Abstract
Objectives
To determine whether poor lower extremity nerve function is associated with more adverse calf muscle characteristics and greater functional impairment in people with and without peripheral arterial disease (PAD).
Design
Cross-sectional
Setting
Three Chicago-area medical centers
Participants
413 participants with PAD (ankle-brachial index (ABI) <0.90) and 271 participants without PAD.
Measurements
Electrodiagnostic testing of the peroneal nerve was performed. Calf muscle cross-sectional area and percent fat were measured using computed tomography at 66.7% of the distance between the distal and proximal tibia. 6-minute walk performance was measured.
Results
Adjusting for age, sex, race, ABI, leg symptoms, smoking, physical activity, comorbidities, and other covariates, lower peroneal nerve conduction velocity (NCV) was associated with lower calf muscle area (1st quartile: 5571.1 mm2, 4th quartile: 4770.3 mm2, p-value<0.001) and poorer 6-minute walk distance (1st quartile: 989.2 ft, 4th quartile: 1210.8 ft, p-value<0.001) in non-diabetic PAD participants. Lower peroneal NCV was associated with lower calf muscle area (1st quartile: 5166.0 mm2, 4th quartile: 6003.8 mm2, p-value=0.014) and poorer 6-minute walk distance (1st quartile: 866.4 ft, 4th quartile: 1082.5 ft, p-value=0.012) in diabetic PAD participants as well. Among non-PAD participants, lower peroneal NCV was not associated with lower calf muscle area but was associated with poorer 6-minute walk distance in non-diabetic participants only (1st quartile 1317.0 ft, 4th quartile 1570.4 ft; p-trend<0.001).
Conclusion
Lower peroneal nerve function is associated with smaller calf muscle area in individuals with PAD and greater functional impairment in individuals with PAD. Future study is needed to determine whether improving peroneal NCV prevents loss of calf muscle and functional decline in PAD.
Keywords: Claudication, Muscles, Peripheral Nervous System, Peripheral Vascular Disease, Physical functioning
INTRODUCTION
Histopathologic studies show that lower extremity peripheral arterial disease (PAD) is associated with pathologic changes and denervation of calf muscle.1–4 Calf muscle pathologic changes associated with PAD include apoptosis, connective tissue proliferation, and type II muscle fiber atrophy.1, 4 However, associations of lower extremity peripheral nerve function with computed tomography (CT)-measured calf muscle area, calf muscle density, and calf muscle percent fat have not been studied in individuals with PAD. Prior study also demonstrates that severe lower extremity ischemia is associated with impaired peroneal nerve function.5 Lower extremity peripheral nerve dysfunction can directly impair calf muscle innervation. As a consequence, affected individuals may have altered calf muscle area and poorer calf muscle characteristics which may lead to functional limitations.6 However, associations of lower extremity nerve function with functional performance have not been studied previously in individuals with PAD.
We studied associations of lower extremity nerve function with calf muscle characteristics and functional performance in men and women with PAD. We hypothesized that poorer lower extremity nerve function would be associated with more adverse lower extremity muscle characteristics and greater functional impairment, respectively, among participants with PAD. We also hypothesized that associations of poorer nerve function with greater functional impairment may be attenuated after adjustment for muscle characteristics, suggesting that associations of nerve impairment with poor functional performance may be mediated by adverse calf muscle characteristics. To determine whether these associations are similar in people with and without PAD, respectively, we also studied these associations in non-PAD participants. Finally, we tested for statistical interactions in the presence vs. absence of PAD on the association of impaired lower extremity nerve function and poor functional performance. Since diabetes mellitus is known to be associated with peripheral neuropathy independent of PAD, we performed analyses separately for those with and without diabetes.
METHODS
Participant identification
The institutional review boards of Northwestern University and Catholic Health Partners Hospital approved the protocol. Participants gave written informed consent. Participants included 368 persons attending their fourth annual follow-up visit in the Walking and Leg Circulation Study (WALCS) and 402 newly identified individuals. These 770 participants comprised the Walking and Leg Circulation Study II (WALCS II).5, 7–9 In this cohort, 478 participants had PAD and 292 participants did not have PAD.
PAD participants were aged ≥59 years and were identified from among consecutive patients diagnosed with PAD in Chicago-area noninvasive vascular laboratories.5, 9 A small number of PAD participants were identified from among consecutive patients in a general internal medicine practice with a low ankle-brachial index (ABI) at their study visit. Approximately half of non-PAD participants were identified consecutively from patients with normal lower extremity arterial studies in the same vascular laboratories. The remainder was identified from consecutive patients in a general medicine practice at Northwestern. PAD was defined as an ABI <0.90. Absence of PAD was defined as an ABI ≥0.90 and <1.30. Participation rates and exclusion criteria for the WALCS II cohort have been described.5, 9 Patients with dementia were excluded because of their inability to answer questions accurately. Nursing home residents, wheelchair-bound patients, and patients with foot or leg amputations were excluded because they have severely impaired functioning. Non-English–speaking patients were excluded because investigators were not fluent in non-English languages. Patients with recent major surgery were also excluded.
