Abstract
Previous studies have shown that an ethylene (ET)-dependent pathway is involved in the cell death signalling triggered by Alternaria alternata f. sp. lycopersici (AAL) toxin in detached tomato (Solanum lycopersicum) leaves. In this study, the role of jasmonic acid (JA) signalling in programmed cell death (PCD) induced by AAL toxin was analysed using a 35S::prosystemin transgenic line (35S::prosys), a JA-deficient mutant spr2, and a JA-insensitive mutant jai1. The results indicated that JA biosynthesis and signalling play a positive role in the AAL toxin-induced PCD process. In addition, treatment with the exogenous ET action inhibitor silver thiosulphate (STS) greatly suppressed necrotic lesions in 35S::prosys leaves, although 35S::prosys leaflets co-treated with AAL toxin and STS still have a significant high relative conductivity. Application of 1-aminocyclopropane-1-carboxylic acid (ACC) markedly enhanced the sensitivity of spr2 and jai1 mutants to the toxin. However, compared with AAL toxin treatment alone, exogenous application of JA to the ET-insensitive mutant Never ripe (Nr) did not alter AAL toxin-induced cell death. In addition, the reduced ET-mediated gene expression in jai1 leaves was restored by co-treatment with ACC and AAL toxin. Furthermore, JA treatment restored the decreased expression of ET biosynthetic genes but not ET-responsive genes in the Nr mutant compared with the toxin treatment alone. Based on these results, it is proposed that both JA and ET promote the AAL toxin-induced cell death alone, and the JAI1 receptor-dependent JA pathway also acts upstream of ET biosynthesis in AAL toxin-triggered PCD.
Keywords: AAL toxin, ethylene (ET), jasmonic acid (JA), PCD, tomato
Introduction
Plants respond to a wide range of biotic and abiotic stresses by evoking reactions for resistance and preventing damage. Phytohormones play a central role in the signalling networks underlying the stress responses (Campos et al., 2009). They act in a modular fashion and the action can be agonistic under some circumstances and antagonistic under others. Different hormones are involved in various stress responses, each leading to a specific set of downstream responses (O'Donnell et al., 2003).
Jasmonic acid (JA) and ethylene (ET) are two of the major plant hormones involved in regulating plant defence responses (Anderson et al., 2004; Melotto et al., 2008). Under O3 stress, ET is necessary for the development of lesions, whereas JA limits O3-induced damage (Castagna et al., 2007). The findings reported by O'Donnell et al. (2001, 2003) indicate that complete disease development of Xanthomonas campestris pv. vesicatoria on tomato requires host hormonal interactions among JA, ET, and salicylic acid (SA). JA and ET act as signalling molecules in resistance against or susceptibility to necrotrophic pathogen attack depending on the plant species and pathogens (Asai et al., 2000; Devadas et al., 2002; Anderson et al., 2004; Egusa et al., 2009; Onkokesung et al., 2010). For instance, the Arabidopsis JA perception mutant coi1, but not JA biosynthesis mutants, exhibited a high level of resistance to a root-infecting fungus Fusarium oxysporum, suggesting that JA signalling mediated through COI1 (CORONATINE-INSENSITIVE1) in Arabidopsis is responsible for susceptibility to this pathogen (Thatcher et al., 2009). There are reports of ET insensitivity leading to increased or decreased disease severity depending on the plant–pathogen combination (O'Donnell et al., 2001). An intact JA–ET signalling pathway is thought to be necessary for the resistance of Arabidopsis to necrotrophic pathogens, such as Botrytis cinerea and Erwinia carotovora (Anderson et al., 2004).
Alternaria alternata f. sp. lycopersici (AAL), a necrotrophic fungal pathogen, causes Alternaria stem canker on susceptible tomato (Solanum lycopersicum) cultivars and produces mycotoxins (Egusa et al., 2009). AAL toxin is a host-specific pathogenicity factor of AAL-induced stem canker disease (Brandwagt et al., 2002). AAL toxins and fumonisins produced by the unrelated fungus Fusarium moniliforme are members of a class of sphinganine analogue mycotoxins (SAMs) (Brandwagt et al., 2002; Yamagishi et al., 2006). SAMs structurally resemble sphinganine, an intermediate of sphingolipid ceramide biosynthesis (Mesbah et al., 2000; Brandwagt et al., 2002). In vivo, AAL toxins inhibit sphingosine N-acyltransferase (i.e. ceramide synthase), a key enzyme in the sphingolipid biosynthetic pathway (Westhuizen et al., 1998). The disruption of sphingolipid biosynthesis causes marked accumulation of free sphingoid bases (Abbas et al., 1994), which induce both programmed cell death (PCD) in susceptible plant cells and neoplastic events in mammals (Wang et al., 1996; Morisseau et al., 1999; Egusa et al., 2009; Zélicourt et al., 2009). Sensitivity to AAL toxin is not common in plants. It has host selectivity within different plant species (Abbas et al., 1995; Mesbah et al., 2000; Zélicourt et al., 2009). Only a small number of Solanaceous species contain SAM-sensitive genotypes (Brandwagt et al., 2002). Insensitivity to SAMs and fungal AAL is determined by the single co-dominant Alternaria Stem Canker (ASC) locus (Brandwagt et al., 2000, 2002). The Asc-1 gene is homologous to the yeast Longevity Assurance Gene 1 (LAG1) (Brandwagt et al., 2002; Zélicourt et al., 2009), which is involved in sphingolipid biosynthesis and can prevent perturbation in sphingolipid metabolism and cell death to a large extent (Gechev et al., 2004). Sensitivity to AAL toxin is limited to tomato genotypes homozygous for the recessive allele (asc/asc) associated with a mutation in the Asc gene (Morisseau et al., 1999; Abbas et al., 2008; Zélicourt et al., 2009).
