Abstract
The incidence of most common cancers increases with age. This occurs in association with, and is possibly caused by a decline in immune function, termed immune senescence. Although the size of the T-cell compartment is quantitatively maintained into older age, several deleterious changes (including significant changes to T-cell subsets) occur over time that significantly impair immunity. This article highlights some of the recent findings regarding the aging immune system, with an emphasis on the T-cell compartment and its role in cancer.
Keywords: aging, cancer, immune senescence, T cell
The aging of the adaptive immune system results in decreased functionality termed immune senescence. Defects arise in both the humoral and cellular arms of the adaptive response, implicating defective T-cell function with age [1–4]. As the thymus is the major site of T-cell development and maturation, thymic involution and the gradual decline in thymic output is considered a primary event in the process of age-associated immune senescence [3]. Furthermore, there is an inverse relationship between immune function and the incidence of many forms of cancer [1–4]; as immune function decreases with age, the incidence of cancer increases. However, a causative link between age-associated immune senescence and increased incidence of cancer remains controversial [1,4].
The global trend for increased cancer incidence with age, especially after the age of 65 years, is a well-established phenomenon. Most common forms of cancer have a peak incidence in the seventh and eighth decades of life [4]. This parallels the age-associated decline in immune function and, by extension, its capacity for immunosurveillance, the proposed process by which the immune system detects and eradicates tumors [4]. Since immune senescence continues to increase with advancing age, it would be expected that cancer incidence would continue this trend as well. A recent population study found a peak incidence of bladder cancer in those >85 years of age [5]. However, the age-associated increase in the incidence of many common cancers is followed by a plateau during the eighth decade and, in some cases, a subsequent decline in incidence in the very old (>85 years of age) [4]. For example, prostate cancer has a peak incidence at age 70–74, and then declines after the age of 80 years [101]. Similar trends are observed in breast cancer, where peak incidence for women occurs from 75 to 79 years followed by a decline in women aged 80 years and older [101].
The decline in incidence of some common cancers is contrary to the theory that the decline in immunity with age has a direct role in increasing cancer incidence, and it suggests that more complex mechanisms underlie the process. However, it may be consistent with respect to views on successful aging. It has been postulated by some that individuals who are genetically predisposed towards weaker inflammatory responses may have a higher likelihood of successful aging provided that they can avoid serious infectious diseases [6]. In addition, individuals with a genetically inherited propensity towards extreme longe vity (survival into the ninth and tenth decades) may have common underlying features relevant to the onset of cancer. A longitudinal study of immune parameters found a reduction in age-associated inflammation, prolonged immune function despite advanced years and a reduction in cytomegalovirus (CMV)-associated immune defects in individuals with a familial history of greater longevity [7]. Consistent with this view, naturally long-lived mice better maintain immune responses to stimuli, have reduced resting state oxidation and have reduced NF-κB activation during resting state compared with aged mice [8]. This same group also found that basal level cytokine secretion of peritoneal leukocytes from aged mice (69–92 weeks old) had a strong proinflamma-tory profile, whereas naturally long-lived mice (125 weeks old) maintained a cytokine profile that was similar to that seen in middle-age mice (44 weeks old) [9]. Aged mice were also impaired in their ability to produce Th1 and Th2 cytokines, including IL-2 compared with middle-aged mice. By contrast, leukocytes from naturally long-lived mice maintained cytokine production similar to that seen in middle-age mice following ex vivo stimulation [9]. These data support the possibility that a subset of individuals survive into late age without cancer as a result of better-maintained immune function, including a reduction in the chronic inflammation typically seen with age. This provides one hypothesis to explain the plateau in incidence during the ninth and tenth decade of life for many cancer types, which is consistent with the immunosurveillance model of cancer prevention. However, it must be stated that the immunosurveillance/immuno senescence argument for the increased incidence of cancer observed with age remains controversial and incompletely understood. An alternative argument has been postulated that as the body ages, cells are increasingly exposed to a variety of potentially carcinogenic and transformational insults that increase the likelihood of malignancy over time [1,4].
Immunosurveillance
The concept of tumor immunosurveillance is important in understanding the role of declining immunity in the increase in cancer incidence. This has been described elsewhere and will only briefly be discussed here [4,10]. Two major systems of tumor suppression by the immune system have been described, namely the production of IFN-γ and the perforin pathway that is used by cytotoxic T cells and natural killer cells [4]. While natural killer cells may kill cancer cells nonspecifically [11], antigen-specific naive CD8+ T cells require CD4+ T-cell help to achieve activation and cytotoxic function [12,13]. CD8+ T cells predictably participate in antigen-specific immunosurveillance through cytotoxic T-lymphocyte killing of tumor cells [14]. Similar to normal T-cell responses to foreign antigens, CD4+ T cells may participate in immunosurveillance by licensing antigen-presenting cells that cross-present tumor-associated antigen to naive CD8+ T cells. In fact, inadequate presentation of tumor-associated antigen to naive CD4+ T cells resulted from downregulated MHC class II on antigen-bearing dendritic cells. This, combined with adequate class I presentation to naive CD8+ T cells, resulted in tolerization of tumor antigen-specific CD8+ T cells rather than activation [15]. This highlights the roles of both CD4+ and CD8+ T cells in this mechanism of antitumor immunity, as well as an immunomodulatory effect of the tumor environment on dendritic cells. Similarly, myeloma-specific CD4+ T cells are able to inhibit the growth of class II negative myeloma through IFN-γ production following interaction with tumor antigen expressing macro phages [16]. Subsequent studies by this group confirmed that naive CD4+ T cells participate in immunosurveillance of class II-negative myeloma only when secreted tumor antigen reaches a critical level in the tumor extracellular matrix, where it is then picked up by macrophages for presentation [17]. In fact, CD4+ T cells can eliminate tumors independently of CD8+ T cells [18–20] and high CD4:CD8 ratios are associated with a good prognosis for patients with lung [21] and liver cancer [22]. In this example, local production of inflammatory and Th1 cytokines by tumor-specific CD4+ T cells mediated cytotoxic activity in tumor infiltrating macrophages [20]. Vella et al. speculate that cyclin B1, which is expressed transiently in normal cells and constitutively in several human cancers, may be a target for immunosurveillance [23]. They note that healthy individuals with no cancer history have memory CD4+ and CD8+ T cells as well as T-cell-dependent serum IgG that are specific for cyclin B1. Murine studies confirmed that priming of the immune system with a cyclin B1 vaccine regimen led to tumor rejection that required participation of CD8+ T cells. Collectively, these findings support a role for both CD4+ and CD8+ T cells in mediating antigen-specific responses that are capable of suppressing tumor growth.
Tumors may evade these mechanisms through a proposed three-phased process, termed immuno editing. The ‘elimination’ phase is immunosurveillance itself, through which the immune system eliminates potentially malignant cells. Transformed cells then achieve an ‘equilibrium’ during which the immune system holds their expansion in check. Finally, tumors ‘escape’ the immune system by deleting immunogenic epitopes and/or suppressing the immune response to the tumor [4,10,24]. This process of removing antigens that the immune system can easily respond to is seen in tumor studies using immune-deficient mice. Tumors developed in these animals were incapable of growing in syngeneic immune competent hosts. Conversely, tumors that develop in immune competent mice could be transferred and successfully grown in syngeneic immune competent hosts [25,26]. The process of immunoediting implies that the immune system is capable of, and necessary for, preventing tumor development. It also implies that cancer initiation requires a means by which the tumor can avoid suppression by the immune system. Consistent with these findings it is predicted that declining immunity would lead to an increased risk of cancer.
