Abstract
Changes in hysteroscopic capabilities have been preceded by technological advances that have enabled them. Modern operative hysteroscopes rely on a variety of different-sized optical, inflow, outflow, and working channels to enable clear visualization of the endometrial cavity as well as the surgical removal of intracavitary lesions such as polyps and myomas. This review examines the relative merits of various hysteroscopic treatment options with a focus on the most recent operative hysteroscopic technique, hysteroscopic morcellation, and how this new technology fits into the armamentarium of the gynecologist.
Key words: Hysteroscopic morcellation, Leiomyoma, Endometrial polyp
The first description of a rudimentary hysteroscope being used to visualize the uterine cavity is credited to D. Commander Pantaleoni in 1869.1 Over the course of the subsequent century, hysteroscopy remained a purely diagnostic endeavor until Neuwirth and Amin used a urologic resectoscope to perform and report the first hysteroscopic resection of a submucous myoma in 1976.2 In more recent years, this transformation has taken on another dimension as operative hysteroscopic procedures have gradually moved from the exclusive domain of the operating room to the physician’s office.
Not surprisingly, changes in hysteroscopic capabilities have been preceded by the technological advances that have enabled them. Whereas the earliest diagnostic hysteroscopes focused solely on optics, later hysteroscopes incorporated nonoptic channels that allowed for the introduction of distention media and instrumentation as well as the removal of media and tissue. Modern operative hysteroscopes rely on a variety of different-sized optical, inflow, outflow, and working channels to enable clear visualization of the endometrial cavity as well as the surgical removal of intracavitary lesions such as polyps and myomas.
This review examines the relative merits of various hysteroscopic treatment options with a focus on the most recent operative hysteroscopic technique, hysteroscopic morcellation, and how this new technology fits into the armamentarium of the gynecologist.
Intrauterine Pathology
Endometrial polyps are one of the most common intrauterine lesions associated with abnormal bleeding symptoms; polyps are found in 10% to 40% of symptomatic women and up to 12% of asymptomatic women.3 The great majority of symptomatic endometrial polyps occur in premenopausal women, with the highest incidence in the fifth decade of life.4 In addition to causing bleeding symptoms such as menorrhagia, metrorrhagia, or intermenstrual spotting, endometrial polyps may be associated with subfertility or premalignant and malignant tissue changes. The use of tamoxifen and conditions such as Lynch syndrome may be associated with additional risk of developing endometrial polyps. Asymptomatic polyps less than 2 cm in premenopausal women may be monitored by the physician. However, in patients with risk factors for endometrial neoplasia (ie, postmenopausal age, personal or family history of ovary/breast/colon/endometrial cancer, tamoxifen use, chronic anovulation, obesity, unopposed estrogen therapy), any lesion should be removed and sent for pathologic examination. In symptomatic patients, it has been reported that polypectomy results in improvement of symptoms in 75% to 100% of women.5
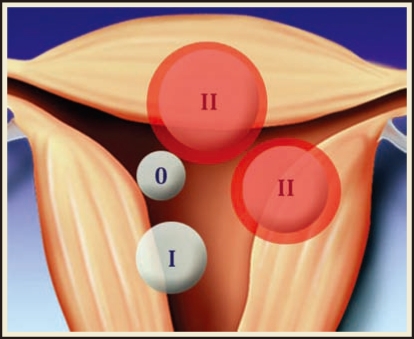
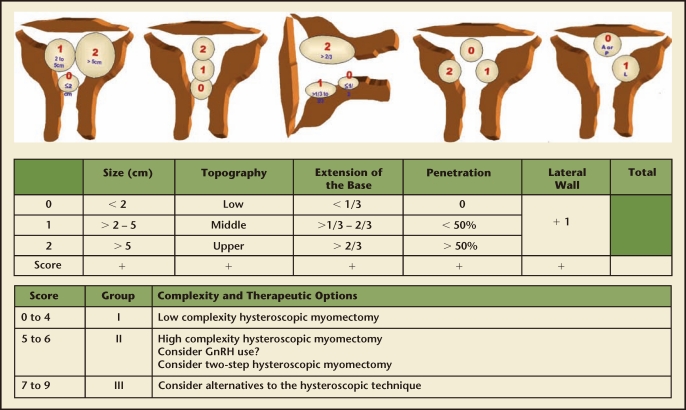
Leiomyomas, the most common gynecologic tumor, are found in up to 70% to 80% of women.6 Risk factors for uterine fibroids include black race, early menarche, and low parity; nonspecific hereditary factors have also been implicated.6,7 Myomas in the submucosal location specifically may cause abnormal uterine bleeding or subfertility, and are amenable to hysteroscopic removal. The European Society of Gynaecological Endoscopy (ESGE) classifies submucosal myomas as Type 0 if the entire lesion is intracavitary, Type I if less than 50% extends into the myometrium, and Type II if greater than 50% of the myoma is intramyometrial (Figure 1).8 A correlation has been found between the depth of