PCD is a genetically determined active suicide process involving a number of regulatory pathways, which ultimately lead to the selective removal of unwanted or severely damaged cells. It occurs at all stages of the plant life cycle and plays an important role in plants exposed to a broad range of biotic and abiotic stress (Orzaez et al., 2001; Gechev et al., 2004; Ma et al., 2010). The cell death process involving AAL toxin and its susceptible tomato host is an excellent model for studying PCD in pathogen response pathways (Moore et al., 1999; Asai et al., 2000). AAL toxin induces the disease symptoms of Alternaria stem canker (i.e. black necrotic spots between and along the veins, with a loss of turgour) on detached leaflets of susceptible tomato plants (Moore et al., 1999; Mesbah et al., 2000; Gechev et al., 2004). The PCD process can be evaluated in this system in the absence of pathogen, which greatly simplifies the analysis (Moore et al., 1999).
Plant mutants impaired in hormonal metabolism or signalling are commonly used to study the complex network of hormone pathways involved in host defence responses (Campos et al., 2009; Egusa et al., 2009). Arabidopsis mutants fad3-2/7-2/8 (with reduced levels of trienoic fatty acids), coi1 [insensitive to phytotoxin coronatine (COR) and JA], and jar1 (with decreased sensitivity to JA) all exhibited enhanced susceptibility to Pythium spp., Alternaria brassicicola, B. cinerea, and Plectosphaerella cucumerina (Egusa et al., 2009). Using the phytohormone mutants, fumonisin B1 (FB1)-induced cell death was found to require JA- and ET-dependent signal transduction pathways (Asai et al., 2000). In tomato, JA-related mutants have also been characterized. For example, jai1 (jasmonic acid insensitive1) contains a mutation in the tomato homologue of Arabidopsis COI1 (Li et al., 2004) and it cannot express JA-regulated genes in response to wounding and methyl jasmonate (MeJA). The jai1 mutation is recessive and female sterile (Li et al., 2004). In addition, a JA-deficient mutant def1 (with a defective octadecanoid synthesis pathway) and a mutant spr2 [a suppressor of (pro)systemin-mediated responses2 mutation with reduced levels of trienoic fatty acids] have also been characterized (Howe et al., 1996; Li et al., 2003). In tomato, an ET-insensitive mutation Never ripe (Nr) caused by a single base substitution in the N-terminal coding region of the ethylene receptor gene LE-ETR3 (i.e., NR) has been identified and it is homologous to Arabidopsis ETR1 (Lanahan et al., 1994; Wilkinson et al., 1995). These mutants provide useful materials to investigate hormone functions in AAL toxin-induced PCD in tomato.
Previous reports have demonstrated that ET biosynthesis and ET perception via the NR receptor play a crucial role in AAL toxin-induced cell death signalling in detached tomato leaves (Moussatos et al., 1994; Moore et al., 1999). During the AAL toxin-induced cell death process in the Arabidopsis loh2 mutant (a T-DNA knockout of a homologue of the tomato Asc gene), ET-responsive genes were among the ones to be up-regulated within seven hours (Gechev et al., 2004). Knowledge of the role of plant hormones other than ET in AAL toxin-induced cell death is limited. Egusa et al. (2009) treated the tomato leaf discs of wild-type (WT) cv. Castlemart (CA) and def1 with AAL toxin in the presence or absence of MeJA, and the results showed that endogenous JA biosynthesis and exogenous MeJA application did not affect the sensitivity of tomato to AAL toxin. After Arabidopsis leaves were infiltrated with AAL toxin, JA marker genes were either not induced or down-regulated, and there was no indication of JA accumulation (Gechev et al., 2004).
Although the action mechanism of ET in AAL toxin-induced tomato PCD has been elucidated, the role of JA in AAL toxin-induced PCD and the relationship to ET remain to be investigated. Here it is reported that JA signalling is involved in the PCD process using intact tomato leaflets of the JA-deficient mutant spr2, the 35S::prosystemin transgenic line (35S::prosys, which accumulates high amounts of JA) (Howe and Ryan 1999), and the JA-insensitive mutant jai1 combined with exogenous JA application. It was observed that JA actually plays a positive role in tomato sensitivity to the fungal toxin. Since both ET and JA signals play important roles in tomato sensitivity to AAL toxin, their interaction in the PCD process was investigated further by exogenous application of 1-aminocyclopropane-1-carboxylic acid (ACC) to the leaves of spr2 and jai1, and silver thiosulphate (STS) to 35S::prosys, as well as treatment of the Nr mutant with exogenous JA in the presence of AAL toxin.
Materials and methods
Plant materials and growth conditions
Tomato cultivar CA is the parental line for JA mutants spr2, def1, and jai1 as well as the transgenic line 35S::prosys (Supplementary Fig. S2 available at JXB online). Cultivar Pearson (PSN) is the parental line for the ET-insensitive Nr mutant. 35S::prosys seeds were collected from a 35S::prosys homozygote that had been backcrossed five times to its WT line cv. CA. They were included as controls where appropriate. The ET-overproducing mutant epinastic (epi), which is constitutively activated in a subset of ET responses (Fujino et al., 1988; Barry et al., 2001), and its WT line VFN8 were obtained from the Tomato Genetics Resource Center (University of California, Davis, CA, USA). Seeds were sown in seedling trays filled with a rich soil mixture after germination on filter paper. Seedlings were grown in a greenhouse, with temperatures ranging from 22°C to 28°C (night and day air temperature, respectively) and a 16 h photoperiod. Three weeks after germination, seedlings were transplanted to plastic pots (12 cm in diameter, 15 cm in depth) filled with perlite and turfy soil [3:1 (v/v)], which were watered daily and fertilized weekly with a half-strength Enshi nutrient solution (Yu and Komada, 1999). All experiments were carried out using fully expanded leaflets from nodes 4–6 (except for the terminal leaflets) of 7-week-old tomato plants.
