Abstract
Introducing various pendant functional groups and building blocks of interest to polypeptides in a highly efficient, controlled manner is crucial to access polypeptide materials with desired structures and functions. In this study, we synthesized γ-(4-vinylbenzyl)-L-glutamate N-carboxyanhydride (VB-Glu-NCA), which was readily obtained and purified in large quantity. VB-Glu-NCA monomer was subsequently used for the synthesis of polypeptides containing conjugation-amenable, pendant vinyl functional groups. Controlled, living polymerizations of VB-Glu-NCA were achieved by using hexamethyldisilazane (HMDS) as the initiator, catalytic amounts of 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) as the co-catalyst, and nitrobenzene as the inhibitor of radical-induced side reactions on the vinyl group of VB-Glu-NCA. The resulting poly(γ-(4-vinylbenzyl)-L-glutamate) (PVBLG) gave rise to polypeptides containing pendant functional groups or moieties through various vinyl chemistries.
Keywords: polypeptides, NCA, ring-opening polymerization, polymer functionalization, N-carboxyanhydride, α-helix, vinyl chemistry
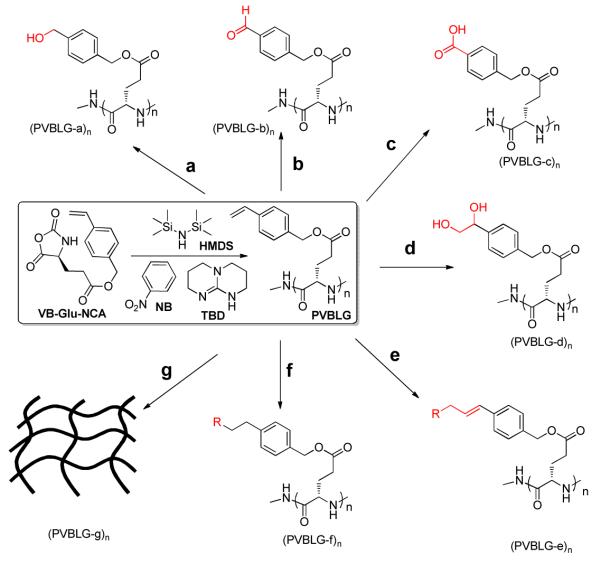
Polypeptides bearing functional side chains have been used in various applications including drug delivery, gene delivery and antimicrobial treatment.1 Preparation of such materials in a highly controlled manner has been a great challenge to polymer chemists. Conventional approaches typically involve the ring-opening polymerizations (ROP) of side-chain protected, multifunctional α-amino acids N-carboxyanhydrides (NCAs),2 followed by removal of the side chain protecting groups and conjugation to desired functional groups/moieties3 or direct aminolysis/transesterification.4 These approaches require the use of harsh deprotection chemistry (e.g., 33% HBr) and may have low grafting efficiency,3 especially for polypeptides with high molecular weights (MWs). To avoid the deprotection step, there has been growing interest in developing new NCA monomers containing conjugation-amenable functional groups that can stay intact during polymerization and be used for the subsequent grafting of desired moieties after polymerization.5 In this study we report the controlled polymerization of γ-(4- vinylbenzyl)-L-glutamate N-carboxyanhydride (VB-Glu-NCA) and utilization of the resulting poly(γ-(4-vinylbenzyl)-L-glutamate) (PVBLG) to easily access a variety of functional polypeptides (PVBLG-a~g) through controlled vinyl chemistries (Scheme 1).
Scheme 1.