Age-associated decline in T-cell function
There are three major trends in T-cell function that have been observed in the aging immune system. The first is a decrease in the number and proportion of naive T cells. This is mainly caused by the progressive decrease in thymic output that begins in childhood and continues into advanced age [27]. Surprisingly, the overall size of the peripheral T-cell population remains consistent well after thymic involution [1,28,29]. That is, the T-cell compartment occupies the same approximate ‘space’ early in life as it does late in life. However, the proportion of various sub-populations changes significantly. The second major trend is the increased proportion of memory T cells. These cells are still capable of proliferative memory responses, but include defects from normal function, including increased type I/type II cytokine production profiles [2]. The third major change in T-cell function is the accumulation of terminally differentiated T cells that are dysfunctional and have extremely limited T-cell receptor (TCR) repertoire diversity [30]. These are mostly CD8+ T cells that arise from chronic exposure to viral antigen [31]. The shift from naive to a preponderance of memory/effector cells with age results in a state of low-level chronic inflammation that is characteristic of the aged immune system. Interestingly, a recent longitudinal study on familial longevity found that individuals genetically predisposed to longer lifespan were less susceptible to common features of age-related immune senescence [7]. Specifically, CMV seropositivity was linked to a reduction in naive T cells and an increase in effector memory T cells for the general population, but not for individuals with familial longevity. A reduced proinflammatory status was also observed in the latter group.
Naive T cells
A diverse TCR repertoire is critical for normal immune function and the recognition of a wide range of antigenic targets. However, with age, the thymus involutes and naive T-cell output decreases. As mentioned previously, this age-associated decrease in thymic output is the primary cause of immunosenescence [3]. Peripheral naive T-cell output is reduced up to 80% [32]. However, despite involution thymic function and naive T-cell output does continue throughout life, albeit at significantly reduced levels [2,27,33]. This decrease is measurable by TCR rearrangement excision circles that indicate recent thymic emigration and are not preserved in proliferating cells, but rather become diluted [33]. TCR rearrangement excision circle-positive CD4+ T cells in the elderly also show signs of aging. The least differentiated naive subset of CD4+ T cells, the TCR rearrangement excision circle-enriched CD45RA+CD31+, have been demonstrated to have shorter telomeres in the elderly [34]. Telomere shortening in T cells is an indicator of reduced proliferative capability [34,35].
The progressive decline in naive T-cell production with aging raises an important question. Are long-term changes in the naive T-cell subpopulation, as seen in the elderly, the result of cell-intrinsic events (e.g., changes in intra cellular signaling caused by the aging process) or cell-extrinsic events (e.g., changes in the environment including thymic involution)? While this remains a topic of discussion there are data to support both suppositions, suggesting that it is a combination of both intrinsic and extrinsic cellular events that contribute to abnormalities in the aged naive T-cell pool.
Cell-intrinsic factors
A reduction in thymus mass versus body mass continues from birth until death [27]. However, naive T cells in the elderly are not only quantitatively reduced, but also qualitatively altered in their function, supporting the presence of cell-intrinsic factors. Response to vaccines diminishes significantly in the elderly [36,37]. Aging naive CD4+ T cells also live longer but proliferate less, produce less IL-2 when stimulated, and are less effective in providing B cell help [38,39]. Using an adoptive transfer model, Eaton et al. demonstrated that the transfer of antigen-specific naive CD4+ T cells from aged mice into young mice resulted in a reduction in cognate B-cell help, germinal center formation and IgG production compared with that seen with the transfer of young naive CD4+ T cells [38]. Conversely, the transfer of young naive CD4+ T cells to either a young or an aged host resulted in similarly elevated levels of B-cell expansion, differentiation and IgG production as compared with that seen in the transfer of aged T cells. This is consistent with age-related changes that are T-cell-intrinsic and not caused by the age of the host environment. However, a subsequent study from this group indicates that bone marrow precursor cells from aged mice do in fact produce normally functioning CD4+ T cells when transferred into lethally irradiated young mice [40]. Memory T cells generated in this process provided potent cognate B-cell help comparable to that seen with the transfer of bone marrow precursor cells from young mice. Taken together, these two studies support cell extrinsic changes (i.e., environmental) in bone marrow precursor cells during the maturation process that lead to intrinsic defects in naive CD4+ T cells [38,40]. However, the model used in these studies relied on transgenic TCRs that bypass the recombination phase in T-cell development and, thus, may not accurately reflect normal changes in T-cell development with age. RAG1 and RAG2 expression, under the control of thymic epithelial cells, does in fact decrease with age [41].
Despite an age-associated decrease in thymic output of new naive T cells, the total number of peripheral T cells does not decline dramatically [1,28,29]. The total T-cell pool is maintained by several means, including TCR-independent homeostatic proliferation and prolonged survival of naive T cells in the periphery. Specifically, increased longevity of naive CD4+ T cells helps maintain homeostasis and also facilitates the development of intrinsic functional defects [42]. Post-thymic longevity of transgenic TCR CD4+ T cells is linked to age-associated defects including reduced in vivo expansion, reduced IL-2 production and impaired cognate B-cell help [43]. In addition, thymectomizing middle-aged mice to mimic thymic involution at an earlier age results in an early onset of age-related defects [42]. In the same study, aged naive CD4+ T cells, when transferred into normal young mice, intrinsically exhibited prolonged survival, decreased antigen-driven proliferation and decreased IL-2 production. Reduced expression of the proapoptotic molecule Bim was associated with intrinsic age-related defects in naive CD4+ T cells [42]. However, the transfer of aged bone marrow into an irradiated young mouse prevented age-associated defects in the newly produced naive CD4+ T cells, indicating that intrinsic age-associated defects in CD4+ T cells do not occur prior to thymic emigration [40,42]. A subsequent study by this group reported that reduced expression of the proapoptotic molecule Bim is the initiating step in increased cellular lifespan and the development of age-associate functional defects in naive CD4+ T-cell function [44]. Collectively, these findings highlight prolonged survival in the periphery as being critical both to T-cell homeostasis as well as the development of functional defects. This facilitates intrinsic changes in naive T cells that are maintained even after transfer into a younger animal. However, the aged environment that promotes these changes results primarily from thymic involution and the subsequent decrease in thymic output associated with aging. Since the thymus provides the microenvironment in which T-cell development occurs, these events are extrinsic to the aged T cell.
Cell-extrinsic factors
Dysfunction in thymic epithelial cells has been implicated as the key cellular event in thymic involution [45,46]. Recently, deterioration of thymic epithelial cells has been attributed to an age-associated decline in the expression of the epithelial gene FoxN1 by these cells. A transgenic model that causes a more rapid, but still gradual, decline in FoxN1 expression demonstrated accelerated thymic involution and age-associated T-cell defects in mice. Interestingly, restoration of intrathymic FoxN1 expression in aged mice partially restored thymic and peripheral CD4+ T-cell function [47].
