Abstract
Based on the extensive investigation of various ways to regenerate bone, bone marrow stromal cells, in conjunction with ceramic scaffolds, show great promise for application in human patients, and are already in use in a limited number of clinical trials. In preparing for clinical trials, scale-up current good manufacturing processes (cGMP) must incorporate the use of appropriate assays to ensure that the resulting cell product has maintained its biological activity. Future developments are needed to identify better scaffolds, and better ways to deliver cells with either injectable carriers, or by developing techniques to aide in their escape from the circulation and their incorporation into the pre-existing tissue. Lastly, development of methods that faithfully direct pluripotent stem cell differentiation into populations of osteogenic precursors (and ideally, containing skeletal stem cells) represents a new challenge in the field of bone regeneration, but also offer new opportunities to not only to study the biology of bone formation, but also to develop a robust cell source for bone regeneration.
Introduction
There is no doubt that there is an increasing need worldwide for the ability of orthopedic and oral surgeons to reproducibly regenerate bone and associated tissues that are lost due to trauma, surgical resection of cancer, or pathologies that affect the skeleton. The field of tissue engineering aims to fulfill this need through a variety of approaches that utilize (1) morphogens, growth factors, and cytokines, (2) scaffolds and carriers, and (3) cells. Various combinations of these different components, tailored for specific applications, have shown great promise in preclinical animal models, and there are a number of small clinical trials underway around the world (http://clinicaltrials.gov/). The ultimate goal is to induce endogenous repair without the need for surgical intervention. However, the right cocktail of factors has yet to be formulated that is long-lasting, without potential unwanted effects (bone where it should not be), and able to regenerate large segments of bone where the number of endogenous cells (either local or recruited) are insufficient to complete the task. Scaffolds, either alone or in combination with factors, can be used to guide regeneration by endogenous cells in certain situations, but again, may not suffice in large skeletal defects. Consequently, cell-based therapy tops the list of potential approaches by supplying sufficient numbers of cells that can not only form bone and associated tissues, but also maintain bone as it undergoes turnover throughout life. What follows is a discussion of the isolation and characterization of potential cell sources and various approaches to cell-based bone regeneration.
Cell Sources: Overview
Based on the pioneering studies of Friedenstein and coworkers,1 and others who followed (reviewed in Ref. 2), it is well established that bone marrow contains a type of nonhematopoietic stem cell, lurking within the sea of blood cells, that is a component of the bone marrow stromal cell (BMSC) population. When populations of cell culture-expanded BMSCs devoid of hematopoietic cells are transplanted in vivo in diffusion chambers (a closed system), they form bone and, in the interior of the chamber, cartilage.3 When transplanted with an appropriate carrier (an open system), a bone/marrow organ is formed, composed of osteocytes, osteoblasts, hematopoiesis-supportive stroma and marrow adipocytes of donor origin, and hematopoietic cells of recipient origin4,5 (Fig. 1A). More recently, it has been determined that these multipotent cells arise from specialized clonogenic BMSCs that are found wrapping around the surfaces of bone marrow sinusoids, otherwise known as pericytes.6 Further, their ability to self-renew was established by serial transplantation assays of clonogenic cells in vivo.6 Based on these findings, it is clear that bone marrow stroma contains a stem cell by the most rigorous criteria: the ability of a single cell to reform and support a complete tissue (bone with marrow), and the ability to self renew.
FIG. 1.
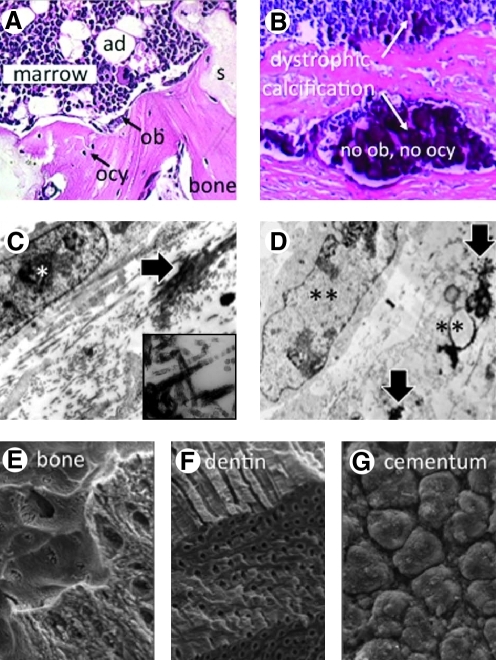
(A) The goal of bone regeneration. The development of bone with morphologically identifiable osteoblasts (ob) and osteocytes (ocy) with the support of hematopoietic marrow with adipocytes (ad), formed in conjunction with an appropriate scaffold (s) upon in vivo transplantation. (B) Dystrophic calcification in vivo is noted for the lack of identifiable osteoblasts and osteocytes, and lack of marrow. (C) In vitro, intact cells (*) and matrix mineralization (large arrow, and inset showing mineral on banded collagen fibrils) characterize physiological mineralization. (D) In cases of prolonged culture in osteogenic conditions, dystrophic calcification (large arrows) is formed in association with dying cells (**). (C and D, courtesy of Dr. Lynda Bonewald,24 inset in C—Heywood and Robey, unpublished data). The organization of mineralized matrix is cell-type specific with distinctive patterns for bone (E), dentin (F), and cementum (G). (E–G, courtesy of Drs. Alan Boyde and Sheila J. Jones34). Color images available online at www.liebertonline.com/teb
