Abstract
Animals, ranging from basal metazoans to primates, are host to complex microbial ecosystems; engaged in a symbiotic relationship that is essential for host physiology and homeostasis. Epithelial surfaces vary in the composition of colonizing microbiota as one compares anatomic sites, developmental stages and species origin. Alterations of microbial composition likely contribute to susceptibility to several distinct diseases. The forces that shape the colonizing microbial composition are the focus of much current investigation, and it is evident that there are pressures exerted both by the host and the external environment to mold these ecosystems. The focus of this review is to discuss recent studies that demonstrate the critical importance of host factors in selecting for its microbiome. Greater insight into host–microbiome interactions will be essential for understanding homeostasis at mucosal surfaces, and developing useful interventions when homeostasis is disrupted.
Keywords: Microbial colonization, Bacteria, Microflora, Intestine, Mucosa, Immunoglobulin A, Antimicrobial peptide, Defensin, Mucus, Dysbiosis, Homeostasis
Introduction
Mammals, including humans, are colonized at epithelial surfaces by a vast and complex microbial ecosystem. Indeed, we are not alone in our co-evolution and intimate association with microbes, as similar surface colonization is observed in lower vertebrates, invertebrates, and even plants. It is becoming increasingly evident that this colonizing microbiota plays a profound role in host physiology [1–4]. Accordingly, the dynamic interplay between host and microbes is a focus of much current research.
In mammals, the intestinal tract contains the majority of colonizing bacteria, reaching 1012 cfu/g of gut contents in the large bowel [5]. Early studies of the microbiota focused on readily cultivable organisms; however, subsequent molecular analyses of the composition of this ecosystem demonstrated much greater complexity than initially deduced. Molecular analysis based on bacterial 16S rRNA sequences has revealed more than 1,000 different species of bacteria in the human gastrointestinal (GI) tract [6]. Revisiting attempts to culture intestinal microbes indicate that a majority of bacterial taxa present in the intestine are actually cultivable under controlled anaerobic conditions [7]. The dominant bacteria have been identified in the intestine, as well as at several other body sites [8] (Fig. 1). Presumably, composition differences reflect, in part, the host’s influences on the microenvironment at each anatomic site.
Fig. 1.
Composition of colonizing bacterial communities at several anatomic surfaces of healthy humans, adapted from Spor et al. [81] with permission. The relative abundances of the six dominant bacterial phyla are based on the analysis of pooled specimens from different body sites. Primary data were from [13, 82–85]. The color designations are: Firmicutes, yellow ( ); Bacteroidetes, green (
); Bacteroidetes, green ( ); Fusobacteria, purple (
); Fusobacteria, purple ( ); Proteobacteria, black (
); Proteobacteria, black ( ); Cyanobacteria, light green (
); Cyanobacteria, light green ( ); Actinobacteria, red (
); Actinobacteria, red ( )
)
The composition of an individual’s colonic microbiota, typically assessed by analysis of bacteria in feces, has been a chief focus of studies in humans [8–14]. Analysis over time has revealed that much of the higher order taxonomy in the colonic microbiota community structure remains relatively fixed in the absence of disease or medication use; however, both transient microbes of low abundance and blooms of various taxa are evident with repetitive sampling [15]. Furthermore, while there is significant variability between individuals [13], a recent analysis indicates that the microbial composition clusters into one of three categories, termed enterotypes [16]. It is not yet clear what drives these patterns, although it appears that it is not a function of age, gender, or body mass index.
The forces that shape each individual’s specific microbiome and those that shape the microbiota at various anatomic sites are not completely understood. Many selective forces influence the composition of these open ecosystems, including nutrients, microbe–microbe interaction, pressures from the external environment, and host-derived factors [17–20]. Greater insight into these mechanisms will be highly relevant to an increased understanding of the impact of the microbiome on host physiology and the best approaches to manipulating the microbiome for the enhancement of health and/or the treatment of disease. The focus of this review is to highlight some recent advances that help elucidate the role of the host in selecting and regulating its own microbiota.
Do genetics trump the environment?
The influence of the host on colonizing microbiota is strikingly evident in studies of Hydra. Fraune and Bosch examined the colonizing microbiota in two Hydra species maintained in laboratory culture for more than two decades under identical conditions of medium, temperature, and diet [21]. Remarkably, the microbial composition from these two species differed greatly in their microbiota. Even more impressive, Hydra of each species living in the wild in their native habitats were colonized by a composition of microbes similar to that of the laboratory organisms (Fig. 2). These data provide compelling support that the host selectively shapes its bacterial community and suggest that genetic factors of the host can outweigh environmental influences in determining microbial surface colonization.
Fig. 2.
