Abstract
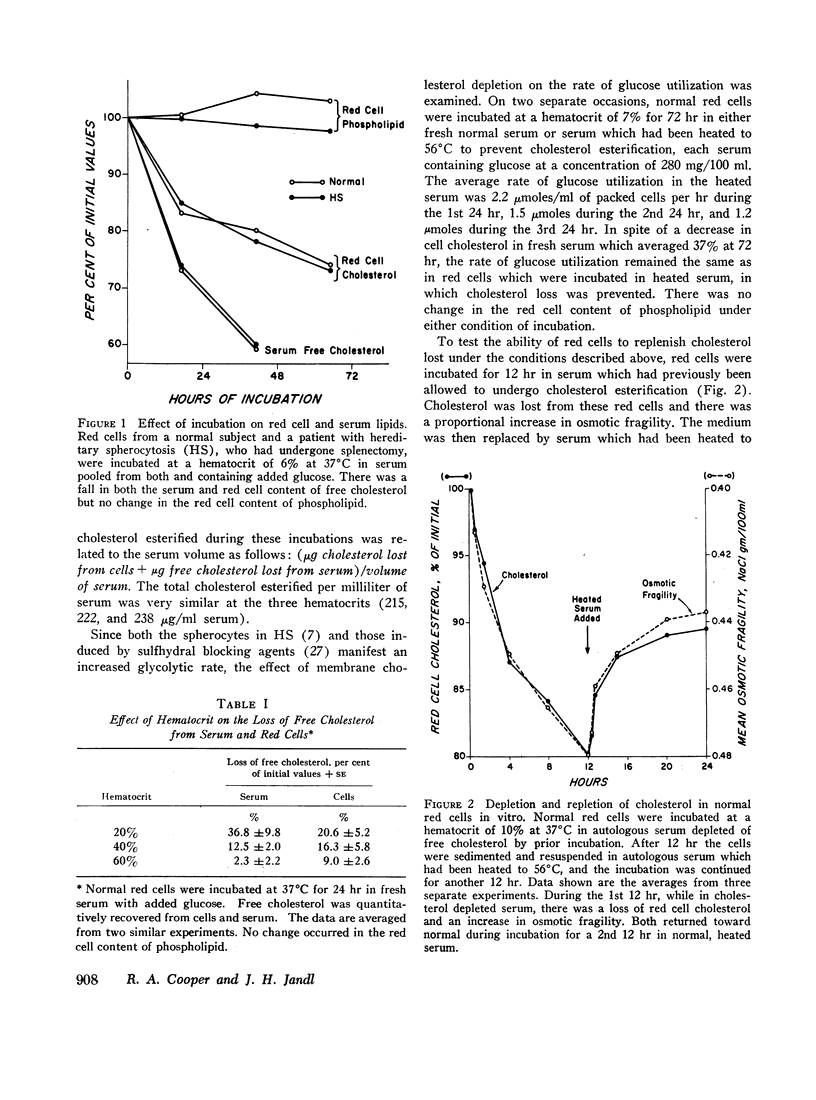
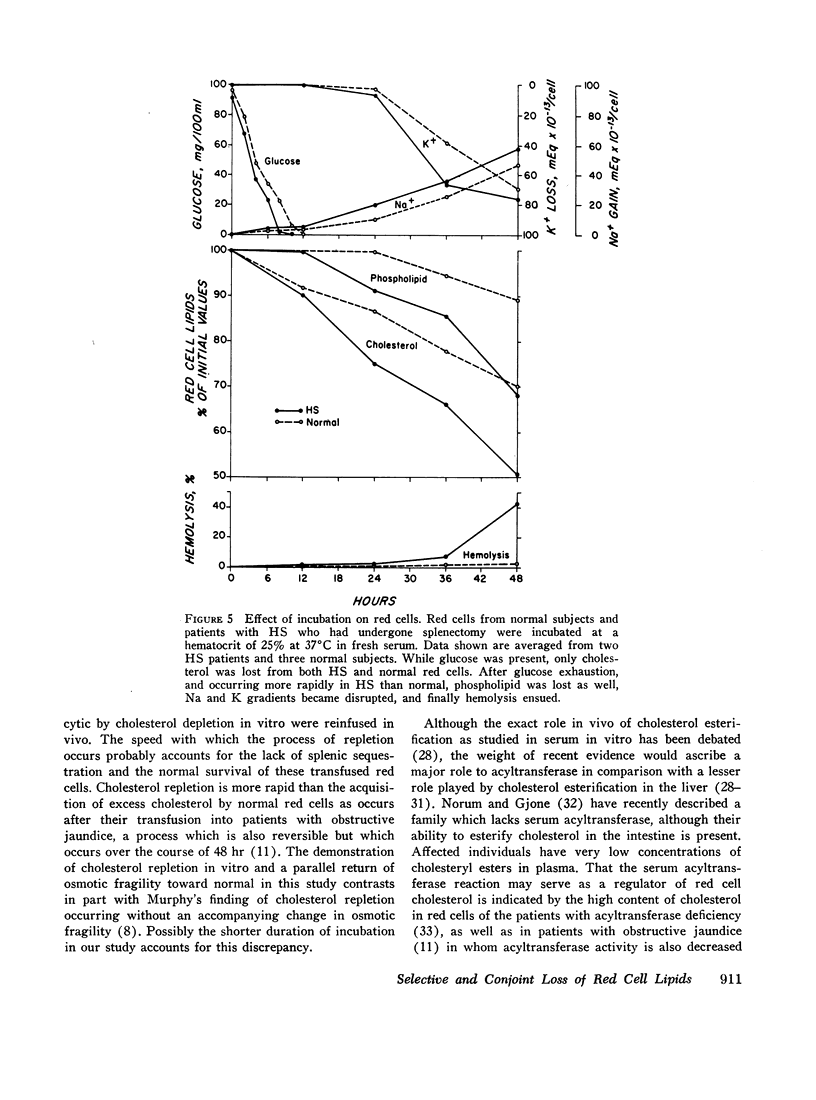
The pattern of lipid loss from the membrane of red cells incubated in serum is influenced by the availability of glucose. Under homeostatic conditions with respect to glucose, cholesterol alone is lost. This results from esterification of free cholesterol in serum by the serum enzyme, lecithin:cholesterol acyltransferase, and is associated with a proportional decrease in membrane surface area, reflected by an increased osmotic fragility. This selective loss of membrane cholesterol also occurs in hereditary spherocytosis (HS) red cells, even after incubation for 65 hr in the