In humans, a study on individuals thymectomized at an early age demonstrates the early onset of age-associated immune defects described as ‘premature immune aging’ [48]. Young adults that were thymectomized at an early age prematurely displayed immune system characteristics typically seen in the aged immune system, including a reduction in the absolute number of CD4+ and CD8+ T cells, reduced naive T-cell populations, and increased populations of oligoclonal memory T cells. Of note was a subset of individuals in the study with exacerbated characteristics of the aged immune system, comparable to that seen in the elderly (age 75 years and older). There was also a correlation between these individuals and CMV infection, including a strong CMV-specific T-cell response. These results are consistent with prior findings that show a reduction in recent thymic emigrant CD4+ T cells in individuals thymectomized at an early aged, compared with healthy age-matched controls [49,50]. As such, both clinical and murine studies strongly support the influence of cell-extrinsic factors in the aging of the immune system.
Naive CD8+ T cells
With age, the naive CD8+ T-cell compartment also decreases in proportion to decreased thymic output [51,52], similar to age-associated changes in the naive CD4+ T-cell compartment. This decrease in size is accompanied by a similar decrease in TCR repertoire. However, in comparison to changes in naive CD4+ T cells, dysregulation in the homeostatic proliferation of naive CD8+ T cells results in clonal expansions similar to that seen in memory/effector CD8+ T cells (discussed later) [51]. One population study of peripheral blood T-cell subsets found an increased sensitivity to aging in CD8+ versus CD4+ T cells caused by differences in homeo-static stability and gene expression [52]. Instability of the naive CD8+ T-cell population has also been attributed to increases in TNF-α- [53] and CD95-mediated [54] apoptosis. Naive and central memory CD8+ T cells in older, but not younger, individuals were more apoptosis sensitive, whereas effector memory CD8+ T cells did not display any age-associated changes in sensitivity to apoptosis. This provides further explanation, beyond decreased thymic output, for the decrease in naive and increase in effector CD8+ T cells observed with age. Importantly, these population changes are accompanied by significant functional changes. In an infection model that requires a strong primary CD8+ T-cell response to clear the intracellular pathogen Listeria monocytogenes, CD8+ T cells in aged B6 mice exhibit reduced proliferation and increased apoptosis in response to novel antigen [55]. Similarly, a study on vaccination efficacy in normal versus aged primates demonstrated that there was a strong correlation between the strength of the early CD8+ T-cell response to novel (vaccine) antigen and the frequency of naive CD8+ T cells in the aged, but not young, immune system [56]. Collectively, these findings indicate that the age-related changes to the naive CD8+ T-cell population result in a decreased functional capacity to respond to novel antigen.
Memory T cells
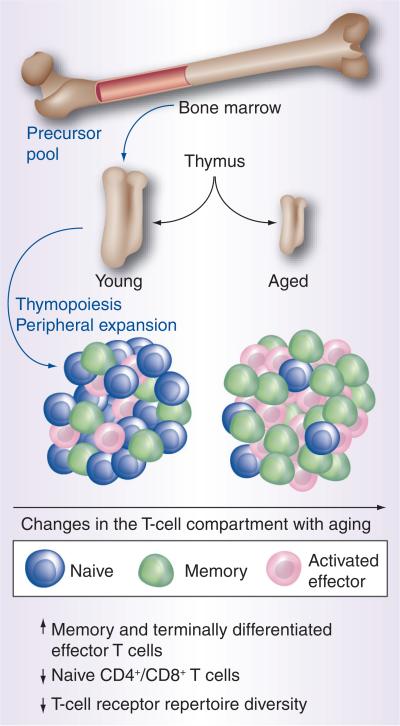
In addition to the observed loss of naive T cells in old age, there is also a well-documented phenotypic transition from naive to memory T cells [57]. As mentioned previously, the size of the overall T-cell compartment remains constant with age [1,28,29], and changes in T-cell sub populations result in a shift from a naive to memory or activated effector T cells (Figure 1). Decreased thymic output and cumulative antigenic exposure all contribute to this transition. Existing memory T cells may also proliferate without antigen-specific TCR engagement through the process of homeostatic proliferation, similar to that seen in naive T cells [29]. The functional changes resulting from this transition are evident in the reduced proliferation and IL-2 production seen in aged T cells stimulated in vitro [57]. Similarly, there is a transition towards the suppression of immune responses as indicated by an increase in Foxp3+ regulatory T cells (Tregs) [58]. The increase in Tregs with age is relevant as this population has been demonstrated to increase with cancer as well as age, and represents an immunosuppressive strategy utilized by tumors [59]. Consistent with the concept of immune surveillance in cancer prevention, patients with colorectal cancer experienced a gradual increase in Tregs with disease progression [60].
Figure 1. Age-associated changes characteristic of immunosenescence occur in T-cell populations.
The young thymus supports more robust thymopoiesis with naive cells, which have the greatest T-cell receptor repertoire diversity and comprise the largest proportion of T cells. With aging, thymus involution occurs and there is progressive loss of T-cell receptor repertoire diversity with the decreased population of naive T cells and there is an enlarged memory component that secretes most type 1 and 2 cytokines. With repeated stimulation, the memory cells give rise to activated effector T cells, which are oligoclonal and have the most restricted T-cell receptor repertoire.
In contrast to an increase in memory T cells is a diminished capacity for normal recall responses. With age there is a progressive loss in primary responses to novel antigen as well as a subsequent decrease in memory formation. For example, seroconversion following influenza vaccination has been reported to be 50% in those aged 61–70 years, and as little as 11% for those 80 years and older [36]. This may, in part, be caused by an increased frequency in central memory CD4+ T cells and decreased frequency of CD4+ effector memory T cells postvaccination in elderly versus younger individuals [37]. Indeed, memory responses that are formed early in life are maintained in the aged immune system and provide better memory responses than memory cells formed in the aged immune system [40]. There are significant implications of this defect for cancer prevention by the immune system. The process of immunoediting indicates that tumor cells, probably caused by their genetic instability, regularly produce novel antigenic targets that are subsequently presented to the immune system. The inability to generate an effective memory response to newly evolving antigenic targets may critically impair, in an age-dependent manner, the immune system's ability to prevent tumorigenesis.
The aforementioned increase in the memory T-cell compartment has significant implications for the cytokine profile. As mentioned previously, there is an increase in inflammatory cytokine production with age. Both accumulating memory CD4+ T cells and terminally differentiated CD8+ T cells may contribute to the inflammatory state. For example, aged CD44hi CD8+ T cells produce IFN-γ in response to IL-12 production, even in the absence of specific TCR stimulation [61]. Similarly, the frequency of CD4+ T cells producing the proinflammatory cytokine IL-17 increases with age [62]. Memory T cells may also act as a source of IL-17 as well [62]. IL-17 has been demonstrated to support the development of cancer in a proinflamma-tory environment [63] and CD4+IL17+ T cells are associated with the progression of certain cancer types [64]. While the precise role of IL-17 in cancer remains controversial [65], inflammatory conditions are associated with the etiology of many forms of cancer [66].
