Abstract
Previous reports have indicated that adenosine A3 receptor (A3R) knockout mice are more sensitive to ischemic or hypoxic brain injury. The purpose of this study was to examine if suppression of A3R expression is associated with increase in sensitivity to injury induced by a high dose of methamphetamine (Meth). Adult male A3R null mutant (−/−) mice and their controls (+/+) were injected with 4 doses (2 hours apart) of Meth (10 mg/kg) or saline. Animals were placed in a behavioral activity chamber, equipped with food and water, for 52 hours starting from one day after injections. The first 4 hours were used for studying exploratory behaviors and the next 48 hours were used to measure locomotor activity. High doses of Meth equally reduced the 4-hour exploratory behavior in −/− and +/+ mice. Meth suppressed locomotor activity between 4 and 52 hours in both groups, with a greater reduction being found in the −/− mice. Brain tissues were collected at 3 days after the Meth or saline injections. Meth treatment reduced striatal dopamine (DA) levels in both +/+ and −/− mice, examined by HPLC, with an increase in DOPAC/DA ratio being found only in −/− animals. Meth also significantly increased ionized calcium-binding adaptor molecule 1 (Iba-1) and cleaved caspase-3 level in striatum as well as Iba-1 and TNFα mRNA expression in nigra in −/−, compared to +/+, mice. Previous studies have shown that pharmacological suppression of VMAT2 by reserpine enhanced Meth toxicity by increasing cytosolic DA and inflammation. A significant reduction in striatal VMAT2 expression was found in −/− mice, compared to +/+ mice, suggesting that increase in sensitivity to Meth injury in −/− mice may be related to a reduction in VMAT2 expression in these mice. In conclusion, our data suggest that A3R −/− mice are more sensitive to high doses of Meth.
Introduction
Adenosine is a purine nucleoside which can be released during injury, activates cell surface receptors, and regulates physiological functions. At least, four G-protein coupled adenosine receptor subtypes (i.e. A1, A2A, A2B, and A3) have been identified in peripheral organs and brain. Amongst these receptors, the adenosine A3 receptor (A3R) was first isolated from rat testis (Meyerhof et al., 1991) and was later identified in the lung, kidneys, heart, and in central nervous system (CNS) regions including the cortex, striatum, and olfactory bulb (Zhou et al., 1992). The density of A3R in the brain is relatively low compared to other adenosine receptor subtypes. A3R was found in neurons by PCR and Western blotting (Lopes et al., 2003) as well as in microglia (Hammarberg et al., 2003) and astrocytes (Di et al., 2002). Using a selective PET tracer, A3R has been identified in adult rat brain (Wadsak et al., 2008). Selective A3R binding was localized in the caudate and nucleus accumbens by autoradiography (Shearman and Weaver, 1997; Wadsak et al., 2008).
A3 receptors (A3Rs) have been found to confer protection against hypoxic or ischemic injury to the lungs (Rivo et al., 2004), heart (Maddock et al., 2003) and also in the CNS. Activation of A3R suppresses lipopolysaccharide-induced tumor necrosis factor-α (TNF-α) production in murine BV2 microglial cells (Lee et al., 2006). In CNS, A3R, but not A1R, was upregulated one hour after 3-nitropropionate administration in hippocampal slices. Such an increase may involve protective preconditioning (von Arnim et al., 2000). In rat cortical pyramidal neurons, the A3R agonist N6-(3-iodobenzyl)-5'-N-methyl carboxoamidoadenosine (IB-MECA) selectively inhibited the amplitude of the excitatory postsynaptic potentials, which has been suggested to contribute to its role in neuroprotection (Brand et al., 2001). We previously reported that pretreatment with the A3R agonist IB-MECA antagonized the hypoxia –mediated decrease in cell viability in cerebral cortical cultures (Chen et al., 2006). Intracerebroventricular pretreatment with IB-MECA reduced density of TUNEL labeling and cerebral infarction in the lesioned cortex in a rodent model of stroke (Chen et al., 2006). These data suggest that A3R plays a neuroprotective role in the CNS.
Methamphetamine (Meth) is a commonly abused drug worldwide. Neurotoxicity induced by Meth has been reported in dopaminergic and non-dopaminergic neurons in various brain regions. In dopaminergic (DA-ergic) neurons, Meth-induced neurodegeneration is seen mainly in nerve terminals. Blocking dopamine (DA) synthesis or uptake protects against Meth toxicity. Similarly, Meth induces less degeneration or free radical formation in the striatum in DA transporter (DAT) knockout mice (Fumagalli et al., 1998), indicating that uptake into DA presynaptic terminals play important roles in Meth neurotoxicity. It has been suggested that Meth redistributes DA to the extra-vesicular space in cytosol which leads to an increase in reactive metabolite formation and degeneration of DA-ergic nerve terminals (Cubells et al., 1994). The significance of cytosolic DA in Meth toxicity is further supported by findings that reduction in vesicular monoamine transport 2 (VMAT2) function, which elevates cytosolic DA level, potentiates Meth toxicity. For example, Meth –induced neurotoxicity is enhanced in transgenic mice with reduced VMAT2 (Fumagalli et al., 1999; Guillot et al., 2008b) or in animals pretreated with the VMAT inhibitor reserpine (Thomas et al., 2009). Furthermore, increasing VMAT2 expression by pituitary adenylyl cyclase activating polypeptide 38 (PACAP38) attenuated Meth toxicity (Guillot et al., 2008a). Taken together, these data suggest that increasing cytosolic DA or suppressing VMAT2 function exacerbates Meth –mediated neurodegeneration in DA-ergic terminals.
We previously reported that adenosine A3 receptor Null mutant (−/−) mice were more vulnerable to ischemic brain injury than wild type (+/+) controls (Chen et al., 2006). Similarly, −/− mice were more sensitive to CO2 –induced neurodegeneration in hippocampus (Fedorova et al., 2003). Although these data suggest that A3Rs act as an endogenous neural protective mechanism, its endogenous role against other types of injuries in CNS is not clear. In this study, we examined behavioral and biochemical responses to Meth in A3R −/− and +/+ mice. Our data suggest that locomotor hypoactivity and changes in 3,4-dihydroxyphenylacetic acid (DOPAC)/DA ratio in striatum as well as increase in TNF-α or ionized calcium-binding adaptor molecule 1 (Iba-1) expression in nigra and striatum after high doses of Meth are potentiated in the −/− mice. This hypersensitivity to high dose of Meth may be attributed to the reduction in VMAT2 expression in −/− mice.
Experimental Procedures
Animals
Adult male adenosine A3 receptor Null mutant mice (−/−) were developed by the the Merck Co. NJ (Lee et al., 2003). Both −/− and wild type control (+/+) mice (C57Bl/6) with the same background were obtained from Taconic Labs. The use of animals was conducted under National Institutes Health (NIH) Guidelines using the NIH handbook “Animals in Research” and was approved by the Institutional Animal Care and Use Committee, NIDA. Animals were housed in a 12-hour dark (6pm to 6 am) and 12-hour light (6 am to 6 pm) cycle. Four doses of (+) Meth (10 mg/kg, s.c.) or saline (0.1 ml/100g body weight, s.c.) were given using a Hamilton’s syringe every 2 hours. This high dose of Meth is required to induce neural toxicity in rodents (Grace et al., 2010; Jayanthi et al., 2005; Zhu et al., 2006) and has been used to examine protection against Meth – mediated neural degeneration in mice (Chou et al., 2008; Harvey et al., 2009). During Meth or saline injections, animals were single-housed at room temperature (25°C) without bedding to prevent aspiration. Tympanic temperatures were taken from a subgroup of −/− and +/+ mice approximately at 30 min before 1st injection and 30 min after 1st, 2nd and 4th injection.
Locomotor behavioral measurement
Animals were placed in an Accuscan activity monitor (Columbus, OH) for 16 or 52 hours (12-hr light and 12-dark/day) starting from 24 hours after the first dose of Meth or saline injection. Food and water were constantly provided in the chambers. Each animal was placed in a 42 × 42 × 31 cm plexiglass open box. Locomotor activities were measured by the number and order of beams broken by the animals. The monitor contained 16 horizontal and 8 vertical infrared sensors spaced 2.5 cm apart. The vertical sensors were situated 10 cm from the floor of the chamber. The first 4 hours were used for habituation and for studying exploratory behavior. The following 12 or 48 hours were used to examine basal (with saline) and post-Meth behavior. Four parameters (i.e. horizontal activity, movement time, movement number, and vertical movement number) were used to analyze the changes in locomotor activity.
