Abstract
Bipolar cells (BCs) are critical relay neurons in the retina that are organized into parallel signaling pathways. The three main signaling pathways in the mammalian retina are the rod, ON cone, and OFF cone BCs. Rod BCs mediate incrementing dim light signals from rods, and ON cone and OFF cone BCs mediate incrementing and decrementing brighter light signals from cones, respectively. The outputs of BCs are shaped by inhibitory inputs from GABAergic and glycinergic amacrine cells in the inner plexiform layer, mediated by three distinct types of inhibitory receptors: GABAA, GABAC, and glycine receptors. The three main BC pathways receive distinct forms of inhibition from these three receptors that shape their light-evoked inhibitory signals. Rod BC inhibition is dominated by slow GABAC receptor inhibition, while OFF cone BCs are dominated by glycinergic inhibition. The inhibitory inputs to BCs are also shaped by serial inhibitory connections between GABAergic amacrine cells that limit the spatial profile of BC inhibition. We discuss our recent studies on how inhibitory inputs to BCs are shaped by receptor expression, receptor properties, and neurotransmitter release properties and how these affect the output of BCs.
Keywords: GABA, Glycine, Light, GABAA receptor, GABAC receptor, glycine receptor, patch-clamp
Bipolar cells (BCs) are retinal relay neurons that are critical for visual signaling. The main signaling pathway through the retina consists of photoreceptors, BCs, and ganglion cells. Vision begins with photoreceptors that transduce light into an electrical signal. Photoreceptors relay light-evoked signals to BCs that in turn transmit information to the ganglion cells that carry visual information to higher visual centers in the brain. However, BCs are not just passive conduits that funnel light-evoked signals from photoreceptors to ganglion cells. Instead, BCs are thought to extract and then transmit different features of the visual scene. The separation of the visual signal into distinct parallel pathways first occurs at the BC dendrites. In all vertebrate retinas, including primates, at least 10 subtypes of BCs have been identified (Wässle, 2004). Each of these BC types subserves a unique functional role.
The 10 subtypes of BCs can be divided into three major classes: the rod, ON cone, and OFF cone BCs. Rod BCs receive inputs from rod photoreceptors and are critical elements for mediating dim light signaling. ON cone and OFF cone BCs receive inputs from cone photoreceptors that sense bright light levels and signal increments and decrements in illumination, respectively. The separation of the ON and OFF BC responses is determined by different glutamate receptors that respond in opposite ways to glutamate released from cone photoreceptors. Rod and ON cone BCs possess dendritic metabotropic glutamate receptors (mGluR6) that close TRPM1 channels when activated by glutamate (Morgans et al., 2009; Shen et al., 2009; van Genderen et al., 2009). This results in a light-evoked depolarization of ON BCs in response to decreased glutamate release from cones. OFF BCs possess dendritic ionotropic AMPA or kainate receptors that open cation channels when activated by glutamate. This results in a light-evoked hyperpolarization of OFF BCs in response to decreased glutamate release from cones. Furthermore, there are multiple subtypes of ON cone and OFF cone BCs that form additional parallel signaling pathways that encode distinct temporal, spatial, and chromatic features of the visual scene (Awatramani & Slaughter, 2000; DeVries, 2000). Photoreceptor inputs to the different BC pathways have distinct temporal properties, with rod signals much slower than cone signals (Ashmore & Copenhagen, 1980; Schnapf & Copenhagen, 1982; Cadetti et al., 2005). Differences in temporal encoding are also attributed to distinct glutamate receptor subtypes at the dendrites of BCs (Li & DeVries, 2006). Thus, the distinct glutamate receptor subtypes temporally filter photoreceptor inputs, allowing different BC classes to extract unique features of the visual input.
Amacrine cell–mediated inhibition shapes BC output
While visual signals received by BCs are shaped by the properties of dendritic glutamate receptors, modulation of transmitter release from BCs is another critical point where the visual signal can be shaped. Amacrine cells contact BC axons and mediate inhibitory signaling in the inner plexiform layer that modulates the output of BCs. BC terminals may receive both GABA- and glycine-mediated inhibition (Lukasiewicz & Werblin, 1994; Pan & Lipton, 1995; Dong & Werblin, 1998; Euler & Masland, 2000). This potential diversity of inhibitory amacrine cell inputs to BC axon terminals may differentially modulate BC outputs. Below, we detail how the major classes of BCs are differentially influenced by GABAergic and glycinergic inhibition.
GABA and glycine signals to BC terminals (Fig. 1) are mediated by two morphologically distinct groups of amacrine cells (Pourcho & Goebel, 1983; Vaney, 1990; Menger et al., 1998). These two groups of amacrine cells have distinct functional roles. GABAergic amacrine cells (ACs) are generally wide field and carry signals laterally across the retina within a single layer of the inner plexiform layer (Pourcho & Goebel, 1983; Vaney, 1990). In contrast, glycinergic ACs are generally narrow field and carry signals vertically across different layers of the inner plexiform layer (Menger et al., 1998). These morphological distinctions are reflected in the differences in GABAergic and glycinergic inhibition observed in ganglion cells.
Fig. 1.
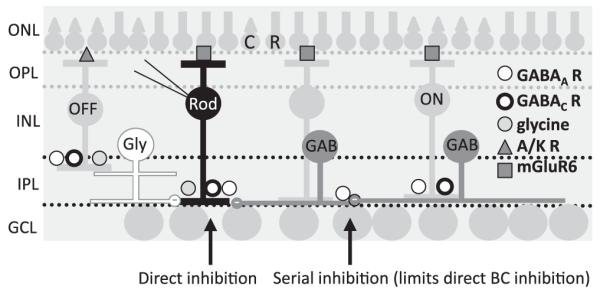
Inhibition to retinal BCs. A cartoon of the retina is shown with the rod (R) and cone (C) photoreceptors in the outer nuclear layer (ONL) that make connections with the BCs in the outer plexiform layer (OPL). BCs and amacrine cells are located in the inner nuclear layer (INL) and make contacts with the ganglion cells (GCL) in the inner plexiform layer (IPL). BCs receive inhibitory inputs from glycinergic (Gly, white) and GABAergic (GAB, dark gray) amacrine cells that have distinct spatial extents in the retina. Rod and OFF cone BCs (OFF) receive inhibitory inputs onto GABAA, GABAC, and glycine receptors (R), while ON cone BCs (ON) receive inputs onto GABAA and GABACRs, all of which is direct inhibition. Glutamatergic inputs to BCs are mediated by two distinct types of glutamate receptors, AMPA/kainate receptors in the OFF pathway (A/K R) and mGluR6s in the ON pathway, including rod and ON cone BCs. GABAergic amacrine cells also receive inhibitory inputs from other GABAergic amacrine cells that are mediated by GABAARs (serial inhibition). The illustrated pathway shows inhibition onto BCs, but the cone BCs receive similar inhibition (see text).
GABAergic inputs are implicated in lateral inhibitory signaling within the inner plexiform layer, typically within a single layer. Blocking GABAergic amacrine cell–mediated inhibition changes the spatial and temporal components of visual processing. Previous studies have shown that signaling from GABAergic amacrine cells is critical for the spatial tuning of ganglion cells (Cook & McReynolds, 1998; Flores-Herr et al., 2001). When GABA signaling was blocked, some types of ganglion cells lost their surround inhibition, resulting in decreased spatial tuning. GABA-mediated inhibition of BC axon terminals may also be important in temporal signaling. Rod BCs receive direct inhibitory feedback from A17 ACs that shapes the time course of glutamate release (Dong & Hare, 2003; Singer & Diamond, 2003; Chavez et al., 2006). Blockade of GABAergic signaling to some BC terminals causes the normally transient BC output to become more sustained (Dong & Werblin, 1998; Sagdullaev et al., 2006), consistent with the idea that inhibition limits the BC glutamate release. However, transient responses were not completely transformed into sustained responses in ganglion cells, indicating that other factors also contribute to the formation of transient responses. Additionally, a study in goldfish (Li et al., 2007) showed that GABAergic signaling onto BCs can undergo paired pulse depression, which can also contribute to differences in kinetics in downstream neuronal responses.