Comparison of microbiota colonizing two species of a basal metazoan, Hydra, adapted from Fraune and Bosch [2] with permission. Analysis of the microbiota of two species, Hydra vulgaris and H. oligactis. The relative abundance of different bacterial divisions within the microbiota is shown in pie charts. Although maintained in the laboratory under standard conditions, including the same diet for more than 20 years, the two species differed dramatically in composition of their microbiota [21]. Analysis also revealed that individuals living in the wild were colonized by a group of microbes similar to that in laboratory-reared counterparts of the same species. The color designations are: α-Proteobacteria, gray ( ); β-Proteobacteria, light blue (
); β-Proteobacteria, light blue ( ); δ-Proteobacteria, white (
); δ-Proteobacteria, white ( ); γ-Proteobacteria, black (
); γ-Proteobacteria, black ( ); Spirochetes, pink (
); Spirochetes, pink ( ); Bacteroidetes, green (
); Bacteroidetes, green ( )
)
In another model system used to examine how the host influences the composition of gut microbial communities, conventional gut microbiota from zebrafish and mice were exchanged between host species (Fig. 3). Accordingly, Rawls et al. [22] colonized germ-free mice with the microbiota of zebrafish, and visa versa, germ-free zebrafish were colonized with mouse intestinal microbiota. Analysis of the microbiota in the recipient hosts revealed that the zebrafish and mice significantly shaped the relative abundance of the different taxa of transferred bacteria to resemble the structure of their native bacterial communities.
Fig. 3.
Comparison of bacterial taxa composition following reciprocal transplantation of intestinal microbiota in gnotobiotic zebrafish and mice by Rawls et al. [22]. Four intestinal bacterial communities were compared: (right to left) conventionally raised zebrafish (top), ex-germ-free mice that had been colonized with a normal zebrafish microbiota, ex-germ free zebrafish that had been colonized with a normal mouse microbiota, and conventional raised C57BL/6 mice. The relative abundance of different bacterial divisions within these four communities is shown in pie charts. The color designations are: Proteobacteria, black ( ); Firmicutes, yellow (
); Firmicutes, yellow ( ); Bacteroidetes, green (
); Bacteroidetes, green ( ); Fusobacteria, purple (
); Fusobacteria, purple ( ); Actinobacteria, red (
); Actinobacteria, red ( ); Planctomycetes, tan (
); Planctomycetes, tan ( ). The tree was based on pairwise differences (weighted UniFrac metric) between the four communities
). The tree was based on pairwise differences (weighted UniFrac metric) between the four communities
Finally, an investigation of hominids in their natural habitats provides evidence that host phylogeny can supercede environment in predicting the community structure of colonizing microbiota of the distal intestine [23]. This study included closely related primate host species (gorillas, bonobos, and three subspecies of chimpanzees) from ten geographic locations in central Africa collected over a 6-year time span. Also included were specimens from two humans from different continents. Analysis of the fecal microbiota from these closely related hosts revealed clear species-specific host signatures in microbial taxonomic structure. Remarkably, the pattern of relationship among the hominids inferred from the analysis of mitochondrial DNA was identical to that inferred from their fecal microbial communities. These data support the notion that host factors are vitally important in shaping the intestinal microbiota of diverging great apes over evolutionary timescales.
Clearly, these investigations in model systems support the idea that the host shapes its colonizing microbiota, which then leads to the question “What are the specific factors that come into play”?
The anaerobic environment of the mammalian intestine
Oxygen tension is well known to be a highly selective factor for bacterial growth. The anaerobic environment of the mammalian intestine is likely to be a principal host factor in determining the composition of the microbiota. Obvious support for this idea comes from the observation that the overwhelming majority of commensal microbiota are strict or facultative anaerobes. Further support comes from observations that changes in oxygen tension can dramatically shift the bacterial composition at this site. Such a shift was revealed by a recent study of the intestinal microbiota following small bowel transplantation, where an ostomy was created at the time of organ transplantation and then maintained to provide access for monitoring possible tissue rejection [24]. The post-transplant microbial communities, sampled via the ostomy, showed a shift from predominantly strict anaerobes (Bacteroides and Clostridia) to dominance by facultative anaerobes (Lactobacillus and Enterobacteria). In the same study, patients with open ostomies for other medical conditions also showed this dramatic shift; these shifts then reverted to the expected anaerobic bacterial composition after surgical closure of the ostomy. The authors hypothesize that the ostomy allowed oxygen into an otherwise anaerobic distal small intestine, thus driving the transition from one microbial community structure to another. These recent investigations, together with the long recognized dominance of strict anaerobes in the mammalian colon, support the view that host regulation of ambient oxygen tension in the intestinal lumen is a key determinant of the community composition of the microbiota.