Selection of jai1 homozygotes
jai1 homozygotes were screened according to Li et al. (2004). Briefly, surface-sterilized tomato seeds were germinated on filter paper until the roots were 2 cm in length. The germinated seedlings were treated with 1 mM MeJA (Sigma, St Louis, MO, USA). Approximately 24 h or 36 h later, MeJA-insensitive seedlings were selected by PCR using genomic DNA.
Detached leaflet bioassay
Treatment of tomato leaflets with AAL toxin was performed as described (Moore et al., 1999; Spassieva et al., 2002). Four excised leaflets from individual plants were incubated for 48 h on one filter paper in one Petri dish containing 4 ml of water or 0.2 μM AAL toxin under continuous light at 25°C (Supplementary Fig. S3 at JXB online). AAL toxin was a gift from Liangcheng Du (Department of Chemistry, University of Nebraska, Lincoln, NE, USA.).
Chemical treatment of different plant materials
To test the effect of exogenous JA on AAL toxin-induced PCD in the leaves of CA, spr2, PSN, and Nr, detached tomato leaflets were treated with solutions containing different concentrations of JA (0, 10, 100, or 500 μM) and 0.2 μM AAL toxin. As JA is an acid solution, to avoid being influenced by pH, different concentrations of sodium phosphate buffer (SPB, pH 7.0) were used as controls. JA at 500 μM was dissolved in 50 mM SPB (pH 7.0). Controls were treated with 0, 1, 10, or 50 mM SPB (pH 7.0) alone, respectively. ACC is an ET precursor, to test the effect of exogenous ET on AAL toxin-induced PCD in the leaves of spr2 and jai1, solutions containing 0, 0.1, or 1 mM ACC (Sigma) and 0.2 μM AAL toxin were applied to the detached leaves. The inhibitor of ET action STS (0, 0.1, 1, and 2 mM) was applied to the leaflets of CA and 35S::prosys in the presence of 0.2 μM AAL toxin. STS solutions were prepared by mixing AgNO3 and sodium thiosulphate at a concentration ratio of 1:4. The concentrations referred to are those of the silver component (Bellés et al., 1993). In all the experiments, unless otherwise stated, the detached leaflets were treated with distilled H2O instead of 0.2 μM AAL toxin as controls. To assess the effect of exogenous chemicals on AAL toxin treatment, AAL toxin was dissolved in various chemical solutions.
Real-time quantitative PCR (qPCR) analysis
Tomato leaflets were sampled following AAL toxin treatment at different time points (0, 2, 6, 12, 24, and 36 h) and immediately immersed in liquid nitrogen. Total RNA extraction was carried out using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Genomic DNA was removed using the RNeasy mini kit (Qiagen, Hilden, Germany). RNA integrity was evaluated on a 1.5% (w/v) agarose gel. cDNA was synthesized using 5 μg of RNA with the RevertAid first-strand cDNA synthesis kit (Fermentas, Canada). cDNA was diluted in 100 μl of water and used as template for qPCR. See Table 1 for primer sequences.
Table 1.
Primer sequences used for real-time quantitative PCR (qPCR)
| Gene | GenBank accession no. | Forward primer 5'–3' | Reverse primer 5'–3' | Product (bp) |
| actin | AB199316 | TGGTCGGAATGGGACAGAAG | CTCAGTCAGGAGAACAGGGT | 190 |
| LoxD | U37840 | GGCTTGCTTTACTCCTGGTC | AAATCAAAGCGCCAGTTCTT | 72 |
| PI-II | K03291 | TGATGAACCCAAGGCAAATA | ACACAACTTGATGCCCACAT | 154 |
| ACO1 | X58273 | TTGCTCATTTCCTTTGTGGA | GGAAGCTAGCAAAGCAAACC | 122 |
| ACS2 | X59139 | ATCCACCTTGTTTGTGACGA | TGTTCATCGAGGATTTCAGC | 86 |
| ETR4 | AF118843 | CTGCAGATTGGAATGAATGG | ATAAGGCACCGTCAACATCA | 123 |
| NR | U38666 | GCGGTTATGGTTCTGGTTCT | TGTCGAGCTACATCCAAAGC | 194 |
| ERF1 | AY044236 | ATTGGAGTTAGAAAGAGGCCAT | CTCATTGATAATGCGGCTTG | 143 |
qPCR was performed in a total volume of 25 μl, using 1 μl of diluted cDNA, 200 μM for each primer, and 12.5 μl of 2× SYBR Green PCR Master Mix (Takara, Japan) on an iCycler (Bio-Rad Inc., CA, USA). The qPCR program included a preliminary step of 30 s at 95°C, followed by 40 cycles of 95°C for 10 s and 58°C for 1 min. Tomato actin (GenBank accession number: AB199316) was used as an internal control (Table 1). Relative gene expression was calculated according to a 2−ΔΔCT method, in which ΔΔCT=(CT, Target–CT, actin)Timex–(CT, Target–CT, actin)Time0 (Livak et al., 2001). Timex is any time point and time0 represents the CT of non-treated control tissues. Three PCR replicates were conducted and the fold change in each target gene of time 0 was set to 1.
Callose assay and measurements of cell death
Leaves were cleared of pigment by vacuum-infiltrating alcoholic lactophenol followed by a 30 min incubation at 65°C. The leaves were then transferred to fresh alcoholic lactophenol solution and incubated overnight at room temperature. Cleared leaves were rinsed briefly in 50% ethanol, then in water, and stained with 0.01% aniline blue. Leaves were examined with a Zeiss Axiophot D-7082 fluorescence microscope with an excitation filter of 365±25 nm, a 400 nm dichroic mirror, and a 450 nm longpass emission filter (Underwood et al., 2007). Cell death was evaluated using an electrolyte leakage assay. After sampling, tomato leaflets were immersed in ultrapure water for 30 min and the conductivity of the solution was measured with an LF-91 conductivity meter. Then the samples were briefly autoclaved in the same solution and the conductivity measured again (total conductivity). The increase in electrolyte leakage (relative conductivity) is expressed as a percentage of the total (Gechev et al., 2004).