presence of glucose. The loss of free cholesterol from red cells relative to its loss from serum, under these conditions, is greatest at higher hematocrits, similar to those found in the spleen.
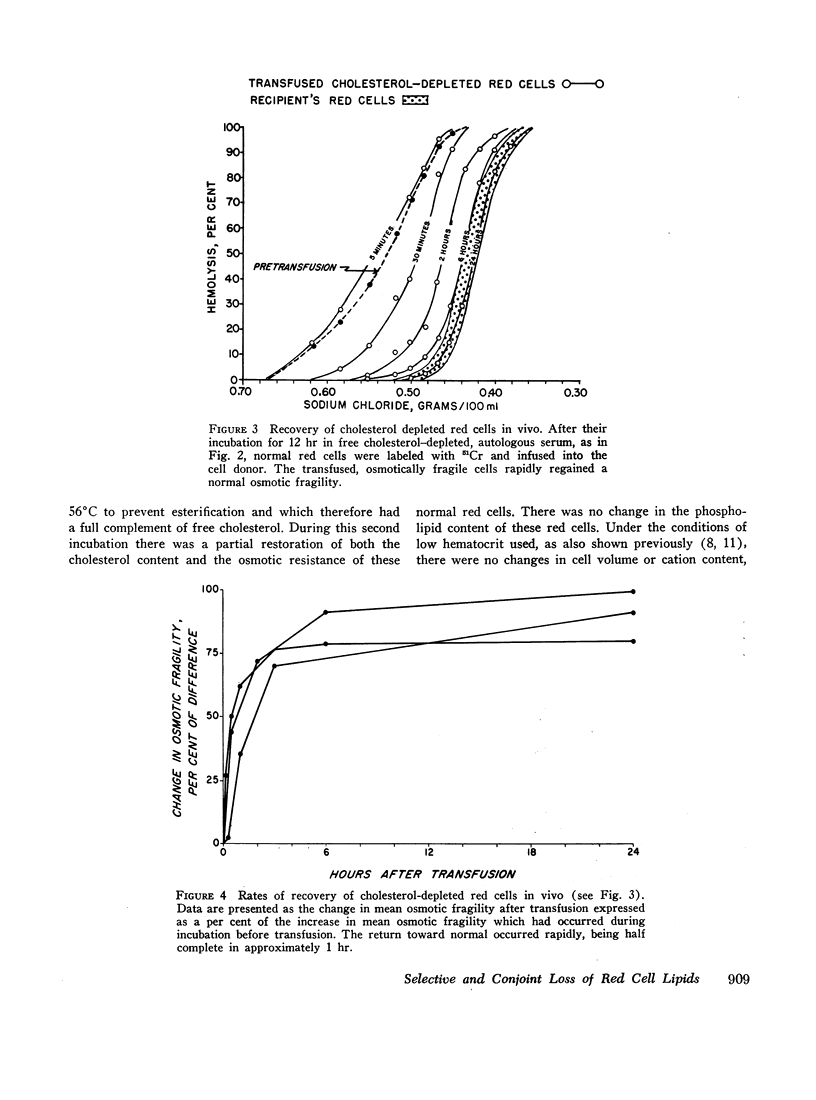
Although the selective loss of membrane cholesterol increases the spherodicity of normal red cells, it does not lead to a change in their rate of glucose consumption, and both the loss of cholesterol and the increase in osmotic fragility are reversible in vitro. Moreover, normal red cells made osmotically fragile by cholesterol depletion in vitro rapidly become osmotically normal and survive normally after their reinfusion in vivo.