Striatal Dopamine and DOPAC measurement
Brain samples were collected on the 3rd day after injury from a subgroup of animals. Striatal tissues were weighed and stored at −80°C until extraction. The tissues obtained from each animal were homogenized in 0.1 M perchloric acid and centrifuged at 13,000 g for 15 min. DA and DOPAC were measured by HPLC with electrochemical detection (Krasnova et al., 2000). The analytical column was a Symmetry C18 3.5 µm (4.6×150.0 mm) from Waters (Milford, MA). The mobile phase consisted of 0.01 M sodium dihydrogenphosphate, 0.01 M citric acid, 2 mM sodium EDTA, 1 mM sodium octylsulfate, 10% methanol, pH 3.5 and was used at flow rate of 0.9 ml/min and a temperature of 25°C. The system consisted of an ESA automated injection system, an ESA 582 pump, and a Coulochem III detector (ESA, Chelmsford, MA). An EZChrom EliteTM chromatography data analysis system (ESA Biosciences, Inc.) was used for data collection and analysis. Contents of DA and DOPAC were calculated as nmole/g of tissue weight.
Quantitative reverse transcription-PCR (qRT-PCR)
Mice were euthanized and the brains were immediately harvested and chilled on ice. The SN was dissected out and total RNA was extracted following the instructions from the manufacturer (RNAqueous, Ambion, Austin, TX). Total RNA (1µg) was reverse transcribed into cDNA using the Superscript III reverse transcriptase kit (Invitrogen, Carlsbad, CA). cDNA levels for hydroxymethylbilane synthase (Hmbs), tyrosine hydroxylase (TH), dopamine transporter (DAT), Iba1, TNFα, and GFAP were determined by quantitative RT-PCR using specific universal probe Library primer probe sets (Roche, Indianapolis, IN). The standard curve method was used to determine the quantities of mRNA levels in samples and each sample were normalized to the house keeping genes noted above. Normalization to both endogenous control genes resulted in similar results. Primers and FAM-labeled probes used in the quantitative RT-PCR for each gene are listed in Table 1.
Table 1.
Oligonucleotide sequences used for quantitative RT-PCR
| Forward Primer | Reverse Primer | universal probe Library #, Roche |
|
|---|---|---|---|
| Hmbs | 5’ – tccctgaaggatgtgcctac | 5’ – acaagggttttcccgtttg | #79 |
| TH | 5’ – cccaagggcttcagaagag | 5’- gggcatcctcgatgagact | #15 |
| DAT | 5’ – caacctgtactggcggctat | 5’- atgctgaccacgaccacata | #38 |
| Iba1 | 5’ – ggatttgcagggaggaaaa | 5’- tgggatcatcgaggaattg | #3 |
| TNFα | 5’ – ctgtagcccacgtcgtagc | 5’- ttgagatccatgccgttg | #25 |
| GFAP | 5’ – tggaggaggagatccagttc | 5’- agctgctcccggagttct | #69 |
Western blot analysis
For Western blot analysis, the frozen tissues were homogenized in RIPA buffer containing 50mM Tris HCl pH 7.4,1% NP40,0.25% Deoxycholic Acid, 150 mM sodium chloride,1mM EDTA and1% Protease inhibitor from Sigma. Lysates were cleared by centrifugation (14,000g at 4 °C for 10 min), and the total protein concentration in each sample was determined by DCA assay using bovine serum albumin (BSA) as a standard curve. Lysates were separated by NuPAGE®Novex Bis-Tris Gels4-12% Gel from Invitrogen and the proteins were transferred to Immobilon-FL membranes (Millipore, Billerica, MA). After pre-blocking in Odyssey blocking buffer from Li-Cor, the membranes were incubated at 4 °C with the primary antibody to synaptophysin (1:10,000, Millipore), actin (1:500, Sigma, ST Louis, MO), VMAT2 (1:500, Sigma), tyrosine hydroxylase (1:2000, Millipore), Iba-1 (1:200, Wako, Richmond, VA), DAT (1:10,000, Millipore), and cleaved Caspase-3 (1:250, BD Pharmingen, San Diego, CA) overnight, then incubated for 1 hr at room temperature in goat anti-rat IR-700nm, donkey-anti-rabbit IR-800nm or donkey anti-mouse IR-700nm secondary antibodies (1:2500, Rockland, Gilbertsville, PA). The membranes were scanned using an Odyssey Infrared Imager (Li-Cor, Lincoln, NE, USA). Immunoblots were quantified with ImageJ.
Statistical analysis
All data were expressed as means ± SEM. Behavioral and biochemical data were analyzed using linear correlation, one or two way ANOVA and post-hoc Newman-Keuls test. All analyses were calculated by SigmaStat software ver 3.0. Statistical significance was determined at p<0.05.
Results
A. Meth-induced changes in tympanic temperature
Tympanic temperature was taken before and after 1st, 2nd and 4th dose of Meth or saline in 23 mice. Mice receiving Meth significantly increased body temperature in comparison to saline challenged animals (p<0.001,F1,74=70.441, three way ANOVA). There were no significant differences in tympanic temperature between −/− and +/+ mice after Meth or saline administration (p=0.941, three way ANOVA, Fig 1).
Fig 1.
Administration of Meth increased tympanic temperature equally during injection in A3R −/− and +/+ mice. Temperatures were measured approximately at 30 min before 1st injection and 30 min after 1st, 2nd and 4th injection. Meth significantly increased body temperature in +/+ and −/− mice (p<0.001,F1,74=70.441, three way ANOVA). There were no significant differences in tympanic temperature between −/− and +/+ mice after Meth or saline administrations (p=0.941, three way ANOVA).
B. Locomotor activity in −/− and +/+ mice
Sixty three mice (A3R −/−, n=31 and +/+, n=32) were treated with saline (n=32) or Meth (n=31). At 24 hours after the first injection, animals were placed in the activity chambers for 52 hours. Behavioral activity was analyzed every four hours. The first 4 hours were used for habituation. Increased locomotor activity, which represents exploratory behavior to a novel environment, was found during this period (Fig 2 vs. Fig 3). The next 48 hours were used to examine locomotor activity.
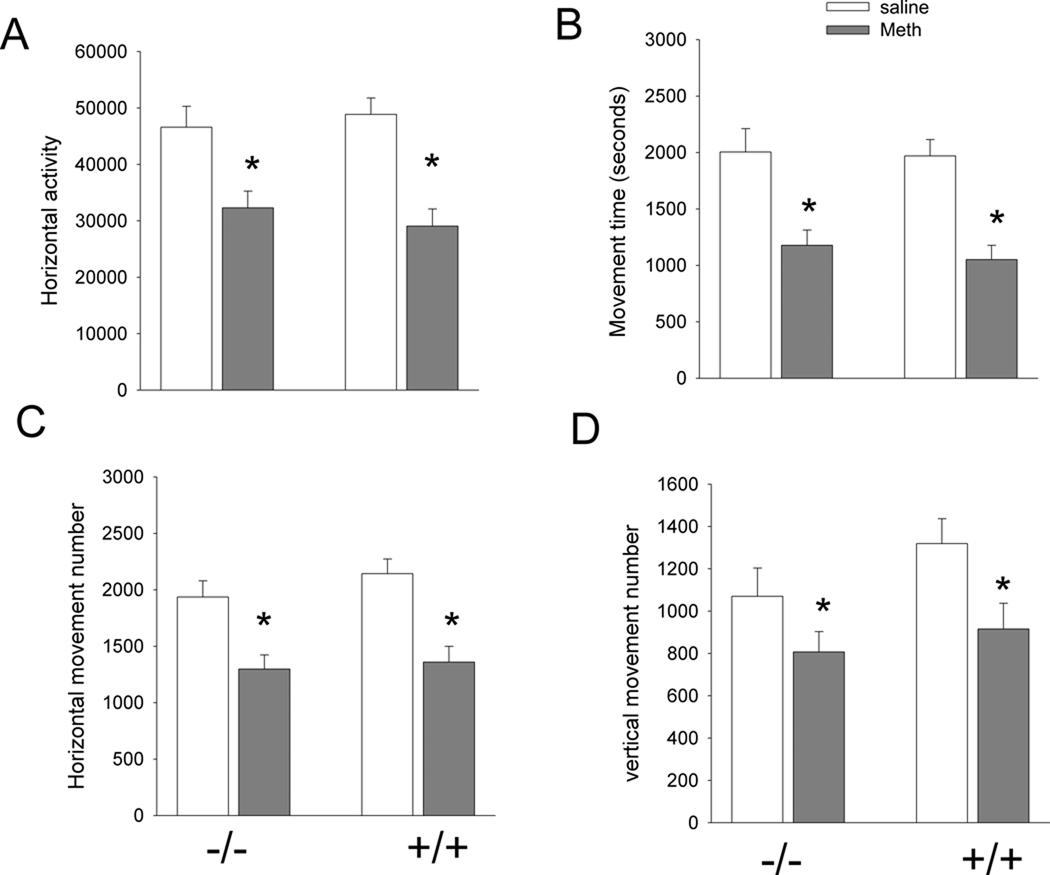
Fig 2. Meth reduces exploratory behavior equally in +/+ and −/− mice.