myometrial involvement and rate of complete resection at time of hysteroscopy; Type II myomas have the lowest rate of complete resection at 61% to 83%.8,9 Large fibroid size may also be associated with risk of recurrence or incomplete resection, with fibroids larger than 3 to 4 cm often requiring repeat procedures10 and myomas larger than 6 cm demonstrating both high recurrence and high complication rates.11 To further refine the preoperative classification of submucosal myomas as a means of predicting complete resection, Lasmer and colleagues introduced the STEPW (size, topography, extension, penetration, wall) Classification system in 2005 (Figure 2) and recently demonstrated significant improvement in its prognostic capabilities as compared with the older, simpler ESGE classification system.12
Figure 1.
European Society of Gynaecological Endoscopy classification. Submucosal myomas are classified as Type 0, Type I, or Type II, depending on the depth of myometrial penetration.
Figure 2.
STEPW (size, topography, extension, penetration, wall) classification system. GnRH, gonadotropin-releasing hormone. Reproduced with permission from Lasmer RB et al.12
Another pathologic entity that is amenable to hysteroscopic removal is retained products of conception. Tissue remaining in the uterus following a pregnancy event, either placental or fetal, may cause abnormal bleeding, pain, or infection. Pelvic ultrasonography may be useful to identify the retained products of conception, although findings of thickened and irregular endometrium do not perfectly correlate with this diagnosis.13 In some cases, hysteroscopy may offer the dual advantage of a sensitive diagnostic tool and concomitant therapeutic intervention for this postpartum complication.14
Traditional Operative Techniques
Many options exist for the treatment of intrauterine lesions. Dilation and curettage, a blind procedure guided by tactile feedback, may be used as a diagnostic procedure to obtain tissue for pathologic examination, a temporizing measure for heavy uterine bleeding, or as a treatment of abortion, abnormal pregnancy event, and retained products of conception. Complications may include recognized or unrecognized uterine perforation or trauma, infection, or formation of intrauterine adhesions. With regard to surgical treatment of endometrial polyps, a hysteroscopically guided procedure has been demonstrated to have superior efficacy compared with the blind approach with sharp curettage or polypectomy forceps.15
Hysteroscopic resectoscopy utilizing a radiofrequency (RF) energy device can be used to remove large polyps and submucosal myomas, or for the treatment of less common conditions such as intrauterine synechia or uterine septa. A monopolar or bipolar energy source may be used depending on surgeon preference. Choice of distending media varies depending on which energy modality is used, but careful fluid management is critical to ensure patient safety in both circumstances. Monopolar electrosurgery requires a nonconducting, electrolyte-poor fluid such as glycine, sorbitol, or mannitol to prevent dispersion of the electrical current. Bipolar electrosurgery may be performed with isotonic solutions such as normal saline or lactated Ringers. The nonconductive distension media carry additional risks of volume overload and electrolyte derangements with brain damage and deaths reported secondary to hyponatremia16; accordingly, there is a lower threshold for hysteroscopic fluid deficit in these cases. Loop or rollerball electrodes that may be used in resectoscopy have the advantage of coagulating bleeding vessels as the procedure progresses, but often leave surgeons battling tissue “chips.” Thin strips of resected tissue, or chips, are created as the case proceeds and need to be periodically removed from the uterine cavity to enhance visualization. With larger myomas, the time spent in chip removal can be significant. These tissue fragments are typically sent for pathologic examination. Vaporizing electrodes may also be used, obviating the need for frequent chip removal but precluding pathologic examination of the specimen.