Glycinergic inputs play critical roles in vertical signaling within the inner plexiform layer. The inner plexiform layer is divided into numerous functional strata that are thought to encode different representations of the visual input (Roska et al., 2006). The ON and OFF signaling pathways are segregated into the inner and outer halves of the inner plexiform layer (IPL), respectively (Famiglietti & Kolb, 1976). The processes of glycinergic amacrine cells typically span many layers of the IPL, suggesting that glycinergic amacrine cells signal between different strata of the IPL. Consistent with this idea, glycinergic signals have recently been shown to mediate crossover inhibition between ON and OFF layers of the IPL (Roska et al., 2006; Chavez & Diamond, 2008; Manookin et al., 2008; Molnar et al., 2009). An important example of this idea is that glycinergic AII amacrine cells receive excitatory input from the ON layers of the IPL and then transmit inhibitory outputs to the OFF layers (Manookin et al., 2008). Glycinergic crossover inhibition has been postulated to act in concert with excitation to linearize signaling in some signaling pathways (Werblin, 2010).
GABA and glycine receptor properties fine-tune inhibitory inputs to BCs
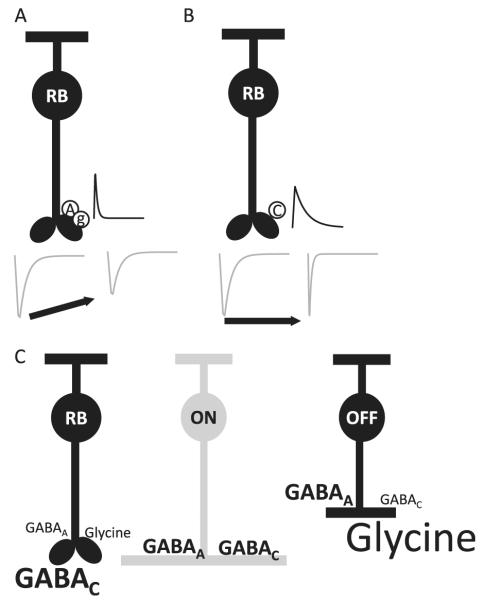
BC inhibition from GABAergic and glycinergic amacrine cells is mediated by three types of inhibitory receptors: GABAC, GABAA, and glycine receptors (R, Fig. 1). These receptors have distinct biophysical properties that could affect how BCs respond to GABA and glycine inputs. GABAARs respond quickly to GABA, rapidly turning on and off in several milliseconds (Eggers & Lukasiewicz, 2006a). Heterologously expressed GABACRs, in contrast, respond more slowly to GABA (Chang & Weiss, 1999), with native GABACRs reaching peak amplitude in several milliseconds and turning off much more slowly than GABAARs in tens to hundreds of milliseconds (Shields et al., 2000; McCall et al., 2002). Typically, the GABAAR responses are over before the GABACR responses have reached their peaks (Shields et al., 2000), as illustrated in Fig. 3. GABACRs are also about 10-fold more sensitive to GABA than GABAARs (Feigenspan & Bormann, 1994; Chang & Weiss, 1999). These distinct GABAR properties suggest that the properties of GABAergic inhibition will vary with the complement of postsynaptic GABAR subtypes. For example, inhibitory responses mediated predominantly by GABACRs should have a slower time course compared to responses mediated mainly by GABAARs. Below, we provide evidence in support of this idea.
Fig. 3.
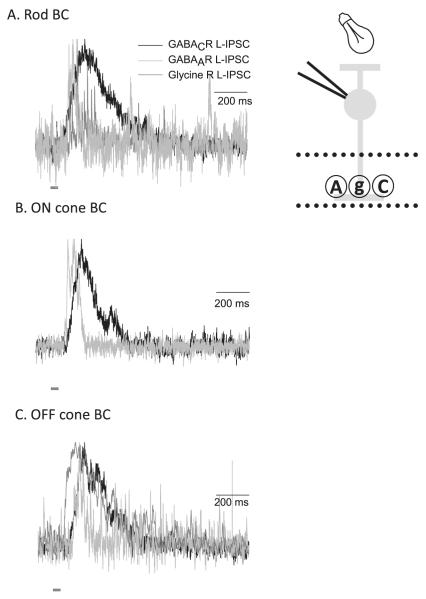
GABACR, GABAAR, and glycineRs mediate L-IPSCs with distinct time courses in all BC classes. L-IPSCs were recorded from BCs voltage clamped to 0 mV, the reversal potential for excitatory currents mediated by nonselective cation channels, and elicited with a 30-ms full-field stimulus (dark gray bar). L-IPSCs were normalized to the peak of the response to illustrate kinetic differences. Receptors were isolated with combinations of antagonists, as in Fig. 2. (A) In rod BCs, GABACR L-IPSCs have a slower decay time and rise time than GABAAR or glycineR L-IPSCs. However, the decay time of glycineR L-IPSCs is slower than GABAAR L-IPSCs. (B) In ON cone BCs, GABAAR and GABACR L-IPSCs show similar kinetics as in rod BCs. (C) OFF cone BC L-IPSCs show similar kinetics differences as ON cone and rod BCs.
GlycineRs are activated by inputs from morphologically and functionally distinct glycinergic amacrine cells. Agonist-evoked and spontaneous responses mediated by glycineRs generally have a fast time course, similar to the responses mediated by GABAARs (Eggers & Lukasiewicz, 2006a). Also like GABAARs, glycineRs have moderate sensitivity for their agonist. Thus, GABAAR and glycineR properties may similarly shape their respective GABA- and glycine-mediated responses, assuming that each transmitter is released with similar kinetics. As we discuss below, differences in transmitter release time courses can also influence the time course of the inhibitory response. Given the diversity of inhibitory receptors on BC terminals, we wanted to know whether the inhibitory inputs of different BCs were distinctly shaped, similar to how their excitatory inputs are shaped by different subtypes of glutamate receptors.
What factors determine the different forms of BC inhibition?
Inhibition to a given BC type is determined by 1) the biophysical properties of the inhibitory receptors present on the BCs, 2) the properties of the amacrine cells that mediate the inhibitory input (i.e., GABA vs. glycine, sustained release vs. phasic release, wide-field integration vs. narrow-field integration, etc.), and 3) network interactions that influence BC inhibition (i.e., serial inhibitory circuits). These three factors interact to determine how inhibition distinctly shapes the output of different BCs. Ultimately, the BC output is shaped by the magnitude and timing of inhibition. The extent of suppression of the BC output is determined by the magnitude of inhibition. The time course of the BC output can be shaped by the timing of inhibition. As noted above, GABA-mediated inhibition can truncate glutamate release, resulting in more phasic excitatory signaling to postsynaptic target cells. Finally, the spatial properties of inhibition depend on the amacrine cell morphology. Wide-field GABAergic amacrine cells mediate spatially extensive surround inhibition, while narrow-field glycinergic amacrine cells mediate spatially compact inhibition that synergistically interacts with excitation from BCs. We have investigated the factors that contribute to the distinct shaping of inhibition for different BC classes. Our results suggest that these distinct forms of BC inhibition may be optimized for parallel BC pathways in the retina. Here, we discuss the evidence for this.
The magnitude of GABAAR-, GABACR-, and glycineR-mediated inhibition varies between BC pathways
BCs receive inputs from glycinergic and GABAergic amacrine cells that are mediated by three types of receptors, GABAARs, GABACRs, and glycineRs. This combination of inputs and postsynaptic receptors suggests that there are potentially at least three distinct inhibitory inputs to BCs. Variations in the magnitudes of each of these inputs between BC pathways can generate even more distinct forms of inhibition. Different forms of inhibition can uniquely modulate the outputs of distinct parallel BC pathways. To determine the roles of these different types of inhibition, we recorded inhibitory responses in BCs that were evoked by applied agonists, by the spontaneous release of inhibitory transmitter, and by light-evoked release of inhibitory transmitter.