While Friedenstein and others have been precise in their terminology by calling this multipotent stem cell a bone marrow stromal stem cell or a skeletal stem cell (SSC) (reviewed in Ref. 7), it has also gone by the name of the “mesenchymal stem cell,” (MSCs) based on its ability to recreate tissues that originate from embryonic mesenchyme (reviewed in Ref.8). By applying some of the techniques developed for the isolation and characterization of BMSCs/SSCs, studies that have followed during the last decade have purported to identify MSCs with the ability to form bone in virtually all connective tissues (Table 1). However, the true differentiation capacity and ability to self-renew of non-bone marrow MSCs has often not been rigorously determined using appropriate differentiation assays, as described below, leading one to believe that any MSC can be used for bone regeneration. Further, while some non-bone marrow MSCs may form bone (usually after encouragement with a bone morphogenetic protein [BMP]), most, if not all, lack the ability to support the formation of marrow. Few publications on non-marrow MSCs comment on the ability to support hematopoiesis; however, examination of the histological results of in vivo transplantation assays highlight the fact that MSCs from adipose tissue (Ref.9; Balakumaran, Cherman, and Robey, unpublished results), dental pulp,10 and periodontal ligament,11 as examples, do not support the formation of hematopoietic marrow.
Table 1.
Potential Cell Sources
| The gooda | The bada | The ugly (but promising) |
|---|---|---|
| Bone marrow stromal cellsb | ||
| Trabecular bone cells | Dental pulp cells | hESCs |
| Periosteal cells | Periodontal ligament cells | iPSCs |
| Cord blood | Adipose derived cells | Direct reprogramming (has not yet been reported for development of osteogenic cells) |
| Amniotic fluid cells | Cells from virtually any connective tissue | |
| Circulating skeletal cells (endogenous cells) |
Mesenchymal stem cells.
To date, the most reliable source for bone regeneration.
hESC, human embryonic stem cell; iPSC, induced pluripotent stem cell.
In considering cell sources for bone regeneration, the ability to form hematopoiesis-supportive stroma could be considered to be essential because the SSC, needed for bone regeneration during bone turnover, resides in the bone marrow stroma that supports hematopoiesis. If the SSC is depleted due to lack of self-renewal, diversion solely to osteogenesis, or is over-diluted by transiently amplifying cells, during ex vivo expansion of the BMSC population, bone turnover, which relies on the presence of the SSC, may be extinguished. Scaffolds could be designed to encourage the ingrowth of marrow stromal elements (containing SSCs), primarily by increasing the pace and extent of creeping substitution. However, in the case of extremely large (critical size) defects, this may not be sufficient to repopulate the entire construct with SSCs derived from surrounding tissues, and may require a long period.
Lastly, when evaluating a potential cell source, it is critical to determine by rigorous assays that bone with identifiable osteocytes and osteoblasts of donor origin is formed (Fig. 1A), rather than dystrophic calcification (Fig. 1B), which can arise from certain pathological conditions. Establishment of marrow, which could be considered as a surrogate marker for the presence of the SSC, would also appear to be essential (Fig. 1A).
Methods of Isolation
BMSCs can be isolated from iliac crest aspirates, core biopsies, and surgical waste. While aspirates are less invasive than core biopsies, aspirates are often contaminated with peripheral blood when large volumes are aspirated, even with frequent repositioning of the aspiration needle, and excess peripheral blood can have a negative impact on growth of BMSCs.12 When core biopsies and surgical waste are available, repeated washing releases high numbers of cells (reviewed in Ref.13). In addition, more mature trabecular bone cells can be isolated using a series of enzymatic treatments, or by explants culture of bone fragments without or with collagenase pretreatment.14–16 Cells from periosteal tissue, and from other soft tissues purported to contain cells with osteogenic capacity, can also be generated via enzymatic treatment, or by explant cultures (e.g., see Refs.17,18) (Fig. 2). Additionally, it has been reported that peripheral blood contains osteogenic progenitors, although they are extremely rare in humans.19,20
FIG. 2.