Animals were treated with Meth (10mg/kg ×4, every 2 hours) or saline. Exploratory behavior was examined one day after the first dose of Meth or saline for 4 hours in 32 +/+ and 31 −/− mice. No significant difference was found between A3R −/− and +/+ mice receiving saline injection in (A) horizontal activity, (B) movement time, (C) horizontal movement number, and (D) vertical movement number. These behavioral parameters were significantly reduced by Meth (horizontal activity: p<0.001; movement time: p<0.001; horizontal movement number: p<0.001, vertical movement number: p=0.007). No difference was found between A3R −/− and +/+ mice after Meth injection.
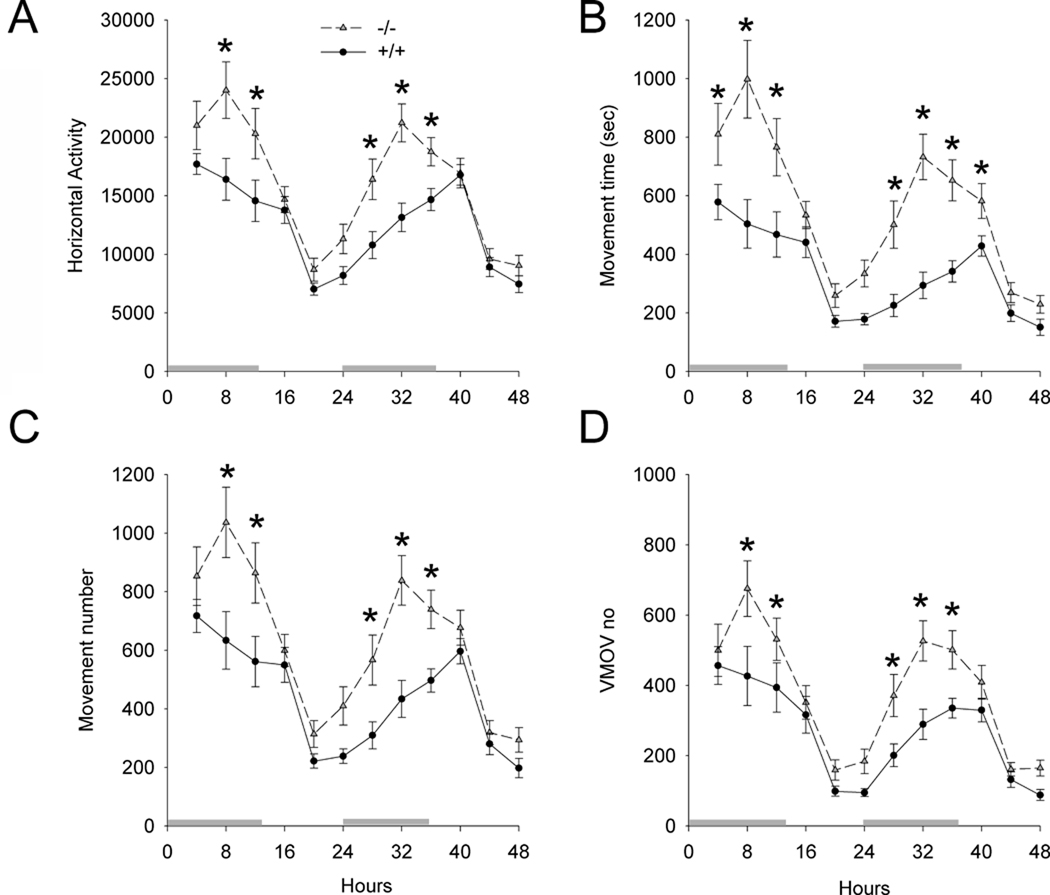
Fig 3. Increased basal locomotor activity after habituation in A3R −/− mice.
Locomotor activity was recorded for 48 hours after a 4-hour habituation period. Both +/+ and −/− mice demonstrated a diurnal motor pattern. A3R −/− mice ( ) had a significant increase in all basal locomotor activities, compared to the +/+ mice (
) had a significant increase in all basal locomotor activities, compared to the +/+ mice ( ). *p<0.05 (2-way ANOVA+ Newman-Keuls test). Horizontal bars above X axis represent dark cycle.
). *p<0.05 (2-way ANOVA+ Newman-Keuls test). Horizontal bars above X axis represent dark cycle.
i. Exploratory behavior in the first 4 hours (+/+ vs. −/−)
The first 4 hours were used for habituation and for studying the exploratory behavior in 32 +/+ and 31 −/− mice. Two variables (genotypes and treatment with Meth or saline) were used to analyze the changes in behaviors using a two way ANOVA. No significant difference was found between A3R −/− and +/+ mice in horizontal activity (Fig 2A, p=0.874, F1,59=0.0252), movement time (Fig 2B, p=0.611, F1,59=0.262), horizontal movement number (Fig 2C, p=0.320, F1,59=1.005), and vertical movement number (Fig 2D, p=0.139, F1,59=2.252). These behavioral parameters were significantly reduced by Meth (Fig 2: horizontal activity: p<0.001, F1,59=29.056; movement time: p<0.001, F1,59=30.756; horizontal movement number: p<0.001, F1,59=27.892, vertical movement number: p=0.007, F1,59=7.895). There was no significant interaction between genotypes and treatment with Meth. These data suggest that Meth suppresses exploratory behavior equally in +/+ and −/− mice.
ii. Locomotor activity in animals receiving saline or Meth injections
Basal locomotor activity was monitored for 2 days after a 4-hour habituation period in animals receiving saline injection. Animals (16 +/+ and 16 −/− mice) were kept in a 12-hr light and 12-hr dark cycle room. Both +/+ and −/− mice demonstrated a diurnal motor pattern (Fig 3). Using a two way ANOVA, we found that A3R −/− mice had a significant increase in horizontal activity (Fig 3A, p<0.001, F1,360=41.412), movement time (Fig 3B, p<0.001, F1,360=76.344), horizontal movement number (Fig 3C, p<0.001, F1,360=48.126), and vertical movement number (Fig 3D, p<0.001, F1,360=33.450), compared to +/+ mice (Fig 3). There is a significant interaction between the genotype and time course (horizontal activity, p=0.026, F11,360=2.015; movement time: p=0.003, F11,360=2.595; horizontal movement number: p=0.037, F11,360=1.907). Post-hoc Newman-Keuls analysis indicates that the difference between +/+ and −/− occurred mainly in the dark cycle (p<0.05, Fig 3).