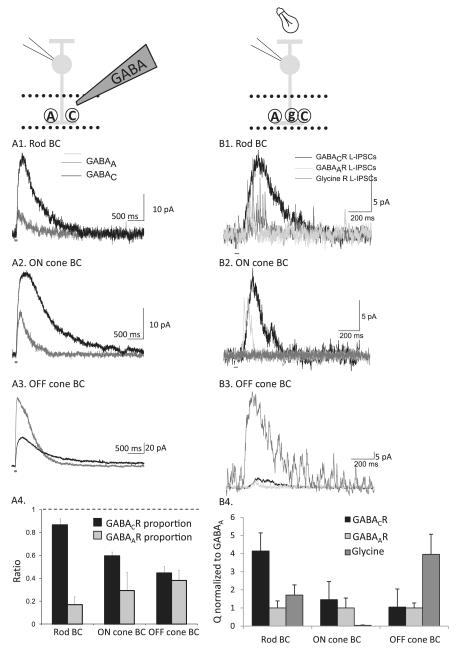
The puff application of GABA revealed that unique combinations of GABAARs and GABACRs mediate inhibition in different classes of BCs. By directly applying GABA to individual BC terminals, we could assess how GABAARs and GABACRs properties shaped response in the absence of transmitter release contributions (Eggers et al., 2007). Specific antagonists were used to assess the separate GABAAR and GABACR contributions in the three major classes of BCs (Fig. 2A). In rod BCs, GABA-evoked currents were mediated primarily by GABACRs. In ON cone BCs, GABACRs mediated most of the response, but there was a larger proportion of GABAAR compared to rod BCs. In OFF cone BCs, there were about equal contributions of GABAARs and GABACRs. The GABA puffs activated different proportions of GABAARs and GABACRs in different classes of mouse BC terminals, which agreed with previous observations in rat and ferret BCs (Euler & Wässle, 1998; Shields et al., 2000). However, the puffed GABA activates both synaptic and extrasynaptic receptors. So, do these observed differences in receptor subtype contribute to light-activated inhibition?
Fig. 2.
The contributions of GABAA, GABAC, and glycineR currents vary across BC class. (A) GABA-evoked currents were measured in BCs by focally applying GABA to the BC axon terminal (inset). GABAAR- or GABACR-mediated currents were isolated and measured using (1,2,5,6-Tetrahydropyridin-4-yl)methylphosphinic acid (TPMPA) (50 μM) or bicuculline (50 μM), respectively in: rod (A1), ON cone (A2), and OFF cone (A3) BCs. (The dark gray bar below each trace indicates the duration of the GABA puff.) (A4) Fractional GABA-evoked current mediated by GABAAR and GABACRs in BC types was calculated by normalizing GABAAR and GABACR charge transfer (Q) to total Q. In rod and ON cone BCs, GABACRs (black bars) mediated significantly more of the total response (Q) than GABAARs (gray bars; P < 0.001 and 0.005, respectively). In OFF cone BCs, the proportion of GABAAR and GABACR contributions was similar (P = 0.3). The error bars represent the s.e.m.(B) L-IPSCs were recorded from BCs voltage clamped to 0 mV, the reversal potential for excitatory currents mediated by nonselective cation channels, and elicited with a 30-ms full-field stimulus (dark gray bar), as shown in the inset. (B1) Rod BCs have large L-IPSCs mediated by GABACRs (in strychnine 500 nM and bicuculline 50 μM) and modest L-IPSCs mediated by glycine (in TPMPA, 50 μM, and bicuculline,50 μM) and GABAARs (in TPMPA 50 μM and bicuculline 50 μM). (B2) ON BCs have moderate GABACR and GABAAR L-IPSCs and no glycinergic currents. (B3) OFF cone BCs have large glycinergic L-IPSCs and smaller GABAAR and GABACRL-IPSCs. (B4) The average Q of GABAAR, GABACR, and glycineR L-IPSCs was normalized to the Q of GABAARL-IPSCs for each BC type. Rod BCs have proportionately the largest GABACR L-IPSCs, and OFF cone BCs have the largest glycinergic L-IPSCs. Portions of this figure were adapted from Eggers et al. (2007), Journal of Physiology.
To determine the contributions of GABAARs, GABACRs, and glycineRs to light-evoked inhibition, we recorded light-evoked inhibitory postsynaptic currents (L-IPSCs) mediated by each pharmacologically isolated inhibitory receptor (Fig. 2B). The response differences to GABA puffs in separate BC pathways were also observed with light-evoked inhibition (Eggers et al., 2007). Rod BCs had a large GABACR-mediated input and a smaller GABAAR-mediated input. ON cone BCs GABACRs mediated most of the L-IPSC, but GABAARs mediated a larger proportion of the response compared to Rod BCs. Finally, OFF cone BCs had about equal amounts of GABAAR- and GABACR-mediated L-IPSCs. We also determined the relative magnitudes of the glycinergic L-IPSCs in the different BC classes. The largest magnitude glycinergic L-IPSCs were recorded in OFF cone BCs. This observation is consistent with previous reports that OFF cone BCs receive large glycinergic inputs from the rod BC-AII AC pathway. By contrast, ON cone BCs did not receive any light-evoked glycine input in agreement with previous glycine application studies (Ivanova et al., 2006). Finally, only small amplitude glycinergic L-IPSCs were recorded in Rod BCs. These results suggest that the magnitude of GABAAR, GABACR, and glycineR inhibition is different across the main BC pathways. However, the differences in magnitude of inhibition are only one factor that distinguishes types of BC inhibition. The timing of inhibition in response to brief light stimuli is also important in determining the BC output.
GABAA, GABAC, and glycine receptors mediate inhibitory inputs with distinct kinetics
To determine how the GABAARs, GABACRs, and glycineRs contribute to the time course of inhibition, we recorded L-IPSCs in response to a brief light stimulus, mediated by each pharmacologically isolated receptor, in the three main classes of BC (Eggers & Lukasiewicz, 2006b; Eggers et al., 2007). In all BC classes, GABACR-mediated L-IPSCs had a slow time to peak and a slow decay. GABAAR-mediated L-IPSCs had a fast time to peak and a fast decay. GlycineR-mediated L-IPSCs had a fast time to peak and a moderately slow decay (Fig. 3). These differences in kinetics suggest that these three receptor inputs have distinct functional properties. Since GABAAR- and glycineR-mediated inputs have a fast rise time, this would suggest that they might influence the peak and initial portion of inhibition in BCs. GABAARs mediate especially brief L-IPSCs that peak well before the GABACR L-IPSCs and in many cases have decayed back to baseline well before the GABACR L-IPSCs reach their maximum. This would suggest that GABACR-mediated inputs would primarily control the decay time of L-IPSCs. However, as we showed in the previous figures, different BC pathways have distinct proportions of these three inhibitory inputs. Therefore, it is likely that the differences in timing and differences in magnitude of inhibitory inputs combine to create distinct inhibition for the BC pathways, an idea we will explore in more detail later.
GABAA, GABAC, and glycine receptors distinctly shape the timing of L-IPSCs
We have shown that GABAAR-, GABACR-, and glycineR-mediated L-IPSCs have distinct magnitudes and time courses in BCs. These response properties could be attributed to the distinct biophysical properties of these three inhibitory receptors. Consistent with this idea, fast agonist application studies of GABA and glycine showed analogous response differences for GABAAR, GABACR, and glycineRs (Jones & Westbrook, 1996; Chang & Weiss, 1999; Morkve & Hartveit, 2009). However, direct comparisons with the light response findings are difficult because some of these studies were performed with nonnative heterologously expressed receptors, and studies with native receptors were unable to distinguish between synaptic and extrasynaptic receptors. Also, L-IPSCs are influenced not only by receptor kinetics but also by neurotransmitter release kinetics. To directly investigate the properties of synaptic receptors, we recorded spontaneously released GABA and glycine currents in BCs (Eggers & Lukasiewicz, 2006a,b; Eggers et al., 2007). In this scenario, currents are elicited by the spontaneous release of one vesicle, so the complexities of release kinetics are eliminated and the response time course is thought to exclusively reflect the postsynaptic receptor properties.