ABI measurement
A hand-held Doppler probe (Nicolet Vascular Pocket Dop II; Nicolet Biomedical Inc, Golden, Colo) was used to obtain systolic pressures in the right and left brachial, dorsalis pedis, and posterior tibial arteries.5, 8–10 Each pressure was measured twice. The ABI was calculated by dividing the mean of the dorsalis pedis and posterior tibial pressures in each leg by the mean of the 4 brachial pressures.5, 8–10 Zero values for the dorsalis pedis and posterior tibial pulses were excluded. Average brachial pressures in the arm with highest pressure were used when 1 brachial pressure was higher than the opposite brachial pressure in both measurement sets and the 2 brachial pressures differed by ≥10 mm Hg in at least 1 measurement set, because subclavian stenosis was possible in these participants.11 The lowest leg ABI was used in analyses.
Peripheral nerve function
Electroneurography is considered the gold standard for measurement of peripheral nerve function and has been previously validated for measuring peripheral nerve function.12–14 Nerve function was measured in both legs in the EMG lab at Northwestern Memorial Hospital. The examiners were blinded to the participants' history, including presence of diabetes or PAD. Peroneal nerve function was measured because it is a measure of motor nerve function and the length of the peroneal nerve increases its susceptibility to arterial obstruction at multiple locations in the lower extremities. Nerve conduction velocity and amplitude were both recorded since they are distinct measures of nerve function. Specifically, nerve conduction velocity reflects the conduction rate of the fastest axon, whereas amplitude is a measure of the number of conducting axons. The testing room was maintained at 25°C or higher. Surface skin temperature was recorded. Peroneal nerve conduction studies
Surface recording electrodes were placed over the extensor digitorum brevis with a belly-tendon fashion. The nerve was stimulated utilizing a constant current stimulator with stimulus duration of 0.2ms over the anterior surface of the ankle, 7cm from the recording electrode, and behind the knee. A ground electrode was positioned between the recording and stimulating electrodes. A mild electrical impulse was applied that progressively increased until the maximum amplitude was obtained.
The time required for action potential onset from the ankle to the recording electrode, distal latency (DL), and from the electrode at the fibular head to the recording electrode, proximal latency (PL), were recorded along with the distance between the 2 pairs of electrodes (distance). The nerve conduction velocity (NCV) was calculated as distance/(PL–DL). Peroneal amplitude was measured from baseline to the negative peak from the distal stimulating electrode site.
Lower extremity functional measures
Six-Minute Walk
Following a standardized protocol15, 16, participants walked up and down a 100-ft hallway for 6 minutes after instructions to cover as much distance as possible.
Repeated Chair Rises
Participants sat in a straight-backed chair with their arms folded across their chests and stood 5 times consecutively as quickly as possible. The time to complete 5 chair rises was measured.
Standing Balance
Participants were asked to hold 3 increasingly difficult standing positions for 10 seconds each: standing with feet together side by side and parallel, standing with feet parallel with the toes of 1 foot adjacent to and touching the heel of the opposite foot, and standing with 1 foot directly in front of the other with both feet in a straight line.17, 18
Four-Meter Walking Velocity
Walking velocity was measured with a 4-m walk performed at “usual” and “fastest” pace. For the usual-paced walk, participants were instructed to walk at their usual pace “as if going down the street to the store.” Each walk was performed twice. The faster walk in each pair was used in analyses.17, 18
Short Physical Performance Battery
The short physical performance battery (SPPB) combines data from the usual-paced 4-m walking velocity, time to rise from a seated position 5 times, and standing balance. Participants received a score of 0 for each task that they are unable to complete. Scores of 1 to 4 were assigned for remaining tasks based on quartiles of performance for >6000 participants in the Established Populations for the Epidemiologic Study of the Elderly.17, 18 Scores were summed to obtain the short physical performance battery score, ranging from 0 to 12.
Calf muscle characteristics
With the use of a computed tomography (CT) scanner (LightSpeed, General Electric Medical Systems, Waukesha, Wis), 2.5-mm cross-sectional images of the calves were obtained at 66.7% of the distance from the distal to the proximal tibia.9 Images were analyzed with the use of BonAlyse (BonAlyse Oy, Jyvaskyla, Finland), a CT imaging processor software that identifies muscle tissue, fat, and bone.9 The muscle outline was traced manually, excluding subcutaneous fat and bone. Intramuscular fat is quantified by summing voxels corresponding to fat within muscle tissue. Cadaver studies demonstrate that these methods provide an estimate of muscle area that is highly correlated with direct anatomic measures.19 Because larger participants require greater muscle mass to support their frame, muscle area was adjusted for the square of individual tibia length.
Other measures
Comorbidities assessed were diabetes mellitus, angina, myocardial infarction, heart failure, cancer, chronic lung disease, spinal stenosis, disc disease, and stroke. Algorithms developed for the Women's Health and Aging Study were used to document comorbidities.20 These algorithms combine data from patient report, physical examination, medical record review, medications, laboratory values, and a primary care physician questionnaire. American College of Rheumatology criteria were used to document presence of knee and hip arthritis.21, 22
Pack-years of cigarette smoking, history of hypertension, number of alcoholic drinks per week, and leg symptoms were determined by patient report. At baseline, participants were asked to report the number of blocks they walked during the previous week with the use of a questionnaire validated previously.23 Height and weight were measured at the study visit. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. History of lower extremity revascularization was determined based on participant report and confirmed by medical record review or the primary care physician questionnaire.