Feeding your friends
While ingested nutrients from the diet can significantly influence the composition of the microbiota, the host can also provide endogenous nutrients for the colonizing microbiota. An example comes from investigations of mechanisms mediating mutualism between the host and a colonizing microbe—Bacteroides thetaiotaomicron, an often-abundant member of the intestinal microbiota in mice and humans. Colonization of germ-free mice with this bacterium led to the induction (likely through a soluble mediator) of pathways controlling host incorporation of fucose into cell surface carbohydrates of enterocytes [25]. The induction occurred under conditions when fucose was otherwise not present in the intestinal environment [26]. In turn, when B. thetaiotaomicron detected the surface expression of fucosylated surface glycans, the bacterium induced the expression of genes facilitating fucose metabolism. The presence of fucose could then serve as a nutrient for the bacteria, and via a negative regulatory loop, the presence of fucose would tap the brakes on host fucosylation of surface carbohydrates. This tightly regulated process has been proposed to facilitate intestinal colonization by these resident bacteria. The model predicts that colonization by other microbes unable to participate in this fucose-dependant interplay would be at a selective disadvantage. Further support for this model comes from the discovery of a significant association of a nonsense mutation in the FUT2 gene with the diversity, richness, and abundance of bifidobacteria in human colonic microbiota. FUT2 encodes the fucosyltransferase-2 enzyme, which underlies cell surface glycosylation to determine ABH histo-blood group classification. The presence or absence of fucosyltransferase-2 activity alters the expression of ABH and Lewis glycan epitopes in the intestinal mucosa, supporting that FUT2 genotype (also termed secretor status) is an important determinant for composition of the intestinal microbiota. Presumably this paradigm may similarly govern other mutualistic interactions between host and colonizing microbiota [27].
An intriguing twist to the theme that host-derived nutrients serve as factors to shape the microbiota comes from analysis of the intestinal microbes in breast-fed human infants [28, 29]. Human breast milk contains substantial quantities of oligosaccharides that are (curiously) indigestible and hence cannot directly nourish the infant (Fig. 4a). Rather, this collection of complex carbohydrates serves as selective nutrients for infant-type bifidobacteria, a group of bacteria commonly observed in the feces of breast-fed infants. Indeed, these bacteria are proficient at utilizing the human milk oligosaccharides (HMOs) as a sole carbon source [30]. Genomic sequencing has revealed that Bifidobacterium longum subsp. infantis, a member of this group, possesses a contiguous (43 kb) gene cluster encoding proteins dedicated to the uptake and catabolism of HMOs (Fig. 4b, c) [31]. Different mechanisms for HMO utilization are present in other infant-associated bifidobacterium, suggesting a convergence of co-evolutionary selection. In contrast, other bacteria, including the closely related adult-type bifidobacteria Bifidobacterium longum subsp. longum, are deficient in this locus and in the ability to utilize HMOs. Thus, it appears that an abundant collection of oligosaccharides present in human milk can selectively nourish genetically compatible bacteria and, accordingly, provide a mechanism for mothers to shape the composition of the gut microbiota in their infant offspring.
Fig. 4.
Elements of a lactation-mediated nutritive strategy to shape the infant intestinal microbiota, adapted from Zivkovic [29] with permission. a Macronutrient composition of human breast milk, with human milk oligosaccharides (HMOs) as an abundant component at an estimated 5–15 g/L. b HMO-related gene cluster 1 from Bifidobacterium longum subsp. infantis contains the necessary glycosidases (sialidase, fucosidase, galactosidase, and hexosaminidase) and carbohydrate transporters necessary for importing and metabolizing HMOs. c Bifidobacterium longum subsp. infantis imports HMO via specific transporters and the carbohydrates are then hydrolyzed by a collection of intracellular glycosidases, which generate monosaccharides that can enter central metabolic pathways. Interestingly, other infant-borne bifidobacteria possess different strain-specific modes for the consumption of HMOs
Microbiota and the immune system: an active dialogue
An intimate relationship exists between the host immune system and its colonizing microbiota [32]. Recent studies, particularly those involving the manipulation and monitored bacterial reconstitution of germ-free mice, have cumulatively shown that the intestinal microbiota is able to drive numerous fundamental aspects of innate and acquired immune responses [33, 34]. In the absence of intestinal microbiota, germ-free animals have stunted intestinal development, including rudimentary Peyer’s patches and intestinal lymphoid follicles [35], fewer CD4 and CD8 T cells, reduced production of immunoglobulin A (IgA) (reviewed in [34]), and reduced expression of some innate immune effector molecules [36, 37]. Reconstitution of germ-free animals with a normal complex microbiota, with specific bacterial mixtures, or in some cases single bacterial species (mono-association), will largely correct these deficiencies. Interestingly, in some cases, it is sufficient to inoculate germ-free animals with specific components of bacteria, such as its surface polysaccharide antigen, to reconstitute pertinent aspects of the mucosal immune system [38]. However, it is also evident that bacterial–host interaction is a two-way street. That is to say, while bacteria induce immune maturation, the host immune system helps regulate the abundance and composition of the microbiota. In addition, even under baseline conditions, the immune system must be vigilant to prevent commensals from indiscriminately gaining access to the mucosal epithelium and the host systemic circulation.