Hysteroscopic Morcellation
Although hysteroscopic loop-electrode resectoscopy provided a reliable method for removing intrauterine pathology for many years, the distension media issues, risks of perforation, and visual field limitation created by resected chips all combined to encourage the development of alternate treatment methods. One such alternative is hysteroscopic morcellation. Using a modified prototype based on an orthopedic arthroscopic tissue shaver, Dr. Mark Hans Emanuel of The Netherlands was able to create a first-generation device that used mechanical energy rather than electrical energy to resect uterine tissue.
TRUCLEAR™ Hysteroscopic Morcellator
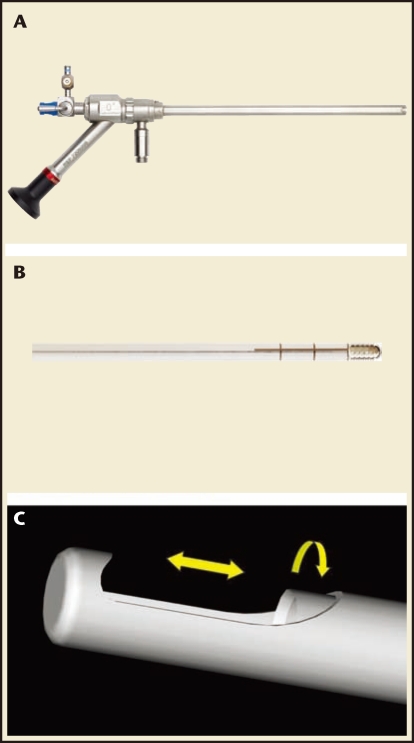
In 2005, the US Food and Drug Administration (FDA) approved the TRUCLEAR™ hysteroscopic morcellator (Smith & Nephew, Andover, MA) as the first mechanical morcellator for intrauterine pathology. This device uses a single-use rigid metal inner tube with cutting edges that rotate and/or reciprocate within a 4-mm rigid metal outer tube. The outer tube incorporates a side-facing cutting window at its distal end (Figure 3). The blade assembly is secured to a reusable hand piece to which a suction tube is attached. The hand piece is also connected to a motor control unit. Suction is applied to the inner tube and tissue is then pulled into the cutting window as the inner tube rotates at 1100 rpm.17 The resected tissue is then aspirated through the device into a collecting pouch for later histopathologic analysis. The entire device is introduced into the uterine cavity with a custom-designed 9-mm outer diameter, rigid, continuous-flow, 0°; hysteroscope that requires a custom-designed high-flow Smith & Nephew fluid pump for proper functioning.
Figure 3.
TRUCLEAR™ hysteroscopic morcellator (Smith & Nephew, Andover, MA) and cutting blades. (A) TRUCLEAR hysteroscope with cutting blade inserted. (B) TRUCLEAR rotating cutting blade. (C) TRUCLEAR reciprocating blade. Note beveling on the inner surface of the blade. Images courtesy of Smith & Nephew, Inc.
MyoSure® Tissue Removal System
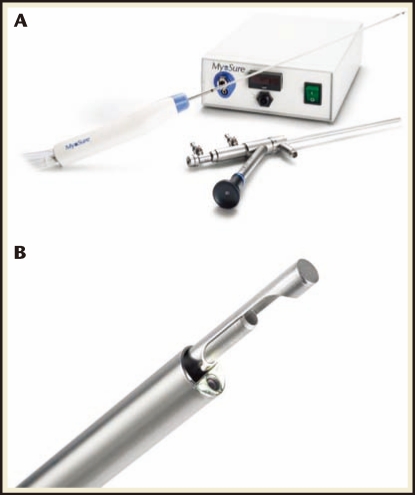
In 2009, the FDA approved a second hysteroscopic morcellation device—the MyoSure® Tissue Removal System (Hologic, Bedford, MA). Like the first generation TRUCLEAR, the second generation MyoSure system relies on a suction-based, mechanical energy, rotating tubular cutter system rather than the high-frequency electrical energy historically used by resectoscopy systems to remove intrauterine tissue. However, the newer MyoSure system has a smaller 2.5-mm inner blade that rotates and reciprocates within a 3-mm outer tube at speeds as high as 6000 rpm and presents an outer bevel rather than an inner bevel on the rotating blade edge (Figure 4).18 The blade and hand piece are combined into a single-use device that is then attached to suction and a motor control unit. The device is introduced into the uterus through a 6.25-mm offset lens, 0°; custom-designed continuous flow hysteroscope that is compatible with all currently available fluid management systems (although the device functions better with higher-flow, higher-pressure pumps). Table 1 provides a comparison between the TRUCLEAR and MyoSure systems.