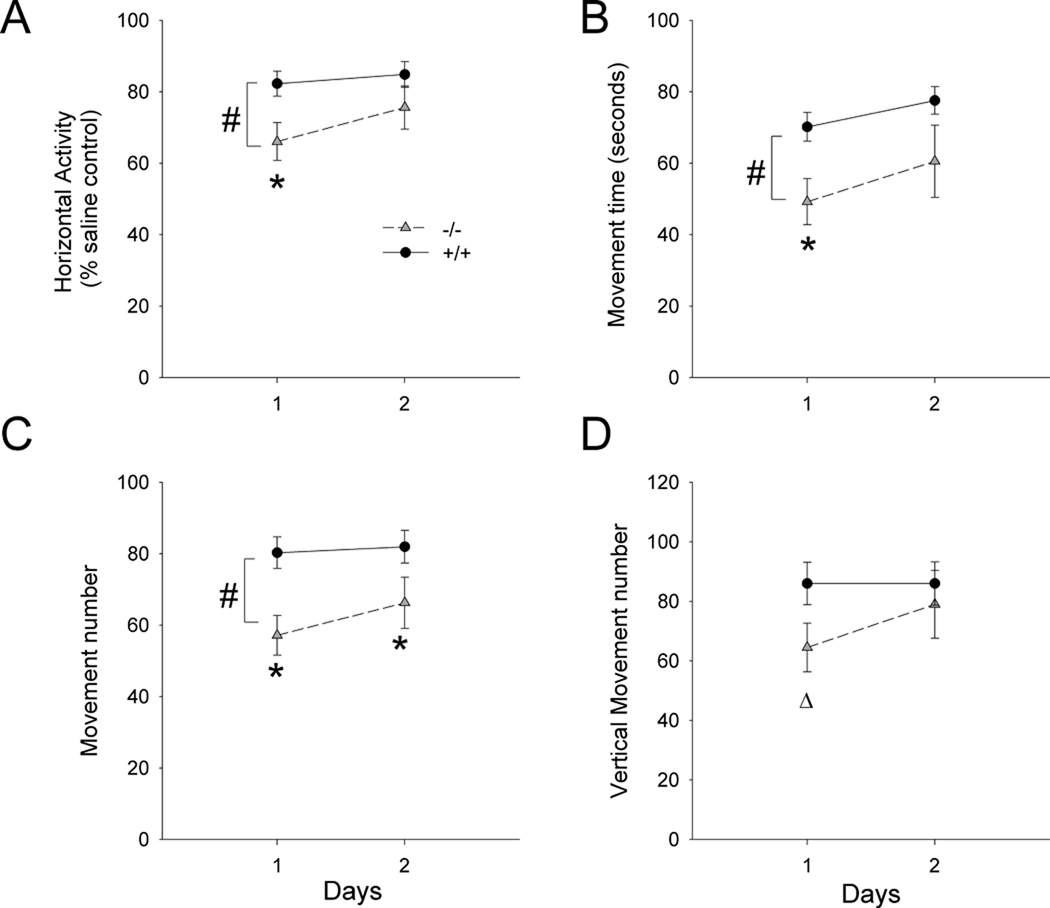
The change in locomotor activity after Meth was examined for 2 days in A3R −/− (n=16) and A3R +/+ (n=15) mice. Because of the difference in basal activity between +/+ and −/− mice (Fig 3), the change in locomotor activity after high dose Meth injection was analyzed by normalizing daily (days 1 and 2) locomotor activity to the mean activity on each day in −/− and +/+ mice receiving saline injection (Fig 4). High doses of Meth significantly suppressed the locomotor activity in both A3R −/− and +/+ animals. Importantly, a greater reduction in horizontal activity (p=0.019, F1,87=5.746), movement time (p=0.005, F1,87=8.490), and movement number (p<0.001, F1,87=12.479), was found in the A3R −/− mice (Fig 4, A–C). There was a marginal significant difference in vertical movement numbers on day 1 (p=0.055, Student’s t test). These data suggest that A3R −/−, compared to +/+, mice are more sensitive to high doses of Meth in reducing horizontal locomotor activity.
Fig 4. Behavioral interactions of Meth in A3R −/− and +/+ mice on days 1 and 2 after injection.
Daily locomotor activity after Meth injection was normalized by comparison to the average activity on each day from mice treated with saline. Meth suppressed the locomotor activity in A3R −/− ( ) and +/+ (
) and +/+ ( ) mice. There is a significant difference in (A) horizontal activity, (B) movement time, and (C) movement number between A3R −/− and +/+ mice, with the −/− mice showing a greater Meth effect (#, p<0.01, between groups, 2-Way ANOVA; *, p<0.05, post-hoc Newman-Keuls test). (D) A marginal difference in vertical movement was found on day 1 after Meth injection using a Student’s t test (p=0.055).
) mice. There is a significant difference in (A) horizontal activity, (B) movement time, and (C) movement number between A3R −/− and +/+ mice, with the −/− mice showing a greater Meth effect (#, p<0.01, between groups, 2-Way ANOVA; *, p<0.05, post-hoc Newman-Keuls test). (D) A marginal difference in vertical movement was found on day 1 after Meth injection using a Student’s t test (p=0.055).
C. Striatal DA, DOPAC, and DOPAC/DA ratio
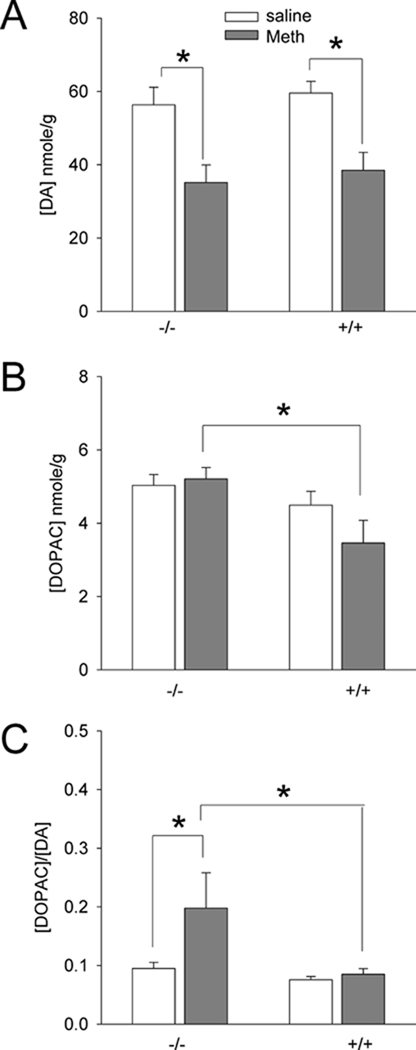
A3R −/− (n=15) and +/+ (n=22) mice were used to examine striatal DA, DOPAC and DOPAC/DA ratios at 3 days after Meth or saline injection. A significant correlation was found between DA or DOPAC level in striatum and locomotor activity on day 1 (Table 2). Two variables (genotypes and treatment with Meth or saline) were used to analyze the biochemical changes using a two way ANOVA. Meth treatment significantly reduced striatal DA levels in both +/+ and −/− mice (Fig 5A, p<0.001, F1,33=23.098). No difference was found between +/+ and −/− (p=0.463, F1,33=0.552). There was a significant increase in DOPAC level in −/−, compared to +/+, after Meth treatment (p=0.012, post-hoc Newman-Keuls test, Fig 5B). A significant difference in DOPAC/DA ratio was found between +/+ and /− (Fig 5C, p=0.013, F1,32=6.923). Post-hoc Newmann Keuls analysis indicates DOPAC/DA was significantly increased after Meth treatment in −/− (p=0.010), but not in +/+ (p=0.776), mice (Fig 5C).
Table 2.
Linear correlation between DA or DOPAC in striatum and locomotor activity in saline and Meth –treated mice
| DA | DOPAC | |||
|---|---|---|---|---|
| r | p | r | p | |
| Horizontal activity | 0.435 | 0.014 | 0.273 | 0.137 |
| movement time | 0.455 | 0.01 | 0.398 | 0.027 |
| movement # | 0.415 | 0.02 | 0.322 | 0.077 |
| vertical movement # | 0.386 | 0.032 | 0.464 | 0.008 |
Fig 5. Striatal DA, DOPAC and DOPAC/DA ratios at 3 days after Meth injection in A3R −/− and +/+ mice.