The mucus barrier: “Good fences make good neighbors”
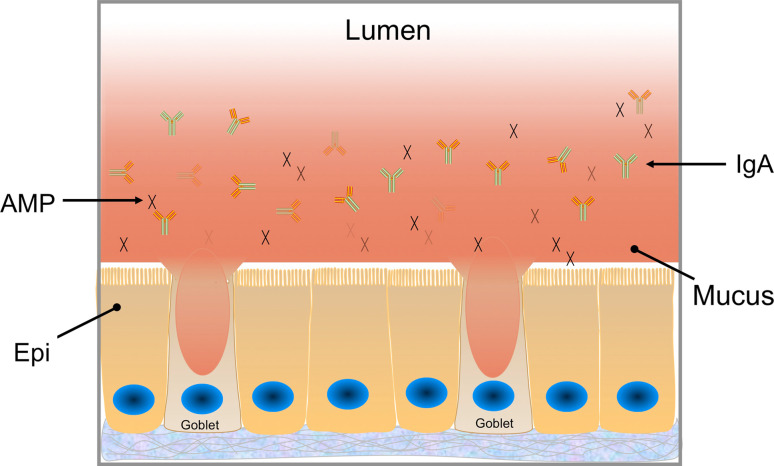
A physical barrier at most body surfaces, including the intestine, is provided by mucus secreted by epithelial goblet cells (Fig. 5) [39]. Similar to the surface glycans discussed above, the mucus provides a food source for bacteria. Although it is likely that the composition of the mucus could selectively enhance the survival of specific bacterial species or ecosystems, experimental evidence to support this hypothesis is limited. Recent investigations of the minimal human gut metagenome has detected a significant contribution by genes involved in secondary metabolism relating to glycan and complex sugar digestion, two processes which are essential for both dietary and endogenous host sugar degradation [14]. This contribution by these genes implies that glycan scavenging is essential for intestinal colonization and provides support for specific glycans provided by the host being able to contribute to the sculpting of the intestinal ecosystem.
Fig. 5.
Mucus produced by goblet cells forms a continuous layer in relatively close proximity to the epithelial cell (Epi) surface of the colon. The mucus layer is discontinuous in the small intestine (not shown). In the colon, there is an inner dense layer as well as a less dense region closer to the lumen [40, 41]. The mucus layer provides a physical barrier to limit contact between the lumenal microbes and host cells. In addition, antimicrobial peptides (AMPs) and secretory immunoglobulin A (IgA) are present at the epithelial surface and embedded in mucus. These immunologically active molecules contribute to the barrier function of mucosal surfaces
In the large intestine, the mucus provides a formidable barrier. It is comprised of an inner dense layer that excludes the majority of bacteria and an outer less dense layer in which the commensal bacteria reside [40–42]. The mucus barrier is a vital contributor to the innate immune barrier in the colon. Evidence suggests that this barrier largely shields the host adaptive immune system from responding to the colonizing microbiota [43, 44]. Defects in this barrier lead to robust immune activation and chronic mucosal inflammation [45, 46]. In the small intestine, the mucus layer is discontinuous, particularly overlying the small intestinal M cells or microfold cells that are commonly found in the epithelium over the dome of the Peyer’s patches, which are mucosal lymphoid structures. Bacteria are taken up by M cells and delivered to macrophages at the basolateral surface of the epithelium. In addition, dendritic cells can directly sample luminal bacteria in close proximity to the epithelial surface. In either scenario, macrophages and dendritic cells can present antigen to initiate adaptive immune responses. In addition to the physical barrier, there are additional immunologically active components of the barrier, including IgA and antimicrobial peptides (Fig. 5), as discussed below.
IgA: the adaptive immune system weighs in
Immunoglobulin A is the most abundant immunoglobulin and is secreted at all mucosal surfaces, where it is engaged in a complex dynamic interaction with the colonizing microbiota. At effecter sites, in the intestine and at other mucosal surfaces, plasma cells secrete IgA, which is transcytosed across the mucosal epithelium and released into the lumen. IgA binding does not destroy bacteria in the lumen, and the majority of colonizing organisms are coated with IgA. Although the mechanisms are not entirely clear, IgA binding may trap bacteria in the mucus or otherwise prevent organisms from gaining access to the epithelial surface.
The interaction between IgA and commensal microbiota is highly dynamic. Using a germ-free mouse model that could be reversibly colonized without the use of antibiotic treatment, Hapfelmeier et al. [47] answered a number of elusive questions regarding IgA–commensal interaction. They determined that high numbers of live bacteria (>109 cfu) were required for IgA induction. The IgA induced is specific to the individual commensal. Interestingly, repeated challenge with the same bacteria induces additive IgA responses rather than a synergistic response. This is in contrast to systemic induction of immunoglobulin responses. In addition, IgA induction does not persist in the context of a complex microbiota, as additional commensal bacterial species induce their own specific IgA, suggesting that the repertoire of IgA is constantly shifting to respond to the specific and changing microbial environment of the intestinal tract. There is evidence that IgA can shift the composition of the microbial environment of the gut as well.