Terminally differentiated effectors
The accumulation of terminally differentiated CD8+ effector T cells represents a third mechanism of age-related immunosenescence. Specifically, there is a progressive loss of CD8+ T-cell repertoire diversity concomitant with the amassing of T-cell clonal expansions with age [2,3,67,68]. Oligoclonal CD8+ T cells, specific for persistent viral antigen, including CMV and Epstein–Barr virus, are dysfunctional and occupy space in the T-cell compartment [2,3,67]. This may include up to 50% of the peripheral T-cell repertoire in the elderly and up to 80% of the repertoire in aged mice [68]. Subsequent loss in immune competence causes complications in clearing new infectious pathogens. Yager et al. demonstrated that aged mice exhibited a five-fold reduction in the CD8+ T-cell response to immunodominant influenza virus epitopes after primary influenza virus infection as compared with young mice. This effect was attributed to reduced thymic output as the study excluded clonal expansions, which, if taken into account, would further diminish the antiviral repertoire diversity [69].
Age-associated CD8+ T-cell dysfunction has been linked to changes in CD28 costimulation and telomerase activity at the cellular level. CD28 costimulation provides a necessary signal for CD8+ T-cell activation and expansion [70]. However, with aging, the expression of CD28 decreases dramatically in the CD8+ subset [71]. This is associated with shorter telomeres and diminished proliferative capacity, a condition referred to as replicative senescence. In addition, CD8+CD28- suppressor T cells can render T-helper cells unreactive, thereby suppressing their activity [72]. Several deleterious clinical outcomes result, including decreased vaccine responsiveness and control over infection [2,70]. Blocking CD28 interaction with its ligand on antigen-presenting cells almost entirely blocks telomerase activity [71]. Recently, a central role of CD28 in replicative senescence and its link to telomerase has been elucidated by Parish et al. This group found that the insertion of the CD28 gene into human CD8+ T cells delays the onset of replicative senescence in cell culture, and observed that telomerase activity and prolifer ation are maintained after subsequent stimulation [70].
Recent findings have shed light on alternative costimulatory pathways and signaling pathways that contribute to CD8+ T-cell senescence. Increased expression of CTLA-4 (CD152), a negative regulator of T-cell activity that can compete with CD28 ligands on antigen-presenting cells, is associated with replicative senescence [70]. This supports the existence of an inverse relationship between CTLA-4 and CD28. The same group also demonstrated the importance of adenosine deaminase (ADA) – an additional costimulatory receptor that converts adenosine to inosine – in modulating the function of T cells [73]. ADA deficiency is an autosomal recessive metabolic disorder that results in severe combined immune deficiency. Exposure to adenosine accelerated the loss of CD28 expression, reduced telo merase activity and reduced IL-2 gene transcription [73]. The CD8+CD28+ADA+ subset had higher telomerase activity than the CD8+CD28+ADA- subset [73]. In addition, it has been demonstrated that adenosine is found at increased levels in certain tumor microenvironments, where it suppresses T-cell function on tumor cells [74]. Thus, deficient ADA function on tumor-specific T cells may allow adenosine to persist, thereby not allowing the initiation of an antitumor response. With regard to specific signaling pathways, decreased Akt (Ser473) phosphorylation is associated with loss of telomerase activity in highly differentiated CD8+CD28-CD27- T cells [75]. These findings highlight the highly complex nature of the pathways that regulate end-stage differentiation of CD8+ T cells.
The implications of CD8+ T-cell senescence and the subsequent accumulation of terminally differentiated effector cells in the T-cell compartment on cancer are twofold. First, the diminution of the CD8+ TCR repertoire with age limits the response of the cytotoxic T-lymphocyte branch of the adaptive immune system to novel cancer antigens. Consistent with this notion, CD8+ T cells with features of senescence/terminal differentiation have been isolated from the tumor microenvironment of several different human cancers that fail to effectively respond to tumor antigen. Particularly, a marked increase in the subset of suppressive CD8+CD28- cells has been found in the peripheral blood of lung cancer patients [76] and large proportions of apoptosis-sensitive CD8+CD28- T cells that lack the ability to kill tumor targets have been isolated from the circulation of patients with head and neck cancer [77]. In addition, tumor-infiltrating lymphocytes lacking CD28 expression were found in endometrial carcinoma [78], cutaneous T-cell lymphoma [79], colon carcinoma [80] and primary melanoma [81]. It is important to note that these findings are correlative and not mechanistic. Furthermore, other features of terminal differentiation, namely a CD8highCD57+ population, have been demonstrated to indicate poor prognosis in terms of recurrence-free survival in renal cancer [82] and bladder carcinoma patients [83]. It has also been speculated that chronic antigenic stimulation of CD28+CD8+ T cells within the tumor microenvironment leads to terminal differentiation, loss of CD28 expression, resulting in diminished costimulation and subsequent sensitivity to activation-induced apoptosis [77]. Second, production of proinflammatory cytokines, including IFN-γ and TNF-α, by CD8+ T cells contributes to the age-associated low-level systemic inflammation frequently seen in the elderly [2]. Increased TNF-α levels correlates with poor prognosis of hematological malignancies and increases the risk of multiple myeloma, hepatocellular carcinoma, and gastric, breast and bladder cancer [84]. Blocking TNF-α through the use of a receptor inhibitor or neutralizing antibody greatly increases the proliferative potential as well as the telomerase activity of CD8+ T cells, thus acting as a novel therapeutic approach to retarding or slowing the process of T-cell senescence [71]. However, there is also evidence that TNF blockers may increase the risk of certain malignancies or cause serious toxicity [85,86].
Terminally differentiated CD8+ T cells that accumulate with age exhibit diminished functionality and have altered cytokine production profiles, including increased IFN-γ and TNF-α [2], that contribute to a proinflammatory state. These dysfunctions also contribute to the decreased ability to control infections, lowered vaccine responsiveness and ability to mount an effective antitumor response in the elderly. Much is still unknown about the specific mechanisms of immune surveillance and cancer in the aging process. However, it is becoming clear that chronic antigenic stimulation leads to extensive proliferation of large, nonfunctional oligoclonal T-cell populations that may contribute to the onset or progression of cancer.
Role of the tumor microenvironment
Recently, there has been increasing interest in the role of the microenvironment with respect to tumor initiation and progression. Once thought to be an inactive bystander, it is now believed that the microenvironment is not only permissive of tumor development but may also actively promote it. As such it is pertinent to ask whether age and/or age-related changes in the immune system alter the local microenvironment in ways that permit or promote cancer. Prostate cancer, and various animal models of it, provides one such example of the importance of the tissue microenvironment. Indeed, an ana lysis of RNA transcripts and secreted proteins from primary prostate stromal fibroblasts from younger (<55 years of age) versus older (>65 years of age) individuals by Begley et al. demonstrate a greater proinflammatory pattern in the older group [87]. A transgenic murine model of prostate cancer and benign prostate hyperplasia demonstrates an age-related increase in TGF-β1 that resulted in altered tissue architecture consistent with local inflammation [88]. This included increased fibrosis and altered matrix deposition associated with the onset of benign prostate hyperplasia and prostate cancer. Normal C57BL/6 mice also display age-dependent disorganization of the prostate stromal extracellular matrix, an increase in inflammatory infiltrates and increased expression of inflammatory mediators [89]. A follow-up study by McDowell et al. using an in vitro model that included aged primary fibroblasts (stroma) to mimic the prostate microenvironment found that the CD4+ T cells attracted to this site promoted the proliferation of prostate epithelial cells whereas CD8+ T cells, macrophages and neutrophils promoted the proliferation of prostate cancer cells [90]. These age-dependent changes in the interaction between T cells and components of the local microenvironment may play a critical role in providing the early inflammatory events necessary for the initiation of cancer while concomitantly failing to adequately respond to newly emerging tumor-specific antigen. As such, further investigation of these interactions may be helpful in developing novel therapeutic strategies.