Methods of isolation and characterization of potential cell sources for bone regeneration. Color images available online at www.liebertonline.com/teb
Characterization
Cell surface markers
FACS is often used to isolate specific populations from freshly isolated cells (lavage of bone fragments, aspirates, and enzymatically released cells) (Fig. 2). A long list of markers has been associated with MSCs purported to have osteogenic differentiation capacity. In humans, these populations are negative for hematopoietic markers (CD34, CD45, CD14, and CD11b) and endothelial markers (CD31 and CD62E), but positive for CD13 (aminopeptidase N), CD29 (β1 integrin subunit), CD44 (hyaluronan receptor), CD49α (α1 integrin subunit and VLA), CD63 (lysosomal membrane-associated glycoprotein 3), CD90 (Thy-1), CD105 (endoglin), CD106 (VCAM-1 and α4 β1 integrin ligand), CD146 (MCAM/MUC18), CD166 (activated leukocyte cell adhesion molecule), and Stro1 (identity unknown to date) (to name a few, reviewed in Ref.13). Of these markers, many are found on connective tissue stromal cells and are involved in cell–matrix interactions (CD29, CD44, and CD49a), but do not specifically define an SSC. However, some of these markers may be critically important in the biological function of BMSCs, such as CD105/endoglin, which is the regulatory subunit of the transforming growth factor (TGF)-β receptor that modulates responses to TGF-β, CD90/Thy-1, thought to mediate hematopoietic stem cell differentiation, and CD 106/VCAM-1 and CD146/MCAM, which may regulate interactions with endothelial cells. From an applicative point of view, it is not practical to achieve sufficient numbers of cells for bone regeneration by FACS without ex vivo expansion, and expression of these markers can be altered with time in culture. Nonetheless, assessment of the cell surface profile provides key information on the nature of the cell population in that absence of expression of key cell surface markers would be indicative of a less than optimal population.
Colony forming efficiency
Clinical bone regeneration protocols are unlikely to rely on the use of clonal strains due to the number of population doublings that would be required to achieve a sufficient number of cells. However, interrogation of a potential population for the presence of SSCs by performing clonogenic assays is warranted, given that SSCs are needed for bone turnover (Fig. 2). Because of the lack of specific markers, an estimate of the number of SSCs comes from colony forming efficiency assays. In this assay, single-cell suspensions of bone marrow are plated at low density (0.14–14.0×103 nucleated cells/cm2 for aspirates, 0.007–3.5×103 nucleated cells/cm2 for core biopsies).13 Of note, when this assay is performed on cells released from tissues by enzymatic digestion, clonal density is 1.63 cells/cm2 because hematopoietic cells, which are nonadherent in human samples, are underrepresented compared to bone marrow. Within 2–3 h of plating, a single cell of fibroblastic character (the colony forming unit-fibroblast [CFU-F]) adheres, and within 24–48 h proliferates to form a colony, demonstrating density-independent growth.21 In human bone marrow, the colony-forming efficiency ranges from 10 to 50 per 105 nucleated cells, and substantial deviations signify either inadequate culture conditions,22 or a potential skeletal pathology (Kuznetsov et al., submitted).
Multipotency
To determine that a multipotent stem cell actually exists within a given population, rigorous clonal analysis of differentiation capacity is required. With respect to bone regeneration, a clone's ability to form bone, hematopoiesis-supportive stroma (where the SSC resides), and marrow adipocytes marks the multipotential nature of the original CFU-F. In vitro, differentiation is induced by various cocktails, and assessed for calcium accumulation by Alizarin Red staining (osteogenesis), and for fat accumulation by Oil Red O or Nile Red (adipogenesis) (reviewed in Ref.13), and the ability to support hematopoiesis is assessed in Dexter-type cultures.23 However, in many cases the results of these in vitro assays, in particular for osteogenesis, are artifactual in nature, and often misleading. The use of prolonged time intervals with cells at high density and high levels of β-glycerolphosphate can induce dystrophic calcification (Fig. 1D) rather than matrix mineralization24 (Fig. 1C). As such, this assay cannot be reliably used to predict osteogenic capacity. Culture conditions for support of complete hematopoiesis (including the hematopoietic stem cell) by human BMSCs in vitro have yet to be optimized. Therefore, osteogenesis and support of hematopoiesis is best determined by in vivo transplantation of clonal cells with an appropriate scaffold. When a series of clonal strains (i.e., the clonal strain arises from a single CFU-F) are transplanted in vivo, only ∼10%–20% of the single-cell-derived strains were found to recreate a bone/marrow organ.25 This points to the fact that SSCs are a fraction of the population, and that not all BMSCs, not even all CFU-Fs, are multipotent. Thus, one does not establish a culture of stem cells, as is often stated in the literature; one establishes a culture in which a subset of cells are stem cells. That is not to say that the BMSC population as a whole, with its subset of SSCs, is inferior in any way, it is only to say that it is necessary to document the existence of multipotent SSCs within the population. In summary, documentation of the presence of SSCs within the population, as evidenced by support of hematopoiesis upon in vivo transplantation (as in Fig. 1A), is necessary when devising ex vivo expansion and scale up procedures to ensure that SSCs have not been eliminated by inappropriate culture conditions, or over-diluted due to excessive passaging.
