain causes depression-like behaviors. Our study had three key findings. First, both SNI and sham surgeries rapidly induced depression-like behaviors. Second, depression induced by SNI was chronic and concurrent with sensory hypersensitivity. Third, a single subanesthetic dose of ketamine provided quick and enduring treatment for neuropathy-induced depression without affecting sensory hypersensitivity.
Using behavioral despair (in FST) and anhedonia (in SPT) as indices for depression and mechanical and cold hypersensitivity as indices for pain, we found that both depression-like behaviors and pain quickly developed after SNI surgery and persisted up to two months. Surprisingly, sham-operated rats also developed reversible post-operative depression. While postoperative depression has been described clinically,5-6,45-46 the underlying mechanisms remain unknown. One possible cause is pain. In our model, it was difficult to test incisional pain after sham surgery using sensory tests, but the time course of depression that we observed (<7 days after surgery) correlated with the time course of postoperative pain in other models.47-49 Other possible causes of depression include surgical stress and inflammation, but these were unlikely causes in our experiments as weight gain and stress hormone levels were unchanged among the test groups. In the future, it will be instructive to test depression in a post-operative pain model such as the paw incision model. At the same time, our results provided an explanation for studies by Kontinen et al.21 and Suzuki et al.,22 where affective changes such as anxiety and depression were not detected until 14 days after the spinal nerve ligation surgery. Thus, in these studies, the control group – sham-operated rats – may have also experienced affective changes in the initial postoperative period, and the difference in FST performance between the test group (spinal nerve ligation) and the control group (sham) may have been obscured as a result.
In rodent studies, pain has traditionally been tested by sensory hypersensitivity. Pain, however, also has cognitive and affective components. Previous studies on the depression-like response to pain have focused on the FST,21-22 but the FST requires some motor competence. We observed that rats in the SNI group used their non-injured legs more often than the injured legs during the FST. This deviation did not affect our conclusions because 1) we measured time of immobility, which did not require motor coordination; and 2) ketamine did not treat neuropathy but reduced immobility associated with the SNI surgery. Increased immobility in the FST could be caused by spontaneous pain, which may make rats less likely to move, independent of depression. We cannot rule out this possibility, but we think this interpretation is unlikely. First, in order for spontaneous pain to increase the time of immobility in the FST, all SNI treated rats would have had to experience spontaneous pain within the five minute interval of the FST (fig 2). Second, ketamine at high doses (50 mg/kg) did not provide relief for evoked pain (fig 5), suggesting that at lower doses (10 mg/kg) it may not provide relief for spontaneous pain either. In contrast, ketamine (10 mg/kg) improved immobility in the FST (fig. 4). Therefore, we believe the increased immobility observed in the FST was unlikely caused by spontaneous pain, but rather, reflected depression-like behaviors. To further confirm this observation, we used SPT, another commonly used test for depression in rodents. Compared with FST, SPT has minimal requirements for motor competence and findings are less likely confounded by motor deficits or spontaneous pain. Combining the SPT with the FST, we are the first group to show that depressive behaviors develop rapidly after surgery and persist in a chronic fashion with neuropathic pain. We argue that depressive behaviors are a key feature of pain in rodents, and hence SPT and FST should be used routinely in pain assessments.
The molecular mechanisms for how pain causes depression are not well studied. However, pain has been shown to cause altered synaptic connectivity at the prefrontal cortex50 and hippocampus,51 as well as altered dopamine signaling from the ventral tegmental area.52-53 These biochemical changes have been known to trigger negative symptoms of depression and may form the link between pain and depression.20,54-60
An alternative explanation for our data is that surgeries induced depression and pain independently. Neuropathy from SNI may create chronic stress that causes depression. However, this is unlikely in our study. First, we did not observe any weight differences – a good measure for general well being – among SNI-, sham-treated and naïve rats. Secondly, we did not detect differences in levels of the stress hormone corticosterone among the three groups. A number of studies have examined stress levels in neuropathic pain models and found no association between pain and chronic stress.13,22 Another possible cause of depression from SNI is neuroinflammation. However, in the sham surgery, nerves were not dissected and we would not expect any neuroinflammation, but sham surgery still caused reversible depression. In our study of neuropathic pain, it was difficult to test the hypothesis that relieving pain would treat depression, because drugs used to treat neuropathic pain (opioids, antidepressants or anticonvulsants) can alter performances in the FST and the SPT independently. In the future, it will be interesting to test depression using a reversible pain model, such as the acute postoperative pain or the inflammatory pain model.