(A) Treatment with Meth significantly reduced tissue DA level in +/+ and −/− mice. (B) After Meth treatment, striatal DOPAC level was significantly increased in −/− mice, compared to +/+ mice. (C) DOPAC/DA ratio was significantly enhanced by Meth in −/−, but not in +/+, mice. *p<0.05, 2-way ANOVA.
D. Iba1 and cleaved caspase-3 immunoreactivity in striatum after Meth administration
Iba1 levels and cleaved caspase-3 activity were measured by Western analysis at 3 days after Meth or saline administration. Similar to previous reports, we also found that Meth treatment enhances Iba-1 and cleaved caspase 3 immunoreactivity in striatum. There is a negative correlation between DA and IBA1 protein expression (p=0.025, R=0.574) in striatum. There is a significant increase in Iba-1/actin level in A3R −/− (n=8), compared to +/+ (n=8), after Meth treatment (p=0.025, t test, Fig 6A and B). In another set of animals, cleaved caspase-3 activity was also significantly increased in −/− (n=5), compared to +/+ (n=5), after Meth (p=0.021, t test, Fig 6C and D). These data suggest that −/− mice are more sensitive to Meth–mediated inflammatory injury and apoptotosis in the striatum.
Fig 6. Meth activates Iba-1 and caspase-3 and in striatum in A3R −/− and +/+ mice.
Striatal tissue was collected at 3 days after Meth or saline administration for Western analysis. (A) Meth significantly potenitates Iba-1/actin expression in −/− mice (p=0.025, t test). (B) An example of Iba-1 Western blotting. (C) Meth significantly increased the production of cleaved caspase-3 activity in A3R −/− mice, compared to +/+ mice (p=0.021, t test). (D) An example of cleaved (18 kD) caspase 3 and actin Western blotting from mice receiving Meth. Data in A and C were normalized to the mean of selective activity in +/+ mice receiving Meth.
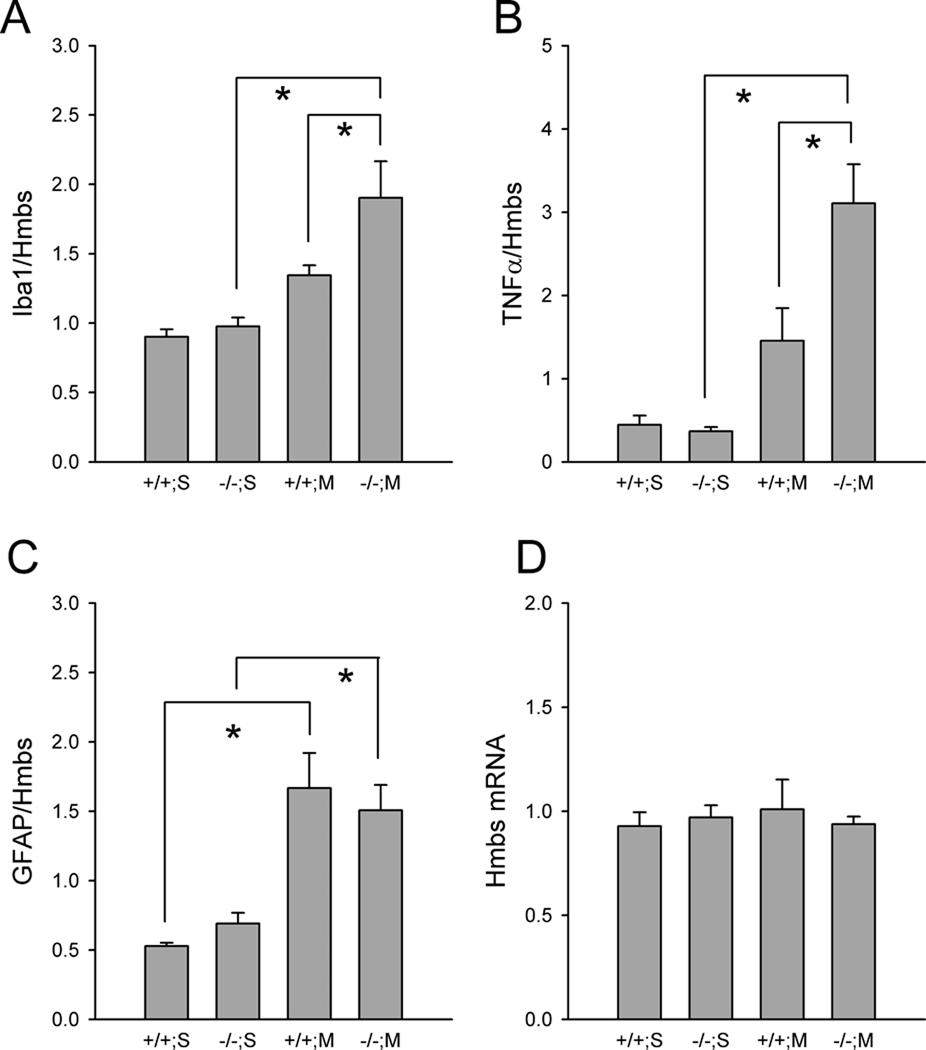
E. Iba-1, TNFα and GFAP in the SN after Meth administration
Nigral tissues were collected at 3 days after Meth or saline administration for qRTPCR analysis. There was no change in reference gene Hmbs (Fig 7D, F1,29=0.0225, p=0.882) or HPRT1 (data not shown) expression between +/+ and −/− mice as well as those receiving Meth or saline treatment (F1,29=0.0624, p=0.804, 2-way ANOVA). The expression of Iba-1, TNFα and GFAP was normalized to the level of Hmbs. Meth treatment significantly upregulated Iba-1 (p=0.001, F1,29=13.668, Fig 7A), TNFα (p<0.001, F1,24=22.720, Fig 7B) and GFAP (p<0.001, F1,26=26.041, Fig 7C) expression. Post-hoc Newman-Keuls analysis indicated that Meth significantly increased Iba-1 (p=0.024, Fig 7A) and TNFα (p=0.003, Fig 7B) mRNA levels in −/−, but not in +/+ mice. No difference was found between +/+ and −/− mice receiving saline injection. GFAP mRNA was significantly increased by MA, compared to saline, in +/+ mice (p<0.001) and −/− mice (p=0.005). No difference was found between +/+ and −/− mice after Meth injection (p=0.517 Fig 7C).
Fig 7. Upregulation of Iba-1 and TNFα mRNA in nigal tissues in A3R −/− mice after Meth treatment.
The expression Iba-1, TNFα and GFAP was normalized to the level of a reference gene Hmbs. Meth significantly upregulated the expression of (A) Iba-1 and (B) TNFα in −/−, but not in +/+ mice. (C) GFAP mRNA was significantly increased by Meth in +/+ mice and −/− mice. No difference was found between +/+ and −/− mice after Meth injection. (D) There is no change in Hmbs expression. * p<0.05, 2-way ANOVA+Newman-Keuls test.