Synthesis and Functionalization of PVBLG
a) i. O3,−78 °C, 1-5 min; ii. NaBH4, rt, 16 h; b) i. O3, −78 °C, 1-5 min; ii. PPh3, rt, 2-3 h; c) OsO4, oxone, rt, 48 h; d) OsO4, NMO, rt, 20 h; e) Grubbs catalyst., cis-RCH=CHR, rt, 24 h; f) i. 9-BBN, rt, 16 h; ii. Ar-Br, Pd(PPh3)4, NaHCO3(aq), N2, 70°C, 20 h; g) UV
VB-Glu-NCA was readily prepared and purified in multi-gram scale in crystalline form (Figure S1).6 The purified VB-Glu-NCA is very stable in moisture-free conditions and can be stored in the freezer of a glove-box at −30°C for more than 6 months without noticeable change of properties. The PVBLGs, the resulting polypeptides, are very soluble in common organic solvents (e.g., THF, CHCl3 and DMF), making study of the polymerization and characterization of its products straightforward by standard techniques (e.g., gel permeation chromatography (GPC)).
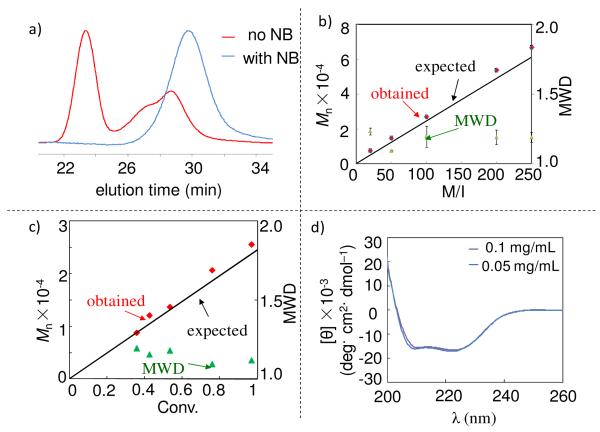
We previously reported that hexamethyldisilazane (HMDS) was an excellent initiator for the ROP of γ-benzyl-L-glutamate NCA (Glu-NCA).2i,2j Along this direction, we first attempted to use HMDS to polymerize VB-Glu-NCA. However, at a monomer/initiator (M/I) ratio of 50, the resulting PVBLG had a Mn value of 1.87× 104 g/mol, which is significantly higher than the expected Mn (1.22 × 104 g/mol, entry 1, Table 1). The molecular-weight distribution (MWD = Mw/Mn) was also fairly broad (2.03). The GPC analysis of the obtained PVBLG gave a bimodal curve with the higher MW peak showing a strong light-scattering signal (red, Figure 1a) but a very weak refractive index signal (data not shown). These GPC results suggest the existence of polymers with very high MWs, presumably due to the inter-chain crosslinking of the vinyl groups of the PVBLG. To eliminate this side reaction, nitrobenzene (NB), a radical retarder, was added to the HMDS-mediated VB-Glu-NCA polymerization solution. As expected, the crosslinking side reaction was completely inhibited, evidenced by the monomodal GPC light-scattering curve of the resulting PVBLG (blue, Figure 1a), which had a MW much closer to the expected value and a narrower MWD (entry 2, Table 1). The amount of NB had very limited effect on polymerization rates and the MWs of the resulting PVBLGs, suggesting that NB functions as a radical inhibitor and does not participate in chain propagation (Figure S2).
Table 1.
HMDS-Mediated VB-Glu-NCA Polymerization.
| entry | monomer | M/HMDS/C ata. |
Cata. | NBa (μL) |
time (h) |
conv. (%) |
Mn (Mn*) (× 10−4) b | MWD |
|---|---|---|---|---|---|---|---|---|
| 1 | VB-Glu-NCA | 50/1/0 | NA | 0 | 30 | >98 | 1.87 (1.22) | 2.03 |
| 2 | VB-Glu-NCA | 50/1/0 | NA | 30 | 30 | >98 | 1.43 (1.22) | 1.10 |
| 3 | VB-Glu-NCA | 200/1/0 | NA | 30 | 40 | 67 | 3.30 (4.9) | 1.08 |
| 4 | VB-Glu-NCA | 200/1/0.1 | TBD c | 30 | 24 | >98 | 4.68 (4.9) | 1.08 |
| 5 | Lys-NCA/ VB-Glu-NCA |
(20/1+50/1) /0.02d |
TBD | 30 | 8+12e | >98 | 0.61/2.10 (0.52 /1.74) f |
1.05/ 1.18g |
NB = nitrobenzene;
Obtained MW (expected MW*);
TBD = 1,5,7-triazabicyclo[4.4.0]dec-5-ene;
Feed ratio of (Lys-NCA/HMDS + VB-Glu-NCA/HMDS)/Cat.;
Lys-NCA polymerization time + VB-Glu-NCA polymerization time;
Obtained MW of PZLL/PZLL-b-PVBLG (expected MW of PZLL/PZLL-b-PVBLG );
MWD of PZLL/PZLL-b-PVBLG.