Statistical analysis
Participants were stratified according to presence or absence of diabetes. Adjusting for age and sex, baseline characteristics for participants with and without PAD in both groups were compared using general linear models for continuous variables and chi-squared tests for categorical variables.
Baseline values for peroneal NCV and peroneal amplitude were categorized into quartiles for diabetic and non-diabetic PAD participants separately. The fourth quartile represented the best nerve function quartiles. Participants with no measurable muscle action potential on nerve stimulation were considered to have the poorest lower extremity nerve function and were placed in the first quartile. Analysis of covariance (ANCOVA) was performed for diabetic and non-diabetic PAD participants to measure the association of baseline calf muscle characteristics and functional performance measures across quartiles of nerve function. We also performed t-test pairwise interquartile comparisons to measure the associations for both calf muscle characteristics and functional performance measures using participants in the lowest (1st) quartile of peroneal nerve function as the reference. This set of analyses was also performed to measure the associations of functional performance measures across quartiles of calf muscle characteristics.
The analyses adjusted for age, sex, and race, ABI, leg symptoms, smoking, BMI, comorbidities, alcohol drinking numbers, blocks walked per week, history of revascularization, and study cohort (WALCS vs. WALCS II). Comorbidities assessed were cancer, pulmonary disease, cardiac or cerebrovascular disease (myocardial infarction, heart failure, angina, stroke), knee or hip arthritis, spinal stenosis, and spinal disc disease. Cardiac and cerebrovascular diseases were grouped together as a categorical variable. The presence of one or more of these conditions was entered as `1' and the absence of any of these conditions was entered as `0'. Knee or hip arthritis, spinal stenosis, and spinal disc disease were grouped together similarly. We repeated associations involving peroneal nerve function and functional performance with additional adjustment for calf muscle characteristics (calf muscle area and calf muscle percent fat). Non-PAD participants were similarly stratified based on the presence or absence of diabetes. The aforementioned analyses were repeated for non-PAD participants but without adjustment for ABI, leg symptoms, and history of lower extremity revascularization. We performed a formal test for interaction of the presence versus absence of PAD on the association of both peroneal nerve conduction velocity and peroneal nerve amplitude with each calf muscle characteristic and functional performance measure. These analyses were performed for diabetic and non-diabetic participants separately. Analyses were performed using SAS statistical software (version 9.1, SAS Institute Inc., Cary, North Carolina).
RESULTS
441 of 478 participants with PAD and 271 of 292 participants without PAD in WALCS II underwent nerve function testing and calf muscle imaging. Of these, 413 participants with PAD (86.4% of entire PAD cohort) and 255 participants without PAD (87.3% of entire non-PAD cohort) completed measures of functional performance. Table 1 shows baseline characteristics of study participants according to presence vs. absence of PAD, among participants with and without diabetes. In both diabetic and non-diabetic participants, participants with PAD had lower calf muscle area, a lower prevalence of spinal stenosis, poorer peroneal nerve function, and lower BMI value compared to those without PAD. In both diabetic and non-diabetic participants, PAD participants had a higher proportion of current and former smokers, a higher proportion with exertional leg symptoms, and a higher prevalence of cardiovascular disease (Table 1). Among diabetic participants, PAD participants had a lower prevalence of knee arthritis, a higher prevalence of pulmonary disease, and a higher percentage of participants with no measurable peroneal nerve conduction velocity (Table 1). Among non-diabetic participants, PAD participants were older and included a higher proportion of males and those with a history of hypertension (Table 1).