Cell Sources Reconsidered
Two factors, in addition to the tissue source, must be taken into consideration in protocols for bone regeneration: (1) autologous (self ) versus allogeneic (nonself ) and (2) embryonic origin. It has been reported that BMSCs are immune privileged; that is, BMSCs can escape rejection when used in an allogeneic setting.26 While this may be true when allogeneic or xenogeneic BMSCs are introduced into developing embryos before establishment of the immune system, there are studies that indicate that allogeneic and xenogeneic BMSCs are indeed rejected when infused systemically into immune competent recipients,27 and are even more likely to be when they are induced to differentiate into osteogenic cells based on their expression of histocompatibility antigens. Thus, for bone regeneration, cells will most likely have to be autologous to avoid the need for immunosuppression of the recipient.
With respect to the embryonic origin, bones in the facial region of the skull derive from neuroectoderm, whereas those in the axial and appendicular skeleton derive from mesoderm (reviewed in Ref.28), and recent studies indicate that cells from these two different embryonic sources are not identical.29 Oral surgeons have long noted that transplantation of iliac crest into the jawbones does not last long-term,30 suggesting some sort of incompatibility, and pointing to the need for a better understanding of bone derived from these two different embryonic origins. With regard to the selection of the tissue sources, evaluation of the potential populations that have been reported for use in bone regeneration based on rigorous cell characterization as outlined above must be carefully considered (Table 1).
The good
It has been shown that periosteal cells and trabecular bone cells form bone upon in vivo transplantation6; however, these populations have yet to be shown to support hematopoiesis in vivo, indicative of a lack of SSCs. Consequently, it is possible that in the face of the need for bone turnover, grafts formed by these cells may ultimately fail. However, it is possible that in certain scenarios that the amount of bone formed would be sufficient for short- and mid-term goals. Furthermore, in-growth of cells from the margins (creeping substitution) may provide the necessary stem cell for bone turnover. Amniotic fluid cells (fetal in origin) can also form bone and perhaps support hematopoiesis (Ref.31, Balakumaran and Robey, unpublished results). Some populations of adherent cells from first trimester cord blood (fetal in origin) have been reported to be osteogenic as well, but have not been tested in vivo.32 Cells from human peripheral blood have been identified that do make bone in vivo, but they are extremely scarce.19,20 Other populations that have been reported from human peripheral blood have not been rigorously tested. Taken together, these findings mark BMSCs as currently the most appropriate cell source (Table 1).
The bad
Some MSCs from non-bone marrow sources do have the capability to form mineralized tissues in vivo, but it is not clear that they can substitute for BMSCs. For example, cells from dental pulp of deciduous teeth make dentin and induce a bone-like structure,11 and cells from adult teeth make dentin and a pulp-like complex,10 as do cells from the apical papilla of extracted teeth.33 Cells from periodontal ligaments make a PDL-like structure and cementum.33 However, the mineral crystal size and shape, and the organization of mineral within the matrix is very different in bone (Fig. 1E), dentin (Fig. 1F), and cementum (Fig. 1G),34 and may not have the same mechanical properties as bone. It is also unlikely that enough cells could be generated from these sources for use in regenerating large segments of bone in humans (Table 1).
Other MSCs have been isolated from a variety of tissues such as fat, muscle, and virtually any connective tissues (reviewed in Ref.35). These cells share certain features with BMSCs, such as the expression of connective tissue cell surface markers. However, their osteogenic potential has been demonstrated primarily by in vitro assays (often with treatment with BMPs), and to date, there is little data showing their capacity to form bone (and maintain bone) with osteocytes and osteoblasts of donor origin, and to support the formation of marrow by in vivo transplantation, making them less suitable for use in bone regeneration (Table 1).
The ugly (but promising)
With the revolutionary derivation of human embryonic stem cells (hESCs),36 and the creation of human induced pluripotent stem cells (iPSCs),37 both with extensive proliferation capacity, there is the hope that they can be coerced into osteoblastic differentiation to produce larger numbers of cells to treat human patients than are achievable from adult stem cells. However, there are a limited number of hESC lines currently available, and their use in bone regeneration would be allogeneic, at best. iPSCs can be generated for autologous use, but there are major issues relating to the method of reprogramming (viral vs. nonviral), completeness of reprogramming, epigenetic changes, and genomic instability (reviewed in Ref.38) (see Table 2 for comparison of different types of stem cells). Efforts are also underway to transdifferentiate cells from one phenotype to another (lineage reprogramming) (both iPSCs and lineage reprogramming are reviewed in Ref.39). There have been several reports on the osteogenic differentiation of murine iPSCs based on adenoviral transduction with runx2 (e.g., Ref.40), or treatment with TGFβ or BMP (e.g., Ref.41). Standard osteogenic differentiation medium has been used to induce bone formation of human iPSCs (e.g., Ref.42), and others have used treatment with basic fibroblast growth factor, platelet-derived growth factor-AB, and epidermal growth factor, followed by cell sorting to generate MSC-like lines (e.g., Ref.43). However, to date, the identification of bone of donor origin formed by in vivo transplantation has yet to be reported for human iPSCs.