Conclusion & future perspective
A causal link between declining immunity with age and increased incidence of cancer, although currently controversial, is suggested by some studies. In some cases, tumors are able to limit the function of T cells, including those specific for tumor antigen. CD8+ T cells specific for prostate tumor antigen, following removal from the tumor microenvironment, were unable to respond to tumor antigen in vitro by IFN-γ production or proliferation in one murine study [91]. Similarly, microvesicles isolated from human tumor cells are able to induce the generation and expansion of Treg that are capable of suppressing T-cell responses, and to convert CD4+CD25- T cells into CD4+CD25highFoxp3+ Tregs [92]. As such, the immunoregulatory effects of the tumor and its environment on T cells, combined with the decreasing ability to replace the naive T-cell population, may allow cancers to progress in the face of a fixed T-cell immune repertoire associated with age-related immunosenescence.
The precise mechanisms that lead to the three main features of declining cellular immunity remain unclear. However, it is apparent that the progressive decline in thymic output is the primary event leading to immune senescence in old age. As production of new naive T cells declines, peripheral T cells undergo repeated rounds of proliferation in order to maintain the overall size of the T-cell compartment. Over time, prolonged survival in the periphery leads to a series of defects in T cells of all activation states (naive through to terminally differentiated). The decline in functional immunity is not only more permissive to tumor formation, but may also actually promote it by contributing to chronic low-level inflammation. Despite a decline in bone marrow precursor cells with age [2], there is evidence to suggest that aged precursor cells will develop into normal T cells in the young thymic environment [40]. As such, improving resistance to cancer in old age may depend on therapeutic options that restore thymic function. For example, IL-7, growth hormone, IGF-1 and KGF have been employed to improve thymopoiesis [3]. Substantial efforts have been made to improve homeostatic peripheral expansion for generating naive T cells outside the thymus in the context of immune reconstitution following bone marrow transplantation [93].
Going forward, many efforts are in progress to improve recovery from malignancy and enhance resistance to it. One approach is the development of effective cancer vaccines, which could significantly reduce the incidence of a specific malignancy. However, given the limited vaccine response observed in old age, it is fair to question whether such a strategy would be effective for this group. Since immunological memory developed at a younger age is more effective than that generated in later years, vaccination strategies that are applied earlier in life could result in better long-term outcomes. Another potential strategy to limit the incidence of cancer in advanced age is to reduce the proinflammatory environment through the use of specific cytokine blockers. Although this has the benefit of improving other inflammatory conditions, including rheumatic diseases, it comes at a cost to the immune system [86]. Certain cytokines have been demonstrated to have a paradoxical role in the context of cancer. As mentioned previously, TNF-α promotes antitumor responses via apoptosis, which has allowed its use in cancer therapies [86]. Conversely, accumulating evidence shows that TNF-α may, in fact, promote tumor formation through several pathways [86]. Further elucidating the exact role of cytokines will allow better targeted therapies to be developed. Finally, replicative senescence in T cells may be delayed if therapeutic strategies that sustain telomerase activity, possibly through the upregulation of CD28 expression, are achieved [71]. While much has been learned about the role of age-related declining immunity as it relates to cancer incidence, the precise cellular mechanisms through which this occurs remain to be fully characterized. As research in the field progresses, from animal models to human clinical trials, new therapeutic strategies to enhance immune function, especially T-cell induction in the aged, may become powerful tools to reduce cancer incidence.
Executive summary.
Changes in the T-cell compartment with aging
Age-associated immune senescence includes three major changes in the T-cell compartment that include a reduction in naive T cells, an expansion of memory T cells and the accumulation of terminally differentiated effector T cells.
The decline in naive T cells is principally caused by the gradual decline in thymic output.
Aged naive CD4+ T cells also exhibit defective function, including reduced IL-2 production, reduced proliferation and impaired B-cell help.
Memory T cells that develop in the aged immune system are less effective than memory cells formed in earlier years, making vaccination less successful in the elderly.
Oligoclonal T cells that accumulate in the elderly have diminished functional capacity against infectious disease and malignancies.
Multiple costimulatory and signaling pathways may contribute to T-cell senescence including CD28 and CTLA4.
Age-related immune senescence & cancer
Defects in both naive and memory T-cell populations impair the immune systems response to specific antigen, including tumors.
Diminished T-cell receptor repertoire diversity compromises response to novel cancer antigen.
The production of inflammatory cytokines by aging memory T cells and terminally differentiated CD8+ T cells contribute to a chronic inflammatory state that may promote tumor development.
Future perspective
Effective cancer vaccines administered at a young age may prevent tumorigenesis during later years.
Strategies aimed at restoring thymopoiesis in the elderly may result in a more ‘youthful’ immune system.
Delaying T-cell replicative senescence by upregulating CD28 and telomerase activity may be useful therapeutic approaches.