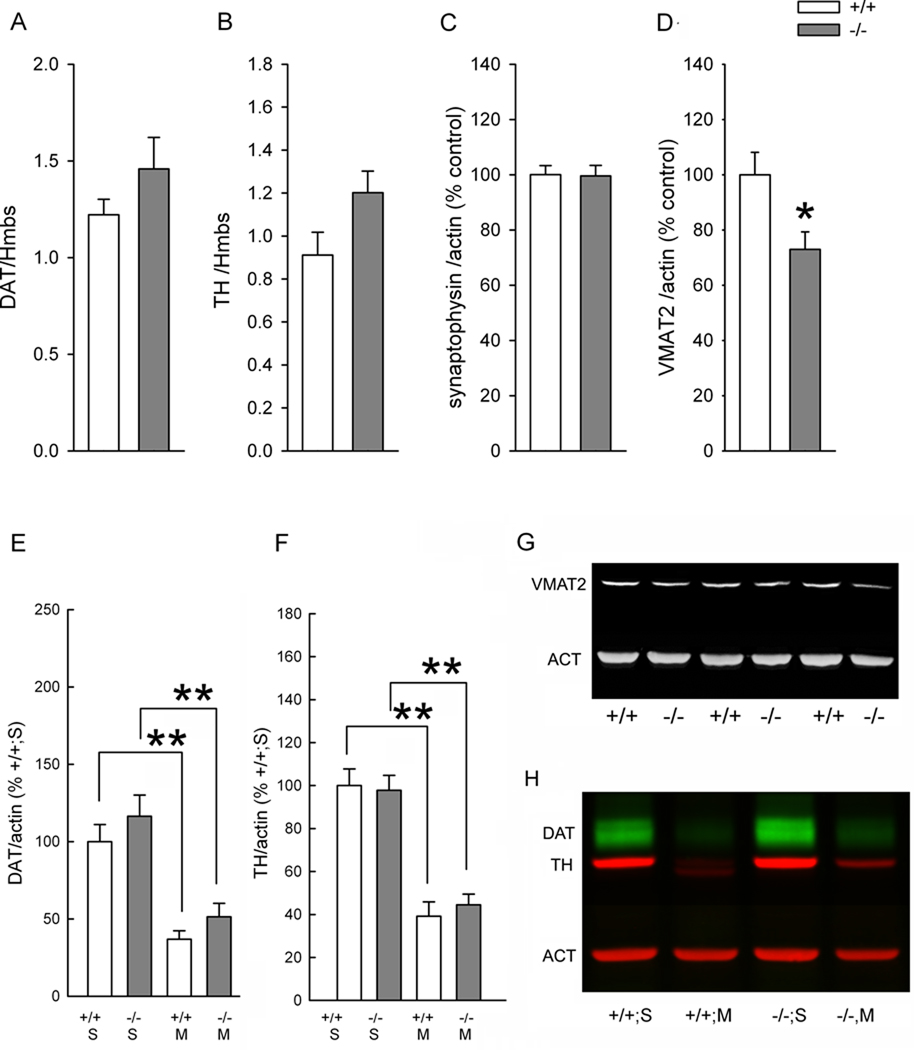
F. TH, DAT, VMAT, and synaptophysin expression in the nigrostriatal system
A total of 12 mice (5 +/+ and 7 −/−) without Meth pretreatment were used to examine the expression of DAT and TH mRNA levels in nigra using RTPCR (Fig 8A & B). There was no difference in the expression of the reference genes Hmbs or HPRT1. Data from each animal was normalized to the level of Hmbs. There was no difference in DAT (p = 0.251, t test, Fig 8A) or TH (p= 0.077, t test, Fig 8B) mRNA expression in the SN.
Fig 8. Selective reduction of VMAT2 expression in A3R −/− mice.
(A–D) Brain samples taken from mice without Meth pretreatment. RTPCR analysis indicates that the mRNA expression of (A) DAT and (B) TH, normalized to a reference gene Hmbs, in nigra was not altered in −/− mice. Western analysis shows that (D) VMAT2, but not (C) synaptophysin, was significantly reduced in −/− animals (*p<0.05). (G) A representing Western blot demonstrating that VMAT2 immunoreactivity was reduced in −/− mice. (E and F) Brain samples taken from mice at 3 days after saline or Meth treatment. Meth significantly reduced DAT (E, **p<0.001; H) and TH (F, **p<0.001) expression in +/+ and −/− mice. There was no difference in DAT or TH expression between +/+ or −/− mice. Data from Panels C,D, E, and F was normalized to the mean of WT controls. S: saline, M: Meth.
Western blotting was used to examine the expression of VMAT-2 and synaptophysin in the striatum in 16 mice (8 +/+ and 8 −/−) without Meth pretreatment. The protein level of VMAT-2 (VMAT/actin) was significantly reduced in −/− mice, compared to +/+ mice (p=0.010, t test, Figs 8D, G). There was no difference in synaptophysin (p= 0.932, t test, Fig 8C) expression in striatum.
The protein expression of TH and DAT in the striatum was in 18 +/+ and 19 −/− mice at 3 days after saline or Meth treatment. Meth significantly reduced DAT (p<0.001, two way ANOVA) and TH (p<0.001, two way ANOVA) expression in +/+ and −/− mice (Fig 8E, 8F, 8H). There was no difference in DAT (p=0.265) or TH (p=0.862) expression between +/+ or −/− mice.
Discussion
Similar to previous reports, we found that high doses of Meth reduced locomotor behavior, increased caspase-3 activation in striatum, and enhanced TNFα expression in nigra (Goncalves et al., 2008; Harvey et al., 2009; Shen et al., 2008). These “neurodegenerative” responses were further potentiated in A3R −/− mice. We previously reported an increase in cerebral infarction in the A3R −/− mice, compared to +/+ mice, after a transient middle cerebral artery occlusion (Chen et al., 2006). Taken together, these data suggest that suppression of A3R expression is associated with increase in sensitivity to injury induced by a dopaminergic toxin as well as by ischemia.
A3 −/− mice have been reported to have an increase in overall locomotor activity. In this study, we further identified that the increase in activity in −/− mice occurred mainly in the dark phase between 4 to 52 hours after a 4-hour habituation to a novel environment. In contrast, difference in the first 4 hours activity was not found between −/− and +/+ animals. Previous studies have shown that behavior during habituation can be affected by non-dopaminergic mechanisms (Mar et al., 2000; Sergeeva et al., 2005; Takeda et al., 2003). These data suggest that there is a differential behavioral response during and after habituation in A3R −/− mice.
The cause of hyperactivity after habituation in −/− mice is still not clear although several attempts have been made to examine the cause of hyperactivity in these animals. It has been shown that A1 and A2A receptors were not altered in the A3R −/− mice and no difference in D1 or D2 receptor binding was found between +/+ and −/− mice (Bjorklund et al., 2008). Our data also indicate that TH and DAT expression, as well as basal dopamine levels in striatum, are not affected after deletion of A3R. In contrast, −/− mice have a significant decrease in the expression of VMAT2 in striatum in mice without Meth pre-treatment. It is not clearly known if deficiency in VMAT2 expression leads to behavioral hyperactivity. One study has shown that heterozygous VMAT2 knockout mice had nearly-normal spontaneous locomotor activity in the 45min test period, similar to the habituation in A3R −/− mice in our experiment. Their locomotor activity after habituation has not been investigated. However, in the same study, a significant enhanced hyperactivity was found in VMAT2 heterozygotes, compared to the wild type controls, after receiving a low dose (1 mg/kg) amphetamine (Takahashi et al., 1997) possibly through increasing cytosolic DA release. These data also suggest that heterozygous VMAT2 mice have a differential behavioral response before and after dopaminergic activation. Since VMAT2 regulates cytosolic DA turnover, a more extensive study is required to elucidate if increase in cytosolic DA facilitates non-vesicular DA release and alters basal locomotor behavior after habituation in A3R −/− animals.
Because of the difference in basal activity between +/+ and −/− mice, relative locomotor activity was used to compare post- Meth response in these animals. Although high doses of Meth had the effect of reducing activity in both A3R −/− and +/+ animals to about the same absolute level of activity, a greater reduction in relative locomotor activity was found in the A3R −/− mice.
Our unpublished observations indicate that Meth treatment slightly (15-17%), but not significantly, reduced VMAT2 protein expression in striatum in both A3R −/− (p=0.085, t test) and +/+ (p=0.265, t test) mice at 3 days after injection. Our data was different from a previous report that VMAT2 expression was reduced at one hour after the last dose of Meth in adult rats (Eyerman and Yamamoto, 2005), which may be attributed to the strain of animals used and/or timing of tissue harvested.
In this study, a prolonged 2-day behavioral effect of Meth in A3R −/− mice was identified. In contrast to hyperactivity induced by low dose amphetamine, high doses of Meth reduces locomotor function through neurodegeneration (Chou et al., 2008). High dose of Meth further suppressed locomotor activity in −/− mice. Similar behavioral response was found in +/+ mice receiving VMAT2 inhibitor reserpine pretreatment, suggesting that inhibition of VMAT2 function potentiates Meth -mediated behavioral deficits. The interaction of VMAT2 and Meth on degeneration is further supported by the fact that Meth –induced neurotoxicity is enhanced in animals pretreated with reserpine (Thomas et al., 2009) or in transgenic mice with reduced VMAT2 (Fumagalli et al., 1999; Guillot et al., 2008b). Under normal conditions, DA is taken up into presynaptic terminals via DAT. VMAT2 transports cytosolic DA into presynaptic vesicles, protects DA from MAO metabolism, and lowers the potential neurotoxicity of DA by reducing DA auto-oxidation and free radical formation in the cytosol. Since A3R −/− mice had less VMAT2 expression and suppression of VMAT2 potentiates Meth—mediated neurodegeneration, the increase neurotoxicity of Meth in −/− mice may be attributed to the deficiency in VMAT2 transport function in DA neurons.