Determination of O2·− production rate
The O2·− production rate was measured by analysing the nitrite formation from hydroxylamine in the presence of O2·−. Frozen leaf segment was homogenized with 3 ml of 65 mM SPB (pH 7.8) and centrifuged at 5000 g for 10 min. The incubation mixture contained 0.9 ml of 65 mM SPB (pH 7.8), 0.1 ml of 10 mM hydroxylamine hydrochloride, and 1 ml of the supernatant. After incubation at 25°C for 20 min, 17 mM sulphanilamide and 7 mM α-naphthylamine were added to the incubation mixture. Ethyl ether in the same volume was added and centrifuged at 1500 g for 5 min. The absorbance in the aqueous solution was read at 530 nm (Zhou et al., 2009).
Experimental design and statistical analysis
Data were analysed using Statistica (SAS Institute Inc., http://www.statsoft.com). Differences in relative conductivity (except for Fig. 2D) and the O2·− production rate in each figure were analysed by one-way analysis of variance (ANOVA); if the ANOVA analysis was significant (P <0.05), Duncan's multiple range test was used to detect significant differences between groups. In Fig. 2D the differences in relative conductivity between VFN8 and epi were analysed by Student's t-tests. Differences in gene expression in the mutant (jai1 or Nr) treated with AAL toxin alone among different time points in each figure were analysed by one-way ANOVA; if the ANOVA analysis was significant (P <0.05), Duncan's multiple range test was used to detect significant differences between groups. Differences in gene expression of AAL toxin treatment alone between the mutant (jai1 or Nr) and its parental line, as well as in gene expression of the mutant (jai1 or Nr) between AAL toxin treatment alone and co-treatment with AAL toxin and exogenous hormone (ACC or JA) at each time point were analysed using Student's t-tests.
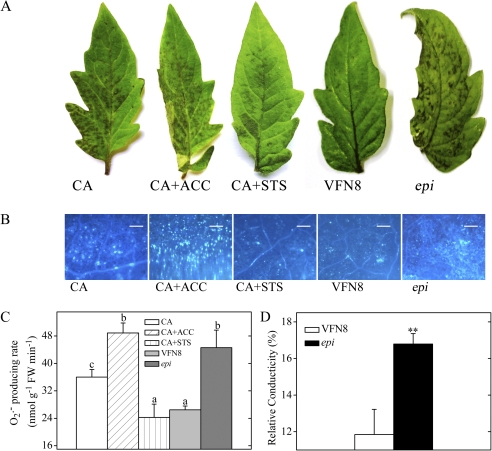
Fig. 2.
Effects of ET levels on the sensitivity of detached tomato leaflets to AAL toxin. Detached leaflets were incubated under continuous light at 25°C for 48 h. (A) Symptoms of tomato leaflets after 0.2 μM AAL toxin application or co-treatment with the toxin and 0.1 mM ACC, an ET precursor, or 1 mM STS, an ET action inhibitor. (B) Callose deposition in tomato leaves in response to AAL toxin or co-treatment with the toxin and 0.1 mM ACC or 1 mM STS. Leaflets were stained with aniline blue to detect callose. Representative microscope images are shown. Callose deposits appear as bright spots on a dark background. Scale bars represent 100 μm. (C) Quantitative measurements of the O2·− level in tomato leaflets after AAL toxin application or co-treatment with the toxin and 0.1 mM ACC or 1 mM STS. Error bars indicate standard deviation. Letters indicate significant differences among treatments (P <0.05, Duncan's multiple range test). (D) Quantitative measurements of relative conductivity in leaflets of VFN8 and epi after AAL toxin application. Error bars indicate standard deviation. Asterisks indicate significant differences in epi leaves compared with VFN8 (**P <0.01; Student's t-test). (This figure is available in colour at JXB online.)
Results and Discussion
AAL toxin-induced PCD in tomato leaves is associated with endogenous JA levels and enhanced by exogenous JA application
The CA tomato cultivar was reported to be sensitive to the host-specific mycotoxin AAL toxin (Egusa et al., 2009). In this study, the leaves of CA exhibited visible necrotic lesions at 36 h after treatment with 0.2 μM AAL toxin (data not shown), and typical necrotic lesions appeared at 48 h (Fig. 1).
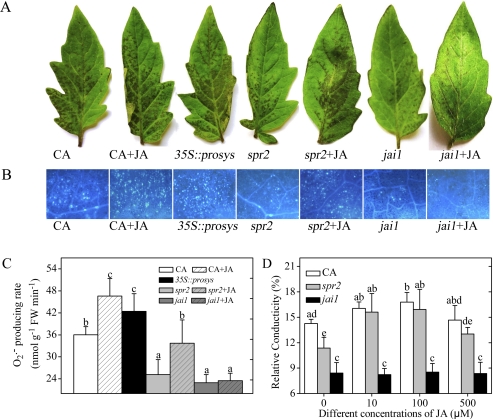
Fig. 1.
Effects of JA levels on the sensitivity of detached tomato leaflets to AAL toxin. Detached leaflets were incubated under continuous light at 25°C for 48 h. (A) Symptoms of CA, 35S::prosys, spr2, and jai1 leaflets after 0.2 μM AAL toxin application or co-treatment with AAL toxin and 100 μM JA. (B) Callose deposition in tomato leaves in response to AAL toxin with or without 100 μM JA. Leaflets were stained with aniline blue to detect callose. Representative microscope images are shown. Callose deposits appear as bright spots on a dark background. Scale bars represent 100 μm. (C) Quantitative measurements of the O2·− level in tomato leaflets after AAL toxin application or co-treatment with the toxin and 100 μM JA. (D) Quantitative measurements of relative conductivity in tomato leaflets after AAL toxin application or co-treatment with the toxin and different concentrations of JA. Error bars indicate standard deviation. For each figure, letters indicate significant differences among treatments (P <0.05, Duncan's multiple range test). (This figure is available in colour at JXB online.)