Other mouse models have examined the role of IgA in the regulation of the composition of the intestinal microbiota, including a knockout model for activation-induced cytidine deaminase (AID−/− mice) [48]. AID is essential for the somatic hypermutation of immunoglobulin genes and immunoglobulin class switching. AID−/− mice accumulate intestinal IgM plasma cells, but no IgA; consequently, they serve as a prominent model for IgA deficiency. The deficiency of IgA in these mice results in alterations of the intestinal microbiota, characterized by the increased presence of Gram-positive anaerobes (Firmicutes) and dominated by the uncultivable organism segmented filamentous bacteria (SFB) [49] (Fig. 6). Similar findings have been noted in other mouse models that lack IgA and isolated lymphoid follicles, including V(D)J recombination activation gene (Rag)−/− [49] and nucleotide-binding oligomerization domain containing 1 (Nod1)−/− mice [35]. If Rag−/− mice are reconstituted with bone marrow from AID−/− mice, the alteration in the microbiota persists. However, if the animals are reconstituted with wild-type bone marrow, their IgA levels normalize and their microbiota shifts back to approximate that of the wild-type control animals. In addition, it is likely that this alteration of the microbiota in AID−/− mice has an impact on the development of enlarged Peyer’s patches and lymphoid follicles, which are characteristic findings in these mice, since treating the animals orally with non-absorbable antibiotics eliminates these pathologies.
Fig. 6.
Impact of α-defensin and IgA expression on colonizing microbiota. a Analysis of microbiota in small intestine of HD5 transgenic mice, adapted from Salzman et al. [54]. Subclone sequence analysis of the bacterial composition of the distal small intestines of HD5 transgenic mice and their wild-type (WT) littermates, presented as the relative percentage of dominant bacterial phyla. b Analysis of microbiota in the AID−/− small intestine, adapted from Suzuki et al. [49]. Analysis of mucosal biopsies obtained from proximal segments of the small intestine of 16-month-old AID−/− and WT mice. Biopsies were pooled from littermates for each genotype, and the microbiota was identified by sequence analyses of the 16S rRNA PCR products. Each color represents a bacterial group identified. AID Activation-induced cytidine deaminase
Antimicrobial peptides: an ancient mediator of peace
Peptides with an antimicrobial activity function in niche protection throughout biology and contribute to the immune barrier in mammals [50, 51]. In addition to their host defense role in protecting metazoans from overt pathogens, newly reported data in tunicates, insects, and mammals show they have a key role in both preventing commensal translocation and regulating the composition of the colonizing microbiota [52–55]. In the small intestine, Paneth cells are the predominant source of antimicrobial peptides [56, 57]. These secretory cells, found at the bases of the crypts of Lieberkühn, have multiple essential functions in both host defense and homeostasis [58]. They produce several classes of molecules with broad-spectrum antimicrobial activity, including α-defensins and lysozyme, which are constitutively expressed and secreted, and RegIIIγ, a bacterial lectin induced via MyD88 (myeloid differentiation primary response gene-88)-mediated pathways [37]. Secreted effector molecules of Paneth cells localize to the mucus layer, where they contribute to the innate barrier against invading microbes [59]. Partial ablation of Paneth cells (by the lineage-specific transgenic expression of toxin) results in increased commensal translocation, but it does not alter the abundance of colonizing intestinal bacteria in the lumen [55]. Mouse models for deficiency of Nod2, a gene encoding a muramyl dipeptide receptor that is prominently expressed in Paneth cells, also show disrupted intestinal homeostasis with alterations in commensal bacterial composition and abundance [60]. Nod2-deficient mice have multiple defects in handling bacterial challenges [61, 62], and their reduced expression of a subset of Paneth cell α-defensins likely contributes to some of these defects.
Complementary mouse models of defensin-excess and defensin-deficiency were used to probe the specific role of Paneth cell α-defensins in shaping the composition of the intestinal microbiota [54]. The expression of physiologically relevant concentrations of human Paneth cell defensin, HD5, in mouse Paneth cells results in significant shifts in the small intestinal microbial composition (Fig. 6). The abundance of Bacteroides increases with the addition of HD5 to the repertoire of endogenous mouse α-defensins, while the abundance of Firmicutes decreases. SFB, a member of the Clostridiales class and Firmicutes phylum, is reduced to undetectable levels in HD5 transgenic mice. SFB are uncultivable bacteria that can intimately adhere to small intestinal epithelial cells and which alone can broadly trigger mucosal immune development [54, 63–65]. In the context of a complex microbiota, SFB can drive Th17 cell differentiation. In the HD5 transgenic mice, the reduced levels of SFB are associated with an absence of Th17 cell differentiation [54]. In wild-type mice, Th17 cell abundance is directly proportional to small intestinal SFB abundance [54].
Reciprocal changes in the composition of the microbiota were observed in a complementary model of defensin-deficiency [54]. Here, mice lacking MMP7 (also called matrilysin) produces a defensin-deficiency because this metalloproteinase is responsible for processing pro-defensins into their active form in mice [66]. These defensin-deficient mice show reductions in the abundance of Bacteroides, with increased numbers of Firmicutes, and show the persistent presence of SFB and lamina propria Th17 differentiation [54]. Thus, Paneth cell α-defensins can regulate the composition of the intestinal microbiota, with far-reaching effects on the mucosal immune system.
