Abstract
Newly developed photoswitchable fluorescent proteins exhibit hundreds to thousands of switching cycles and facilitate live-cell super-resolution imaging at low light intensity.
Recent years have witnessed rapid development in super-resolution fluorescence microscopy techniques that peer beneath the ~200 nm resolution barrier imposed by the diffraction of light. Unlike conventional fluorescence imaging, where bright, unwavering probes are typically desired, many super-resolution techniques rely upon the ability to switch fluorophores between “bright”, emissive states and “dark”, nonemissive states. In this issue of Nature Biotechnology and a concurrent issue of Nature, Stefan Hell, Stefan Jacobs and coworkers report two engineered photoswitchable fluorescent protein variants which surpass existing genetically encoded probes in that they can robustly switch between bright and dark states hundreds to thousands of times (Fig. 1a)1,2. These probes substantially improve live-cell super-resolution imaging capabilities and enable high-density data storage.
Figure 1.
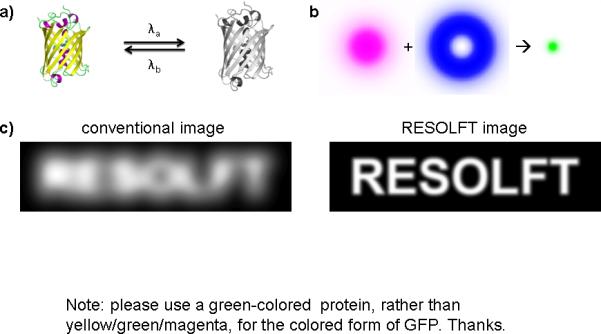
Fatigue-resistant photoswitchable fluorescent proteins enable RESOLFT imaging of living cells. (a) A reversibly photoswitchable fluorescent protein can be converted between bright and dark forms by illumination at two different wavelengths. In the case of Dreiklang, 365 nm and 405 nm light switches the fluorophore on and off, respectively, while 515 nm light excites fluorescence from the bright form. rsEGFP is switched on and off by 405 nm and 491 nm light, respectively, while 491 nm also excites fluorescence from the bright form. (b) RESOLFT imaging with switchable fluorophores. Fluorophores in the region of interest are first switched on by a diffraction-limited Gaussian beam (magenta). A donut-shaped beam (blue) is then used to switch off fluorophores at the periphery of the spot, leaving on only those molecules in a very small area at the center of the donut beam, where the light intensity is zero. Subsequently, another Gaussian beam excites fluorescence from the remaining on-state fluorophores (green). Scanning the three beams across the sample generates a super-resolution RESOLFT image. (c) Comparison of a simulated diffraction-limited image (left) and corresponding RESOLFT image (right).
The reason that photoswitchable probes greatly facilitate sub-diffraction-limit imaging lies in the principle underlying far-field super-resolution imaging techniques, namely the ability to distinguish probes within a diffraction-limited volume by turning them on at different times. In particular, stimulated emission depletion (STED) microscopy3 uses a donut-shaped beam to trigger a stimulated emission process by interacting with the excited state molecules and consequently turning off fluorescence in the beam-covered region. As a result, only molecules in the small area at the center of the STED beam remain fluorescent, and scanning this small area across the sample then produces a super-resolution image (Fig. 1b, c). Because of the short excited-state lifetimes of most fluorophores, high intensity STED beams are required to ensure that STED beam photons interact with excited state fluorophores. On the other hand, if the fluorophores can be switched off from a long-lived state, the power required for the donut-shaped off-switching beam would be dramatically reduced. This is the idea behind RESOLFT (reversible saturable optical fluorescence transition) microscopy4, which uses switching between metastable fluorescent and dark states of fluorophores, instead of stimulated emission, to accomplish sub-diffraction-limit imaging (Fig. 1b, c).
A key requirement of probes for RESOLFT is that they can be robustly switched back and forth between the fluorescent and dark states many times. Roughly speaking, to enable a 10-fold improvement over the diffraction-limited resolution requires the probes to switch at least 100 times for two-dimensional (2D) imaging and 1000 times for 3D imaging. Until now, the lack of such “fatigue-resistant” switchable probes has prevented RESOLFT imaging from being used for cellular imaging. This impasse is now broken by Brakemann et al. and Grotjohann et al1,2.
Brakemann et al. introduce a new yellow fluorescent protein (YFP) variant named Dreiklang (the German word for a musical three-note chord) which operates by a novel photoswitching mechanism. Like YFP, Dreiklang emits fluorescence at 530 nm upon illumination with 515 nm light. However, Dreiklang can be switched between a nonfluorescent and a fluorescent form by illumination with 405 nm and 365 nm light, respectively (Fig. 1a). Through a series of X-ray crystallography and mass spectrometry measurements, Brakemann et al. show that the photochemical transformations occur as a result of a reversible hydration reaction of the tripeptide chromophore at the center of the fluorescent protein, mediated by key side-chain interactions that stabilize a water molecule close to the hydration site. The optically controlled hydration-dehydration cycle can be repeated >160 times.