JA has important roles in resistance against and susceptibility to pathogen attack. For example, enhanced resistance against the necrotrophic pathogen B. cinerea in the JA-insensitive Arabidopsis mutant jin1 showed that JA is essential for susceptibility of Arabidopsis plants to this pathogen (Lorenzo et al., 2004). JA has a promotional effect on susceptibility of tomato to the fungal pathogen AAL (Egusa et al., 2009). To investigate whether endogenous JA levels are involved in tomato defence against AAL toxin, a tomato JA-deficient mutant spr2 and a 35S::prosys transgenic line were treated with AAL toxin. Compared with CA, development of necrotic lesions on spr2 leaves was markedly decreased, while there was extensive cell death on 35S::prosys leaves (Fig. 1A). Callose deposition is a typical early defence response. Superoxide anion (O2·−) is one of the key reactive oxygen species (ROS). Relative conductivity is an indicator of plasma membrane damage. Callose deposition, O2·− production rate, and relative conductivity from leaflets were detected to measure further the extent of cell death. The leaflets of spr2 exhibited a significant reduction in callose deposition compared with CA (Fig. 1B). The O2·− producition rate and the relative conductivity in spr2 were also obviously lower than that in cv. CA (Fig. 1C, D). However, the callose deposition, O2·− levels, and relative conductivity in 35S::prosys were markedly higher (Figs 1B, C, 4E). The results are consistent with the above cell death phenotypes.
The results showed that SPB (pH 7.0) application alone did not alter the relative conductivity in tomato leaves compared with H2O treatment alone, and there were no significant differences between the toxin treatment alone and co-treatment with the toxin and buffer (data not shown). Besides, the relative conductivity in tomato leaves did not change significantly after treatment with different concentrations of STS, JA, or ACC compared with H2O treatment alone (Supplementary Fig. S4 at JXB online). Thus, SPB, STS, JA, or ACC solutions were not toxic to detached tomato leaves. When CA and spr2 leaves were treated with AAL toxin in the presence of different concentrations of JA, the result showed that 100 μM JA application caused a significant propagation of necrotic cell death in CA and spr2 leaves (Fig. 1A), which was also reflected by the increased callose accumulation and O2·− levels (Fig. 1B, C). Accordingly, the relative conductivity of CA and spr2 leaves increased to different extents after 10 μM and 100 μM JA application (Fig. 1D). The above results suggested that an impaired JA biosynthesis pathway attenuated the extent of tissue damage. However, higher endogenous JA levels in 35S::prosys and exogenous JA application to CA increased the sensitivity to AAL toxin as compared with the isogenic WT parent CA (Fig. 1D, 0 mM JA bar; Fig. 4E, 0 mM STS bar) during this response.
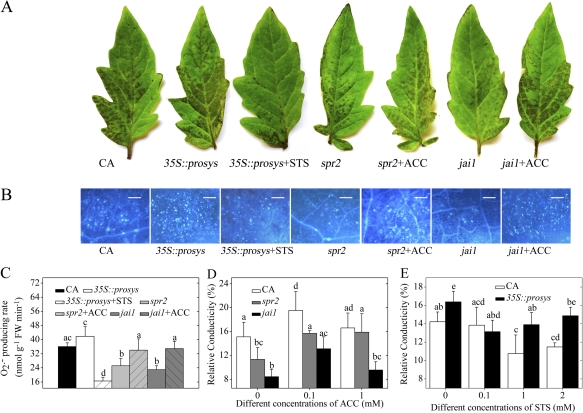
Fig. 4.
Effects of exogenous ET levels on the sensitivity of detached leaflets of JA mutants to AAL toxin. (A) Symptoms of CA, 35S::prosys, spr2, and jai1 leaflets after 0.2 μM AAL toxin application or co-treatment with AAL toxin and 0.1 mM ACC or 1 mM STS. (B) Callose deposition in detached leaves of JA mutants after 0.2 μM AAL toxin application or co-treatment with AAL toxin and 0.1 mM ACC or 1 mM STS. Leaflets were stained with aniline blue to detect callose. Representative microscope images are shown. Callose deposits appear as bright spots on a dark background. Scale bars represent 100 μm. (C) Quantitative measurements of the O2·− level in detached leaflets of JA mutants after 0.2 μM AAL toxin application or co-treatment with AAL toxin and 0.1 mM ACC or 1 mM STS. (D) Quantitative measurements of relative conductivity in leaflets of CA, spr2, and jai1 after AAL toxin application or co-treatment with the toxin and different concentrations of ACC. (D) Quantitative measurements of relative conductivity in leaflets of CA, spr2, and jai1 after AAL toxin application or co-treatment with the toxin and different concentrations of ACC. (E) Quantitative measurements of relative conductivity in leaflets of CA and 35S::prosys after AAL toxin application or co-treatment with the toxin and different concentrations of STS. Error bars indicate standard deviation. For each figure, letters indicate significant differences among treatments (P <0.05, Duncan's multiple range test). (This figure is available in colour at JXB online.)