Table 1.
Baseline characteristics of study participants*
| Diabetes Mellitus | No Diabetes Mellitus | |||||
|---|---|---|---|---|---|---|
| Baseline Characteristics | Peripheral Arterial Disease (n=130) | No Peripheral Arterial Disease (n=59) | p-value | Peripheral Arterial Disease (n=283) | No Peripheral Arterial Disease (n=196) | p-value |
| Age, (years) | 73.1 (7.8) | 71.0 (7.7) | 0.094 | 75.8 (8.1) | 71.7 (7.6) | <0.001 |
| Male, (%) | 60 | 50.9 | 0.239 | 48.8 | 40.3 | 0.068 |
| Black race, (%) | 23.9 | 33.9 | 0.149 | 13.8 | 15.8 | 0.535 |
| Ankle-brachial index | 0.63 (0.15) | 1.09 (0.10) | <0.001 | 0.64 (0.16) | 1.09 (0.09) | <0.001 |
| Cigarette smoking, (pack-years) | 30.7 (36.1) | 18.9 (25.2) | 0.025 | 38.4 (36.4) | 14.6 (24.7) | <0.001 |
| BMI, (kg/m2) | 30.3 (5.5) | 32.4 (6.7) | 0.022 | 26.6 (4.4) | 28.1 (5.2) | 0.001 |
| Number of alcoholic drinks per week | 2.8 (6.7) | 1.6 (3.1) | 0.171 | 3.8 (6.1) | 3.7 (5.6) | 0.927 |
| Pulmonary disease, (%) | 45.4 | 28.8 | 0.031 | 43.8 | 36.7 | 0.121 |
| Angina, (%) | 42.6 | 32.2 | 0.174 | 31.2 | 16.1 | <0.001 |
| Myocardial infarction, (%) | 35.4 | 13.6 | 0.002 | 22.3 | 16.3 | 0.109 |
| Heart failure, (%) | 40.0 | 23.7 | 0.030 | 23.7 | 11.7 | 0.001 |
| Stroke, (%) | 28.5 | 13.6 | 0.026 | 17.7 | 6.6 | <0.001 |
| Hypertension, (%) | 79.7 | 81.4 | 0.790 | 72.9 | 58.7 | 0.001 |
| Hip arthritis, (%) | 5.4 | 5.1 | 1.000 | 3.2 | 3.6 | 0.815 |
| Knee arthritis, (%) | 13.8 | 27.1 | 0.028 | 11.7 | 16.3 | 0.143 |
| Disk disease, (%) | 41.5 | 28.8 | 0.094 | 41.7 | 36.7 | 0.275 |
| Spinal stenosis, (%) | 13.8 | 49.2 | <0.001 | 16.6 | 40.3 | <0.001 |
| Cancer, (%) | 16.2 | 23.7 | 0.214 | 20.5 | 24.0 | 0.365 |
| Leg symptoms, (%) | ||||||
| Classic symptoms of intermittent claudication | 25.4 | 1.7 | <0.001 | 22.3 | 2.6 | <0.001 |
| No exertional leg pain | 19.2 | 47.5 | <0.001 | 25.4 | 62.2 | <0.001 |
| Any exertional leg pain | 55.4 | 50.8 | 0.637 | 52.3 | 35.2 | <0.001 |
| Peroneal nerve function | ||||||
| Nerve conduction velocity, (meters/second) | 33.7(16.6) | 40.2(12.2) | 0.008 | 40.8(11.9) | 43.9 (9.0) | 0.003 |
| Amplitude, (μV) | 2.0(2.1) | 2.7(2.2) | 0.043 | 3.2 (2.3) | 4.0 (9.0) | 0.014 |
| Lower extremity revascularization, (%) | 36.2 | 0.0 | NA | 31.4 | 0.0 | NA |
| Calf muscle characteristics | ||||||
| Calf muscle area, (mm2) | 5635.4 (1386.4) | 6209 (1650.0) | 0.014 | 5324.8 (1417.6) | 5949.9 (1497.1) | <0.001 |
| Calf muscle fat, (%) | 14.4 (13.6) | 13.8 (13.5) | 0.778 | 9.7 (11.5) | 8.6 (10.8) | 0.320 |
| Percent with no measurable nerve conduction, (%) | ||||||
| Peroneal nerve | 18.0 | 6.9 | 0.047 | 6.8 | 3.1 | 0.076 |
| Sural nerve | 52.0 | 46.6 | 0.494 | 26.4 | 21.9 | 0.259 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared)
Values shown are mean (SD) unless otherwise indicated. Cardiac or cerebrovascular diseases included myocardial infarction, heart failure, angina, and stroke. Arthritis diseases included knee arthritis, hip arthritis, hip fracture, spinal stenosis, and disk disease.
Non-diabetic PAD participants
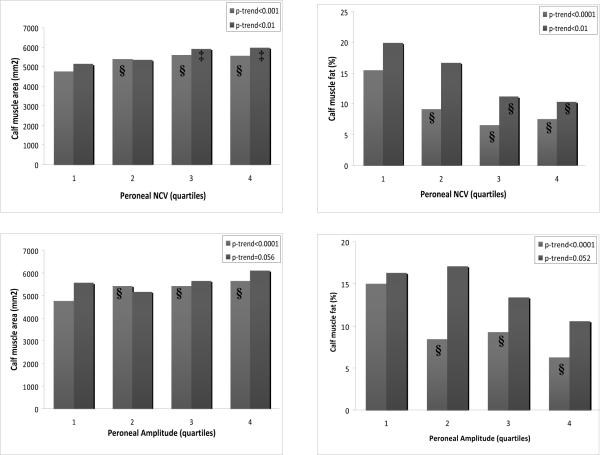
Adjusting for age, sex, race, ABI, smoking, BMI, physical activity, and comorbidities, lower peroneal nerve function was associated with more adverse calf muscle characteristics in non-diabetic PAD participants (Figure 1). Lower peroneal NCV was associated with lower calf muscle area (p-trend<0.001) and higher calf muscle percent fat (p-trend<0.001). Lower peroneal amplitude was associated with lower calf muscle area (p-trend<0.001) and higher calf muscle percent fat (p-trend<0.001).
Figure 1.