Acknowledgments
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Fulop T, Kotb R, Fortin CF, Pawelec G, De Angelis F, Larbi A. Potential role of immunosenescence in cancer development. Ann. NY Acad. Sci. 2010;1197:158–165. doi: 10.1111/j.1749-6632.2009.05370.x. [DOI] [PubMed] [Google Scholar]
- 2.Hakim FT, Flomerfelt FA, Boyiadzis M, Gress RE. Aging, immunity and cancer. Curr. Opin. Immunol. 2004;16(2):151–156. doi: 10.1016/j.coi.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Hakim FT, Gress RE. Immunosenescence: deficits in adaptive immunity in the elderly. Tissue Antigens. 2007;70(3):179–189. doi: 10.1111/j.1399-0039.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 4.Sportès C, Hakim FT. Aging, immunity and cancer. In: Fulop T, Franceschi C, Hirokawa K, Pawelec G, editors. Handbook on Immunosenescence: Basic Understanding and Clinical Applications. Springer; Berlin, Germany: 2009. [Google Scholar]
- 5.Schultzel M, Saltzstein SL, Downs TM, Shimasaki S, Sanders C, Sadler GR. Late age (85 years or older) peak incidence of bladder cancer. J. Urol. 2008;179(4):1302–1305. doi: 10.1016/j.juro.2007.11.079. discussion 1305–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Candore G, Balistreri CR, Listi F, et al. Immunogenetics, gender, and longevity. Ann. NY Acad. Sci. 2006;1089:516–537. doi: 10.1196/annals.1386.051. [DOI] [PubMed] [Google Scholar]
- 7.Derhovanessian E, Maier AB, Beck R, et al. Hallmark features of immunosenescence are absent in familial longevity. J. Immunol. 2010;185(8):4618–4624. doi: 10.4049/jimmunol.1001629. [DOI] [PubMed] [Google Scholar]
- 8.Arranz L, Caamano JH, Lord JM, De la Fuente M. Preserved immune functions and controlled leukocyte oxidative stress in naturally long-lived mice: possible role of nuclear factor κB. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65(9):941–950. doi: 10.1093/gerona/glq101. [DOI] [PubMed] [Google Scholar]
- 9.Arranz L, Lord JM, De la Fuente M. Preserved ex vivo inflammatory status and cytokine responses in naturally long-lived mice. Age (Dordr.) 2010;32(4):451–466. doi: 10.1007/s11357-010-9151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schreiber TH, Podack ER. A critical ana lysis of the tumour immunosurveillance controversy for 3-MCA-induced sarcomas. Br. J. Cancer. 2009;101(3):381–386. doi: 10.1038/sj.bjc.6605198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bedel R, Thiery-Vuillemin A, Grandclement C, et al. Novel role for STAT3 in transcriptional regulation of NK immune cell targeting receptor MICA on cancer cells. Cancer Res. 2011;71(5):1615–1626. doi: 10.1158/0008-5472.CAN-09-4540. [DOI] [PubMed] [Google Scholar]
- 12.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393(6684):474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 13.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297(5589):2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 14.Matsui S, Ahlers JD, Vortmeyer AO, et al. A model for CD8+ CTL tumor immunosurveillance and regulation of tumor escape by CD4 T cells through an effect on quality of CTL. J. Immunol. 1999;163(1):184–193. [PubMed] [Google Scholar]
- 15.Gerner MY, Casey KA, Mescher MF. Defective MHC class II presentation by dendritic cells limits CD4 T cell help for antitumor CD8 T cell responses. J. Immunol. 2008;181(1):155–164. doi: 10.4049/jimmunol.181.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corthay A, Skovseth DK, Lundin KU, et al. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22(3):371–383. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Corthay A, Lundin KU, Lorvik KB, Hofgaard PO, Bogen B. Secretion of tumor-specific antigen by myeloma cells is required for cancer immunosurveillance by CD4+ T cells. Cancer Res. 2009;69(14):5901–5907. doi: 10.1158/0008-5472.CAN-08-4816. [DOI] [PubMed] [Google Scholar]
- 18.Levitsky HI, Lazenby A, Hayashi RJ, Pardoll DM. In vivo priming of two distinct antitumor effector populations: the role of MHC class I expression. J. Exp. Med. 1994;179(4):1215–1224. doi: 10.1084/jem.179.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogen B, Munthe L, Sollien A, et al. Naive CD4+ T cells confer idiotype-specific tumor resistance in the absence of antibodies. Eur. J. Immunol. 1995;25(11):3079–3086. doi: 10.1002/eji.1830251114. [DOI] [PubMed] [Google Scholar]
- 20.Haabeth OA, Lorvik KB, Hammarstrom C, et al. Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat. Comm. 2011;2:240. doi: 10.1038/ncomms1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura H, Saji H, Ogata A, et al. Immunologic parameters as significant prognostic factors in lung cancer. Lung Cancer. 2002;37(2):161–169. doi: 10.1016/s0169-5002(02)00100-9. [DOI] [PubMed] [Google Scholar]
- 22.Unitt E, Marshall A, Gelson W, et al. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J. Hepatol. 2006;45(2):246–253. doi: 10.1016/j.jhep.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 23.Vella LA, Yu M, Fuhrmann SR, El-Amine M, Epperson DE, Finn OJ. Healthy individuals have T-cell and antibody responses to the tumor antigen cyclin B1 that when elicited in mice protect from cancer. Proc. Natl Acad. Sci. USA. 2009;106(33):14010–14015. doi: 10.1073/pnas.0903225106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koebel CM, Vermi W, Swann JB, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450(7171):903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 25.Engel AM, Svane IM, Rygaard J, Werdelin O. MCA sarcomas induced in SCID mice are more immunogenic than MCA sarcomas induced in congenic, immunocompetent mice. Scand. J. Immunol. 1997;45(5):463–470. doi: 10.1046/j.1365-3083.1997.d01-419.x. [DOI] [PubMed] [Google Scholar]
- 26.Shankaran V, Ikeda H, Bruce AT, et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410(6832):1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 27.Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Ann. Rev. Immunol. 2000;18:529–560. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 28.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat. Immunol. 2003;4(7):680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 29.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol. Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 30.Ivory K, Martin R, Hughes DA. Significant presence of terminally differentiated T cells and altered NF-κB and I-κBα interactions in healthy ageing. Exp. Gerontol. 2004;39(4):567–576. doi: 10.1016/j.exger.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 31.Ouyang Q, Wagner WM, Wikby A, et al. Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. J. Clin. Immunol. 2003;23(4):247–257. doi: 10.1023/a:1024580531705. [DOI] [PubMed] [Google Scholar]
- 32.Arnold CR, Wolf J, Brunner S, Herndler-Brandstetter D, Grubeck-Loebenstein B. Gain and loss of T cell subsets in old age – age-related reshaping of the T cell repertoire. J. Clin. Immunol. 2011;31(2):137–146. doi: 10.1007/s10875-010-9499-x. [DOI] [PubMed] [Google Scholar]
- 33.Sempowski GD, Gooding ME, Liao HX, Le PT, Haynes BF. T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol. Immunol. 2001;38(11):841–848. doi: 10.1016/s0161-5890(01)00122-5. [DOI] [PubMed] [Google Scholar]
- 34.Kilpatrick RD, Rickabaugh T, Hultin LE, et al. Homeostasis of the naive CD4+ T cell compartment during aging. J. Immunol. 2008;180(3):1499–1507. doi: 10.4049/jimmunol.180.3.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Globerson A, Effros RB. Ageing of lymphocytes and lymphocytes in the aged. Immunol. Today. 2000;21(10):515–521. doi: 10.1016/s0167-5699(00)01714-x. [DOI] [PubMed] [Google Scholar]