Figure 1.
a) GPC (Multi-Angle Laser Light Scattering (MALLS) detector) curves overlay of HMDS-mediated VB-Glu-NCA polymerizations at M/I ratio of 50/1 in the presence (blue) and absence (red) of NB; b) Plot of MW and MWD versus M/I in the HMDS/TBD initiated VB-Glu-NCA polymerization; the experiment was repeated three times at each M/I ratio and the error bars were presented as the standard deviation; c) Plot of MW and MWD versus conversion in the HMDS/TBD initiated VB-Glu-NCA polymerization; (d) CD curves of PVBLG-d70 at the concentration of 0.05 mg/mL (blue) and 0.1 mg/mL in water (purple).
Although HMDS-mediated VB-Glu-NCA polymerization in the presence of NB gave controlled polymerization, VB-Glu-NCA has fairly low reactivity as compared to the parent Glu-NCA (Figure S4), making HMDS/NB-mediated polymerization undesirable for the synthesis of high MW PVBLG (Figure S5). Polymerization at high M/I ratio generally gave low monomer conversion even with extended reaction time (entry 3, Table 1). Our previous study indicated that HMDS-mediated Glu-NCA polymerization proceeds via a trimethylsilyl carbamate (TMS-CBM) terminal group. Polypeptide chains were propagated through the transfer of the TMS group from the terminal TMS-CBM to the incoming monomer to form a new TMS-CBM terminal propagating group.2i,2j In the case of VB-Glu-NCA, the TMS-transfer process could have been retarded due to the low reactivity of VB-Glu-NCA.
We next screened various substrates that have been used as nucleophilic organic catalysts in various acyl-transfer or acyl-activation reactions.7 We found that 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) assisted faster polymerizations with excellent control over MWs when it was used in conjunction with HMDS for VB-Glu NCA polymerization. At a VB-Glu-NCA/HMDS molar ratio of 200:1 along with a catalytic amount of TBD (0.1 equiv.), the polymerization was noticeably faster and completed within 20 h with quantitative conversion of VB-Glu-NCA (entry 4 vs. 3, Table 1). The GPC analysis of the polymerization solution in situ revealed that the resulting PVBLG had a narrow MWD (Mw/Mn = 1.08) and an Mn value of 4.7 × 104 g/mol, which was very close to the expected Mn of 4.9 × 104 g/mol (entry 4, Table 1). As shown in Figure 1b, the obtained Mn’s, which were the average of the Mn’s of the PVBLGs prepared in three separate polymerization experiments at the corresponding M/I ratios, agreed almost perfectly with the expected Mn’s. Furthermore, the very small error bars of Mn’s indicate that the polymerizations were highly reproducible. The resulting PVBLGs all had very narrow MWDs (1.08-1.27). The MWs of PVBLG also showed linear correlation with the conversions of VB-Glu-NCA and agreed well with the expected MWs (Figure 1c), demonstrating that PVBLG chains were propagated through living chain ends. Block co-polypeptides, such as poly(ε-cdzL-lysine)-block-PVBLG (PZLL-b-PVBLG), can be readily prepared with predictable MWs and narrow MWDs (entry 5, Table 1). These experiments demonstrated that the HMDS/TBD mediated well controlled, living polymerizations of VB-Glu-NCA. It is unclear if HMDS/TBD can be applied to other NCAs for accelerated polymerization and the mechanism of action of TBD. These studies are underway and will be reported later.