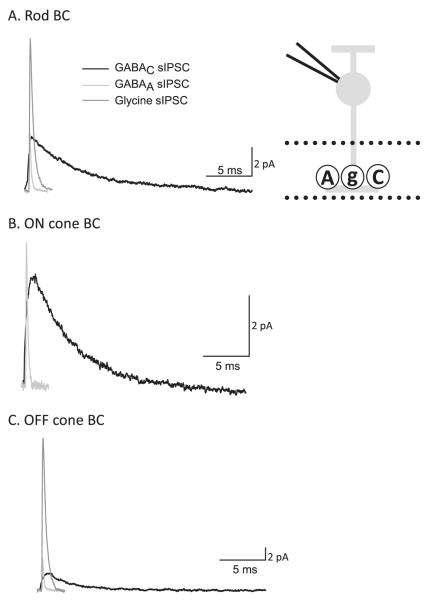
We measured the average spontaneous (s)IPSCs mediated by GABAAR, GABACR, and glycineRs to determine how the receptor properties differ in BCs. GABAAR-mediated sIPSCs have a fast rise and decay (Fig. 4). GABACR sIPSCs have a slow rise and decay. GlycineR sIPSCs have kinetics in between GABAAR and GABACR, with a fast rise but a slower decay than GABAAR. The relative differences in the kinetics of the sIPSCs were similar to the differences observed with light-evoked IPSCs in all BC pathways. Similar differences in the kinetics of GABAAR and GABACR have also been seen in goldfish BCs (Palmer, 2006). Also note that OFF cone BCs had large glycinergic sIPSCs, consistent with their L-IPSCs being dominated by glycinergic input. We conclude that receptor properties are a major contributor to the time course of light-evoked currents. In parts of the central nervous system (CNS), receptor kinetics is the primary determinant of synaptic signal time course. However, the sIPSCs we measured had time courses that were more than 10 times faster than the L-IPSCs we recorded (see Fig. 3 vs. Fig. 4). This suggested that properties of neurotransmitter release from amacrine cells might also be important in determining the time course of L-IPSCs.
Fig. 4.
Spontaneous IPSCs (sIPCs) indicate that GABAAR, GABACR, and glycineRs show distinct biophysical properties. Shown are average sIPSCs from rod, ON cone, and OFF cone BCs (inset). (A) Rod BCs show spontaneous currents mediated by GABAAR, GABACR, and glycineRs, with distinct decay times. (B) ON cone BCs show sIPSCs mediated by only GABAAR and GABACRs, with no currents mediated by glycineRs. GABACR currents have a significantly longer decay time than GABAAR currents. (C) OFF cone BCs show sIPSCs mediated by GABAAR, GABACR, and glycineRs, with distinct decay times. The amplitude of glycineR sIPSCs is significantly larger than other sIPSCs consistent with the primary role of glycinergic inhibition in OFF cone BCs.
Do differences in transmitter release kinetics contribute to differences in inhibitory time courses?
While differences in receptor kinetics are a major contributor to L-IPSC time course, differences in neurotransmitter release can also contribute to L-IPSC time course. There are many subtypes of GABAergic and glycinergic amacrine cells in the retina (Werblin et al., 2001). Different subtypes of amacrine cells may release GABA or glycine with distinct time courses, possibly contributing to differences in L-IPSC time course. To determine the contributions of transmitter release kinetics, we estimated neurotransmitter release using convolution analysis (Diamond & Jahr, 1995; Eggers & Lukasiewicz, 2006b). This analysis is based on the premise that light-evoked release is the convolution of the spontaneous current (attributable to the release of a single vesicle) and the time course of neurotransmitter release. For the pharmacologically isolated GABAA, GABAC, and glycine receptors, we measured both the light-evoked currents and the spontaneous currents in the three major classes of BCs. Thus, by deconvolving the L-IPSCS with the sIPSCs for each receptor type in rod BCs, we can estimate the time course of transmitter release for each case.
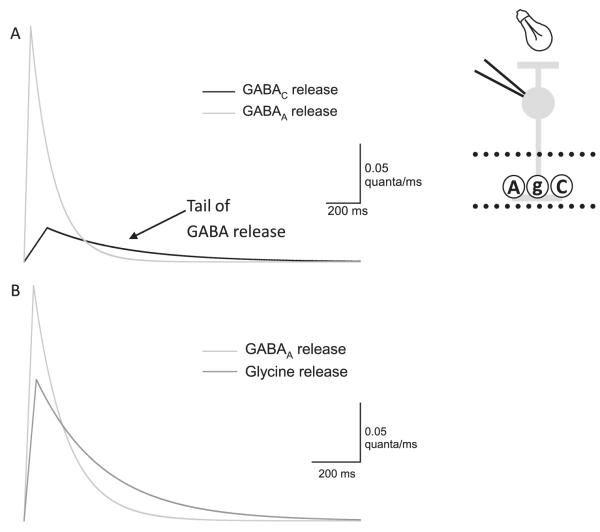
We found that the release time courses estimated from convolution analysis were distinct for GABAAR-, GABACR-, and glycineR-mediated light-evoked currents (Fig. 5). The release time course onto GABACRs was longer than the release time course onto GABAARs. There are several potential explanations for these differences, but one intriguing possibility is that GABACRs and GABAARs receive inputs from distinct cells with different release kinetics. Another possibility is that GABA spills over from neighboring synapses and then preferentially activates the high-sensitivity GABACRs, prolonging the time course of neurotransmitter release (Eggers & Lukasiewicz, 2006b). Additionally, recent simulations of GABACR activation during synaptic release suggested that GABACRs are not significantly activated by the release of only one vesicle of neurotransmitter, which generally has a concentration of ~1 mM (Chavez et al., 2010). If this is the case, then it is possible that the GABACR sIPSC we used to estimate the release time course onto GABACRs is actually composed of the response to several vesicles of GABA. This could partially explain the difference in the magnitude of the release estimated between GABAARs and GABACRs (Fig. 5). This is also supported by the necessity of adding kainate to activate presynaptic amacrine cells before GABACR sIPSCs were evident (Eggers & Lukasiewicz, 2006b).
Fig. 5.
GABAAR-, GABACR-, and glycineR-mediated L-IPSCs have distinct apparent release functions and receptor kinetics, both of which contribute to L-IPSC kinetics. (A) Release functions computed by deconvolving idealized GABAAR- and GABACR-mediated L-IPSCs. GABAAR-mediated L-IPSCs have a much larger release function than GABACRs, likely because of the much smaller Q of GABAAR sIPSCs versus GABACR sIPSCs. The GABACR release function has a prolonged tail not shown by the GABAAR release function. Scale bars are 0.05 quanta/ms and 200 ms. (B) The release functions calculated from the deconvolution of the L-IPSCs and sIPSC traces for GABAAR and glycineRs are shown. The glycine release function has much slower decay time than the GABAAR release function. This suggests that part of the differences between GABAAR- and glycineR-mediated L-IPSCs are due to distinct release kinetics. This figure was adapted from Eggers and Lukasiewicz (2006b), Journal of Neuroscience.
Convolution analysis indicates that glycine release is slower than GABA release onto GABAARs. Thus, for glycinergic L-IPSCs, both receptor kinetics and transmitter release kinetics make major contributions to the slower decay time we observe (Fig. 3). These large differences in release kinetics have not often been observed in other studies (but see Hefft & Jonas, 2005). This is likely attributable to differences in how transmitter release is evoked. In previous studies in other parts of the CNS, release was evoked by electrical shocks, which likely synchronized release. In our studies, we utilized light, the natural stimulus for these circuits, to activate release. As photoreceptors and BCs use graded release instead of all or nothing action potential–mediated release, this could lead to more desynchronized release from amacrine cells, accounting for the slow time course of release underlying light-evoked currents. Additionally, it is possible that amacrine cells have inherently desynchronized release in order to match the time course of the excitatory inputs that BCs are receiving. Our experiments described here have not distinguished between these two possibilities.
Taken together, our results show that distinct biophysical inhibitory receptor properties shape light-evoked inhibition to BCs. In addition, distinct neurotransmitter release time courses, either from different presynaptic amacrine cells or from spillover of GABA to neighboring synapses, shape the light-evoked inhibition to BCs. These unique temporal properties of light-evoked inhibition shape the BC output, potentially leading to distinct BC output pathways.
Magnitude and timing differences of GABAA, GABAC, and glycine receptors shape BC L-IPSCs
The photoreceptor inputs to the distinct BC pathways are filtered by different glutamate receptor subtypes to shape their response time courses. Our results show that GABAA, GABAC, and glycine receptors also mediate inhibitory input with distinct time courses and that BC classes that possess different combinations of these receptors have differing inhibitory inputs. These findings suggest that the modulation of BC output may be as diverse as that reported for their inputs, allowing for additional computational complexity among the parallel pathways.
We found that the terminals of different classes of BCs have different proportions of GABAAR and GABACR-mediated inputs. How does this diversity of inputs contribute to BC inhibition? The differing time courses of GABAAR- and GABACR-mediated L-IPSCs predict that inhibition mediated by fast GABAARs might preferentially contribute to the earliest phase of inhibition, such as the time to peak of inhibition, while the more slowly responding GABACRs would contribute to the decay time of inhibition.