In agreement with previous reports (Kuhn et al., 2008), we also found that striatal DA level was reduced after high doses of Meth and was further attenuated by the pretreatment with reserpine in +/+ mice (unpublished observation). We found that A3R −/− or reserpinized +/+ mice had an increase DOPAC/DA ratios at 3 days after Meth administration. The elevation of tissue DOPAC content, which was also found in the heterozygous VMAT2 knockout mice (Takahashi et al., 1997), represents a typical response of vesicular inhibition which catecholamines redistribute from vesicles to cytosol and are subsequently metabolized by MAO (Colzi et al., 1993). It is likely that the increase in DOPAC or DOPAC/DA ratio in A3R −/− mice or in +/+ receiving reserpine treatment is attributed to the deficiency in VMAT2 function. Cytosolic DA cannot be efficiently taken to the vesicle in these mice. The excess DA molecules in cytosol are then metabolized by MAO to DOPAC and resulting in an increase in DOPAC/DA ratio.
Activation of A3R reduces inflammation and pro-inflammatory cytokine production after injury. In cultured cells, treatment with the A3R agonist Cl-IB-MECA suppressed lipopolysaccharide (LPS) -induced NF-κB activation and TNF-α production in murine BV2 microglial cells (Lee et al., 2006) and in RAW 264.7 cells (Martin et al., 2006). In peripheral tissue, A3R agonists CF101 and Cl-IB-MECA reduced inflammation by suppression of TNF-α production in murine autoimmune arthritis models (Baharav et al., 2005). In the CNS, treatment with Cl-IB-MECA inhibited microglial activation and reduced the expression of TNF-α and interleukin-1β after subarachnoid haemorrhage (Luo et al., 2010). Several studies have indicated a strong association between microglial activation and Meth neurotoxicity in DA-ergic terminals (Thomas et al., 2004) and in brains of human Meth abusers (Sekine et al., 2008). Increases in cytoplasmic DA by treatment with reserpine increased microglial activation in the nucleus accumbens (Thomas et al., 2009) and in striatum (Kuhn et al., 2008) after Meth. In this study, an overexpression of Iba-1 was found in striatum in A3R −/− mice receiving Meth. Similar to previous reports that Meth alone did not induce microglia activation in the substanita nigra (Thomas et al., 2004), we also found that IBA-1 and TNFα mRNA expression in the nigra region was not altered at 3 days after Meth treatment in +/+ mice; however, was significantly upregulated in A3R −/− mice. These data suggest that deficiency in A3R expression can potentiate Meth –mediated inflammation in striatum and nigra cells and endogenous A3R may be associated with the anti-inflammatory properties against Meth –mediated neurodegeneration. Whether potentiation of Iba-1 and TNFα in −/− is due to the increase in cytosolic DA requires further investigation.
A3R has been found in brain at low concentration (Lopes et al., 2003). Selective Cl-IB-MECA or [125I]AB-MECA binding was found in caudate and nucleus accumbens and other brain regions by autoradiography (Wadsak et al., 2008). It is likely that the increased sensitivity to high dose Meth in A3R −/− is due to the absence of A3R in adult brain. It has been shown that pretreatment with the developmental toxicant methylmercury during pregnancy and lactation enhanced the exploratory horizontal behavior in A3R −/−, but not in +/+, offspring mice (Bjorklund et al., 2008). These data suggest a possible role of A3 receptors during development. It is also likely that A3R function during development may indirectly alter the expression of VMAT2 and the sensitivity to Meth.
In conclusion, we have found that deletion of A3R enhanced vulnerability to injury induced by high dose of Meth. The cause of this finding may be attributed to the reduction in VMAT2 expression in dopaminergic neurons because VMAT knockout mice also suffer from enhanced Meth toxicity.
Highlights.
Suppression of A3R expression increases sensitivity to injury induced by high doses of Meth in dopaminergic neurons.
Meth significantly increased the expression of Iba-1, cleaved caspase-3, and TNFα in A3R −/− mice, compared to +/+, mice.
The increase in sensitivity to Meth injury in A3R −/− mice may be attributed to a reduction in VMAT2 expression.
Acknowledgment
This study was supported by NIDA IRP. We would like to thank Dr Jean Lud Cadet for his critical suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Baharav E, Bar-Yehuda S, Madi L, Silberman D, Rath-Wolfson L, Halpren M, Ochaion A, Weinberger A, Fishman P. Antiinflammatory effect of A3 adenosine receptor agonists in murine autoimmune arthritis models. J Rheumatol. 2005;32:469–476. [PubMed] [Google Scholar]
- Bjorklund O, Halldner-Henriksson L, Yang J, Eriksson TM, Jacobson MA, Dare E, Fredholm BB. Decreased behavioral activation following caffeine, amphetamine and darkness in A3 adenosine receptor knock-out mice. Physiol Behav. 2008;95:668–676. doi: 10.1016/j.physbeh.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Brand A, Vissiennon Z, Eschke D, Nieber K. Adenosine A(1) and A(3) receptors mediate inhibition of synaptic transmission in rat cortical neurons. Neuropharmacology. 2001;40:85–95. doi: 10.1016/s0028-3908(00)00117-9. [DOI] [PubMed] [Google Scholar]
- Chen GJ, Harvey BK, Shen H, Chou J, Victor AI, Wang Y. Activation of adenosine A3 receptors reduces ischemic brain injury in rodents. J Neurosci Res. 2006;84:1848–1855. doi: 10.1002/jnr.21071. [DOI] [PubMed] [Google Scholar]
- Chou J, Luo Y, Kuo CC, Powers K, Shen H, Harvey BK, Hoffer BJ, Wang Y. Bone morphogenetic protein-7 reduces toxicity induced by high doses of methamphetamine in rodents. Neuroscience. 2008;151:92–103. doi: 10.1016/j.neuroscience.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzi A, D'Agostini F, Cesura AM, Borroni E, Da PM. Monoamine oxidase-A inhibitors and dopamine metabolism in rat caudatus: evidence that an increased cytosolic level of dopamine displaces reversible monoamine oxidase-A inhibitors in vivo. J Pharmacol Exp Ther. 1993;265:103–111. [PubMed] [Google Scholar]
- Cubells JF, Rayport S, Rajendran G, Sulzer D. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J Neurosci. 1994;14:2260–2271. doi: 10.1523/JNEUROSCI.14-04-02260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di IP, Kleywegt S, Ciccarelli R, Traversa U, Andrew CM, Crocker CE, Werstiuk ES, Rathbone MP. Mechanisms of apoptosis induced by purine nucleosides in astrocytes. Glia. 2002;38:179–190. doi: 10.1002/glia.10055. [DOI] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. Lobeline attenuates methamphetamine-induced changes in vesicular monoamine transporter 2 immunoreactivity and monoamine depletions in the striatum. J Pharmacol Exp Ther. 2005;312:160–169. doi: 10.1124/jpet.104.072264. [DOI] [PubMed] [Google Scholar]
- Fedorova IM, Jacobson MA, Basile A, Jacobson KA. Behavioral characterization of mice lacking the A3 adenosine receptor: sensitivity to hypoxic neurodegeneration. Cell Mol Neurobiol. 2003;23:431–447. doi: 10.1023/A:1023601007518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Valenzano KJ, Caron MG. Role of dopamine transporter in methamphetamine-induced neurotoxicity: evidence from mice lacking the transporter. J Neurosci. 1998;18:4861–4869. doi: 10.1523/JNEUROSCI.18-13-04861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Wang YM, Valenzano KJ, Miller GW, Caron MG. Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter 2 knock-out mice. J Neurosci. 1999;19:2424–2431. doi: 10.1523/JNEUROSCI.19-07-02424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves J, Martins T, Ferreira R, Milhazes N, Borges F, Ribeiro CF, Malva JO, Macedo TR, Silva AP. Methamphetamine-induced early increase of IL-6 and TNF-alpha mRNA expression in the mouse brain. Ann N Y Acad Sci. 2008;1139:103–111. doi: 10.1196/annals.1432.043. [DOI] [PubMed] [Google Scholar]