Table 2.
Comparison of Different Types of Stem Cells
| Adult stem cells that form mineralized tissues | Embryonic stem cells | Induced pluripotent stem cells | |
|---|---|---|---|
| Derivation | Tissue-specific protocols from different tissues | Inner cell mass of the blastocyst | By viral transduction, or plasmids, or small molecules, or mRNAs of adult cells |
| Advantages | Moderate division, commitment to specific cell types, can be autologous | Unlimited division, pluripotent, no molecular alteration | Unlimited division (?), pluripotent, no destruction of a blastocyst, autologous |
| Disadvantages | May not be able to generate enough cells needed for therapy, may take too long to generate cells | Destruction of embryo, incomplete differentiation, possibility of teratoma, allogeneic | Viral integration, equivalence to hESCs (?), genetic memory, incomplete differentiation, possibility of teratoma |
In spite of the fact that it is doubtful that hESCs would be used for bone regeneration in humans due to histocompatibility issues, they are a pure form of pluripotent stem cells (i.e., have not been genetically manipulated) and provide a valuable tool to establish methods for bone formation that can be adapted to iPSCs. Several reports have shown promise,44,45 whereas others have failed to demonstrate bone of donor origin (with identifiable human osteocytes and osteoblasts). However, to date (1) bone was not the only tissue formed upon in vivo transplantation, (2) hematopoiesis was not supported, and (3) long-term studies have not proven that these unpurified populations were free of hESCs, which could subsequently form a teratoma. Further refinement is needed to coax the cells through a developmental process starting with differentiation into mesoderm (or neuroectoderm for facial bones), and from there, into osteoprogenitors.
Currently, techniques devised for hESC differentiation are being applied to iPSCs derived from a variety of sources such as skin fibroblasts and BMSCs, but there is much to be done. First, derivation of zero footprint (no viral integration) iPSCs in xeno-free culture conditions need to be developed, and methods for expansion in a simpler fashion (monolayers vs. colonies) would be desirable. Lastly, embryoid bodies (spheres that contain tissues from all three germ layers), followed by liberation of cells for FACS to isolate specific populations for subsequent differentiation, may not yield the number of cells needed for bone regeneration. Consequently, scale-up represents a major hurdle. Nonetheless, the possibility of directing iPSCs into osteogenic differentiation would mark a major advance in bone tissue engineering, providing the potential to generate larger numbers of autologous cells than what can be obtained with BMSCs.
Cell Therapy for Bone Regeneration
Cell processing
While some applications have envisioned using freshly isolated, concentrated bone marrow (containing BMSCs) for treatment of local bone loss such as avascular necrosis as an example,46 treatment of large defects calls for the generation of large numbers of cells by ex vivo expansion in a current good manufacturing processes (cGMP) facility. However, care must be taken to maintain the stem cell within the BMSC population. Excessive passaging will dilute the stem cell (if one thinks of self-renewing asymmetric division and the generation of transiently amplifying cells). Further, addition of growth factors may also alter the kinetics of stem cell division (symmetric division resulting in loss of the stem cell), leading to the generation of less than optimal populations. In generating clinical-grade populations of cells, assessment of colony-forming efficiency, cell surface properties, and in vitro (if done appropriately) and in vivo differentiation of nonclonal populations defines the potency of the cell product (a term used extensively by the U.S. Food and Drug Administration [FDA] in validation of the processing procedure). Ex vivo expansion also provides the opportunity to improve the performance of defective BMSCs (i.e., from patients with genetic bone disease) through the use of molecular engineering to correct gene defects (see Ref.47 as an example) (Fig. 3).
FIG. 3.
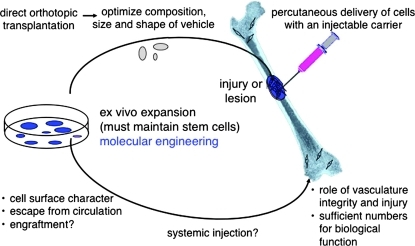
Cell therapy for bone regeneration, requiring ex vivo expansion that maintains the stem cells within the population, but also allowing for molecular engineering to improve performance or correct gene defects. Appropriate scaffolds for direct orthotopic application and for percutaneous delivery are required. Systemic injection may be used to treat generalized skeletal disease; however, the cell surface characteristics of bone marrow stromal cells prevent their escape from the circulation, even in the face of vascular defects, and sufficient numbers may not be able to incorporate at a sufficient level to have a biological effect. Color images available online at www.liebertonline.com/teb
Orthotopic applications
There have been a large number of preclinical studies and a number of small human trials showing the efficacy of using ex vivo expanded BMSCs in conjunction with an appropriate scaffold for direct orthotopic delivery into large segmental defects (reviewed in Ref.48). In some approaches, cells are precultured on a scaffold before transplantation. However, it is not clear that this is an advantage, especially if osteogenesis is induced, which may preclude the cell population's ability to maintain the stem cells. Additionally, the nature of the scaffold plays a major role in the performance of the cell population. While many synthetic and natural scaffolds have been fabricated, to date, three-dimensional (3D) scaffolds that contain ceramics (usually hydroxyapatite/tricalcium phosphate) as part of their formulation (reviewed in Ref.49), appear to be the most reliable with respect to the formation of bone and the support of hematopoiesis when seeded with BMSCs (Fig. 3). However, many of these scaffolds are resorbed very poorly and can persist for long periods of time in vivo. Consequently, scaffolds composed of polymers such as poly(lactic-co-glycolic acid) and poly(ɛ-caprolactone), with and without calcium phosphate components or further functionalization, have been developed (reviewed in Refs.50,51). However, it is not clear whether these scaffolds also maintain the BMSC population's ability to support hematopoiesis. Of note, many studies to identify new scaffolds rely on in vitro assays to evaluate the performance of newly developed scaffolds; however, these assays may not adequately determine osteogenesis or support of hematopoiesis, and in vivo transplantation is required.