To test the relative roles of GABACR- and GABAAR-mediated inputs, we used GABACR null mice that lack GABACRs (McCall et al., 2002; Eggers & Lukasiewicz, 2006b; Eggers et al., 2007). Since rod BCs have the most GABACR-mediated input, we would expect them to show the largest change in L-IPSC kinetics when GABACRs were removed, while OFF cone BCs that have the least GABACR-mediated input would only show small changes in L-IPSC kinetics. Elimination of GABACRs decreased the time course of the L-IPSCs, consistent with GABACRs mediating the later phases of L-IPSCs. As predicted, rod BCs response time course was dramatically shortened in mice that lacked GABACRs. However, the elimination of GABACRs did not significantly change the peak of the response, consistent with the notion that GABAARs and glycineRs, and not GABACRs, mediate the early components of the L-IPSC (Fig. 6). For ON cone BCs, the time course of the L-IPSCs was moderately shortened in mice lacking GABACRs, consistent with the somewhat smaller GABACR contributions to this BC class. For OFF cone BCs, there was no change in time course observed in mice without GABACRs, suggesting that GABACRs did not significantly contribute to the kinetics of these responses.
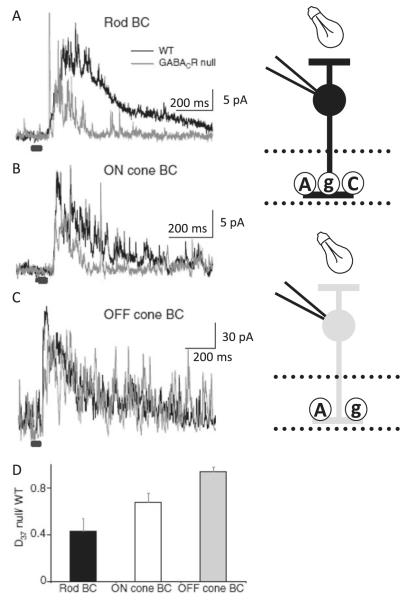
Fig. 6.
GABACRs shape L-IPSCs in ON cone and rod BCs but not OFF cone BCs. (A–C) Representative L-IPSCs (30-ms light stimulus duration, gray bar) from rod (A), ON cone (B), and OFF cone (C) BCs in WT (black) and GABACR null (gray) mice. (D) The histogram plots the average L-IPSCs decay (D37) from GABACR null BCs normalized to WT. GABACR null L-IPSCs in rod BCs (WT n = 43, null n = 15, P < 0.0001) and ON cone BCs (WT n = 17, null n = 4, P < 0.05) were significantly briefer than WT. There was no significant difference in L-IPSC decays from GABACR null and WT OFF cone BCs (WT n = 13, null n = 6, P = 0.7). Error bars in (D) represent propagated s.e.s of the average null to WT values. Adapted from Eggers et al. (2007), Journal of Physiology.
Our studies with the GABACR null mice indicate that the GABACRs are the primary determinant of response time course, especially in rod BCs and ON cone BCs. This idea was further supported by our recordings of isolated GABAergic L-IPSCs from BCs in wild-type mice (in the presence of strychnine to block glycineRs). L-IPSCs from rod BCs had the slowest decay time, consistent with the largest GABACR-mediated input, while OFF cone BCs had the fastest decay time, consistent with the least GABACR-mediated input (Eggers et al., 2007). Together, the responses from null and wild-type (WT) animals show that differing proportions of GABAAR- and GABACR-mediated input shape L-IPSCs in BCs. Fast GABAAR-mediated inputs control the peak of the L-IPSC and slower GABACR-mediated inputs control the duration of the GABAergic L-IPSC. These differences in inhibitory input between BC pathways correlate with the differences discussed earlier for excitatory inputs to the BC pathways.
Glycinergic inhibition also differs between the major BC classes (Eggers et al., 2007). We compared the glycinergic L-IPSCs in different BC classes (Fig. 2B) and found that rod BCs receive a small amount of glycinergic inhibition, ON cone BCs receive none, and OFF cone BC inhibition is dominated by glycinergic input. How does glycinergic inhibition shape the time course of L-IPSCs in OFF cone BCs? Glycinergic inhibition has a slow time course in OFF cone BCs comparable to the slow time course found in rod BCs that is attributable to GABACRs. Thus, if we record L-IPSCs mediated by all the receptor types (Fig. 7), OFF cone BCs and rod BCs have similar L-IPSC time courses. However, the slow time courses are attributable to large GABACR inputs to rod BCs and large glycineR inputs to OFF cone BCs. Therefore, in spite of little GABACR input to OFF cone BCs, their L-IPSCs still show a slow time course. Does this contradict our idea of a correlation between inhibitory and excitatory input timing? In all cases, recordings were obtained from dark-adapted retinas and signaling was mediated primarily by the rod circuitry. The slow glycinergic input to the OFF BCs originates from AII amacrine cells in the rod signaling pathway. The slow GABACR-dominated input to rod BCs may originate from A17 amacrine cells, also part of the rod signaling pathway (Hartveit, 1999). Therefore, L-IPSCs in both classes of BC are slow and suitably matched to the slow time course of rod input signals.
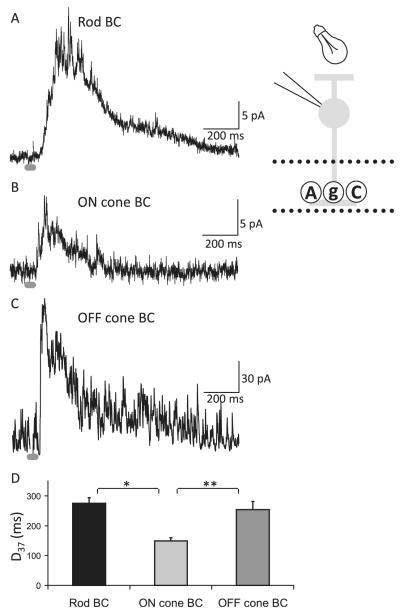
Fig. 7.
The decay of combined glycinergic and GABAergic L-IPSCs varies with WT BC class. (A–C) Representative total L-IPSCs (glycinergic + GABAergic) from rod (A), ON cone (B), and OFF cone (C) BCs evoked by a light stimulus (30-ms light stimulus, dark gray bar). (D) The histogram plots the average decay (D37) from each BC class. L-IPSCs from rod BCs were significantly slower than ON cone BCs (rod 5 43, ON cone n = 17, ANOVA P < 0.001, rod vs. ON Scheffe post hoc P < 0.001, *) but similar to OFF cone BCs (n = 13, P = 0.86). The decay of L-IPSCs from OFF cone BCs also was significantly slower than ON cone BCs (P < 0.05, **). Adapted from Eggers et al. (2007), Journal of Physiology.
GABAA, GABAC, and glycine receptors shape distinct components of BC output
We have shown that different combinations of GABACR, GABAAR, and glycineR inputs uniquely shape the kinetics of L-IPSCs in different BC pathways. These amacrine cell inputs directly contact BC axon terminals and are optimally placed to shape the output of BCs. We assessed how inhibition to BCs affected retinal signaling by measuring BC outputs in the retinal slice preparation, where synaptic connections are maintained and can be physiologically activated with light (Eggers & Lukasiewicz, 2006a,b). We determined the BC output by recording light-evoked excitatory postsynaptic currents (L-EPSCs) in postsynaptic amacrine or ganglion cells. As noted above, GABACRs control the decay of BC inhibition, while GABAARs control the peak, and glycineRs affect both the peak and the decay of BC inhibition. How does each of these inhibitory inputs regulate the output of BCs?