- Grace CE, Schaefer TL, Herring NR, Graham DL, Skelton MR, Gudelsky GA, Williams MT, Vorhees CV. Effect of a neurotoxic dose regimen of (+)-methamphetamine on behavior, plasma corticosterone, and brain monoamines in adult C57BL/6 mice. Neurotoxicol Teratol. 2010;32:346–355. doi: 10.1016/j.ntt.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot TS, Richardson JR, Wang MZ, Li YJ, Taylor TN, Ciliax BJ, Zachrisson O, Mercer A, Miller GW. PACAP38 increases vesicular monoamine transporter 2 (VMAT2) expression and attenuates methamphetamine toxicity. Neuropeptides. 2008a;42:423–434. doi: 10.1016/j.npep.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot TS, Shepherd KR, Richardson JR, Wang MZ, Li Y, Emson PC, Miller GW. Reduced vesicular storage of dopamine exacerbates methamphetamine-induced neurodegeneration and astrogliosis. J Neurochem. 2008b;106:2205–2217. doi: 10.1111/j.1471-4159.2008.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarberg C, Schulte G, Fredholm BB. Evidence for functional adenosine A3 receptors in microglia cells. J Neurochem. 2003;86:1051–1054. doi: 10.1046/j.1471-4159.2003.01919.x. [DOI] [PubMed] [Google Scholar]
- Harvey BK, Chou J, Shen H, Hoffer BJ, Wang Y. Diadenosine tetraphosphate reduces toxicity caused by high-dose methamphetamine administration. Neurotoxicology. 2009;30:436–444. doi: 10.1016/j.neuro.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Ladenheim B, McCoy MT, Cluster A, Cai NS, Cadet JL. Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 2005;102:868–873. doi: 10.1073/pnas.0404990102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DM, Francescutti-Verbeem DM, Thomas DM. Dopamine disposition in the presynaptic process regulates the severity of methamphetamine-induced neurotoxicity. Ann N Y Acad Sci. 2008;1139:118–126. doi: 10.1196/annals.1432.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HT, Ota-Setlik A, Xu H, D'Agati VD, Jacobson MA, Emala CW. A3 adenosine receptor knockout mice are protected against ischemia- and myoglobinuria-induced renal failure. Am J Physiol Renal Physiol. 2003;284:F267–F273. doi: 10.1152/ajprenal.00271.2002. [DOI] [PubMed] [Google Scholar]
- Lee JY, Jhun BS, Oh YT, Lee JH, Choe W, Baik HH, Ha J, Yoon KS, Kim SS, Kang I. Activation of adenosine A3 receptor suppresses lipopolysaccharide-induced TNF-alpha production through inhibition of PI 3-kinase/Akt and NF-kappaB activation in murine BV2 microglial cells. Neurosci Lett. 2006;396:1–6. doi: 10.1016/j.neulet.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Lopes LV, Rebola N, Pinheiro PC, Richardson PJ, Oliveira CR, Cunha RA. Adenosine A3 receptors are located in neurons of the rat hippocampus. Neuroreport. 2003;14:1645–1648. doi: 10.1097/00001756-200308260-00021. [DOI] [PubMed] [Google Scholar]
- Luo C, Yi B, Tao G, Li M, Chen Z, Tang W, Zhang JH, Feng H. Adenosine A3 receptor agonist reduces early brain injury in subarachnoid haemorrhage. Neuroreport. 2010;21:892–896. doi: 10.1097/WNR.0b013e32833dbd13. [DOI] [PubMed] [Google Scholar]
- Maddock HL, Gardner NM, Khandoudi N, Bril A, Broadley KJ. Protection from myocardial stunning by ischaemia and hypoxia with the adenosine A3 receptor agonist, IB-MECA. Eur J Pharmacol. 2003;477:235–245. doi: 10.1016/j.ejphar.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Mar A, Spreekmeester E, Rochford J. Antidepressants preferentially enhance habituation to novelty in the olfactory bulbectomized rat. Psychopharmacology (Berl) 2000;150:52–60. doi: 10.1007/s002130000400. [DOI] [PubMed] [Google Scholar]
- Martin L, Pingle SC, Hallam DM, Rybak LP, Ramkumar V. Activation of the adenosine A3 receptor in RAW 264.7 cells inhibits lipopolysaccharide-stimulated tumor necrosis factor-alpha release by reducing calcium-dependent activation of nuclear factor-kappaB and extracellular signal-regulated kinase 1/2. J Pharmacol Exp Ther. 2006;316:71–78. doi: 10.1124/jpet.105.091868. [DOI] [PubMed] [Google Scholar]
- Meyerhof W, Muller-Brechlin R, Richter D. Molecular cloning of a novel putative G-protein coupled receptor expressed during rat spermiogenesis. FEBS Lett. 1991;284:155–160. doi: 10.1016/0014-5793(91)80674-r. [DOI] [PubMed] [Google Scholar]
- Rivo J, Zeira E, Galun E, Matot I. Activation of A3 adenosine receptors attenuates lung injury after in vivo reperfusion. Anesthesiology. 2004;101:1153–1159. doi: 10.1097/00000542-200411000-00015. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeeva OA, Schulz D, Doreulee N, Ponomarenko AA, Selbach O, Borsch E, Kircheis G, Huston JP, Haussinger D, Haas HL. Deficits in cortico-striatal synaptic plasticity and behavioral habituation in rats with portacaval anastomosis. Neuroscience. 2005;134:1091–1098. doi: 10.1016/j.neuroscience.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Weaver DR. [125I]4-aminobenzyl-5'-N-methylcarboxamidoadenosine (125I)AB-MECA) labels multiple adenosine receptor subtypes in rat brain. Brain Res. 1997;745:10–20. doi: 10.1016/s0006-8993(96)01120-1. [DOI] [PubMed] [Google Scholar]
- Shen H, Luo Y, Kuo CC, Wang Y. BMP7 reduces synergistic injury induced by methamphetamine and ischemia in mouse brain. Neurosci Lett. 2008;442:15–18. doi: 10.1016/j.neulet.2008.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Miner LL, Sora I, Ujike H, Revay RS, Kostic V, Jackson-Lewis V, Przedborski S, Uhl GR. VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc Natl Acad Sci U S A. 1997;94:9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H, Tsuji M, Fujisawa Y, Yamada T, Tsuji K, Egashira T, Matsumiya T. Intracerebroventricular administration of endothelin-1 impairs the habituation of rats to a novel environment in conjunction with brain serotonergic activation. Neuroscience. 2003;117:449–460. doi: 10.1016/s0306-4522(02)00871-0. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. Increases in cytoplasmic dopamine compromise the normal resistance of the nucleus accumbens to methamphetamine neurotoxicity. J Neurochem. 2009;109:1745–1755. doi: 10.1111/j.1471-4159.2009.06094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther. 2004;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- von Arnim CA, Timmler M, Ludolph AC, Riepe MW. Adenosine receptor up-regulation: initiated upon preconditioning but not upheld. Neuroreport. 2000;11:1223–1226. doi: 10.1097/00001756-200004270-00016. [DOI] [PubMed] [Google Scholar]
- Wadsak W, Mien LK, Shanab K, Ettlinger DE, Haeusler D, Sindelar K, Lanzenberger RR, Spreitzer H, Viernstein H, Keppler BK, Dudczak R, Kletter K, Mitterhauser M. Preparation and first evaluation of [(18)F]FE@SUPPY: a new PET tracer for the adenosine A(3) receptor. Nucl Med Biol. 2008;35:61–66. doi: 10.1016/j.nucmedbio.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Zhou QY, Li C, Olah ME, Johnson RA, Stiles GL, Civelli O. Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc Natl Acad Sci U S A. 1992;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JP, Xu W, Angulo N, Angulo JA. Methamphetamine-induced striatal apoptosis in the mouse brain: comparison of a binge to an acute bolus drug administration. Neurotoxicology. 2006;27:131–136. doi: 10.1016/j.neuro.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]