In addition to the use of 3D scaffolds, it would also be of benefit to develop techniques for the use of injectable scaffolds that would hold cells in place, and support their differentiation, and thereby avoid the need for open surgery.52 Injection of concentrated bone marrow aspirates has been shown to be beneficial in the treatment of avascular necrosis,46 but it would be of interest to determine if their efficacy could be enhanced through the use of an injectable carrier. It could also be envisioned that this approach would be useful for the treatment of unicameral bone cysts and nonunions (Fig. 3).
Systemic delivery
It has been envisioned that systemic administration of BMSCs could be used as a way to treat generalized skeletal disease. In the intact animal, systemically administered BMSCs (either autologous or allogeneic) are predominantly trapped in the lungs based on their size,53 and because they lack cell surface properties that allow them to associate with the luminal surface of endothelial cells and migrate into the extravascular spaces. Recent studies have shown that BMSC cell surface modification with a fucosyltransferase converts CD44 on the BMSC surface into a variant that is able to bind to E-selectin on endothelial surfaces, thereby allowing escape from the circulation into the extravascular space.54 In the case of injury or disease, small numbers of cells can escape due to vascular damage. However, it is not clear whether either when using surface-modified cells or in the case of vascular leak that cells can escape in sufficient numbers to have a biological effect; that is, to incorporate into a pre-existing 3D structure (Fig. 3).
In spite of the fact that systemic infusion of autologous or allogeneic BMSCs does not lead to a broad distribution of cells that persist for long periods, it has been noted that there is a beneficial effect in a wide variety of diseases and disorders, such as in acute graft versus host disease and inflammatory bowel disease (reviewed in Ref.55). It was initially thought that BMSCs transdifferentiate into nonskeletal cell types to bring about repair; however, this occurs very rarely, if at all. However, it appears that the beneficial effect emanates from the BMSCs' ability to secrete copious amounts of a large repertoire of growth factors and cytokines. In the bone marrow microenvironment, these factors support hematopoiesis, but in areas of disease or injury, these factors are immunomodulatory, and appear to allow local cells to begin the repair process. In some cases it has been determined that cell-to-cell contact is necessary for reasons that have yet to be determined.56 However, given the pericyte nature of BMSCs, it is a possibility that they associate with vascular cells, thereby stabilizing them during blood vessel ingrowth into the diseased or injured tissue. It can further be envisioned that the immunomodulatory properties of BMSCs would also play a role in bone formation in a site under reconstruction by reducing inflammation, and other local factors that may oppose endogenous bone regeneration.
In summary, the field of tissue engineering, specifically for bone regeneration, has made great strides in the past several decades. While a number of cell sources would appear to be available, currently BMSCs lead the way. However, the development of ex vivo expansion facilities that not only meet FDA requirements, but also maintain the biological properties of the cells as assessed by several critical assays will be essential for clinical translation. Newer and better scaffolds and carriers are also needed to move the field forward. Finally, the rising of pluripotent stem cell technology on the horizon offers that possibility to create large numbers of cells that will be required for most types of bone regeneration applications, and defining their differentiation conditions remains a challenge to cell biologist and tissue engineer alike.
Acknowledgments
The author would like to acknowledge all past and present members of the Craniofacial and Skeletal Diseases Branch and collaborators for their contributions to the field of bone regeneration reviewed in this article. Supported in part by the Division of Intramural Research of the NIDCR, a part of the Intramural Research Program, NIH, DHHS.
Disclosure Statement
No competing financial interests exist.