The results are contradictory to the results reported by Egusa et al. (2009), who showed that reduced endogenous JA levels in def1 and 100 μM MeJA treatment of CA and def1 did not affect the sensitivity of the leaf discs to AAL toxin. This contradiction may be due to the use of intact leaflets in the present study versus the use of leaf discs. The experiments of Egusa et al. (2009) were repeated here and the same results as they reported were obtained (Supplementary Fig. S1 at JXB online). It is possible that compared with intact leaflets, the smaller leaf discs produced after severe mechanical wounding have a high background of diverse effects of JAs. In addition, JAs have several bioactive forms in plants. Compared with WT cv. CA, there may be free JA levels in def1 as well as other conjugated forms of JA or analogues of JAs, which promote the AAL toxin-triggered cell death. Furthermore, after wounding for 1 h, the JA levels in def1 and spr2 were 20% and 6% of those of WT plants, respectively (C Li et al., unpublished data). The product encoded by the DEF1 gene is unknown (Howe et al., 1996); however, the SPR2 gene is known to encode a fatty acid desaturase (Li et al., 2003). Therefore, it was decided to use the spr2 mutant instead of def1 to study the effect of endogenous JAs in the AAL toxin-induced PCD process.
Impaired perception of JA via the JAI1 receptor reduced the AAL toxin-induced cell death
The insensitivity of coi1 mutants of Arabidopsis and tomato to COR, which is a phytotoxin produced by some strains of Pseudomonas syringae and exerts its virulence effects by activating the host's JA pathway, demonstrated that COI1 is required for the action of the toxin (Feys et al., 1994; Zhao et al., 2003). To elucidate further the role of JA signalling in the AAL toxin-elicited PCD process, the detached leaflets of the JA-insensitive mutant jai1 (Li et al., 2004) were treated with the toxin. Similarly to spr2, the toxin-treated jai1 leaves displayed minor necrotic lesions at 48 h compared with CA (Fig. 1A), which was consistent with callose deposition, O2·− levels, and the relative conductivity in jai1 and CA leaflets (Fig. 1B, C, D 0 mM bars). Exogenous JA application did not significantly alter the AAL toxin-induced cell death, callose deposition, O2·− levels, and relative conductivity in jai1 leaves (Fig. 1A–D), which was different from the situation in spr2, suggesting that the perception of JA requires the JAI1 receptor in this PCD process. The above results indicate that tomato JAI1 has a crucial role in mediating the complex response of tomato leaves to AAL toxin. Based on these results, it can be concluded that a JA signalling pathway positively regulates the sensitivity of tomato leaflets to AAL toxin.
Exposure to O3 leads to JA biosynthesis in Arabidopsis plants, and pre-treatment of O3-sensitive Arabidopsis plants with exogenous MeJA alleviates O3-induced PCD (Rao et al., 2000; Rao and Davis, 2001). Asai et al. (2000) reported that FB1-induced PCD is alleviated in the protoplasts of the Arabidopsis JA-insensitive mutant jar1, suggesting that the role of JA may be unique to certain stimuli.
ET biosynthesis and signalling through NR in tomato is responsible for sensitivity to AAL toxin-induced cell death
The essential role of ET in plant PCD is well known. For instance, O3 induces ET biosynthetic gene expression, and treatment with the ET inhibitor aminoethoxyvinylglycine (AVG) decreases transcript abundances of these genes in Arabidopsis (Overmyer et al., 2000; Tuominen et al., 2004). FB1-induced PCD is depressed in the Arabidopsis ET perceptional mutant ein2 (Asai et al., 2000). Transcription profiles of AAL toxin-induced cell death in the Arabidopsis loh2 mutant plant suggested that ET-responsive genes are among the earliest to be up-regulated and AVG application reduced AAL toxin-induced PCD in loh2 plants (Gechev et al., 2004). Previous studies suggested that treatment of susceptible tomato leaflets with AAL toxin increases ACC and ET levels (Moussatos et al., 1994). Inhibitors of ET biosynthesis or action markedly reduce the AAL toxin-induced lesion size and number, while the addition of exogenous ACC results in increased tissue necrosis and ET evolution (Moussatos et al., 1994; Moore et al., 1999). Consistent with these reports, it was found that the tomato ET-overproducing mutant epi evidently promoted AAL toxin-elicited PCD compared with the WT VFN8 (Fig. 2A–D). ACC application markedly promoted AAL toxin-induced cell death, while application of the inhibitor of ET action STS markedly inhibits AAL toxin-induced cell death in CA leaves (Fig. 2A–C).
Plants under abiotic and biotic stresses produce increased levels of ET, which is perceived by ET receptors and triggers cellular responses further downstream (Kim et al., 2003). Six tomato ET receptors, designated LeETR1, LeETR2, LeETR3 (NR), LeETR4, LeETR5, and LeETR6, have been isolated and characterized (Klee, 2002, 2004). Each receptor gene has a distinct pattern of expression throughout development and in response to external stimuli. NR and LeETR4, but not the other genes, are induced by pathogen infection (Klee, 2002). Compared with WT PSN, the ET-insensitive mutant Nr, which carries a semi-dominant mutation in NR, showed markedly less necrosis and chlorosis in response to AAL toxin, suggesting that NR may be important in this PCD process (Moore et al., 1999).
The ET pathway acts downstream of JA during the AAL toxin-induced PCD process
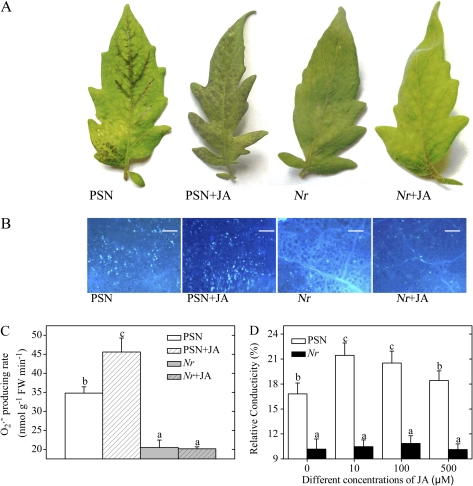
Since JA and ET responses are both important in cell death, it was of interest to test whether JA and ET act independently or cooperatively by addition of hormones or hormonal inhibitors to the leaves of spr2, jai1, and Nr mutants or 35S::prosys, as well as their isogenic parents. The result showed that the degree of necrotic cell death and callose deposition, O2·− levels, and the relative conductivity in Nr leaves co-treated with JA and AAL toxin were similar to those of leaves treated with toxin alone (Fig. 3A–D). However, exogenous JA application markedly increased the degree of cell death and callose deposition, O2·− levels, and the relative conductivity in the leaves of PSN (Fig. 3A–D), consistent with those of the cv. CA.