Ketamine treats both depression and pain. Clinically, ketamine has not consistently demonstrated long-lasting analgesic effects at low doses. Rodent studies have shown >25mg/kg is necessary for anti-nociception.40-42 The duration of anti-nociception is also variable, but most often limited to within 24 hrs.27,41 It is possible that ketamine relieved spontaneous pain at low doses, but higher doses are required to treat evoked pain (tested by mechanical and cold hypersensitivities). However, even at higher doses of ketamine (20 and 50 mg/kg), there was no relief of evoked pain 24 hr after administration (fig. 5). Thus, ketamine did not likely provide long-lasting analgesia for evoked or spontaneous pain at subanesthetic doses. In contrast, both clinical and animal studies have shown that ketamine provides antidepressant effects that last from days to weeks.32-34 At 10mg/kg ketamine is known to treat depression38 without causing psychomotor side-effects.27,41 We observed statistically significant relief of depression beginning one day after ketamine administration and lasting 5 days. Therefore, at this dose ketamine appeared to illustrate a distinction between the sensory and affective responses to pain. This result has three-fold significance. First, it further validates the use of FST and SPT in pain studies. A concern with higher order behavioral tests is that their results may be confounded by spontaneous or movement-associated pain. Ketamine reduced immobility without changing hypersensitivity, implying that immobility in our study was not likely caused by the sensation of painful movement. Rather, it more likely reflected behavioral despair, an index for depression. The same is true for the sucrose preference test. The second significance of this finding is that the effective dosage (10mg/kg) and time course (at least 5 days) of ketamine therapy in our model was highly compatible with other models of depression.32,38,61 This suggests that the underlying mechanism of ketamine’s antidepressant function may be conserved among different models of depression. Whereas analgesic properties of ketamine are mediated at spinal and peripheral level,26-28 its antidepressant effects have been shown to be mediated at cortical and limbic areas.37-38 Our result argues that ketamine likely ameliorates pain-induced depression at the supraspinal level. Thirdly, our result shows that we can selectively target the depressive symptoms of pain without altering the sensory component. This concept has high clinical relevance, especially when the sensory component of pain is difficult to treat such as the case with complex regional pain syndrome or phantom limb pain. In clinical studies, ketamine has been shown to improve mood in patients with phantom limb pain long after its analgesia effects have worn off.62 Mood improvements are critical for physical rehabilitation during the postoperative period and for functional recovery among chronic pain patients.46,63-65 Ketamine may have an important role in this area of pain treatment, given its quick onset of action and enduring activity. Our preclinical data, therefore, advocates further investigation into the clinical use of ketamine as an effective and specific antidepressant in pain patients.
In conclusion, we found that pain rapidly and stably induces depression, but pain-induced depression can be treated by ketamine at low doses without treating the sensory input. These data argue that standard tests of depression such as the SPT and the FST should be applied to the battery of pain tests in rodent studies. They also suggest that the affective component of pain can the selectively targeted successfully. Clinically, our results encourage careful evaluation and aggressive treatment of depression in both acute and chronic pain patients; they also show that low-dose ketamine may have a unique role in treating the depressive symptoms of neuropathic pain.
Summary Statement.
What we already know about this topic
Ketamine reduces depressive symptoms in patients in small reports, but whether this effect is independent of pain relief in patients with chronic pain has not been addressed
What this article tells us that is new
In rats with neuropathic hypersensitivity, low dose ketamine reduced behavioral measures of depression without altering hypersensitivity
Acknowledgments
Supported by Foundations for Anesthesia Research and Education (FAER) grant 67750-15-C0001-01387, Rochester, MN (to J.W.) and National Institute of Health grant MH067229, Bethesda, MD (to E.B.Z.)
Contributor Information
Jing Wang, Department of Anesthesiology, New York University School of Medicine, New York, NY.
Yossef Goffer, Department of Anesthesiology, New York University School of Medicine, New York, NY.
Duo Xu, Department of Anesthesiology, New York University School of Medicine, New York, NY.
David S. Tukey, Department of Biochemistry, New York University School of Medicine, New York, NY.
D. B. Shamir, Sackler Graduate Program, New York University School of Medicine, New York, NY.
Sarah E. Eberle, Department of Anesthesiology, New York University School of Medicine, New York, NY.
Anthony H. Zou, Columbia College, New York, NY.