We determined how each inhibitory input affected the BC output by assaying the effects of specific pharmacological blockers of inhibition upon the rod BC output. Because A17 amacrine cells receive excitatory input exclusively from rod BCs, we recorded L-EPSCs from these neurons and determined how they were affected by inhibitory inputs to BCs. Since inhibition mediated by GABAARs produces a fast rising and decaying current, this form of inhibition is likely to limit the initial component of glutamate release and reduce the peak of the L-EPSCs. GABACRs mediate slow rising and decaying L-IPSCs, so inhibition mediated by these receptors should primarily reduce the later components of glutamate release and enhance the decay of L-EPSCs. We found that blocking GABAARs with bicuculline increased the peak of BC output while leaving the decay unchanged (Fig. 8), confirming that this component of inhibition mainly limited the initial component of the BC output. Eliminating GABACR function either genetically or pharmacologically prolonged the decay time of the L-EPSCs, indicating that this component of GABAergic inhibition limited the late sustained components of BC output. These findings demonstrate that GABAergic input to rod BCs affects the early and late phases of BC output by acting through GABAARs and GABACRs, respectively. Glycinergic inhibition to BCs is mediated by amacrine cells that are functionally and morphologically distinct from GABAergic amacrine cells. GlycineR-mediated L-IPSCs have relatively fast onset kinetics and moderately slow offset kinetics, suggesting that inhibition by these receptors will affect both the early and the late phases of BC output. Consistent with these properties, we found that blocking glycineRs with strychnine both increased the peak amplitude and prolonged the decay of L-EPSCs.
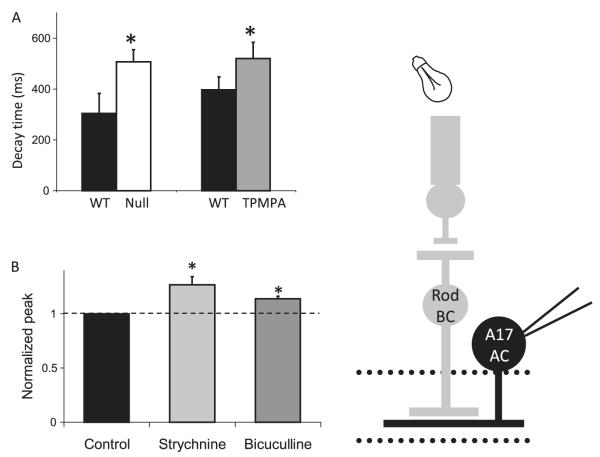
Fig. 8.
Presynaptic GABACR limit release, making L-EPSCs from A17 amacrine cells more transient. Glutamate release from rod BCs was monitored by recording L-EPSCs from postsynaptic A17 ACs. (A) The absence of GABACRs in GABACR null mice causes the L-EPSC to have a longer decay and larger charge transfer. A similar effect was observed in WT mice when TPMPA was added to block GABACRs. The decay (D37) of A17 amacrine cells from GABACR null mice (P < 0.05) and WT mice in TPMPA (P < 0.01) was significantly longer than WT mice in control conditions. (B) Presynaptic glycine and GABAARs decrease the peak response of L-EPSCs from A17 amacrine cells. The peak amplitude of L-EPSCs from A17 amacrine cells from GABACR null mice was increased by the addition of strychnine to block glycineRs, but the decay time of the response was unaffected. Similarly, the peak amplitude of L-EPSCs from A17 amacrine cells from GABACR null mice was increased by addition of bicuculline to block GABAARs, but the response decay was unaffected. The amplitude of L-EPSCs was significantly larger with the addition of both strychnine (P < 0.05, *) and bicuculline (P < 0.05, *). Peak values in bicuculline and strychnine are normalized to control values, represented by the dotted line.
ON cone and OFF cone BCs receive strong and weak GABACR-mediated inhibition, respectively. These observations suggest that the output of ON and OFF BCs is differentially influenced by GABACR-mediated L-IPSCs. To determine whether this was the case, we assayed ON cone and OFF cone BC outputs onto ganglion cells in normal mice and in mice that lacked GABACRs (Sagdullaev et al., 2006). We recorded excitatory responses in ON and OFF ganglion cells to assay each form of BC output. In mice lacking GABACRs, excitatory drive increased in ON ganglion cells but not OFF ganglion cells, indicating that GABACRs normally limited the output of ON cone BCs but not OFF cone BCs. These results suggest that the differences we see in L-IPSCs between BC pathways are important for shaping the output of BCs and that unique combinations of GABACR, GABAAR, and glycineR inputs can create distinct types of BC inhibition.
Serial inhibition modulates spatial signaling to BCs
As noted above, BCs receive direct presynaptic inhibition from GABAergic amacrine cells onto GABAARs and GABACRs. In addition, there are also serial inhibitory signals between GABAergic amacrine cells that are mediated by GABAARs (Zhang et al., 1997; Roska et al., 1998; Eggers & Lukasiewicz, 2006a, 2010). Serial inhibition limits the direct GABAergic inhibition to BCs (Fig. 1). Serial inhibition has been shown to affect the kinetics of transmission between BCs and ganglion cells (Zhang et al., 1997; Roska et al., 1998). However, the role of serial inhibitory circuits in spatial processing is less well understood. Because direct and serial inhibitory inputs are spatially narrow and extensive, respectively, different sizes of light stimuli preferentially activate them. Narrow-field light stimuli may preferentially activate direct inhibition, while wide-field light stimuli that activate the more extensive amacrine cell network may preferentially activate serial inhibition.
To determine the effects of serial inhibition on the direct inhibitory inputs to BCs, we recorded L-IPSCs in response to narrow-field and wide-field light stimuli under conditions when serial inhibition was either present or absent (Fig. 9) (Eggers & Lukasiewicz, 2010). Serial inhibition was eliminated by adding bicuculline to the bath to block GABAAR serial inhibitory connections between GABAergic amacrine cells. The direct inhibitory input to BCs was assayed by recording the GABACR-mediated component of the L-IPSCs. We found that wide-field L-IPSCs increased after GABAARs were blocked, suggesting that serial inhibitory connections between amacrine cells limit wide-field light-activated L-IPSCs. If GABAARs were present only on BCs, then the wide-field L-IPSCs, attributed to direct inhibition, would decrease because a component of the direct input was blocked. This was never observed with wide-field light stimuli, indicating that the net effect of bicuculline was the blockade of serial inhibition. However, when we used narrow-field light stimuli, L-IPSCs decreased when GABAARs were blocked, suggesting that narrow-field light only activated direct connections. These findings suggest that serial connections are spatially regulated; these connections are activated by wide-field stimulation but not narrow-field stimulation, which fails to activate the amacrine cell network.
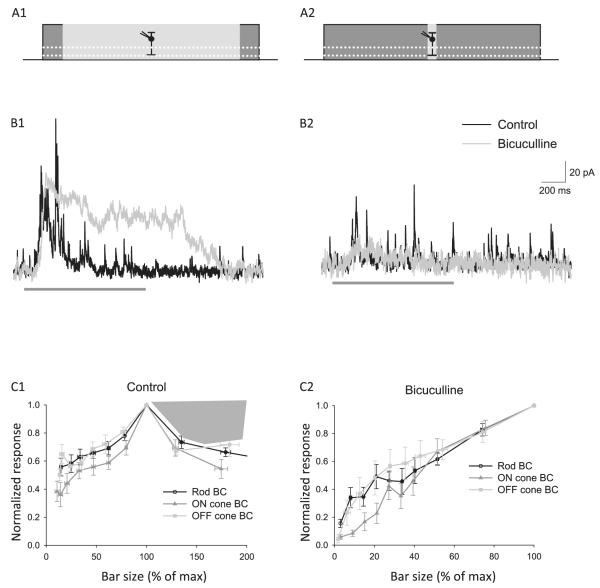
Fig. 9.
(A) Serial inhibitory connections limit wide-field but not narrow-field BC L-IPSCs. (A) Wide-field (825 μm, A2) and narrow-field (25 μm, A2) light stimuli (A1, A2, and thick dark gray bar in traces) were applied to BCs. Bicuculline (50 μM) was added to block GABAARs. (B) In all BC types (OFF cone BC shown), blocking GABAARs increased the charge transfer (Q) of wide-field L-IPSCs (B1), suggesting that serial inhibitory connections between ACs limit wide-field light activated L-IPSCs (rod P < 0.05, ON P < 0.05, OFF P < 0.05). In contrast, blocking GABAARs decreased the Q of narrow-field L-IPSCs (B2), suggesting that narrow-field light activated only direct connections (rod n = 10, P < 0.001; ON n = 6, P < 0.01; OFF n = 5, P < 0.05). (C) The spatial responses of BC L-IPSCs are suppressed at large light stimulus sizes in control conditions but not when serial connections are blocked by bicuculline. Light stimuli of 10 different sizes (25–825 μm) were applied to BCs in control and bicuculline, and the Q of each L-IPSC was measured. The average area response function (ARFs) of all BCs recorded were normalized to the maximum light response for each BC and to the light size where the maximum response was elicited. ARFs showed a peak at an intermediate-sized light stimuli for all BC types in control (C1, rod n = 19, ON n = 14, OFF n = 9). When GABAAR-mediated serial connections are blocked, BC L-IPSCs show no suppression. The average ARFs of all BCs recorded in bicuculline were calculated (C2) and showed an increasing light response with increasing light stimulus size (rod n = 11, ON n = 5, OFF n = 5). This suggests that the spatial tuning of BC L-IPSCs seen in control conditions is limited by serial connections. Adapted from Eggers and Lukasiewicz (2010), Journal of Neurophysiology.