With the successful establishment of ROP of VB-Glu-NCA for the controlled synthesis of PVBLG, we next performed the post-polymerization reactions as illustrated in Scheme 1, aiming to explore the scope and versatility of PVBLG for the synthesis of functional polypeptides. The efficiencies of side-chain functionalization illustrated in Scheme 1 were found to be at least 90% as confirmed by the NMR. The separated yields of all the reactions were between 60% and 90% (See SI). To generate PVBLG-a~g, the N-terminus of PVBLG70, a 70mer of PVBLG, was first protected by a CBZ group immediately after polymerization to prevent undesired side reactions. The PVBLG70 was then treated with ozone and the vinyl group was converted to alcohol in (PVBLG-a)70 in 72% yield (route a, Scheme 1) and aldehyde in (PVBLG-b)70 in 78% yield (route b), when sodium borohydride and triphenylphosphine were used as the reductive reagent, respectively. Notably, the aldehyde functionalized PVBLG-b was very reactive and could be used for further grafting of various functional moieties with amines, hydrazides, and oxyamines through condensation/reductive amination.8 The vinyl group of PVBLG70 was also converted to carboxylic acid (PVBLG-c)70 in 81% yield under mild condition by osmium tetroxide-promoted catalytic, oxidative cleavage of the olefin (route c).9 1,2-Bishydroxylation of the vinyl of PVBLG70 was performed by following osmium tetraoxide catalyzed oxidative addition in the presence of N-methylmorpholine N-oxide (route d), resulting in (PVBLG-d)70 in 79% yield. Remarkably, PVBLG-d is very soluble in water, has very low toxicity (IC50 > 1 mM in HeLa cells, see Figure S6), and adopts a helical conformation in aqueous solution (Figure 1d). The molar ellipticity at both 208 nm and 222 nm remained unchanged when the CD analyses were carried out at two different concentrations of PVBLG-d, indicating that PVBLG-d stays in its monomeric form in water. Thus, we can readily convert PVBLG, a water-insoluble polypeptide, to a water-soluble PVBLG-d via a one-step post-modification reaction, and PVBLG-d can potentially be used as water-soluble, non-charged, rod-like structures in self-assembly or biological applications. Further studies of PVBLG-d are underway in our laboratory. We also performed the metathesis reaction of PVBLG70 (route e, Scheme 1). By mixing the polymer solution in dichloromethane with excessive cis-1,4-dichlorobutene in the presence of the second-generation Grubbs catalyst, allyl chloride functionalized polypeptide (PVBLG-e)70 was exclusively generated in 78% yield. By treating PVBLG70 with 9-borabicyclo[3.3.1]nonane (9-BBN) followed by reaction with 4′-bromoacetophenone and tetrakis(triphenylphosphine)-palladium, (PVBLG-f)70 was derived in 60% separated yield via the Suzuki reaction. UV-induced crosslinking reaction of the vinyl group of PVBLG70 resulted in formation of an organogel ((PVBLG-g)70).10 Thus, the functionalization of PVBLG’s side-chain vinyl group can be a very useful approach to generate a large number of polypeptide materials with a variety of side chain functionalities and moieties through versatile vinyl chemistry.
In conclusion, we report the preparation of polypeptides with poly(L-glutamate) backbone and a variety of different side chains via controlled polymerization of VB-Glu-NCA followed by a variety of highly efficient post-functionalization reactions. VB-Glu-NCA is readily available in large scale (tens of grams) with satisfactory purity after crystallization. The initiator (HMDS) and the co-catalyst (TBD) of the polymerization are both commercially available and inexpensive and can be used as received to prepare PVBLGs with precisely controlled MWs and narrow MWDs. The side-chain post-functionalization reactions are also straightforward with high efficiency. We believe this streamlined strategy will find widespread utility for the synthesis of a large number of functional polypeptides with tailored side-chain structures and desired functions.