- 36.Bouree P. Immunity and immunization in elderly. Pathol. Biol. (Paris) 2003;51(10):581–585. doi: 10.1016/j.patbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Kang I, Hong MS, Nolasco H, et al. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J. Immunol. 2004;173(1):673–681. doi: 10.4049/jimmunol.173.1.673. [DOI] [PubMed] [Google Scholar]
- 38.Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J. Exp. Med. 2004;200(12):1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haynes L, Linton PJ, Eaton SM, Tonkonogy SL, Swain SL. Interleukin 2, but not other common γ chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J. Exp. Med. 1999;190(7):1013–1024. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Eaton SM, Maue AC, Swain SL, Haynes L. Bone marrow precursor cells from aged mice generate CD4 T cells that function well in primary and memory responses. J. Immunol. 2008;181(7):4825–4831. doi: 10.4049/jimmunol.181.7.4825. [Demonstrates that memory T cells formed from aged bone marrow precursor cells transferred into young hosts function normally. This supports T-cell-extrinsic factors in age-associated dysfunction.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yehuda AB, Friedman G, Wirtheim E, Abel L, Globerson A. Checkpoints in thymocytopoiesis in aging: expression of the recombination activating genes RAG-1 and RAG-2. Mech. Ageing Dev. 1998;102(2–3):239–247. doi: 10.1016/s0047-6374(98)00021-9. [DOI] [PubMed] [Google Scholar]
- 42.Tsukamoto H, Clise-Dwyer K, Huston GE, et al. Age-associated increase in lifespan of naive CD4 T cells contributes to T-cell homeostasis but facilitates development of functional defects. Proc. Natl Acad. Sci. USA. 2009;106(43):18333–18338. doi: 10.1073/pnas.0910139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones SC, Clise-Dwyer K, Huston G, et al. Impact of post-thymic cellular longevity on the development of age-associated CD4+ T cell defects. J. Immunol. 2008;180(7):4465–4475. doi: 10.4049/jimmunol.180.7.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Tsukamoto H, Huston GE, Dibble J, Duso DK, Swain SL. Bim dictates naive CD4 T cell lifespan and the development of age-associated functional defects. J. Immunol. 2010;185(8):4535–4544. doi: 10.4049/jimmunol.1001668. [Identified reduced BIM expression as being responsible for prolonged survival of aged naive CD4 T cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gui J, Zhu X, Dohkan J, Cheng L, Barnes PF, Su DM. The aged thymus shows normal recruitment of lymphohematopoietic progenitors but has defects in thymic epithelial cells. Int. Immunol. 2007;19(10):1201–1211. doi: 10.1093/intimm/dxm095. [DOI] [PubMed] [Google Scholar]
- 46.Zhu X, Gui J, Dohkan J, Cheng L, Barnes PF, Su DM. Lymphohematopoietic progenitors do not have a synchronized defect with age-related thymic involution. Aging Cell. 2007;6(5):663–672. doi: 10.1111/j.1474-9726.2007.00325.x. [DOI] [PubMed] [Google Scholar]
- 47•.Sun L, Guo J, Brown R, Amagai T, Zhao Y, Su DM. Declining expression of a single epithelial cell-autonomous gene accelerates age-related thymic involution. Aging Cell. 2010;9(3):347–357. doi: 10.1111/j.1474-9726.2010.00559.x. [Identified declining FoxN1 expression as a critical mediator of thymic epithelial cell degeneration.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Sauce D, Larsen M, Fastenackels S, et al. Evidence of premature immune aging in patients thymectomized during early childhood. J. Clin. Invest. 2009;119(10):3070–3078. doi: 10.1172/JCI39269. [Demonstrates accelerated aging of the immune system in individuals thymectomized at an early age.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madhok AB, Chandrasekran A, Parnell V, Gandhi M, Chowdhury D, Pahwa S. Levels of recent thymic emigrant cells decrease in children undergoing partial thymectomy during cardiac surgery. Clin. Diagn. Lab. Immunol. 2005;12(5):563–565. doi: 10.1128/CDLI.12.5.563-565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ogle BM, West LJ, Driscoll DJ, et al. Effacing of the T cell compartment by cardiac transplantation in infancy. J. Immunol. 2006;176(3):1962–1967. doi: 10.4049/jimmunol.176.3.1962. [DOI] [PubMed] [Google Scholar]
- 51.Ahmed M, Lanzer KG, Yager EJ, Adams PS, Johnson LL, Blackman MA. Clonal expansions and loss of receptor diversity in the naive CD8 T cell repertoire of aged mice. J. Immunol. 2009;182(2):784–792. doi: 10.4049/jimmunol.182.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Czesnikiewicz-Guzik M, Lee WW, Cui D, et al. T cell subset-specific susceptibility to aging. Clin. Immunol. 2008;127(1):107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta S, Gollapudi S. TNF-α-induced apoptosis in human naive and memory CD8+ T cells in aged humans. Exp. Gerontol. 2006;41(1):69–77. doi: 10.1016/j.exger.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Gupta S, Gollapudi S. CD95-mediated apoptosis in naive, central and effector memory subsets of CD4+ and CD8+ T cells in aged humans. Exp. Gerontol. 2008;43(4):266–274. doi: 10.1016/j.exger.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 55.Smithey MJ, Renkema KR, Rudd BD, Nikolich-Zugich J. Increased apoptosis, curtailed expansion and incomplete differentiation of CD8+ T cells combine to decrease clearance of L. monocytogenes in old mice. Eur. J. Immunol. 2011;41(5):1352–1364. doi: 10.1002/eji.201041141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cicin-Sain L, Smyk-Pearson S, Currier N, et al. Loss of naive T cells and repertoire constriction predict poor response to vaccination in old primates. J. Immunol. 2010;184(12):6739–6745. doi: 10.4049/jimmunol.0904193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linton PJ, Li SP, Zhang Y, Bautista B, Huynh Q, Trinh T. Intrinsic versus environmental influences on T-cell responses in aging. Immunol. Rev. 2005;205:207–219. doi: 10.1111/j.0105-2896.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 58.Cusi MG, Martorelli B, Di Genova G, Terrosi C, Campoccia G, Correale P. Age related changes in T cell mediated immune response and effector memory to respiratory syncytial virus (RSV) in healthy subjects. Immun. Ageing. 2010;7:14. doi: 10.1186/1742-4933-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watanabe MA, Oda JM, Amarante MK, Cesar Voltarelli J. Regulatory T cells and breast cancer: implications for immunopathogenesis. Cancer Metastasis Rev. 2010;29(4):569–579. doi: 10.1007/s10555-010-9247-y. [DOI] [PubMed] [Google Scholar]
- 60.Hirokawa K, Utsuyama M, Ishikawa T, et al. Decline of T cell-related immune functions in cancer patients and an attempt to restore them through infusion of activated autologous T cells. Mech. Ageing Dev. 2009;130(1–2):86–91. doi: 10.1016/j.mad.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 61.Rottinghaus EK, Vesosky B, Turner J. Interleukin-12 is sufficient to promote antigen-independent interferon-γ production by CD8 T cells in old mice. Immunology. 2009;128(Suppl. 1):e679–e690. doi: 10.1111/j.1365-2567.2009.03061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ouyang X, Yang Z, Zhang R, et al. Potentiation of Th17 cytokines in aging process contributes to the development of colitis. Cell Immunol. 2011;266(2):208–217. doi: 10.1016/j.cellimm.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L, Yi T, Zhang W, Pardoll DM, Yu H. IL-17 enhances tumor development in carcinogen-induced skin cancer. Cancer Res. 2010;70(24):10112–10120. doi: 10.1158/0008-5472.CAN-10-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iida T, Iwahashi M, Katsuda M, et al. Tumor-infiltrating CD4+ Th17 cells produce IL-17 in tumor microenvironment and promote tumor progression in human gastric cancer. Oncol. Rep. 2011;25(5):1271–1277. doi: 10.3892/or.2011.1201. [DOI] [PubMed] [Google Scholar]