Figure 4.
MyoSure® Tissue Removal System (Hologic, Bedford, MA). (A) MyoSure system hysteroscope, hand piece, and motor drive. (B) MyoSure system blade inserted through hysteroscope. Note beveling on the outer surface of the blade. Photos courtesy of Hologic.
Table 1.
Comparison of Device Characteristics of TRUCLEAR™ Hysteroscopic Morcellator and MyoSure® Tissue Removal System
| Morcellator Characteristic | TRUCLEAR | MyoSure |
| Device outer diameter | 4 mm | 3 mm |
| Hysteroscope outer diameter | 9 mm | 6.25 mm |
| Pump compatibility | Smith & Nephew pump | Any fluid management system |
| Blade rotational speed | 1100 rpm | 6000 rpm |
| Blade edge | Inner bevel | Outer bevel |
| Maximum rate of suction | 200 mm Hg | 400 mm Hg |
TRUCLEAR™ hysteroscopic morcellator (Smith & Nephew, Andover, MA)
MyoSure® Tissue Removal System (Hologic, Bedford, MA)
Hysteroscopic Morcellation Technique
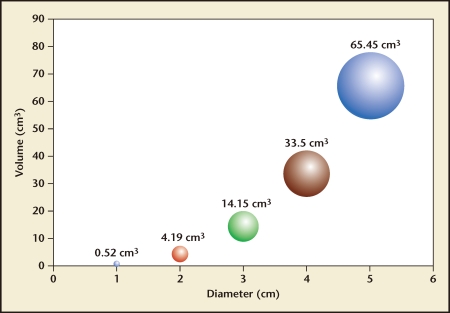
Regardless of the methodology used to resect intrauterine pathology, it is important to remember that resected tissue must be thought of in terms of three-dimensional rather than two-dimensional measurements. Thus, increasing pathology diameter yields a exponential rather than linear increase in volume following the equation ν = πd3/6 (see Figure 5). This mathematical consideration becomes important as one plans a surgical approach for submucous myomas in which the resection rate and procedure time will be a function of the volume, density, and type of myoma tissue. With loop resectoscopy, the amount of tissue removed per minute will depend on (1) how quickly the surgeon deploys each pass of the loop, (2) how much tissue each bite with the loop resects, and (3) how quickly the tissue chips can be removed from the uterine cavity. On the other hand, with hysteroscopic morcellation, the amount of tissue removed per minute will only be a function of (1) how much contact the cutting window maintains with the myoma and (2) how quickly the device can cut tissue and aspirate it out. Because the devices’ cutting speeds are relatively fixed by their design characteristics, minimizing procedure time mostly depends on maintaining tissue contact between the cutting window and the pathology. Learning the correct resection technique, although not difficult, is of prime importance with hysteroscopic morcellation.
Figure 5.