Thomas J.J. Blanck, Dorothy Reaves Spatz, M.D. Professor and Chair, Department of Anesthesiology, Professor of Physiology and Neurosciences, New York University School of Medicine, New York, NY
Edward B. Ziff, Department of Biochemistry, New York University School of Medicine, New York, NY.
References
- 1.Dworkin RH, Gitlin MJ. Clinical aspects of depression in chronic pain patients. Clin J Pain. 1991;7:79–94. doi: 10.1097/00002508-199106000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Miller LR, Cano A. Comorbid chronic pain and depression: Who is at risk? J Pain. 2009;10:619–27. doi: 10.1016/j.jpain.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Ohayon MM, Schatzberg AF. Using chronic pain to predict depressive morbidity in the general population. Arch Gen Psychiatry. 2003;60:39–47. doi: 10.1001/archpsyc.60.1.39. [DOI] [PubMed] [Google Scholar]
- 4.Romano JM, Turner JA. Chronic pain and depression: Does the evidence support a relationship? Psychol Bull. 1985;97:18–34. [PubMed] [Google Scholar]
- 5.Scott CE, Howie CR, MacDonald D, Biant LC. Predicting dissatisfaction following total knee replacement: A prospective study of 1217 patients. J Bone Joint Surg Br. 2010;92:1253–8. doi: 10.1302/0301-620X.92B9.24394. [DOI] [PubMed] [Google Scholar]
- 6.Edwards RR, Haythornthwaite JA, Smith MT, Klick B, Katz JN. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain Res Manag. 2009;14:307–11. doi: 10.1155/2009/273783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baune BT, Miller R, McAfoose J, Johnson M, Quirk F, Mitchell D. The role of cognitive impairment in general functioning in major depression. Psychiatry Res. 2010;176:183–9. doi: 10.1016/j.psychres.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Chronic pain-associated depression: Antecedent or consequence of chronic pain? A review. Clin J Pain. 1997;13:116–37. doi: 10.1097/00002508-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Dickens C, McGowan L, Dale S. Impact of depression on experimental pain perception: A systematic review of the literature with meta-analysis. Psychosom Med. 2003;65:369–75. doi: 10.1097/01.psy.0000041622.69462.06. [DOI] [PubMed] [Google Scholar]
- 10.Lautenbacher S, Spernal J, Schreiber W, Krieg JC. Relationship between clinical pain complaints and pain sensitivity in patients with depression and panic disorder. Psychosom Med. 1999;61:822–7. doi: 10.1097/00006842-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Schwier C, Kliem A, Boettger MK, Bar KJ. Increased cold-pain thresholds in major depression. J Pain. 2010;11:287–90. doi: 10.1016/j.jpain.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Loggia ML, Mogil JS, Bushnell MC. Experimentally induced mood changes preferentially affect pain unpleasantness. J Pain. 2008;9:784–91. doi: 10.1016/j.jpain.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Shi M, Wang JY, Luo F. Depression shows divergent effects on evoked and spontaneous pain behaviors in rats. J Pain. 2010;11:219–29. doi: 10.1016/j.jpain.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ang DC, Chakr R, France CR, Mazzuca SA, Stump TE, Hilligoss J, Lengerich A. Association of Nociceptive Responsivity With Clinical Pain and the Moderating Effect of Depression. J Pain. 2010;12:384–9. doi: 10.1016/j.jpain.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Hao JX, Shi TJ, Xu IS, Kaupilla T, Xu XJ, Hokfelt T, Bartfai T, Wiesenfeld-Hallin Z. Intrathecal galanin alleviates allodynia-like behaviour in rats after partial peripheral nerve injury. Eur J Neurosci. 1999;11:427–32. doi: 10.1046/j.1460-9568.1999.00447.x. [DOI] [PubMed] [Google Scholar]
- 16.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 17.Leite-Almeida H, Almeida-Torres L, Mesquita AR, Pertovaara A, Sousa N, Cerqueira JJ, Almeida A. The impact of age on emotional and cognitive behaviours triggered by experimental neuropathy in rats. Pain. 2009;144:57–65. doi: 10.1016/j.pain.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 18.Okun A, DeFelice M, Eyde N, Ren J, Mercado R, King T, Porreca F. Transient inflammation-induced ongoing pain is driven by TRPV1 sensitive afferents. Mol Pain. 2011;7:4. doi: 10.1186/1744-8069-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasnie FS, Breuer J, Parker S, Wallace V, Blackbeard J, Lever I, Kinchington PR, Dickenson AH, Pheby T, Rice AS. Further characterization of a rat model of varicella zoster virus-associated pain: Relationship between mechanical hypersensitivity and anxiety-related behavior, and the influence of analgesic drugs. Neuroscience. 