References
- 1.Friedenstein A.J. Piatetzky-Shapiro I.I. Petrakova K.V. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381. [PubMed] [Google Scholar]
- 2.Bianco P. Riminucci M. Gronthos S. Robey P.G. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 3.Friedenstein A.J. Chailakhyan R.K. Gerasimov U.V. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 4.Friedenstein A.J. Chailakhyan R.K. Latsinik N.V. Panasyuk A.F. Keiliss-Borok I.V. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Krebsbach P.H. Kuznetsov S.A. Satomura K. Emmons R.V. Rowe D.W. Robey P.G. Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;63:1059. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- 6.Sacchetti B. Funari A. Michienzi S. Di Cesare S. Piersanti S. Saggio I., et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 7.Bianco P. Robey P.G. Simmons P.J. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caplan A.I. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 9.Hicok K.C. Du Laney T.V. Zhou Y.S. Halvorsen Y.D. Hitt D.C. Cooper L.F., et al. Human adipose-derived adult stem cells produce osteoid in vivo. Tissue Eng. 2004;10:371. doi: 10.1089/107632704323061735. [DOI] [PubMed] [Google Scholar]
- 10.Gronthos S. Mankani M. Brahim J. Robey P.G. Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miura M. Gronthos S. Zhao M. Lu B. Fisher L.W. Robey P.G., et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kharlamova L.A. [Colony formation inhibition in human bone marrow stromal cells exposed to a factor formed in vitro by peripheral blood leukocytes] Biull Eksp Biol Med. 1975;80:89. [PubMed] [Google Scholar]
- 13.Bianco P. Kuznetsov S.A. Riminucci M. Gehron Robey P. Postnatal skeletal stem cells. Methods Enzymol. 2006;419:117. doi: 10.1016/S0076-6879(06)19006-0. [DOI] [PubMed] [Google Scholar]
- 14.Howard G.A. Turner R.T. Sherrard D.J. Baylink D.J. Human bone cells in culture metabolize 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 and 24, 25-dihydroxyvitamin D3. J Biol Chem. 1981;256:7738. [PubMed] [Google Scholar]
- 15.Beresford J.N. Gallagher J.A. Poser J.W. Russell R.G. Production of osteocalcin by human bone cells in vitro. Effects of 1, 25(OH)2D3, 24, 25(OH)2D3, parathyroid hormone, and glucocorticoids. Metab Bone Dis Relat Res. 1984;5:229. doi: 10.1016/0221-8747(84)90064-x. [DOI] [PubMed] [Google Scholar]
- 16.Robey P.G. Termine J.D. Human bone cells in vitro. Calcif Tissue Int. 1985;37:453. [PubMed] [Google Scholar]
- 17.Nakahara H. Goldberg V.M. Caplan A.I. Culture-expanded human periosteal-derived cells exhibit osteochondral potential in vivo. J Orthop Res. 1991;9:465. doi: 10.1002/jor.1100090402. [DOI] [PubMed] [Google Scholar]
- 18.O'Driscoll S.W. Recklies A.D. Poole A.R. Chondrogenesis in periosteal explants. An organ culture model for in vitro study. J Bone Joint Surg Am. 1994;76:1042. doi: 10.2106/00004623-199407000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Kuznetsov S.A. Mankani M.H. Gronthos S. Satomura K. Bianco P. Robey P.G. Circulating skeletal stem cells. J Cell Biol. 2001;153:1133. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuznetsov S.A. Mankani M.H. Leet A.I. Ziran N. Gronthos S. Robey P.G. Circulating connective tissue precursors: extreme rarity in humans and chondrogenic potential in guinea pigs. Stem Cells. 2007;25:1830. doi: 10.1634/stemcells.2007-0140. [DOI] [PubMed] [Google Scholar]
- 21.Friedenstein A.J. Precursor cells of mechanocytes. Int Rev Cytol. 1976;47:327. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- 22.Kuznetsov S.A. Mankani M.H. Bianco P. Robey P.G. Enumeration of the colony-forming units-fibroblast from mouse and human bone marrow in normal and pathological conditions. Stem Cell Res. 2009;2:83. doi: 10.1016/j.scr.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dexter T.M. Allen T.D. Lajtha L.G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977;91:335. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- 24.Bonewald L.F. Harris S.E. Rosser J. Dallas M.R. Dallas S.L. Camacho N.P., et al. von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcif Tissue Int. 2003;72:537. doi: 10.1007/s00223-002-1057-y. [DOI] [PubMed] [Google Scholar]
- 25.Kuznetsov S.A. Krebsbach P.H. Satomura K. Kerr J. Riminucci M. Benayahu D., et al. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- 26.Liechty K.W. MacKenzie T.C. Shaaban A.F. Radu A. Moseley A.M. Deans R., et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 27.Eliopoulos N. Stagg J. Lejeune L. Pommey S. Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106:4057. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- 28.Olsen B.R. Reginato A.M. Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- 29.Akintoye S.O. Lam T. Shi S. Brahim J. Collins M.T. Robey P.G. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38:758. doi: 10.1016/j.bone.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 30.Burnette E.W., Jr Fate of an iliac crest graft. J Periodontol. 1972;43:88. doi: 10.1902/jop.1972.43.2.88. [DOI] [PubMed] [Google Scholar]