Adjusted associations between peroneal nerve function and calf muscle characteristics in diabetic and non-diabetic individuals with PAD (n=441).*
*■Non-diabetic individuals with PAD (n=301) ■Diabetic individuals with PAD (n=140) Analyses were adjusted for age, sex, race, ankle-brachial index, body-mass index, smoking, alcohol consumption, leg symptoms, comorbidities, physical activity, leg revascularization, and the study cohort. ‡P<0.05 for pairwise comparison to reference group. §P<0.01 for pairwise comparison to reference group. NCV=nerve conduction velocity, PAD=peripheral arterial disease
Adjusting for age, sex, race, ABI, smoking, BMI, physical activity, and comorbidities, we found significant associations between lower peroneal nerve function and poorer functional performance in non-diabetic participants with PAD (Table 2). Compared to participants in the 1st quartile of peroneal NCV, non-diabetic PAD participants in the 2nd, 3rd, & 4th quartiles performed better in the six minute walk distance, usual-paced & fast-paced 4-m walking velocity, and SPPB. Compared to participants in the lowest quartile of peroneal amplitude, non-diabetic PAD participants in the 2nd, 3rd, & 4th quartiles of performed better in the six minute walk distance, usual-paced & fast-paced 4-m walking velocity, and SPPB. After additionally adjusting these associations for calf muscle area and calf muscle percent fat, the associations between peroneal nerve function and functional performance were attenuated among non-diabetic PAD participants. Associations between peroneal NCV and both the six minute walk distance (p-trend=0.036) and SPPB (p-trend=0.021) remained statistically significant. In these fully adjusted analyses, lower calf muscle area was associated with greater functional impairment for non-diabetic PAD participants for six minute walk distance (p-trend=0.004), usual-paced 4-m walking velocity (p-trend<0.001), fast-paced 4-m walking velocity (p-trend<0.001), and SPPB (p-trend<0.001). Greater calf muscle percent fat was associated with more impairment in the fast-paced 4-m walking velocity (p-trend=0.015) and the SPPB (p-trend=0.014).
Table 2.
Adjusted associations between peroneal nerve function and functional performance in peripheral arterial disease (n=413).*
| Functional performance measure | ||||
|---|---|---|---|---|
| 6 minute walk distance (feet) | Usual 4m walk velocity (meters/second) | Fast 4m walk velocity (meters/second) | SPPB† (0–12 scale, 12=best) | |
|
| ||||
| PAD persons without diabetes (n=283) | ||||
|
| ||||
| Peroneal nerve conduction velocity | ||||
|
| ||||
| 1st quartile | 989.2 | 0.80 | 1.04 | 8.77 |
| 2nd quartile | 1200.3§ | 0.89§ | 1.19§ | 9.83§ |
| 3rd quartile | 1192.1§ | 0.91§ | 1.23§ | 10.74§ |
| 4th quartile | 1210.8§ | 0.89§ | 1.24§ | 10.38§ |
| P-trend | <0.001 | 0.017 | <0.001 | <0.001 |
| Peroneal amplitude | ||||
| 1st quartile | 1010 | 0.81 | 1.05 | 9.06 |
| 2nd quartile | 1231.5§ | 0.90§ | 1.22§ | 10.18§ |
| 3rd quartile | 1187.2§ | 0.88‡ | 1.19§ | 9.92‡ |
| 4th quartile | 1182.9§ | 0.91§ | 1.24§ | 10.53§ |
| P-trend | 0.171 | 0.028 | 0.003 | 0.011 |
| 1st quartile | 866.4 | 0.74 | 1.00 | 6.77 |
| 2nd quartile | 1092.1§ | 0.82 | 1.11 | 9.29§ |
| 3rd quartile | 1065.8‡ | 0.83 | 1.08 | 8.95§ |
| 4th quartile | 1082.5‡ | 0.83 | 1.13 | 9.29§ |
| P-trend | 0.008 | 0.059 | 0.047 | <0.001 |
| Peroneal amplitude | ||||
| 1st quartile | 881.5 | 0.76 | 1.01 | 7.28 |
| 2nd quartile | 976.5 | 0.78 | 1.05 | 7.96 |
| 3rd quartile | 1066.5‡ | 0.86 | 1.12 | 9.43§ |
| 4th quartile | 1166.5§ | 0.83 | 1.13 | 9.43§ |
| P-trend | <0.001 | 0.103 | 0.053 | 0.005 |
Adjusted for age, sex, race, ankle-brachial index, body mass index, smoking, alcohol consumption, leg symptoms, comorbidities, physical activity, leg revascularization, and the study cohort.
SPPB=short performance physical battery.
P<0.05 for pairwise comparison to reference group.
P<0.01 for pairwise comparison to reference group.
Diabetic PAD participants
Adjusting for age, sex, race, ABI, smoking, BMI, physical activity, and comorbidities, poorer peroneal nerve function was associated with more adverse calf muscle characteristics and greater impairment in lower extremity functional performance among diabetic PAD participants (Figure 1 & Table 2). Lower peroneal NCV was associated with lower calf muscle area (p-trend<0.01) and higher calf muscle percent fat (p-trend<0.01). Compared to participants in the 1st quartile of peroneal NCV, diabetic PAD participants in the 2nd, 3rd, and 4th quartiles performed better in the six minute walk distance and SPPB. Compared to diabetic PAD participants in the 1st quartile of peroneal amplitude, diabetic PAD participants in the 3rd and 4th quartiles performed better in the six minute walk distance and SPPB.