Volume as a function of diameter (ν = πd3/6).23
Morcellation Versus Resectoscopy
For polyps and Type I and Type II submucous myomas, hysteroscopic morcellation has been demonstrated to be both faster and easier to learn than traditional resectoscopy. The earliest published trial with a hysteroscopic morcellation device by Emanuel and colleagues showed a significant reduction in operating room time when removing polyps and Type I and Type II submucous myomas. In that study, polyps were removed with a 72% reduction in operating room time with a morcellator as compared with a resectoscope (8.7 min vs 30.9 min), whereas Type 0 and Type I myomas were removed in 61% less time, respectively (16.4 min vs 42.2 min).19 Similarly, in a 2008 trial by van Dongen and associates, 60 patients with intrauterine pathology consisting of either a polyp or a Type 0 myoma or Type I myoma smaller than 30 mm were randomized to either hysteroscopic morcellation or loop-electrode resection. All the procedures were performed by residents in training under the direct guidance of an attending physician. The morcellation group demonstrated a 38% reduction in operating room (OR) time (17 min vs 10.6 min; P = .008) as well as a 32% reduction in distention media used (5050 mL vs 3413 mL; P = .041). Not surprisingly, the trial also demonstrated a marked reduction in the number of insertions and reinsertions of the hysteroscope to remove chips when the morcellator was used (number of insertions = 1 [range, 1–2]) compared with the resectoscope (number of insertions = 7 [range, 3–50]).20
Looking again at polyps and Type I and Type II submucous myomas but using the newer MyoSure device, Miller and coworkers reported average polyp morcellation times of 37 seconds and average myoma morcellation times of 6.4 minutes for Type 0, I, and II myomas with a mean diameter of 31.7 mm.21 These data were further validated in a recent abstract by Lukes, who reported using the MyoSure device to remove 6 myomas (≤ 3 cm) and 20 polyps in 13 women with a mean resection time of 84 seconds. All 13 procedures were performed in an office setting using local anesthesia with average pain scores < 1 using the Wong-Baker Faces Rating Scale (no pain = 0; worst pain = 10).22
MyoSure Device Versus TRUCLEAR System
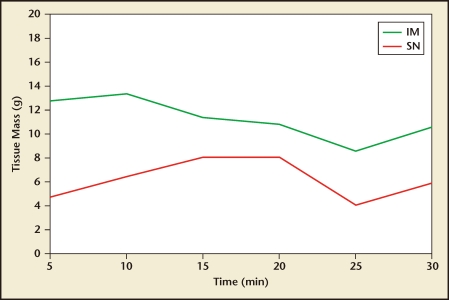
Although in vivo accurate measurements of tissue resection speed are challenging to conclusively determine due to surgeon and pathology variations, in vitro measurements have been performed to assess the tissue resection characteristics of the different devices. As part of an IRB-approved FDA submission study in 2008, the author (JAG) compared a working MyoSure device prototype with a TRUCLEAR device to assess tissue resection speed. Fresh, discarded uterine leiomyoma tissue was placed in a saline-filled container and each device was placed directly on the tissue in alternating 5-minute intervals for 30 minutes. The trial was repeated on three different myoma specimens. The study was designed to compare tissue cutting on identical tissue and to assess decline of cutting speed over time as a result of blade dulling. The results are presented in Table 2 and demonstrated graphically in Figure 6.23 As these data demonstrate, both devices are capable of resecting submucous myomas 3 cm in diameter (∼15 cc3) in 15 minutes or less, although the MyoSure device was consistently faster at tissue removal at every time interval despite its smaller diameter.
Table 2.
MyoSure® Tissue Removal System Versus TRUCLEAR™ Hysteroscopic Morcellator Tissue Cutting Performance
| Tissue (g) Test | ||||||
| System | Time (min) | 1 | 2 | 3 | Avg | |
| IM | 5 | 12.9 | 10.6 | 14.9 | 12.8 | |
| 10 | 15.9 | 11.0 | 13.0 | 13.3 | SD | |
| 15 | 12.2 | 4.8 | 17.2 | 11.4 | 3.3 | |
| 20 | 10.1 | 7.8 | 14.4 | 10.8 | ||
| 25 | 11.3 | 7.6 | 6.8 | 8.6 | ||
| 30 | 10.7 | 12.5 | 8.4 | 10.5 | ||
| SN | 5 | 6.4 | 2.7 | 5.2 | 4.8 | |
| 10 | 4.6 | 8.5 | 6.1 | 6.4 | SD | |
| 15 | 10.5 | 8.7 | 4.9 | 8.0 | 3.2 | |
| 20 | 10.6 | 1.6 | 13.0 | 8.0 | ||
| 25 | Clog | 5.8 | 2.3 | 4.1 | ||
| 30 | Clog | 6.5 | 5.3 | 5.9 | ||
Clog, morcellator could not be cleared; IM, Interlace Medical; SD, standard deviation; SN, predicate device.
Data from Greenberg JA et al.23
Figure 6.