Supplementary Material
ACKNOWLEDGMENT
J.C. acknowledges support from the NSF (CHE-0809420), the NIH (NIH Director’s New Innovator Award 1DP2OD007246-01, 1R21EB009486A and 1R21CA139329Z), and the Center for Nanoscale Science and Technology of UIUC.
Footnotes
Supporting Information Available. Supporting figures, tables, and experimental methods. These materials are available free of charge via the internet at http://pubs.acs.org.
References
- (1).(a) Shim MS, Kwon YJ. Biomaterials. 2010;31:3404–3413. doi: 10.1016/j.biomaterials.2010.01.019. [DOI] [PubMed] [Google Scholar]; (b) Engler AC, Shukla A, Puranam S, Buss HG, Jreige N, Hammond PT. Biomacromolecules. 2011;12:1666–1674. doi: 10.1021/bm2000583. [DOI] [PubMed] [Google Scholar]; (c) Tang HY, Li YC, Lahasky SH, Sheiko SS, Zhang DH. Macromolecules. 2011;44:1491–1499. [Google Scholar]
- (2).(a) Deming TJ. Nature. 1997;390:386–389. doi: 10.1038/37084. [DOI] [PubMed] [Google Scholar]; (b) Yu M, Nowak AP, Deming TJ, Pochan DJ. J. Am. Chem. Soc. 1999;121:12210–12211. [Google Scholar]; (c) Bhaw-Luximon A, Jhurry D, Belleney J, Goury V. Macromolecules. 2003;36:977–982. [Google Scholar]; (d) Dimitrov I, Schlaad H. Chem. Commun. 2003:2944–2945. doi: 10.1039/b308990h. [DOI] [PubMed] [Google Scholar]; (e) Aliferis T, Iatrou H, Hadjichristidis N. Biomacromolecules. 2004;5:1653–1656. doi: 10.1021/bm0497217. [DOI] [PubMed] [Google Scholar]; (f) Vayaboury W, Giani O, Cottet H, Deratani A, Schue F. Macromol. Rapid Commun. 2004;25:1221–1224. [Google Scholar]; (g) Deming TJ. Adv. Polym. Sci. 2006;202:1–18. [Google Scholar]; (h) Gibson MI, Hunt GJ, Cameron NR. Org. Biomol. Chem. 2007;5:2756–2757. doi: 10.1039/b707563d. [DOI] [PubMed] [Google Scholar]; (i) Lu H, Cheng JJ. J. Am. Chem. Soc. 2007;129:14114–14115. doi: 10.1021/ja074961q. [DOI] [PubMed] [Google Scholar]; (j) Lu H, Cheng JJ. J. Am. Chem. Soc. 2008;130:12562–12563. doi: 10.1021/ja803304x. [DOI] [PubMed] [Google Scholar]; (k) Peng YL, Lai SL, Lin CC. Macromolecules. 2008;41:3455–3459. [Google Scholar]; (l) Wang Y, Zou S, Kim KT, Manners I, Winnik MA. Chem-Eur J. 2008;14:8624–8631. doi: 10.1002/chem.200800762. [DOI] [PubMed] [Google Scholar]; (m) Gibson MI, Cameron NR. J. Polym. Sci. Part A. 2009;47:2882–2891. [Google Scholar]; (n) Hadjichristidis N, Iatrou H, Pitsikalis M, Sakellariou G. Chem. Rev. 2009;109:5528–5578. doi: 10.1021/cr900049t. [DOI] [PubMed] [Google Scholar]; (o) Klok HA. Macromolecules. 2009;42:7990–8000. [Google Scholar]; (p) Pickel DL, Politakos N, Avgeropoulos A, Messman JM. Macromolecules. 2009;42:7781–7788. [Google Scholar]; (q) Kramer JR, Deming TJ. J. Am. Chem. Soc. 2010;132:15068–15071. doi: 10.1021/ja107425f. [DOI] [PubMed] [Google Scholar]