After additionally adjusting these associations for calf muscle characteristics, the associations of impaired peroneal nerve function with poorer functional performance among diabetic PAD participants were attenuated. Associations between poorer peroneal NCV and greater impairment in both the six minute walk distance (p-trend=0.043) and SPPB (p-trend=0.010) remained statistically significant. Similarly, the association for poorer peroneal amplitude and greater impairment in six minute walk distance (p-trend=0.009) remained significant after additional adjustment for calf muscle characteristics. In these fully adjusted analyses, lower calf muscle area was associated with greater functional impairment for diabetic PAD participants for six minute walk distance (p-trend=0.009), usual-paced 4-m walking velocity (p-trend=0.003), fast-paced 4-m walking velocity (p-trend=0.005), and SPPB (p-trend<0.001). Greater calf muscle percent fat was associated with more impairment in the six minute walk distance (p-trend=0.004), usual-paced 4-m walking velocity (p-trend=0.004), fast-paced 4-m walking velocity (p-trend=0.002) and the SPPB (p-trend=0.001).
Diabetic and non-diabetic participants without PAD
Adjusting for age, sex, race, smoking, BMI, physical activity, and comorbidities, lower peroneal NCV was not associated with lower calf muscle area or higher calf muscle percent fat among non-PAD participants. In non-PAD participants without diabetes, lower peroneal nerve amplitude was associated with higher calf muscle percent fat (4th quartile 6.9%, 1st quartile 12.1%; p-trend=0.011). Additionally, lower peroneal amplitude was associated with higher calf muscle percent fat (4th quartile 11.1%, 1st quartile 22.4%; p-trend=0.039) and lower calf muscle area (4th quartile 6542.72 mm2, 1st quartile 5388.2 mm2; p-trend=0.035) in diabetic participants without PAD.
Lower peroneal nerve conduction velocity was associated with greater functional impairment in non-PAD participants without diabetes. Among non-PAD participants without diabetes, lower peroneal nerve conduction velocity was associated with poorer six-minute walk distance (4th quartile 1570.4 ft, 1st quartile 1317.0 ft; p-trend<0.001), slower usual-paced 4m velocity (4th quartile 0.97 m/s, 1st quartile 0.91 m/s; p-trend=0.002), slower fast-paced 4m velocity (4th quartile 1.35 m/s; 1st quartile 1.18 m/s; p-trend<0.001), and a lower SPPB score (4th quartile 10.98, 1st quartile 9.12; p-trend<0.001). No significant associations were observed between lower peroneal nerve amplitude and greater functional impairment for either diabetic or non-diabetic participants without PAD.
Additional adjustment for calf muscle characteristics did not significantly attenuate the associations between lower peroneal nerve conduction velocity and greater functional impairment in non-diabetic participants without PAD. In these fully adjusted analyses, lower calf muscle area was associated with greater functional impairment for non-diabetic participants without PAD for six minute walk distance (p-trend<0.001), usual-paced 4-m walking velocity (p-trend<0.001), fast-paced 4-m walking velocity (p-trend<0.001), and SPPB (p-trend<0.001). Greater calf muscle percent fat was associated with more impairment in the six minute walk distance (p-trend=0.002), usual-paced 4-m walking velocity (p-trend<0.001), fast-paced 4-m walking velocity (p-trend<0.001) and the SPPB (p-trend<0.001).
Among non-diabetic participants, a significant interaction was observed for the presence of PAD in the association between peroneal nerve amplitude and both calf muscle area (p-trend=0.007) and calf muscle percent fat (p-trend=0.003). We also found a significant interaction among non-diabetic participants for the presence of PAD in the association between peroneal nerve conduction velocity and both the six-minute walk (p-trend=0.037) and SPPB (p-trend=0.009). Our results suggest that associations of peroneal nerve function and calf muscle characteristics as well as functional performance are stronger in PAD than in non-PAD participants.
DISCUSSION
Among 413 participants with PAD, lower peroneal nerve function was associated with smaller calf muscle area, higher calf muscle percent fat, and poorer functional performance. These associations were independent of potential confounders, including the ABI. Associations of lower peroneal nerve function with greater functional impairment were attenuated after additional adjustment for calf muscle characteristics. Our findings demonstrate that poorer lower extremity nerve function is associated with greater functional impairment and more adverse calf muscle characteristics in individuals with PAD.
In addition, our results demonstrated a significant interaction for the presence vs. absence of PAD and the association of peroneal NCV with six-minute walk performance and the SPPB among participants without diabetes mellitus. We also identified a significant interaction for the presence vs. absence of PAD and the association of peroneal nerve amplitude with calf muscle area and calf muscle percent fat. Our results demonstrate that among non-diabetic participants, the association of impaired peroneal NCV with poorer six-minute walk and SPPB performance are stronger in participants with PAD than in those without PAD. Similarly, among non-diabetic participants, the association of impaired peroneal amplitude with smaller calf muscle area and greater calf muscle percent fat are stronger in participants with PAD than in those without PAD.
Previous study in individuals without PAD demonstrates that poorer lower extremity nerve function is associated with greater functional impairment.24 However, there are several unique features of the current study. First, to our knowledge, no prior studies have assessed whether poorer lower extremity nerve function is associated with more adverse calf muscle characteristics in people with or without PAD. Second, to our knowledge, no prior studies have assessed associations of the degree of impairment in lower extremity nerve function with the degree of lower extremity functional impairment in people with PAD. Third, to our knowledge, no prior studies have assessed whether an interaction exists between the presence vs. absence of PAD and the association of lower extremity nerve function with the degree of functional impairment. Because previous study demonstrates that people with PAD have more severe lower extremity nerve function, more adverse muscle characteristics, and greater functional impairment than those without PAD, it is conceivable that associations of impaired lower extremity nerve function with calf muscle characteristics and lower extremity functional impairment may differ among those with PAD compared to those without PAD. Furthermore, many clinicians presume that lower extremity arterial obstruction is the primary factor contributing to functional impairment in PAD. Our findings may have implications for development of new therapies to improve functional performance in individuals with PAD.