MyoSure® Tissue Removal System (Hologic, Bedford, MA) versus TRUCLEAR™ hysteroscopic morcellator (Smith & Nephew, Andover, MA) tissue cutting performance in average grams per minute over 30 minutes. IM, Interlace Medical; SN, predicate device. Data from Greenberg JA et al.23
In addition, the smaller diameter of the MyoSure hysteroscope (6.25 mm) compared with the TRUCLEAR hysteroscope (9.0 mm) makes the MyoSure device potentially more compatible with an oral sedation/cervical block anesthesia protocol and therefore amenable to office-based treatments of polyps and Type 0 or I submucosal fibroids.22
Summary
Endometrial polyps and submucous myomas represent a common gynecologic problem that frequently requires surgical intervention. Although traditional hysteroscopic resection with a loop electrode has been a reliable tool for the gynecologic surgeon, its use typically requires a large diameter hysteroscope (7–9 mm outer diameter), hypotonic distension media, and a well-anesthetized patient. Further, the loop resectoscopy process invariably produces vision-obscuring tissue chips and introduces a significant risk for uterine perforation.
Conversely, in patients with polyps or Type 0 and Type I submucous myomas, hysteroscopic morcellation allows for the use of smaller diameter hysteroscopes that require less cervical dilation and less anesthesia without sacrificing procedure time. Moreover, with these newer, smaller morcellating devices, hysteroscopic procedures that were once confined to the OR may slowly gravitate into the office where patients, physicians, and third-party payers can all realize significant cost savings and efficiency improvements.
Main Points.
In patients with risk factors for endometrial neoplasia (ie, postmenopausal age, personal or family history of ovary/breast/colon/endometrial cancer, tamoxifen use, chronic anovulation, obesity, unopposed estrogen therapy), any lesion should be removed and sent for pathologic examination.
Myomas in the submucosal location specifically may cause abnormal uterine bleeding or subfertility, and are amenable to hysteroscopic removal.
Another pathologic entity that is amenable to hysteroscopic removal is retained products of conception. Tissue remaining in the uterus following a pregnancy event, either placental or fetal, may cause abnormal bleeding, pain, or infection.
Although hysteroscopic loop-electrode resectoscopy provided a reliable method for removing intrauterine pathology for many years, the distention media issues, risks of perforation, and visual field limitation created by resected chips all combined to encourage the development of alternate treatment methods. One such alternative is hysteroscopic morcellation.
With hysteroscopic morcellation, the amount of tissue removed per minute will only be a function of (1) how much contact the cutting window maintains with the myoma and (2) how quickly the device can cut tissue and aspirate it out. Because the devices’ cutting speeds are relatively fixed by their design characteristics, minimizing procedure time mostly depends on maintaining tissue contact between the cutting window and the pathology. Learning the correct resection technique, although not difficult, is of prime importance with hysteroscopic morcellation.
Hysteroscopic morcellation allows for the use of smaller diameter hysteroscopes that require less cervical dilation and less anesthesia without sacrificing procedure time. Moreover, with these newer, smaller morcellating devices, hysteroscopic procedures that were once confined to the operating room may slowly gravitate into the office where patients, physicians, and third-party payers can all realize significant cost savings and efficiency improvements.
Footnotes
Dr. Greenberg was a paid consultant on the medical advisory board of Interlace Medical, Inc., the original manufacturers of the MyoSure device.