To determine how serial connections affect the total spatial response properties of L-IPSCS in BCs, we measured the area–response function elicited by light stimuli of increasing sizes (25–825 μm). In all BC types, the response increased as a function of light size up to an intermediate-sized light stimulus, and then the response decreased as the area further increased. These findings show that the L-IPSCs were suppressed by large area spots, which activate serial inhibitory circuitry. However, when bicuculline blocked serial connections, the L-IPSCs were not suppressed by large spot stimuli and, unlike the control conditions, the area–response functions increased as a function of light size. These findings suggest that the spatial tuning seen in control conditions is attributable to serial inhibition limiting BC L-IPSCs. When serial inhibition is active, larger spot areas activate GABAAR-mediated serial connections between amacrine cells that result in spatial tuning of direct inhibition to BCs. These results show that the spatial modulation of inhibition by serial connections is another level of modulation of BC inhibition.
Future directions
Our studies show that BC inhibition is both diverse and complex (Fig. 10). Inhibition varies between different BC pathways. This variation is largely attributable to differing proportions of GABAAR-,GABACR-, and glycineR-mediated inhibitory inputs that mediate L-IPSCs with distinct time courses. These distinct inhibitory inputs interact to shape the timing of BC inhibition, which, in turn, modulates the BC output, glutamate release. BC inhibition is also spatially tuned by serial inhibitory connections between ACs. While these studies provide new insights into BC inhibition, several open questions remain open.
Fig. 10.
GABAAR, GABACR, and glycineRs control distinct properties of glutamate release from BCs. (A) GABAAR and glycineRs mediated light-evoked currents with a fast rise and decay, which primarily limit the peak of glutamate release from BCs. (B) GABACRs mediated light-evoked inhibition with slow decay, which primarily limits prolonged glutamate release from BCs. The time course of light-evoked BC inhibition is controlled both by biophysical receptor properties as well as prolonged GABA and glycine release from amacrine cells. (C) The magnitude of inhibition varies between BC pathways. Rod BCs receive large GABACR-mediated inhibition and less GABAAR and glycineR inhibition. ON cone BCs receive moderate amounts of GABAAR and GABACR inhibition with no glycinergic inhibition. OFF cone BCs receive little GABACR inhibition, some GABAAR inhibition, and are dominated by glycinergic inhibition. The magnitude of GABAergic inhibition is also controlled by GABAAR-mediated synapses between GABAergic amacrine cells that serve to limit inhibition to BCs.
Our data suggest that the distinct kinetics of inhibition mediated by varying contributions of GABACRs across BC class may be well matched to the excitatory inputs to BC classes that are shaped by differing rod and cone kinetics and distinct glutamate receptors. However, this idea has never been tested directly by correlating the kinetics of both excitatory and inhibitory inputs in distinct BC types. Although we have divided BCs into three main classes, there are many subtypes of ON and OFF BCs (Ghosh et al., 2004). Agonist application studies have suggested that the proportions of GABA and glycine receptors may vary between these subtypes of BCs, as well as between the major classes (Euler & Wässle, 1998; Ivanova et al., 2006). Previous studies have also suggested differences between subtypes of OFF BCs based on their distinct glutamate receptor complement (Devries & Schwartz, 1999; DeVries, 2000; Li & DeVries, 2004). Experiments that look specifically at the light-evoked excitatory and inhibitory inputs in the subtypes of BCs are needed to determine if the idea of similarly shaped inhibitory and excitatory inputs is correct. This could serve several functions in the retina. First, if the goal of inhibition in BCs is to shape glutamate output, then the inhibition has to be temporally well matched to the excitation to be effective. However, it is possible that inhibition also has other functions, like enforcing a nonresponsive period after a stimulus. In this case, the ideal inhibition might last for much longer time than the excitatory inputs.
We have also shown that serial inhibitory connections between amacrine cells can shape the spatial activation of inhibition to BCs. Determining the spatial properties of inhibition of BCs is important for establishing how inhibitory inputs to BCs contribute to the inhibitory surround of ganglion cells. In addition to serial connections shaping of BC inhibition, differences between GABAergic and glycinergic amacrine cells may also contribute to the spatial sensitivity of BC inhibition. The wide-field GABAergic cells suggest that GABAergic inhibition has a larger spatial extent, while narrow-field glycinergic cells suggest that glycinergic inhibition has a smaller spatial extent. However, serial inhibitory connections between GABAergic amacrine cells and electrical coupling between some glycinergic amacrine cells suggest that the spatial extent of GABA- and glycine-mediated inhibition is more complex. Therefore it is yet to be determined if these two inhibitory inputs specifically mediate distinct spatial sensitivities of BC inhibition.
References
- Ashmore JF, Copenhagen DR. Different postsynaptic events in two types of retinal bipolar cell. Nature. 1980;288:84–86. doi: 10.1038/288084a0. [DOI] [PubMed] [Google Scholar]
- Awatramani GB, Slaughter MM. Origin of transient and sustained responses in ganglion cells of the retina. The Journal of Neuroscience. 2000;20:7087–7095. doi: 10.1523/JNEUROSCI.20-18-07087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadetti L, Tranchina D, Thoreson WB. A comparison of release kinetics and glutamate receptor properties in shaping rod-cone differences in EPSC kinetics in the salamander retina. The Journal of Physiology. 2005;569:773–788. doi: 10.1113/jphysiol.2005.096545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Weiss DS. Channel opening locks agonist onto the GABAC receptor. Nature Neuroscience. 1999;2:219–225. doi: 10.1038/6313. [DOI] [PubMed] [Google Scholar]
- Chavez AE, Diamond JS. Diverse mechanisms underlie glycinergic feedback transmission onto rod bipolar cells in rat retina. The Journal of Neuroscience. 2008;28:7919–7928. doi: 10.1523/JNEUROSCI.0784-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AE, Grimes WN, Diamond JS. Mechanisms underlying lateral GABAergic feedback onto rod bipolar cells in rat retina. The Journal of Neuroscience. 2010;30:2330–2339. doi: 10.1523/JNEUROSCI.5574-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AE, Singer JH, Diamond JS. Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature. 2006;443:705–708. doi: 10.1038/nature05123. [DOI] [PubMed] [Google Scholar]
- Cook PB, McReynolds JS. Lateral inhibition in the inner retina is important for spatial tuning of ganglion cells. Nature Neuroscience. 1998;1:714–719. doi: 10.1038/3714. [DOI] [PubMed] [Google Scholar]
- DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28:847–856. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- Devries SH, Schwartz EA. Kainate receptors mediate synaptic transmission between cones and ‘Off’ bipolar cells in a mammalian retina. Nature. 1999;397:157–160. doi: 10.1038/16462. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Asynchronous release of synaptic vesicles determines the time course of the AMPA receptor-mediated EPSC. Neuron. 1995;15:1097–1107. doi: 10.1016/0896-6273(95)90098-5. [DOI] [PubMed] [Google Scholar]
- Dong CJ, Hare WA. Temporal modulation of scotopic visual signals by A17 amacrine cells in mammalian retina in vivo. Journal of Neurophysiology. 2003;89:2159–2166. doi: 10.1152/jn.01008.2002. [DOI] [PubMed] [Google Scholar]