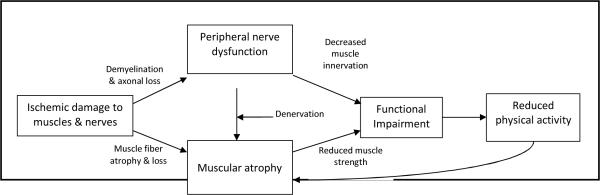
Figure 2 illustrates a proposed causal pathway for how poor lower extremity nerve function might lead to muscular atrophy and functional limitations in PAD. Ischemic damage to lower extremity nerve fibers can cause demyelination and axonal loss, resulting in a loss of nerve function. Poor lower extremity nerve function can reduce motor nerve innervation and cause calf muscle denervation. Impaired lower extremity motor nerve innervation to muscles may directly lead to functional limitations.6 Calf muscle denervation has been associated with gastrocnemius muscle weakness in those with PAD.3 Data reported here demonstrates that poorer peroneal nerve function is associated with more adverse calf muscle characteristics in people with PAD. Impaired leg strength and smaller calf muscle area are associated with impaired lower extremity functional performance and greater functional decline in individuals with PAD.9, 26 Similarly, lower physical activity levels can potentially contribute to muscular atrophy and subsequently functional impairment. Among individuals with PAD, poorer functional performance is associated with lower physical activity levels,27 which in turn is associated with more unfavorable calf muscle characteristics in PAD persons.28
Figure 2.
Development of functional impairment in individuals with peripheral arterial disease
Proposed framework for the associations of PAD with impaired nerve function, adverse calf muscle characteristics, and functional impairment and decline in PAD. Denervation refers to damage to the distal motor nerve axons supplying muscle resulting in pathologic changes in muscle. Decreased muscle innervation refers to the loss of nerve conduction resulting in reduced muscle function.
In the current study, additional adjustment for calf muscle characteristics attenuated the identified associations between lower peroneal nerve function and functional impairment performance measures in both diabetic and non-diabetic participants with PAD. Based on these results, further study is needed to determine whether calf muscle characteristics mediate relationships between impaired peroneal nerve function and greater impairment in functional performance. Associations between lower peroneal nerve function and more adverse calf muscle characteristics were also observed in non-PAD participants. Additional adjustment for calf muscle characteristics, however, did not attenuate relationships between poorer peroneal nerve function and impaired functional performance in non-PAD participants.
Our study has limitations. Since this study was cross-sectional, associations reported here cannot be construed as causal. We did not collect information on fasting glucose. However, since associations were generally stronger in the non-diabetic PAD participatns, any misclassification of PAD participants who were diabetic likely only attenuated our findings. We also did not collect information on glycosylated hemoglobin levels or duration of diabetes, and, consequently, could not adjust for these variables in our analyses involving diabetic participants. We did not collect data on the anatomic location of lower extremity arterial obstruction. Finally, our sample sizes for non-PAD participants may not have been sufficient enough to detect significant associations of poorer lower extremity nerve function with either adverse calf muscle characteristics or functional impairment.
In summary, poor lower extremity nerve function is associated with more adverse calf muscle characteristics and greater functional impairment in participants with PAD, even after adjusting for PAD severity. Future longitudinal studies should focus on establishing the role of calf muscle characteristics in the relationship between lower extremity nerve function and functional performance and determine whether peripheral neuropathy contributes to the causal pathway for functional decline in PAD individuals.
ACKNOWLEDGMENTS
Financial Disclosure: This study was supported by grants R01-HL58099, R01-HL64739, and R01-HL71223, and R01-HL076298 from the National Heart Lung and Blood Institute and by grant RR-00048 from the National Center for Research Resources, National Institutes of Health.
Funding sources: This study was supported by grants R01-HL58099, R01-HL64739, and R01-HL71223, and R01-HL076298 from the National Heart Lung and Blood Institute and by grant RR-00048 from the National Center for Research Resources, National Institutes of Health.
Sponsors' Role: The funding agency played no role in the study design, methods, subject recruitment, data collection, data analyses, or paper preparation.
Footnotes
Author Contributions: Parveen K Garg, Kiang Liu, Luigi Ferrucci, Jack M. Guralnik, Michael H Criqui, Mary McDermott: study conception and design. Kiang Liu, Luigi Ferrucci, Michael H. Criqui, and Mary McDermott: acquisition of subjects and data. Parveen K Garg, Kiang Liu, Luigi Ferrucci, Jack M Guralnik, Michael H Criqui, Lu Tian, Robert Sufit, Takashi Nishida, Huimin Tao, Yihua Liao, Mary McDermott: data analysis and interpretation. Parveen K Garg, Kiang Liu, Luigi Ferrucci, Jack M Guralnik, Michael H Criqui, Lu Tian, Robert Sufit, Takashi Nishida, Huimin Tao, Yihua Liao, Mary McDermott: manuscript preparation.