References
- 1.Siegle AM. The early history of hysteroscopy. J Am Assoc Gynecol Laparosc. 1998;5:329–332. doi: 10.1016/s1074-3804(98)80044-3. [DOI] [PubMed] [Google Scholar]
- 2.Neuwirth RS, Amin HK. Excision of submucous fibroids with hysteroscopic control. Am J Obstet Gynecol. 1976;126:95–99. doi: 10.1016/0002-9378(76)90471-3. [DOI] [PubMed] [Google Scholar]
- 3.Lieng M, Istre O, Qvigstad E. Treatment of endometrial polyps: a systematic review. Acta Obstet Gynecol Scand. 2010;89:992–1002. doi: 10.3109/00016349.2010.493196. [DOI] [PubMed] [Google Scholar]
- 4.Van Bogaert LJ. Clinicopathologic findings in endometrial polyps. Obstet Gynecol. 1988;71:771–773. [PubMed] [Google Scholar]
- 5.Nathani F, Clark TJ. Uterine polypectomy in the management of abnormal uterine bleeding: a systematic review. J Minim Invasive Gynecol. 2006;13:260–268. doi: 10.1016/j.jmig.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Day Baird D, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 7.Wise LA, Palmer JR, Harlow BL, et al. Reproductive factors, hormonal contraception, and risk of uterine leiomyomata in African-American women: a prospective study. Am J Epidemiol. 2004;159:113–123. doi: 10.1093/aje/kwh016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wamsteker K, Emanuel MH, de Kruif JH. Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: results regarding the degree of intramural extension. Obstet Gynecol. 1993;82:736–740. [PubMed] [Google Scholar]
- 9.Van Dongen H, Emanuel MH, Smeets MJ, et al. Follow-up after incomplete hysteroscopic removal of uterine fibroids. Acta Obstet Gynecol Scand. 2006;85:1463–1467. doi: 10.1080/00016340600984647. [DOI] [PubMed] [Google Scholar]
- 10.Hart R, Molnár BG, Magos A. Long term follow up of hysteroscopic myomectomy assessed by survival analysis. Br J Obstet Gynaecol. 1999;106:700–705. doi: 10.1111/j.1471-0528.1999.tb08370.x. [DOI] [PubMed] [Google Scholar]
- 11.Camanni M, Bonino L, Delpiano EM, et al. Hysteroscopic management of large symptomatic submucous uterine myomas. J Minim Invasive Gynecol. 2010;17:59–65. doi: 10.1016/j.jmig.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Lasmar RB, Xinmei Z, Indman PD, et al. Feasibility of a new system of classification of submucous myomas: a multicenter study. Fertil Steril. 2011;95:2073–2077. doi: 10.1016/j.fertnstert.2011.01.147. [DOI] [PubMed] [Google Scholar]
- 13.McEwing RL, Anderson NG, Meates JB, et al. Sonographic appearances of the endometrium after termination of pregnancy in asymptomatic versus symptomatic women. J Ultrasound Med. 2009;28:579–586. doi: 10.7863/jum.2009.28.5.579. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg JA, Miner JD, O’Horo SK. Uterine artery embolization and hysteroscopic resection to treat retained placenta accreta: a case report. J Minim Invasive Gynecol. 2006;13:342–344. doi: 10.1016/j.jmig.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Gebauer G, Hafner A, Siebzehnrübl E, Lang N. Role of hysteroscopy in detection and extraction of endometrial polyps: results of a prospective study. Am J Obstet Gynecol. 2001;184:59–63. doi: 10.1067/mob.2001.108332. [DOI] [PubMed] [Google Scholar]
- 16.Cooper BC, Murray CA. Syndrome of inappropriate antidiuretic hormone in a healthy woman after diagnostic laparoscopy and hysteroscopy: a case report. J Reprod Med. 2006;51:199–201. [PubMed] [Google Scholar]
- 17.Instructions for use: disposable hysteroscopic morcellators. Andover, MA: Smith & Nephew, Inc.; 2007. [Google Scholar]
- 18.MyoSURE™ Hysteroscopic Tissue Removal System 501(k) summary. Framingham, MA: Interlace Medical; [Accessed June 23, 2011]. prepared March 30, 2009. http://www.accessdata.fda.gov/cdrh_docs/pdf9/K091100.pdf. [Google Scholar]
- 19.Emanuel MH, Wamsteker K. The Intra Uterine Morcellator: a new hysteroscopic operating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12:62–66. doi: 10.1016/j.jmig.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 20.van Dongen H, Emanuel MH, Wolterbeek R, et al. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15:466–471. doi: 10.1016/j.jmig.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Miller C, Glazerman L, Roy K, Lukes A. Clinical evaluation of a new hysteroscopic morcellator—retrospective case review. J Med. 2009;2:163–166. [Google Scholar]
- 22.Lukes AS. MyoSure® tissue removal system—Comparative sedation study in an office setting. J Minim Invasive Gynecol. 2007;17(6 suppl):S67. [Google Scholar]
- 23.Greenberg JA, Adam R, Chin A, Sullivan R. Ex-vivo myoma morcellation. Interlace Medical, Inc.; 2008. Data on file with. [Google Scholar]