- 31.Peister A. Deutsch E.R. Kolambkar Y. Hutmacher D.W. Guldberg R.E. Amniotic fluid stem cells produce robust mineral deposits on biodegradable scaffolds. Tissue Eng Part A. 2009;15:3129. doi: 10.1089/ten.tea.2008.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erices A. Conget P. Minguell J.J. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 33.Sonoyama W. Liu Y. Fang D. Yamaza T. Seo B.M. Zhang C., et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyde A. Jones S.J. Development and structure of teeth and periodontal tissues. In: Rosen C.J., editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 7th. Washington, D.C.: American Society for Bone and Mineral Research; 2008. pp. 491–496. [Google Scholar]
- 35.Porada C.D. Zanjani E.D. Almeida-Porad G. Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther. 2006;1:365. doi: 10.2174/157488806778226821. [DOI] [PubMed] [Google Scholar]
- 36.Thomson J.A. Itskovitz-Eldor J. Shapiro S.S. Waknitz M.A. Swiergiel J.J. Marshall V.S., et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 38.Zhou H. Ding S. Evolution of induced pluripotent stem cell technology. Curr Opin Hematol. 2010;17:276. doi: 10.1097/MOH.0b013e328339f2ee. [DOI] [PubMed] [Google Scholar]
- 39.Illich D.J. Demir N. Stojkovic M. Scheer M. Rothamel D. Neugebauer J., et al. Concise review: induced pluripotent stem cells and lineage reprogramming: prospects for bone regeneration. Stem Cells. 2011;29:555. doi: 10.1002/stem.611. [DOI] [PubMed] [Google Scholar]
- 40.Tashiro K. Inamura M. Kawabata K. Sakurai F. Yamanishi K. Hayakawa T., et al. Efficient adipocyte and osteoblast differentiation from mouse induced pluripotent stem cells by adenoviral transduction. Stem Cells. 2009;27:1802. doi: 10.1002/stem.108. [DOI] [PubMed] [Google Scholar]
- 41.Li F. Bronson S. Niyibizi C. Derivation of murine induced pluripotent stem cells (iPS) and assessment of their differentiation toward osteogenic lineage. J Cell Biochem. 2010;109:643. doi: 10.1002/jcb.22440. [DOI] [PubMed] [Google Scholar]
- 42.Bilousova G. Hyun Jun D. King K.B. Delanghe S. Chick W.S. Torchia E.C., et al. Osteoblasts derived from induced pluripotent stem cells form calcified structures in scaffolds both in vitro and in vitro. Stem Cells. 2011;29:206. doi: 10.1002/stem.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lian Q. Zhang Y. Zhang J. Zhang H.K. Wu X. Lam F.F., et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 44.Arpornmaeklong P. Brown S.E. Wang Z. Krebsbach P.H. Phenotypic characterization, osteoblastic differentiation, and bone regeneration capacity of human embryonic stem cell-derived mesenchymal stem cells. Stem Cells Dev. 2009;18:955. doi: 10.1089/scd.2008.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuznetsov S.A. Cherman N. Robey P.G. In vivo bone formation by progeny of human embryonic stem cells. Stem Cells Dev. 2011;20:269. doi: 10.1089/scd.2009.0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hernigou P. Poignard A. Manicom O. Mathieu G. Rouard H. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J Bone Joint Surg Br. 2005;87:896. doi: 10.1302/0301-620X.87B7.16289. [DOI] [PubMed] [Google Scholar]
- 47.Piersanti S. Remoli C. Saggio I. Funari A. Michienzi S. Sacchetti B., et al. Transfer, analysis and reversion of the fibrous dysplasia cellular phenotype in human skeletal progenitors. J Bone Miner Res. 2010;25:1103. doi: 10.1359/jbmr.091036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chatterjea A. Meijer G. van Blitterswijk C. de Boer J. Clinical application of human mesenchymal stromal cells for bone tissue engineering. Stem Cells Int. 2010;2010:215625. doi: 10.4061/2010/215625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oh S. Oh N. Appleford M. Ong J.L. Bioceramics for tissue engineering applications—a review. Am J Biochem Biotechnol. 2006;2:49. [Google Scholar]
- 50.Rezwan K. Chen Q.Z. Blaker J.J. Boccaccini A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 51.Kretlow J.D. Mikos A.G. Review: mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue Eng. 2007;13:927. doi: 10.1089/ten.2006.0394. [DOI] [PubMed] [Google Scholar]
- 52.Mankani M.H. Kuznetsov S.A. Marshall G.W. Robey P.G. Creation of new bone by the percutaneous injection of human bone marrow stromal cell and HA/TCP suspensions. Tissue Eng Part A. 2008;14:1949. doi: 10.1089/ten.tea.2007.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schrepfer S. Deuse T. Reichenspurner H. Fischbein M.P. Robbins R.C. Pelletier M.P. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 54.Sackstein R. Merzaban J.S. Cain D.W. Dagia N.M. Spencer J.A. Lin C.P., et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- 55.Nauta A.J. Fibbe W.E. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 56.Nemeth K. Leelahavanichkul A. Yuen P.S. Mayer B. Parmelee A. Doi K., et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]