Fig. 3.
Effects of exogenous JA treatment on the sensitivity of detached PSN and Nr leaflets to AAL toxin. Detached leaflets were incubated under continuous light at 25°C for 48 h. (A) Symptoms of PSN and Nr leaflets after 0.2 μM AAL toxin application with or without 100 μM JA. (B) Callose deposition in PSN and Nr leaves in response to AAL toxin with or without 100 μM JA. Leaflets were stained with aniline blue to detect callose. Representative microscope images are shown. Callose deposits appear as bright spots on a dark background. Scale bars represent 100 μm. (C) Quantitative measurements of the O2·− level in PSN and Nr leaflets after AAL toxin application with or without 100 μM JA. (D) Quantitative measurements of relative conductivity in leaflets of PSN and Nr after AAL toxin application or co-treatment with the toxin and different concentrations of JA. Error bars indicate standard deviation. For each figure, letters indicate significant differences among treatments (P <0.05, Duncan's multiple range test). (This figure is available in colour at JXB online.)
Subsequently, 35S::prosys leaves were co-treated with STS and AAL toxin. As shown in Fig. 4A, blocking ET perception with 1 mM STS in the presence of AAL toxin markedly reduced the amount of tissue damage in 35S::prosys relative to the toxin treatment alone. In addition, exogenous 0.1 mM ACC application rescued AAL toxin-elicited development of disease symptoms in spr2 and jai1 leaflets (Fig. 4A). The callose deposition and O2·− production rate measured were consistent with the visible damage on spr2, jai1, and 35S::prosys leaflets (Fig. 4B, C). Application of 0.1 mM ACC significantly increased the relative conductivity in CA and jai1 leaves, and 0.1 mM and 1 mM ACC significantly increased the relative conductivity in spr2 leaves (Fig. 4D). Application of 1 mM and 2 mM STS markedly decreased the relative conductivity in CA leaves, and 0.1 mM and 1 mM STS markedly decreased the relative conductivity in 35S::prosys leaves (Fig. 4E). In addition, Fig. 4E shows that 35S::prosys leaflets co-treated with AAL toxin and different concentrations of STS still have a significant high relative conductivity, suggesting an ET-independent pathway for AAL toxin-induced PCD. Therefore, both JA and ET pathways independently promote AAL toxin-induced PCD. Furthermore, the cell death-promoting function of ET seems to be downstream of JA signalling during the AAL toxin-induced PCD process.
AAL toxin-induced JA-related gene expression decreased in jai1 leaves and was not restored by exogenous ACC application
To investigate the underlying molecular mechanisms in this cell death process, the temporal expression patterns of some of the genes involved in the JA- and ET-related responses were examined by qPCR in jai1 and Nr mutants, as well as their isogenic WT parents after treatment with AAL toxin with or without exogenous hormones. After Arabidopsis loh2 mutant leaves were infiltrated with AAL toxin, expression of JA marker genes was reduced (Gechev et al., 2004). Here, expression of the LoxD gene encoding lipoxygenase (Li et al., 2004) and the proteinase inhibitor II (PI-II) gene, a marker of wounding or JA signalling (Ryan, 2000), was analysed by qPCR. As shown in Fig. 5A, the LoxD mRNA content in CA leaflets increased and reached a maximum (73-fold) at 2 h following toxin application, after which the transcript abundance decreased slightly, although it remained significantly higher than at 0 h. The expression of PI-II in CA leaflets was elevated slightly at 2 h after toxin treatment. Subsequently, it increased greatly at 6, 12, and 24 h; thereafter the expression declined slightly at 36 h (Fig. 5B). PI-II mRNA in the water-treated controls was also increased starting from 6 h, but to a much lesser extent than in the toxin-treated leaves (Fig. 5B). This result suggested that the accumulation of PI-II mRNA in the controls is more likely in response to another factor, possibly wounding. PI-II mRNA levels in the toxin-treated leaves were noticeably higher than those in the related water control at all time points (Fig. 5B).
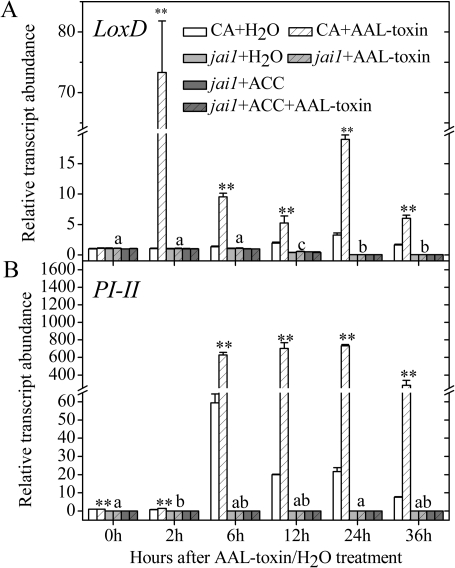
Fig. 5.
Transcription patterns of the JA-biosynthetic gene LoxD (A) and the JA-regulated gene PI-II (B) in the leaflets of CA and jai1 after AAL toxin application or co-treatment with AAL toxin and ACC. Error bars indicate standard deviation. Letters indicate significant differences in gene expression of jai1 treated with AAL toxin alone among different time points (P <0.05, Duncan's multiple range test); asterisks indicate significant differences in gene expression of AAL toxin treatment alone between CA and jai1 as well as in gene expression of jai1 between treatment with AAL toxin alone and co-treatment with AAL toxin and ACC at each time point (*P <0.05; **P <0.01; Student's t-test).