Recent studies of another antimicrobial peptide, human β-defensin 1 (hBD-1), encourage speculation on a different (in this case, indirect) mechanism by which oxygen tension may influence the composition of the microbiota. hBD-1 is broadly expressed at most human epithelial surfaces, including the large intestine. The primary structure and tissue expression patterns of hBD-1 are conserved in many mammals. Curiously, however, its in vitro antibiotic activity is very weak, raising the question of whether its antibiotic functions are significant in vivo. The paradox appears to have been solved by the discovery that the reduction of the hBD-1 disulfide bonds dramatically enhances its antimicrobial activity, particularly against anaerobic commensal species [67]. While further studies are needed to ascertain details regarding the relative amounts of reduced and oxidized hBD-1 present in vivo, and to fully understand both regulation and consequences at mucosal surfaces, it appears that the redox state of this defensin peptide might play a key role in regulating the composition of microbiota at the epithelial surface, and perhaps in preventing translocation of the mucosa-associated bacteria.
Pathogens cleverly exploit host immunity to favorably alter the microbiota
The evidence discussed here indicates that the host’s immune system is intimately involved in shaping the composition of the intestinal microbiota during homeostasis. There is also evidence to support that defects in mucosal immunity can result in profoundly altered commensal colonization of the intestine, termed dysbiosis [35, 46, 60, 68–71]. However, even a normal immune response to infectious challenge can induce dysbiosis. Typically, the disruption is transient, and with resolution of the infection, the changed microbiota returns to baseline [72]. However, in some cases, the changes in the microbiota can favor survival of the invading pathogen, providing an interesting twist to the host–microbiota dialogue.
Salmonella enterica serovar Typhimurium is a well-characterized enteric pathogen which causes enteritis that is characterized by an inflammatory diarrhea. In mouse gastroenteritis models, virulent S. Typhimurium triggers local mucosal immune responses that cause disruption of the intestinal bacterial ecosystem [73]. In this situation, the host response to Salmonella drives the disruption of the microbiota, not Salmonella directly [73, 74]. Disruption of the microbiota enhances pathogen colonization and persistence, suggesting that the bacterial-dependent induction of mucosal inflammation functions to the pathogen’s advantage [73–76]. Additional studies have shown that S. Typhimurium directly exploits other aspects of the host response to enhance its own survival in the course of infection [77, 78].
Studies using the intestinal pathogens Citrobacter rodentium and Campylobacter jejuni reported similar findings, in that host inflammation was responsible for the disruption of the intestinal microbiota [72]. Interestingly, these studies also demonstrated that inflammation and resulting diarrhea resulted in characteristic changes in the microbiota composition, with loss of the highly abundant Firmicutes phylum and replacement by the Proteobacteria phylum, dominated by bacteria of the Enterobacteriacea family. These changes appear to be similar in models of chronic intestinal inflammation [79] and in humans with inflammatory bowel disease [80], suggesting that host innate mucosal inflammatory responses also select for a characteristic microbiota composition.
Concluding comments
The intestinal microbiota is a complex and multifunction ecosystem that is essential to the development, protection, and overall health of its host. The microbiota functions as an extra organ, to which the host has outsourced numerous crucial metabolic, nutritional, and protective functions. Yet, what is driving this symbiotic relationship? Do our microbes shape their environment, or adapt to the host environment? What is the relative significance of our intrinsic environment (driven by genetics, aging, and possibly epigenetics) versus our extrinsic environment (driven by nutrition, behavior, pathogens, and toxins)? In this review, we have examined new evidence suggesting that the host selects and shapes its ecosystem (Fig. 7). The resulting ecosystem is a critical driver of host physiology, and disruption consequently may have significant health consequences. The study of organisms on the simpler end of the evolutionary scale suggests that the host’s role far outweighs other environmental factors in molding the composition of the microbiota. In mammals, the role of the host is also clearly evident, although countless underlying mechanisms surely remain unresolved. In humans, the situation is further complicated by our ability to relentlessly and deliberately manipulate our environment (both external and internal), ultimately affecting the host–microbe balance of the super-organism. In many cases, the disruptions may contribute to disease pathogenesis.
Fig. 7.
Sculpting of the colonizing microbiota. Our central tenet is that the host actively shapes the composition of its colonizing microbiota. The mechanisms include non-immune factors, as well as innate and adaptive immune factors. Together, these host factors combine to create a discriminating environment, provide selective nutrients, and generate specific antimicrobial factors
Acknowledgments
The authors apologize that, because of the selective focus, many interesting investigations and reviews were not included. The authors’ work related to this topic was supported in part by AI57757, AI32738, AI50843, AI76246, and HD59127 from the National Institutes of Health, and by the Crohn’s and Colitis Foundation of America.