2007;144:1495–508. doi: 10.1016/j.neuroscience.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–9. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kontinen VK, Kauppila T, Paananen S, Pertovaara A, Kalso E. Behavioural measures of depression and anxiety in rats with spinal nerve ligation-induced neuropathy. Pain. 1999;80:341–6. doi: 10.1016/s0304-3959(98)00230-9. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki T, Amata M, Sakaue G, Nishimura S, Inoue T, Shibata M, Mashimo T. Experimental neuropathy in mice is associated with delayed behavioral changes related to anxiety and depression. Anesth Analg. 2007;104:1570–7. doi: 10.1213/01.ane.0000261514.19946.66. [DOI] [PubMed] [Google Scholar]
- 23.Garcia LS, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:140–4. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 24.Correll GE, Maleki J, Gracely EJ, Muir JJ, Harbut RE. Subanesthetic ketamine infusion therapy: A retrospective analysis of a novel therapeutic approach to complex regional pain syndrome. Pain Med. 2004;5:263–75. doi: 10.1111/j.1526-4637.2004.04043.x. [DOI] [PubMed] [Google Scholar]
- 25.Jorum E, Warncke T, Stubhaug A. Cold allodynia and hyperalgesia in neuropathic pain: The effect of N-methyl-D-aspartate (NMDA) receptor antagonist ketamine--a double-blind, cross-over comparison with alfentanil and placebo. Pain. 2003;101:229–35. doi: 10.1016/S0304-3959(02)00122-7. [DOI] [PubMed] [Google Scholar]
- 26.Sawynok J, Reid A. Modulation of formalin-induced behaviors and edema by local and systemic administration of dextromethorphan, memantine and ketamine. Eur J Pharmacol. 2002;450:153–62. doi: 10.1016/s0014-2999(02)02119-2. [DOI] [PubMed] [Google Scholar]
- 27.Koizuka S, Obata H, Sasaki M, Saito S, Goto F. Systemic ketamine inhibits hypersensitivity after surgery via descending inhibitory pathways in rats. Can J Anaesth. 2005;52:498–505. doi: 10.1007/BF03016530. [DOI] [PubMed] [Google Scholar]
- 28.Oatway M, Reid A, Sawynok J. Peripheral antihyperalgesic and analgesic actions of ketamine and amitriptyline in a model of mild thermal injury in the rat. Anesth Analg. 2003;97:168–73. doi: 10.1213/01.ane.0000067406.52093.bf. [DOI] [PubMed] [Google Scholar]
- 29.Cohen ML, Chan SL, Way WL, Trevor AJ. Distribution in the brain and metabolism of ketamine in the rat after intravenous administration. Anesthesiology. 1973;39:370–6. doi: 10.1097/00000542-197310000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Christoph T, Schiene K, Englberger W, Parsons CG, Chizh BA. The antiallodynic effect of NMDA antagonists in neuropathic pain outlasts the duration of the in vivo NMDA antagonism. Neuropharmacology. 2006;51:12–7. doi: 10.1016/j.neuropharm.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Max MB, Byas-Smith MG, Gracely RH, Bennett GJ. Intravenous infusion of the NMDA antagonist, ketamine, in chronic posttraumatic pain with allodynia: A double-blind comparison to alfentanil and placebo. Clin Neuropharmacol. 1995;18:360–8. doi: 10.1097/00002826-199508000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA., Jr Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther. 2009;123:143–50. doi: 10.1016/j.pharmthera.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 34.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 35.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA., Jr A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, Carver F, Holroyd T, DiazGranados N, Machado-Vieira R, Grillon C, Drevets WC, Zarate CA., Jr Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35:1415–22. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–7. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ricci V, Martinotti G, Gelfo F, Tonioni F, Caltagirone C, Bria P, Angelucci F. Chronic ketamine use increases serum levels of brain-derived neurotrophic factor. Psychopharmacology (Berl) 2010;215:143–8. doi: 10.1007/s00213-010-2121-3. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki R, Dickenson AH. Differential pharmacological modulation of the spontaneous stimulus-independent activity in the rat spinal cord following peripheral nerve injury. Exp Neurol. 2006;198:72–80. doi: 10.1016/j.expneurol.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 41.Qian J, Brown SD, Carlton SM. Systemic ketamine attenuates nociceptive behaviors in a rat model of peripheral neuropathy. Brain Res. 1996;715:51–62. doi: 10.1016/0006-8993(95)01452-7. [DOI] [PubMed] [Google Scholar]