- 65.Wilke CM, Kryczek I, Wei S, et al. Th17 cells in cancer: help or hindrance? Carcinogenesis. 2011;32(5):643–649. doi: 10.1093/carcin/bgr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahmad A, Banerjee S, Wang Z, Kong D, Majumdar AP, Sarkar FH. Aging and inflammation: etiological culprits of cancer. Curr. Aging Sci. 2009;2(3):174–186. doi: 10.2174/1874609810902030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haynes L, Maue AC. Effects of aging on T cell function. Curr. Opin. Immunol. 2009;21(4):414–417. doi: 10.1016/j.coi.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maue AC, Yager EJ, Swain SL, Woodland DL, Blackman MA, Haynes L. T-cell immunosenescence: lessons learned from mouse models of aging. Trends Immunol. 2009;30(7):301–305. doi: 10.1016/j.it.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J. Exp. Med. 2008;205(3):711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parish ST, Wu JE, Effros RB. Sustained CD28 expression delays multiple features of replicative senescence in human CD8 T lymphocytes. J. Clin. Immunol. 2010;30(6):798–805. doi: 10.1007/s10875-010-9449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71•.Effros RB. Telomere/telomerase dynamics within the human immune system: effect of chronic infection and stress. Exp. Gerontol. 2010;46(2–3):135–140. doi: 10.1016/j.exger.2010.08.027. [Highlights recent findings in the T lymphocyte replicative senescence model, the role of latent viral infections and also addresses therapeutic approaches to retarding the process of senescence.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cortesini R, Lemaoult J, Ciubotariu R, Cortesini NS. CD8+CD28- T suppressor cells and the induction of antigen-specific, antigen-presenting cell-mediated suppression of Th reactivity. Immunol. Rev. 2001;182:201–206. doi: 10.1034/j.1600-065x.2001.1820116.x. [DOI] [PubMed] [Google Scholar]
- 73.Parish ST, Kim S, Sekhon RK, Wu JE, Kawakatsu Y, Effros RB. Adenosine deaminase modulation of telomerase activity and replicative senescence in human CD8 T lymphocytes. J. Immunol. 2010;184(6):2847–2854. doi: 10.4049/jimmunol.0903647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mackenzie WM, Hoskin DW, Blay J. Adenosine suppresses α(4)β(7) integrin-mediated adhesion of T lymphocytes to colon adenocarcinoma cells. Exp. Cell Res. 2002;276(1):90–100. doi: 10.1006/excr.2002.5514. [DOI] [PubMed] [Google Scholar]
- 75.Plunkett FJ, Franzese O, Finney HM, et al. The loss of telomerase activity in highly differentiated CD8+CD28-CD27- T cells is associated with decreased Akt (Ser473) phosphorylation. J. Immunol. 2007;178(12):7710–7719. doi: 10.4049/jimmunol.178.12.7710. [DOI] [PubMed] [Google Scholar]
- 76.Meloni F, Morosini M, Solari N, et al. Foxp3 expressing CD4+ CD25+ and CD8+CD28- T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Hum. Immunol. 2006;67(1–2):1–12. doi: 10.1016/j.humimm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 77.Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8+CD28- T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol. Immunother. 2003;52(10):599–607. doi: 10.1007/s00262-003-0395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang WC, Li CH, Huang SC, Chang DY, Chou LY, Sheu BC. Clinical significance of regulatory T cells and CD8+ effector populations in patients with human endometrial carcinoma. Cancer. 2010;116(24):5777–5788. doi: 10.1002/cncr.25371. [DOI] [PubMed] [Google Scholar]
- 79.Urbaniak-Kujda D, Kapelko-Slowik K, Wolowiec D, et al. Increased percentage of CD8+CD28- suppressor lymphocytes in peripheral blood and skin infiltrates correlates with advanced disease in patients with cutaneous T-cell lymphomas. Postepy. Hig. Med. Dosw. (Online) 2009;63:355–359. [PubMed] [Google Scholar]
- 80.Kruger K, Buning C, Schriever F. Activated T lymphocytes bind in situ to stromal tissue of colon carcinoma but lack adhesion to tumor cells. Eur. J. Immunol. 2001;31(1):138–145. doi: 10.1002/1521-4141(200101)31:1<138::aid-immu138>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 81.Becker JC, Vetter CS, Schrama D, Bröcker EB, thor Straten P. Differential expression of CD28 and CD94/NKG2 on T cells with identical TCR β variable regions in primary melanoma and sentinel lymph node. Eur. J. Immunol. 2000;30(12):3699–3706. doi: 10.1002/1521-4141(200012)30:12<3699::AID-IMMU3699>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 82.Characiejus D, Pasukoniene V, Kazlauskaite N, et al. Predictive value of CD8highCD57+ lymphocyte subset in interferon therapy of patients with renal cell carcinoma. Anticancer Res. 2002;22(6B):3679–3683. [PubMed] [Google Scholar]
- 83.Characiejus D, Pasukoniene V, Jacobs JJ, et al. Prognostic significance of peripheral blood CD8highCD57+ lymphocytes in bladder carcinoma patients after intravesical IL-2. Anticancer Res. 2011;31(2):699–703. [PubMed] [Google Scholar]
- 84.Malaguarnera L, Cristaldi E, Malaguarnera M. The role of immunity in elderly cancer. Crit. Rev. Oncol. Hematol. 2010;74(1):40–60. doi: 10.1016/j.critrevonc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 85.Diak P, Siegel J, La Grenade L, Choi L, Lemery S, McMahon A. Tumor necrosis factor a blockers and malignancy in children: forty-eight cases reported to the food and drug administration. Arthritis Rheum. 2010;62(8):2517–2524. doi: 10.1002/art.27511. [DOI] [PubMed] [Google Scholar]
- 86.Zidi I, Mestiri S, Bartegi A, Amor NB. TNF-α and its inhibitors in cancer. Med. Oncol. 2009;27(2):185–198. doi: 10.1007/s12032-009-9190-3. [DOI] [PubMed] [Google Scholar]
- 87.Begley LA, Kasina S, MacDonald J, Macoska JA. The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine. 2008;43(2):194–199. doi: 10.1016/j.cyto.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barron DA, Strand DW, Ressler SJ, et al. TGF-β1 induces an age-dependent inflammation of nerve ganglia and fibroplasia in the prostate gland stroma of a novel transgenic mouse. PLoS One. 2010;5(10):e13751. doi: 10.1371/journal.pone.0013751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bianchi-Frias D, Vakar-Lopez F, Coleman IM, Plymate SR, Reed MJ, Nelson PS. The effects of aging on the molecular and cellular composition of the prostate microenvironment. PLoS One. 2010;5(9):e12501. doi: 10.1371/journal.pone.0012501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McDowell KL, Begley LA, Mor-Vaknin N, Markovitz DM, Macoska JA. Leukocytic promotion of prostate cellular proliferation. Prostate. 2010;70(4):377–389. doi: 10.1002/pros.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anderson MJ, Shafer-Weaver K, Greenberg NM, Hurwitz AA. Tolerization of tumor-specific T cells despite efficient initial priming in a primary murine model of prostate cancer. J. Immunol. 2007;178(3):1268–1276. doi: 10.4049/jimmunol.178.3.1268. [DOI] [PubMed] [Google Scholar]
- 92.Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (T). PLoS One. 2010;5(7):e11469. doi: 10.1371/journal.pone.0011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fry TJ, Sinha M, Milliron M, et al. Flt3 ligand enhances thymic-dependent and thymic-independent immune reconstitution. Blood. 2004;104(9):2794–2800. doi: 10.1182/blood-2003-11-3789. [DOI] [PubMed] [Google Scholar]
- 101.National Cancer Institute website Surveillance, Epidemiology and End Results (SEER) database. http://seer.cancer.gov/