References
- 1.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7(7):688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraune S, Bosch TC. Why bacteria matter in animal development and evolution. Bioessays. 2010;32(7):571–580. doi: 10.1002/bies.200900192. [DOI] [PubMed] [Google Scholar]
- 3.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 4.Chow J, Mazmanian SK. A pathobiont of the microbiota balances host colonization and intestinal inflammation. Cell Host Microbe. 2010;7(4):265–276. doi: 10.1016/j.chom.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 6.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodman AL, Kallstrom G, Faith JJ, Reyes A, Moore A, Dantas G, et al. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc Natl Acad Sci USA. 2011;108(15):6252–6257. doi: 10.1073/pnas.1102938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer C, Bik EM, digiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biology. 2007;5(7):e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rrna sequencing. PLoS Biology. 2008;6(11):e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterson J, Garges S, Giovanni M, mcinnes P, Wang L, Schloss JA, et al. The NIH human microbiome project. Genome Res. 2009;19(12):2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326(5960):1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caporaso JG, Lauber CL, Costello EK, Berg-Lyons D, Gonzalez A, Stombaugh J, et al. Moving pictures of the human microbiome. Genome Biol. 2011;12(5):R50. doi: 10.1186/gb-2011-12-5-r50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 18.Brugman S, Nieuwenhuis EE. Mucosal control of the intestinal microbial community. J Mol Med. 2010;88(9):881–888. doi: 10.1007/s00109-010-0639-9. [DOI] [PubMed] [Google Scholar]
- 19.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10(3):159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 20.Chung H, Kasper DL. Microbiota-stimulated immune mechanisms to maintain gut homeostasis. Curr Opin Immunol. 2010;22:455–460. doi: 10.1016/j.coi.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Fraune S, Bosch TC. Long-term maintenance of species-specific bacterial microbiota in the basal metazoan Hydra . Proc Natl Acad Sci USA. 2007;104(32):13146–13151. doi: 10.1073/pnas.0703375104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rawls JF, Mahowald MA, Ley RE, Gordon JI. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell. 2006;127:423–433. doi: 10.1016/j.cell.2006.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochman H, Worobey M, Kuo CH, Ndjango JB, Peeters M, Hahn BH, et al. Evolutionary relationships of wild hominids recapitulated by gut microbial communities. PLoS Biology. 2010;8(11):e1000546. doi: 10.1371/journal.pbio.1000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartman AL, Lough DM, Barupal DK, Fiehn O, Fishbein T, Zasloff M, et al. Human gut microbiome adopts an alternative state following small bowel transplantation. Proc Natl Acad Sci USA. 2009;106(40):17187–17192. doi: 10.1073/pnas.0904847106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bry L, Falk PG, Midtvedt T, Gordon JI. A model of host-microbial interactions in an open mammalian ecosystem. Science. 1996;273(5280):1380–1383. doi: 10.1126/science.273.5280.1380. [DOI] [PubMed] [Google Scholar]
- 26.Hooper LV, Xu J, Falk PG, Midtvedt T, Gordon JI. A molecular sensor that allows a gut commensal to control its nutrient foundation in a competitive ecosystem. Proc Natl Acad Sci USA. 1999;96:9833–9838. doi: 10.1073/pnas.96.17.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wacklin P, Makivuokko H, Alakulppi N, Nikkila J, Tenkanen H, Rabina J, et al. Secretor genotype (FUT2 gene) is strongly associated with the composition of Bifidobacteria in the human intestine. PLoS One. 2011;6(5):e20113. doi: 10.1371/journal.pone.0020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sela DA, Mills DA. Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol. 2010;18(7):298–307. doi: 10.1016/j.tim.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zivkovic AM, German JB, Lebrilla CB, Mills DA. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4653–4658. doi: 10.1073/pnas.1000083107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.locascio RG, Ninonuevo MR, Freeman SL, Sela DA, Grimm R, Lebrilla CB, et al. Glycoprofiling of bifidobacterial consumption of human milk oligosaccharides demonstrates strain specific, preferential consumption of small chain glycans secreted in early human lactation. J Agric Food Chem. 2007;55(22):8914–8919. doi: 10.1021/jf0710480. [DOI] [PubMed] [Google Scholar]
- 31.Sela DA, Chapman J, Adeuya A, Kim JH, Chen F, Whitehead TR, et al. The genome sequence of Bifidobacterium longum subsp. Infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci USA. 2008;105(48):18964–18969. doi: 10.1073/pnas.0809584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen J, Gulati A, Sartor RB. The role of mucosal immunity and host genetics in defining intestinal commensal bacteria. Curr Opin Gastroenterol. 2010;26(6):564–571. doi: 10.1097/MOG.0b013e32833f1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev. 1998;62(4):1157–1170. doi: 10.1128/mmbr.62.4.1157-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456(7221):507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 36.Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4:269–273. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 37.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313(5790):1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122(1):107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 39.McGuckin MA, Linden SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. 2011;9(4):265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 40.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105(39):15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansson GC, Johansson ME. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Gut Microbes. 2010;1(1):51–54. doi: 10.4161/gmic.1.1.10470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng T, Elson CO. Adaptive immunity in the host-microbiota dialog. Mucosal Immunol. 2011;4(1):15–21. doi: 10.1038/mi.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci USA. 2009;106(46):19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathogens. 2010;6(5):e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu J, Wei B, Wen T, Johansson ME, Liu X, Bradford E, et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J Clin Invest. 