- 42.De Vry J, Kuhl E, Franken-Kunkel P, Eckel G. Pharmacological characterization of the chronic constriction injury model of neuropathic pain. Eur J Pharmacol. 2004;491:137–48. doi: 10.1016/j.ejphar.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 43.Decosterd I, Woolf CJ. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–58. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 44.Bourquin AF, Suveges M, Pertin M, Gilliard N, Sardy S, Davison AC, Spahn DR, Decosterd I. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain. 2006;122:14 e1–14. doi: 10.1016/j.pain.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 45.Zieger M, Schwarz R, Konig HH, Harter M, Riedel-Heller SG. Depression and anxiety in patients undergoing herniated disc surgery: Relevant but underresearched - a systematic review. Cen Eur Neurosurg. 2010;71:26–34. doi: 10.1055/s-0029-1225325. [DOI] [PubMed] [Google Scholar]
- 46.Kendel F, Gelbrich G, Wirtz M, Lehmkuhl E, Knoll N, Hetzer R, Regitz-Zagrosek V. Predictive relationship between depression and physical functioning after coronary surgery. Arch Intern Med. 2010;170:1717–21. doi: 10.1001/archinternmed.2010.368. [DOI] [PubMed] [Google Scholar]
- 47.Beitz AJ, Newman A, Shepard M, Ruggles T, Eikmeier L. A new rodent model of hind limb penetrating wound injury characterized by continuous primary and secondary hyperalgesia. J Pain. 2004;5:26–37. doi: 10.1016/j.jpain.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 49.Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: A model for postoperative pain. Anesthesiology. 2004;101:191–203. doi: 10.1097/00000542-200407000-00030. [DOI] [PubMed] [Google Scholar]
- 50.Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: Abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–81. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kodama D, Ono H, Tanabe M. Altered hippocampal long-term potentiation after peripheral nerve injury in mice. Eur J Pharmacol. 2007;574:127–32. doi: 10.1016/j.ejphar.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 52.Wood PB. Mesolimbic dopaminergic mechanisms and pain control. Pain. 2006;120:230–4. doi: 10.1016/j.pain.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 53.Saade NE, Atweh SF, Bahuth NB, Jabbur SJ. Augmentation of nociceptive reflexes and chronic deafferentation pain by chemical lesions of either dopaminergic terminals or midbrain dopaminergic neurons. Brain Res. 1997;751:1–12. doi: 10.1016/s0006-8993(96)01164-x. [DOI] [PubMed] [Google Scholar]
- 54.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 55.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–98. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 57.Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, Staib LH, Charney DS. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–9. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 58.Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev. 2009;61:105–23. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009;30:563–9. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–13. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elizalde N, Pastor PM, Garcia-Garcia AL, Serres F, Venzala E, Huarte J, Ramirez MJ, Del Rio J, Sharp T, Tordera RM. Regulation of markers of synaptic function in mouse models of depression: Chronic mild stress and decreased expression of VGLUT1. J Neurochem. 2010;114:1302–14. doi: 10.1111/j.1471-4159.2010.06854.x. [DOI] [PubMed] [Google Scholar]
- 62.Wilson JA, Nimmo AF, Fleetwood-Walker SM, Colvin LA. A randomised double blind trial of the effect of pre-emptive epidural ketamine on persistent pain after lower limb amputation. Pain. 2008;135:108–18. doi: 10.1016/j.pain.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 63.Bremander AB, Holmstrom G, Bergman S. Depression and age as predictors of patient-reported outcome in a multidisciplinary rehabilitation programme for chronic musculoskeletal pain. Musculoskeletal Care. 2011;9:41–8. doi: 10.1002/msc.198. [DOI] [PubMed] [Google Scholar]
- 64.Edmond SL, Werneke MW, Hart DL. Association between centralization, depression, somatization, and disability among patients with nonspecific low back pain. J Orthop Sports Phys Ther. 2010;40:801–10. doi: 10.2519/jospt.2010.3334. [DOI] [PubMed] [Google Scholar]
- 65.Brander V, Gondek S, Martin E, Stulberg SD. Pain and depression influence outcome 5 years after knee replacement surgery. Clin Orthop Relat Res. 2007;464:21–6. doi: 10.1097/BLO.0b013e318126c032. [DOI] [PubMed] [Google Scholar]