In contrast to this selective loss of membrane cholesterol, red cells incubated in the absence of glucose lose both cholesterol and phospholipid. This occurs more rapidly in HS than normal red cells and is followed by a disruption of cation gradients and then by hemolysis. Cholesterol and phospholipid lost under these conditions is not restored during subsequent incubations in vitro.
Selective loss of membrane cholesterol is a physiologic event secondary to an altered state of serum lipids. It is reversible both in vitro and in vivo and neither influences cellular metabolism nor impairs viability. Conjoint loss of phospholipid and cholesterol, however, results from intrinsic injury to the red cell membrane which results from prolonged metabolic depletion.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashworth L. A., Green C. Plasma membranes: phospholipid and sterol content. Science. 1966 Jan 14;151(3707):210–211. doi: 10.1126/science.151.3707.210. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. Phospholipids as ion exchangers: implications for a possible role in biological membrane excitability and anesthesia. Biochim Biophys Acta. 1967 Sep 9;135(4):653–668. doi: 10.1016/0005-2736(67)90096-x. [DOI] [PubMed] [Google Scholar]
- Cooper R. A., Jandl J. H. Bile salts and cholesterol in the pathogenesis of target cells in obstructive jaundice. J Clin Invest. 1968 Apr;47(4):809–822. doi: 10.1172/JCI105775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOMSET J. A. The mechanism of the plasma cholesterol esterification reaction: plasma fatty acid transferase. Biochim Biophys Acta. 1962 Nov 19;65:128–135. doi: 10.1016/0006-3002(62)90156-7. [DOI] [PubMed] [Google Scholar]
- GRIGGS R. C., WEISMAN R., Jr, HARRIS J. W. Alterations in osmotic and mechanical fragility related to in vivo erythrocyte aging and splenic sequestration in hereditary spherocytosis. J Clin Invest. 1960 Jan;39:89–101. doi: 10.1172/JCI104032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjone E., Torsvik H., Norum K. R. Familial plasma cholesterol ester deficiency. A study of the erythrocytes. Scand J Clin Lab Invest. 1968;21(4):327–332. doi: 10.3109/00365516809077001. [DOI] [PubMed] [Google Scholar]
- Glomset J. A. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968 Mar;9(2):155–167. [PubMed] [Google Scholar]
- Goodman D. S. Cholesterol ester metabolism. Physiol Rev. 1965 Oct;45(4):747–839. doi: 10.1152/physrev.1965.45.4.747. [DOI] [PubMed] [Google Scholar]
- HAGERMAN J. S., GOULD R. G. The in vitro interchange of cholesterol between plasma and red cells. Proc Soc Exp Biol Med. 1951 Oct;78(1):329–332. doi: 10.3181/00379727-78-19064. [DOI] [PubMed] [Google Scholar]
- HOKIN L. E., HOKIN M. R. Phosphatidic acid metabolism and active transport of sodium. Fed Proc. 1963 Jan-Feb;22:8–18. [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. Effects of sulfhydryl inhibition on red blood cells. I. Mechanism of hemolysis. J Clin Invest. 1962 Apr;41:779–792. doi: 10.1172/JCI104536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. INCREASED CELL MEMBRANE PERMEABILITY IN THE PATHOGENESIS OF HEREDITARY SPHEROCYTOSIS. J Clin Invest. 1964 Aug;43:1704–1720. doi: 10.1172/JCI105046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H. S., Karnovsky M. L. Concomitant alterations of sodium flux and membrane phospholipid metabolism in red blood cells: studies in hereditary spherocytosis. J Clin Invest. 1967 Feb;46(2):173–185. doi: 10.1172/JCI105520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H. S. Membrane lipid depletion in hyperpermeable red blood cells: its role in the genesis of spherocytes in hereditary spherocytosis. J Clin Invest. 1967 Dec;46(12):2083–2094. doi: 10.1172/JCI105695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandl J. H., Aster R. H. Increased splenic pooling and the pathogenesis of hypersplenism. Am J Med Sci. 1967 Apr;253(4):383–398. doi: 10.1097/00000441-196704000-00001. [DOI] [PubMed] [Google Scholar]