2011;121(4):1657–1666. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328(5986):1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298(5597):1424–1427. doi: 10.1126/science.1077336. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, Honjo T, et al. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci USA. 2004;101(7):1981–1986. doi: 10.1073/pnas.0307317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boman HG. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 51.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 52.Fraune S, Augustin R, Anton-Erxleben F, Wittlieb J, Gelhaus C, Klimovich VB, et al. In an early branching metazoan, bacterial colonization of the embryo is controlled by maternal antimicrobial peptides. Proc Natl Acad Sci USA. 2010;107(42):18067–18072. doi: 10.1073/pnas.1008573107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryu JH, Kim SH, Lee HY, Bai JY, Nam YD, Bae JW, et al. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 2008;319(5864):777–782. doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- 54.Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjoberg J, Amir E, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11(1):76–82. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105(52):20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Porter EM, Bevins CL, Ghosh D, Ganz T. The multifaceted Paneth cell. Cell Mol Life Sci. 2002;59(1):156–170. doi: 10.1007/s00018-002-8412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ouellette AJ. Paneth cells and innate mucosal immunity. Curr Opin Gastroenterol. 2010;26(6):547–553. doi: 10.1097/MOG.0b013e32833dccde. [DOI] [PubMed] [Google Scholar]
- 58.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9(5):356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 59.Meyer-Hoffert U, Hornef MW, Henriques-Normark B, Axelsson LG, Midtvedt T, Putsep K, et al. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut. 2008;57(6):764–771. doi: 10.1136/gut.2007.141481. [DOI] [PubMed] [Google Scholar]
- 60.Petnicki-Ocwieja T, Hrncir T, Liu YJ, Biswas A, Hudcovic T, Tlaskalova-Hogenova H, et al. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci USA. 2009;106(37):15813–15818. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307(5710):731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 62.Biswas A, Liu YJ, Hao L, Mizoguchi A, Salzman NH, Bevins CL, et al. Induction and rescue of Nod2-dependent Th1-driven granulomatous inflammation of the ileum. Proc Natl Acad Sci USA. 2010;107(33):14739–14744. doi: 10.1073/pnas.1003363107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, Mulder I, Lan A, Bridonneau C, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31(4):677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 66.Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, Lopez-Boado YS, Stratman JL, et al. Regulation of intestinal a-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 67.Schroeder BO, Wu Z, Nuding S, Groscurth S, Marcinowski M, Beisner J, et al. Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. Nature. 2011;469(7330):419–423. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- 68.Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, Petras RE, et al. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc Natl Acad Sci USA. 2005;102(50):18129–18134. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328(5975):228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sokol H, Vasquez N, Hoyeau-Idrissi N, Seksik P, Beaugerie L, Lavergne-Slove A, et al. Crypt abscess-associated microbiota in inflammatory bowel disease and acute self-limited colitis. World J Gastroenterol. 2010;16(5):583–587. doi: 10.3748/wjg.v16.i5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saleh M, Elson CO. Experimental inflammatory bowel disease: insights into the host-microbiota dialog. Immunity. 2011;34(3):293–302. doi: 10.1016/j.immuni.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2(2):119–129. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 73.Barman M, Unold D, Shifley K, Amir E, Hung K, Bos N, et al. Enteric Salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun. 2008;76(3):907–915. doi: 10.1128/IAI.01432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthei M, Kremer M, et al. Salmonella enteric serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biology. 2007;5(10):e244. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Croswell A, Amir E, Teggatz P, Barman M, Salzman NH. Prolonged impact of antibiotics on intestinal microbial ecology and susceptibility to enteric Salmonella infection. Infect Immun. 2009;77(7):2741–2753. doi: 10.1128/IAI.00006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sekirov I, Tam NM, Jogova M, Robertson ML, Li Y, Lupp C, et al. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infect Immun. 2008;76(10):4726–4736. doi: 10.1128/IAI.00319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, et al. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe. 2009;5(5):476–486. doi: 10.1016/j.chom.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467(7314):426–429. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010;140(6):859–870. doi: 10.1016/j.cell.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peterson DA, Frank DN, Pace NR, Gordon JI. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe. 2008;3(6):417–427. doi: 10.1016/j.chom.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spor A, Koren O, Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol. 2011;9(4):279–290. doi: 10.1038/nrmicro2540. [DOI] [PubMed] [Google Scholar]
- 82.Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, et al. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci USA. 2006;103(3):732–737. doi: 10.1073/pnas.0506655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Price LB, Liu CM, Johnson KE, Aziz M, Lau MK, Bowers J, et al. The effects of circumcision on the penis microbiome. PLoS ONE. 2010;5(1):e8422. doi: 10.1371/journal.pone.0008422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim TK, Thomas SM, Ho M, Sharma S, Reich CI, Frank JA, et al. Heterogeneity of vaginal microbial communities within individuals. J Clin Microbiol. 2009;47(4):1181–1189. doi: 10.1128/JCM.00854-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pei Z, Bini EJ, Yang L, Zhou M, Francois F, Blaser MJ. Bacterial biota in the human distal esophagus. Proc Natl Acad Sci USA. 2004;101(12):4250–4255. doi: 10.1073/pnas.0306398101. [DOI] [PMC free article] [PubMed] [Google Scholar]