REFERENCES
- 1.Mitchell RG, Duscha BD, Robbins JL, et al. Increased levels of apoptosis in gastrocnemius skeletal muscle in patients with peripheral arterial disease. Vasc Med. 2007;12:285–290. doi: 10.1177/1358863X07084858. [DOI] [PubMed] [Google Scholar]
- 2.England JD, Regensteiner JG, Ringel SP, et al. Muscle denervation in peripheral arterial disease. Neurology. 1992;42:994–999. doi: 10.1212/wnl.42.5.994. [DOI] [PubMed] [Google Scholar]
- 3.Regensteiner JG, Wolfel EE, Brass EP, et al. Chronic changes in skeletal muscle histology and function in peripheral arterial disease. Circulation. 1993;87:413–421. doi: 10.1161/01.cir.87.2.413. [DOI] [PubMed] [Google Scholar]
- 4.Hedberg B, Angquist KA, Henriksson-Larsen K, et al. Fibre loss and distribution in skeletal muscle from patients with severe peripheral arterial insufficiency. Eur J Vasc Surg. 1989;3:315–322. doi: 10.1016/s0950-821x(89)80067-2. [DOI] [PubMed] [Google Scholar]
- 5.McDermott MM, Sufit R, Nishida T, et al. Lower extremity nerve function in patients with lower extremity ischemia. Arch Intern Med. 2006;166:1986–1992. doi: 10.1001/archinte.166.18.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Resnick HE, Vinik AI, Schwartz AV, et al. Independent effects of peripheral nerve dysfunction on lower-extremity physical function in old age: The Women's Health and Aging Study. Diabetes Care. 2000;23:1642–1647. doi: 10.2337/diacare.23.11.1642. [DOI] [PubMed] [Google Scholar]
- 7.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 8.McDermott MM, Greenland P, Liu K, et al. The ankle brachial index is associated with leg function and physical activity: The Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 9.McDermott MM, Hoff F, Ferrucci L, et al. Lower extremity ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2007;55:400–406. doi: 10.1111/j.1532-5415.2007.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDermott MM, Liu K, Greenland P, et al. Functional decline in peripheral arterial disease: Associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 11.Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–623. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 12.Magladery JW, Mc Dougal DB, Jr., Stoll J. Electrophysiological studies of nerve and reflex activity in normal man. I. Identification of certain reflexes in the electromyogram and the conduction velocity of peripheral nerve fibers. Bull Johns Hopkins Hosp. 1950;86:265–290. [PubMed] [Google Scholar]
- 13.Lambert EH. Diagnostic value of electrical stimulation of motor nerves. Electroencephalogr Clin Neurophysiol. 1962;22(suppl):9–16. [Google Scholar]
- 14.Gilliatt RW, Sears TA. Sensory nerve action potentials in patients with peripheral nerve lesions. J Neurol Neurosurg Psychiatry. 1958;21:109–118. doi: 10.1136/jnnp.21.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc. 1998;46:706–711. doi: 10.1111/j.1532-5415.1998.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: A new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 17.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 2. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 19.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 20.Fried LP, Kasper JD, Williamson JD, et al. Disease ascertainment algorithms. In: Guralnik JM, Fried LP, Simonsick EM, et al., editors. The Women's Health and Aging Study: Health and Social Characteristics of Older Women with Disability. National Institute on Aging; Bethesda: 1995. [Google Scholar]
- 21.Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505–514. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 22.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 23.Simonsick EM, Guralnik JM, Volpato S, et al. Just get out the door! Importance of walking outside the home for maintaining mobility: Findings from the women's health and aging study. J Am Geriatr Soc. 2005;53:198–203. doi: 10.1111/j.1532-5415.2005.53103.x. [DOI] [PubMed] [Google Scholar]
- 24.Strotmeyer ES, de Rekeneire N, Schwartz AV, et al. The relationship of reduced peripheral nerve function and diabetes with physical performance in older white and black adults: The Health, Aging, and Body Composition (Health ABC) study. Diabetes Care. 2008;31:1767–1772. doi: 10.2337/dc08-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDermott MM, Kerwin DR, Liu K, et al. Prevalence and significance of unrecognized lower extremity peripheral arterial disease in general medicine practice*. J Gen Intern Med. 2001;16:384–390. doi: 10.1046/j.1525-1497.2001.016006384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDermott MM, Criqui MH, Greenland P, et al. Leg strength in peripheral arterial disease: Associations with disease severity and lower-extremity performance. J Vasc Surg. 2004;39:523–530. doi: 10.1016/j.jvs.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 27.McDermott MM, Greenland P, Ferrucci L, et al. Lower extremity performance is associated with daily life physical activity in individuals with and without peripheral arterial disease. J Am Geriatr Soc. 2002;50:247–255. doi: 10.1046/j.1532-5415.2002.50055.x. [DOI] [PubMed] [Google Scholar]
- 28.McDermott MM, Guralnik JM, Ferrucci L, et al. Physical activity, walking exercise, and calf skeletal muscle characteristics in patients with peripheral arterial disease. J Vasc Surg. 2007;46:87–93. doi: 10.1016/j.jvs.2007.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]