- Dong CJ, Werblin FS. Temporal contrast enhancement via GABAC feedback at bipolar terminals in the tiger salamander retina. Journal of Neurophysiology. 1998;79:2171–2180. doi: 10.1152/jn.1998.79.4.2171. [DOI] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. GABA(A), GABA(C) and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. The Journal of Physiology. 2006a;572:215–225. doi: 10.1113/jphysiol.2005.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Receptor and transmitter release properties set the time course of retinal inhibition. The Journal of Neuroscience. 2006b;26:9413–9425. doi: 10.1523/JNEUROSCI.2591-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Interneuron circuits tune inhibition in retinal bipolar cells. Journal of Neurophysiology. 2010;103:25–37. doi: 10.1152/jn.00458.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. The Journal of Physiology. 2007;582:569–582. doi: 10.1113/jphysiol.2007.131763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Masland RH. Light-evoked responses of bipolar cells in mammalian retina. Journal of Neurophysiology. 2000;83:1817–1829. doi: 10.1152/jn.2000.83.4.1817. [DOI] [PubMed] [Google Scholar]
- Euler T, Wässle H. Different contributions of GABAA and GABAC receptors to rod and cone bipolar cells in a rat retinal slice preparation. Journal of Neurophysiology. 1998;79:1384–1395. doi: 10.1152/jn.1998.79.3.1384. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV, Jr., Kolb H. Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science. 1976;194:193–195. doi: 10.1126/science.959847. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Bormann J. Differential pharmacology of GABA-A and GABA-C receptors on rat retinal bipolar cells. European Journal of Pharmacology. 1994;288:97–104. doi: 10.1016/0922-4106(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Flores-Herr N, Protti DA, Wässle H. Synaptic currents generating the inhibitory surround of ganglion cells in the mammalian retina. The Journal of Neuroscience. 2001;21:4852–4863. doi: 10.1523/JNEUROSCI.21-13-04852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wässle H. Types of bipolar cells in the mouse retina. The Journal of Comparative Neurology. 2004;469:70–82. doi: 10.1002/cne.10985. [DOI] [PubMed] [Google Scholar]
- Hartveit E. Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. Journal of Neurophysiology. 1999;81:2923–2936. doi: 10.1152/jn.1999.81.6.2923. [DOI] [PubMed] [Google Scholar]
- Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nature Neuroscience. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- Ivanova E, Muller U, Wässle H. Characterization of the glycinergic input to bipolar cells of the mouse retina. The European Journal of Neuroscience. 2006;23:350–364. doi: 10.1111/j.1460-9568.2005.04557.x. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. The impact of receptor desensitization on fast synaptic transmission. Trends in Neuroscience. 1996;19:96–101. doi: 10.1016/s0166-2236(96)80037-3. [DOI] [PubMed] [Google Scholar]
- Li GL, Vigh J, von Gersdorff H. Short-term depression at the reciprocal synapses between a retinal bipolar cell terminal and amacrine cells. The Journal of Neuroscience. 2007;27:7377–7385. doi: 10.1523/JNEUROSCI.0410-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, DeVries SH. Separate blue and green cone networks in the mammalian retina. Nature Neuroscience. 2004;7:751–756. doi: 10.1038/nn1275. [DOI] [PubMed] [Google Scholar]
- Li W, DeVries SH. Bipolar cell pathways for color and luminance vision in a dichromatic mammalian retina. Nature Neuroscience. 2006;9:669–675. doi: 10.1038/nn1686. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD, Werblin FS. A novel GABA receptor modulates synaptic transmission from bipolar to ganglion and amacrine cells in the tiger salamander retina. The Journal of Neuroscience. 1994;14:1213–1223. doi: 10.1523/JNEUROSCI.14-03-01213.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, Demb JB. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. The Journal of Neuroscience. 2008;28:4136–4150. doi: 10.1523/JNEUROSCI.4274-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall MA, Lukasiewicz PD, Gregg RG, Peachey NS. Elimination of the 1 subunit abolishes GABAC receptor expression and alters visual processing in the mouse retina. The Journal of Neuroscience. 2002;22:4163–4174. doi: 10.1523/JNEUROSCI.22-10-04163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menger N, Pow DV, Wässle H. Glycinergic amacrine cells of the rat retina. The Journal of Comparative Neurology. 1998;401:34–46. doi: 10.1002/(sici)1096-9861(19981109)401:1<34::aid-cne3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Molnar A, Hsueh HA, Roska B, Werblin FS. Crossover inhibition in the retina: Circuitry that compensates for nonlinear rectifying synaptic transmission. Journal of Computational Neuroscience. 2009;27:569–590. doi: 10.1007/s10827-009-0170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Zhang J, Jeffrey BG, Nelson SM, Burke NS, Duvoisin RM, Brown RL. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19174–19178. doi: 10.1073/pnas.0908711106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morkve SH, Hartveit E. Properties of glycine receptors underlying synaptic currents in presynaptic axon terminals of rod bipolar cells in the rat retina. The Journal of Physiology. 2009;587:3813–3830. doi: 10.1113/jphysiol.2009.173583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MJ. Functional segregation of synaptic GABAA and GABAC receptors in goldfish bipolar cell terminals. The Journal of Physiology. 2006;577:45–53. doi: 10.1113/jphysiol.2006.119560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z-H, Lipton SA. Multiple GABA receptor subtypes mediate inhibition of calcium influx at rat retinal bipolar cell terminals. The Journal of Neuroscience. 1995;15:2668–2679. doi: 10.1523/JNEUROSCI.15-04-02668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcho RG, Goebel DJ. Neuronal subpopulations in cat retina which accumulate the GABA agonist, (3H)muscimol: A combined Golgi and autoradiographic study. The Journal of Comparative Neurology. 1983;219:25–35. doi: 10.1002/cne.902190104. [DOI] [PubMed] [Google Scholar]
- Roska B, Molnar A, Werblin FS. Parallel processing in retinal ganglion cells: How integration of space-time patterns of excitation and inhibition form the spiking output. Journal of Neurophysiology. 2006;95:3810–3822. doi: 10.1152/jn.00113.2006. [DOI] [PubMed] [Google Scholar]
- Roska B, Nemeth E, Werblin FS. Response to change is facilitated by a three-neuron disinhibitory pathway in the tiger salamander retina. The Journal of Neuroscience. 1998;18:3451–3459. doi: 10.1523/JNEUROSCI.18-09-03451.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagdullaev BT, McCall MA, Lukasiewicz PD. Presynaptic inhibition modulates spillover, creating distinct dynamic response ranges of sensory output. Neuron. 2006;50:923–935. doi: 10.1016/j.neuron.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Schnapf JL, Copenhagen DR. Differences in the kinetics of rod and cone synaptic transmission. Nature. 1982;296:862–864. doi: 10.1038/296862a0. [DOI] [PubMed] [Google Scholar]
- Shen Y, Heimel JA, Kamermans M, Peachey NS, Gregg RG, Nawy S. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. The Journal of Neuroscience. 2009;29:6088–6093. doi: 10.1523/JNEUROSCI.0132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields CR, Tran MN, Wong RO, Lukasiewicz PD. Distinct ionotropic GABA receptors mediate presynaptic and postsynaptic inhibition in retinal bipolar cells. The Journal of Neuroscience. 2000;20:2673–2682. doi: 10.1523/JNEUROSCI.20-07-02673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. The Journal of Neuroscience. 2003;23:10923–10933. doi: 10.1523/JNEUROSCI.23-34-10923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Genderen MM, Bijveld MM, Claassen YB, Florijn RJ, Pearring JN, Meire FM, McCall MA, Riemslag FC, Gregg RG, Bergen AA, Kamermans M. Mutations in TRPM1 are a common cause of complete congenital stationary night blindness. American Journal of Human Genetics. 2009;85:730–736. doi: 10.1016/j.ajhg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney DI. The mosaic of amacrine cells in the mammalian retina. Progress in Retinal Research. 1990;9:49–100. [Google Scholar]
- Wässle H. Parallel processing in the mammalian retina. Nature Reviews. Neuroscience. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Werblin F, Roska B, Balya D. Parallel processing in the mammalian retina: Lateral and vertical interactions across stacked representations. Progress in Brain Research. 2001;131:229–238. doi: 10.1016/s0079-6123(01)31019-1. [DOI] [PubMed] [Google Scholar]
- Werblin FS. Six different roles for crossover inhibition in the retina: Correcting the nonlinearities of synaptic transmission. Visual Neuroscience. 2010;27:1–8. doi: 10.1017/S0952523810000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chang-Sub J, Slaughter MM. Serial inhibitory synapses in retina. Visual Neuroscience. 1997;14:553–563. doi: 10.1017/s0952523800012219. [DOI] [PubMed] [Google Scholar]