- (3).(a) Li T, Lin JP, Chen T, Zhang SN. Polymer. 2006;47:4485–4489. [Google Scholar]; (b) Feuz L, Strunz P, Geue T, Textor M, Borisov O. Eur. Phys. J. E. 2007;23:237–245. doi: 10.1140/epje/i2007-10180-9. [DOI] [PubMed] [Google Scholar]
- (4).(a) Watanabe J, Fukuda Y, Gehani R, Uematsu I. Macromolecules. 1984;17:1004–1009. [Google Scholar]; (b) Watanabe J, Ono H, Uematsu I, Abe A. Macromolecules. 1985;18:2141–2148. [Google Scholar]; (c) Watanabe J, Goto M, Nagase T. Macromolecules. 1987;20:298–304. [Google Scholar]; (d) Inomata K, Shimizu H, Nose T. J. Polym. Sci. Part B. 2000;38:1331–1340. [Google Scholar]; (e) Tang DM, Lin JP, Lin SL, Zhang SN, Chen T, Tian XH. Macromol. Rapid Commun. 2004;25:1241–1246. [Google Scholar]; (f) Kulkarni RK, Blout ER. J. Am. Chem. Soc. 1962;84:3971–3972. [Google Scholar]; (g) Guo JS, Huang YB, Jing XB, Chen XS. Polymer. 2009;50:2847–2855. [Google Scholar]
- (5).(a) Xiao CS, Zhao CW, He P, Tang ZH, Chen XS, Jing XB. Macromol. Rapid Commun. 2010;31:991–997. doi: 10.1002/marc.200900821. [DOI] [PubMed] [Google Scholar]; (b) Tang HY, Zhang DH. Biomacromolecules. 2010;11:1585–1592. doi: 10.1021/bm1002174. [DOI] [PubMed] [Google Scholar]; (c) Sun J, Schlaad H. Macromolecules. 2010;43:4445–4448. [Google Scholar]; (d) Huang J, Habraken G, Audouin F, Heise A. Macromolecules. 2010;43:6050–6057. [Google Scholar]; (e) Engler AC, Lee H-I, Hammond PT. Angew. Chem. Int. Ed. 2009;48:9334–9338. doi: 10.1002/anie.200904070. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Poche DS, Thibodeaux SJ, Rucker VC, Warner IM, Daly WH. Macromolecules. 1997;30:8081–8084. [Google Scholar]
- (6).(a) Vanheeswijk WAR, Eenink MJD, Feijen J. Synthesis. 1982:744–747. [Google Scholar]; (b) Luijten J, Groeneveld DY, Nijboer GW, Vorenkamp EJ, Schouten AJ. Langmuir. 2007;23:8163–8169. doi: 10.1021/la7005106. [DOI] [PubMed] [Google Scholar]; (c) Mabuchi M, Kobata S, Ito S, Yamamoto M, Schmidt A, Knoll W. Langmuir. 1998;14:7260–7266. [Google Scholar]
- (7).(a) Kamber NE, Jeong W, Waymouth RM, Pratt RC, Lohmeijer BGG, Hedrick JL. Chem. Rev. 2007;107:5813–5840. doi: 10.1021/cr068415b. [DOI] [PubMed] [Google Scholar]; (b) Chuma A, Horn HW, Swope WC, Pratt RC, Zhang L, Lohmeijer BGG, Wade CG, Waymouth RM, Hedrick JL, Rice JE. J. Am. Chem. Soc. 2008;130:6749–6754. doi: 10.1021/ja0764411. [DOI] [PubMed] [Google Scholar]
- (8).Lu H, Wang J, Bai YG, Lang JW, Liu SY, Lin Y, Cheng JJ. Nat. Commun. 2011;2:206. doi: 10.1038/ncomms1209. [DOI] [PubMed] [Google Scholar]
- (9).Travis BR, Narayan RS, Borhan B. J. Am. Chem. Soc. 2002;124:3824–3825. doi: 10.1021/ja017295g. [DOI] [PubMed] [Google Scholar]
- (10).Ajayaghosh A, Praveen VK, Vijayakumar C. Chem. Soc. Rev. 2008;37:109–122. doi: 10.1039/b704456a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.