Grotjohann et al. introduce another new fluorescent protein, named reversibly switchable enhanced green fluorescent protein, or rsEGFP. The protein uses a photoswitching scheme similar to that of previously known reversibly switchable fluorescent proteins5,6, where illumination with blue light both excites fluorescence and converts the protein to a nonfluorescent state, but illumination with near ultraviolet light returns the fluorophore to the fluorescent form. However, unlike any previous fluorescent proteins, rsEGFP is extremely fatigue-resistant and, remarkably, is capable of >1,000 switching cycles.
Both Dreiklang and rsEGFP enabled live-cell RESOLFT imaging with a low light intensity of ~1 kW/cm2, nearly one million times lower that the typical light intensity used in STED. In particular, using rsEGFP, Grotjohann et al. demonstrate RESOLFT imaging of various living samples at ~40 nm resolution, including bacteria, mammalian cells, and organotypic tissue slices. Time-resolved RESOLFT measurements of living hippocampal tissue revealed fine structural rearrangements of dendritic spines. The impressive capability of RESOLFT to monitor dynamics in living samples at ultra-high resolution and with minimal photo-toxicity will dramatically enhance our ability to investigate the inner workings of the cell.
It is not difficult to imagine that, by replacing the donut-shaped beam with other illumination patterns, such as the sinusoidal stripes as used in structured illumination microscopy, these fatigue-resistant switchable fluorescent proteins should allow parallel detection schemes for super-resolution imaging with patterned illumination4,7, which could help increase imaging speed.
Moreover, the remarkable photoswitching properties of these new probes should also greatly benefit another type of sub-diffraction-limit imaging approach which is based on single-molecule localization rather than patterned illumination. In this approach, typically referred to as STORM8, PALM9 or FPALM10, only a sparse, optically resolvable subset of probes is switched on at any time to allow precise localization of these probes by finding the centroid positions of their images. Iteration of this process allows numerous molecules to be localized, and consequently a sub-diffraction-limit image to be constructed from the molecular coordinates. Because each localization is subject to significant random noise, the use of probes that can be switched on many times can substantially reduce this random error by allowing the same structure to be sampled many times to average out some of the noise. Indeed, images taken with probes that switch more cycles are often smoother and less jagged. Perhaps even more importantly, the ability to sample the same structure many times will allow many snapshots to be taken for a time-dependent target, greatly facilitating imaging of dynamic processes. We thus also anticipate that probes with so many switching cycles, like rsEGFP and Dreiklang, could potentially make substantial improvements to live-cell imaging with the STORM type of apprach11. For this type of super-resolution imaging, another essential property of the probe is the number of photons emitted per switching cycle, with more photons giving better resolution. The powerful mutagenesis and imaging-based selection procedures used in these two papers, which allowed a large number of mutants (30,000-70,000) to be efficiently screened, show great potential for improving this aspect of fluorescent proteins as well.
These novel fluorescent proteins are bound to have broad applications. In addition to super-resolution imaging, rsEGFP and Dreiklang can also be used as “optical highlighers”6 or for data storage, as demonstrated by Brakemann et al. and Grotjohann et al.
Moving forward, it will be of particular interest to extend these superb multi-cycle photoswitches to fluorescent proteins in other spectral ranges, such as red fluorescent proteins, for multi-color imaging. The insights gained from Dreiklang and rsEGFP may even be applicable to recently developed RNA aptamers which bind chromophores similar to that found in fluorescent proteins12, potentially enabling super-resolution imaging of nucleic acids in living cells. As the technology of super-resolution imaging continues to evolve, new and improved fluorescent probes will play major roles both in improving the imaging capabilities and in bringing the technology to the point of routine use by nonspecialist laboratories, thereby allowing a wide range of biological problems to be solved. The work by Hell, Jacobs and coworkers sets two excellent examples.
References
- 1.Brakeman T, et al. Nat. Biotech. 2011 [Google Scholar]
- 2.Grotjohann T, et al. Nature. 2011 [Google Scholar]
- 3.Klar TA, Hell SW. Opt. Lett. 1999;24:954–956. doi: 10.1364/ol.24.000954. [DOI] [PubMed] [Google Scholar]
- 4.Hell SW. Nat. Biotechnol. 2003;21:1347–1355. doi: 10.1038/nbt895. [DOI] [PubMed] [Google Scholar]
- 5.Dickson RM, Cubitt AB, Tsien RY, Moerner WE. Nature. 1997;388:355–358. doi: 10.1038/41048. [DOI] [PubMed] [Google Scholar]
- 6.Lippincott-Schwartz J, Patterson GH. Trends Cell. Biol. 2009;19:555–565. doi: 10.1016/j.tcb.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heintzmann R, Gustafsson MGL. Nat. Photonics. 2009;3:362–364. [Google Scholar]
- 8.Rust MJ, Bates M, Zhuang X. Nat. Methods. 2006;3:793–795. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betzig E, et al. Science. 2006;313:1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 10.Hess ST, Girirajan TPK, Mason MD. Biophys. J. 2006;91:4258–4272. doi: 10.1529/biophysj.106.091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones S, Shim S-H, He J, Zhuang X. Nat. Methods. 2011;8:499–505. doi: 10.1038/nmeth.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paige JS, Wu KY, Jaffrey SR. Science. 2011;333:642–646. doi: 10.1126/science.1207339. [DOI] [PMC free article] [PubMed] [Google Scholar]