- Langley G. R., Axell M. Changes in erythrocyte membrane and autohaemolysis during in vitro incubation. Br J Haematol. 1968 Jun;14(6):593–603. doi: 10.1111/j.1365-2141.1968.tb00365.x. [DOI] [PubMed] [Google Scholar]
- MOHLER D. N. ADENOSINE TRIPHOSPHATE METABOLISM IN HEREDITARY SPHEROCYTOSIS. J Clin Invest. 1965 Aug;44:1417–1424. doi: 10.1172/JCI105247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURPHY J. R. ERYTHROCYTE METABOLISM. VI. CELL SHAPE AND THE LOCATION OF CHOLESTEROL IN THE ERYTHROCYTE MEMBRANE. J Lab Clin Med. 1965 May;65:756–774. [PubMed] [Google Scholar]
- McConnell D. G., Tzagoloff A., MacLennan D. H., Green D. E. Studies on the electron transfer system. LXV. Formation of membranes by purified cytochrome oxidase. J Biol Chem. 1966 May 25;241(10):2373–2382. [PubMed] [Google Scholar]
- Murphy J. R. The influence of pH and temperature on some physical properties of normal erythrocytes and erythrocytes from patients with hereditary spherocytosis. J Lab Clin Med. 1967 May;69(5):758–775. [PubMed] [Google Scholar]
- Nestel P. J., Couzens E. A. Turnover of individual cholesterol esters in human liver and plasma. J Clin Invest. 1966 Jul;45(7):1234–1240. doi: 10.1172/JCI105429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestel P. J., Monger E. A. Turnover of plasma esterified cholesterol in normocholesterolemic and hypercholesterolemic subjects and its relation to body build. J Clin Invest. 1967 Jun;46(6):967–974. doi: 10.1172/JCI105603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRANKERD T. A. Studies on the pathogenesis of haemolysis in hereditary spherocytosis. Q J Med. 1960 Apr;29:199–208. [PubMed] [Google Scholar]
- ROSE H. G., OKLANDER M. IMPROVED PROCEDURE FOR THE EXTRACTION OF LIPIDS FROM HUMAN ERYTHROCYTES. J Lipid Res. 1965 Jul;6:428–431. [PubMed] [Google Scholar]
- Reed C. F., Swisher S. N. Erythrocyte lipid loss in hereditary spherocytosis. J Clin Invest. 1966 May;45(5):777–781. doi: 10.1172/JCI105392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAIFER A., GERSTENFELD S. The photometric microdetermination of blood glucose with glucose oxidase. J Lab Clin Med. 1958 Mar;51(3):448–460. [PubMed] [Google Scholar]
- SELWYN J. G., DACIE J. V. Autohemolysis and other changes resulting from the incubation in vitro of red cells from patients with congenital hemolytic anemia. Blood. 1954 May;9(5):414–438. [PubMed] [Google Scholar]
- SMITH J. A., LONERGAN E. T., STERLING K. SPUR-CELL ANEMIA: HEMOLYTIC ANEMIA WITH RED CELLS RESEMBLING ACANTHOCYTES IN ALCOHOLIC CIRRHOSIS. N Engl J Med. 1964 Aug 20;271:396–398. doi: 10.1056/NEJM196408202710804. [DOI] [PubMed] [Google Scholar]
- Silber R., Amorosi E., Lhowe J., Kayden H. J. Spur-shaped erythrocytes in Laennec's cirrhosis. N Engl J Med. 1966 Sep 22;275(12):639–643. doi: 10.1056/NEJM196609222751204. [DOI] [PubMed] [Google Scholar]
- TURNER K. B., McCORMACK G. H., Jr, RICHARDS A. The cholesterol-esterifying enzyme of human serum. I. In liver disease. J Clin Invest. 1953 Sep;32(9):801–806. doi: 10.1172/JCI102795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed R. I., Bowdler A. J. Metabolic dependence of the critical hemolytic volume of human erythrocytes: relationship to osmotic fragility and autohemolysis in hereditary spherocytosis and normal red cells. J Clin Invest. 1966 Jul;45(7):1137–1149. doi: 10.1172/JCI105420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed R. I., Reed C. F. Membrane alterations leading to red cell destruction. Am J Med. 1966 Nov;41(5):681–698. doi: 10.1016/0002-9343(66)90030-1. [DOI] [PubMed] [Google Scholar]
- YOUNG L. E. Hereditary spherocytosis. Am J Med. 1955 Mar;18(3):486–497. doi: 10.1016/0002-9343(55)90229-1. [DOI] [PubMed] [Google Scholar]
- ZLATKIS A., ZAK B., BOYLE A. J. A new method for the direct determination of serum cholesterol. J Lab Clin Med. 1953 Mar;41(3):486–492. [PubMed] [Google Scholar]