The transcript levels of these two JA-related genes were greatly depressed in jai1 leaves compared with CA leaves. In addition, when jai1 leaves were co-treated with AAL toxin and exogenous ACC, no significant changes were observed in the transcript abundance of LoxD and PI-II compared with those treated with AAL toxin alone (Fig. 5). The above results suggested that the alleviated PCD symptoms in jai1 leaves are associated with decreased JA signalling compared with its WT line cv. CA. Furthermore, the degree of restored PCD by exogenous ACC application in jai1 leaves is independent of the JA pathway.
Decreased sensitivity to AAL toxin in jai1 leaves was associated with decreased expression of ET-modulated genes
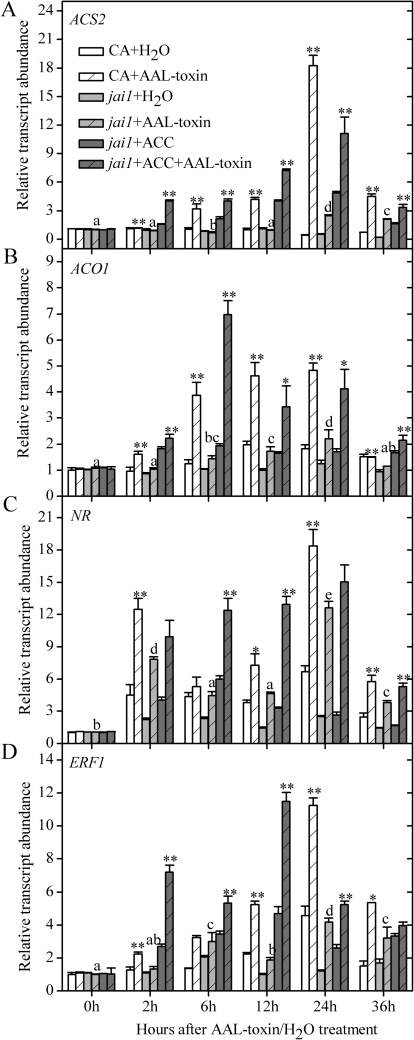
ACC synthase (ACS) catalyses the first committed step of ET biosynthesis and is generally considered as rate limiting for ET production (Rottmann et al., 1991), and ACC oxidase (ACO) catalyses the final step of ET biosynthesis (Yang and Hoffman, 1984). Accelerated ET biosynthesis in plants is frequently associated with the induction of ACS and ACO gene activation (Kim et al., 2003). Moore et al. (1999) reported that treatment of susceptible tomato leaflets with AAL toxin increased LeACS2 and LeACO mRNA levels. ERF1 is an ET-inducible gene encoding an ERF transcription factor in tomato (Huang et al., 2004). The expression of ERF1 is induced synergistically by JA or ET in Arabidopsis (Lorenzo et al., 2003). Berrocal-Lobo et al. (2004) reported that Fusarium oxysporum-induced ERF1 expression depends on both functionally intact ET and JA pathways. To explore whether the decreased sensitivity to AAL toxin in jai1 leaves is associated with the ET response, the transcript levels of ET biosynthetic genes (ACS2 and ACO1) as well as ET-responsive genes (NR and ERF1) were tested in jai1 and CA leaves following AAL toxin application. In general, AAL toxin greatly induced the expression of the four ET-related genes in cv. CA leaves. The expression of ACS2, ACO1, and ERF1 in CA increased gradually after AAL toxin application, peaked at 24 h, and then declined at 36 h (Fig. 6A, B, D). Similarly, AAL toxin greatly induced the expression of NR in CA and there were two peaks: the first at 2 h and the second at 24 h (Fig. 6C). After AAL toxin treatment, the expression of all the ET-related genes tested noticeably declined in jai1 leaves relative to cv. CA (Fig. 6). Furthermore, compared with AAL toxin treatment alone, co-treatment of jai1 leaves with the toxin and exogenous ACC drastically restored the expression of ACS2, ACO1, NR, and ERF1 genes. Taken together, the decreased disease symptoms in jai1 leaves were associated with the depression of the ET pathway, and exogenous ACC restored AAL toxin-induced lesion development through the enhanced ET pathway, suggesting that the action of the JAI1-dependent JA pathway must precede ET signalling.
Fig. 6.
Transcription patterns of ET-biosynthetic genes (ACS2 and ACO1) and ET-regulated genes (NR and ERF1) in the leaflets of CA and jai1 after AAL toxin application or co-treatment with AAL toxin and ACC. Error bars indicate standard deviation. Letters indicate significant differences in gene expression of jai1 treated with AAL toxin alone among different time points (P <0.05, Duncan's multiple range test); asterisks indicate significant differences in gene expression of AAL toxin treatment alone between CA and jai1 as well as in gene expression of jai1 between treatment with AAL toxin alone and co-treatment with AAL toxin and ACC at each time point (*P <0.05; **P <0.01; Student's t-test).
ET-related gene expression was decreased in Nr leaves and exogenous JA restored the expression of ET-biosynthetic genes but not the ET-responsive genes
AAL toxin induced the expression of all the ET-related genes tested, including ACS2, ACO1, ETR4, and ERF1, in cv. PSN leaves, although the trends of induction were different (Fig. 7). The expression of the four genes in PSN leaves started to increase significantly at 6 h after toxin treatment. The ACS2 transcript peaked at 24 h and the transcript abundance of ACO1 and ERF1 reached a maximum at 12 h, while the ETR4 transcript exhibited a peak at 6 h after toxin treatment and decreased gradually afterwards (Fig. 7).
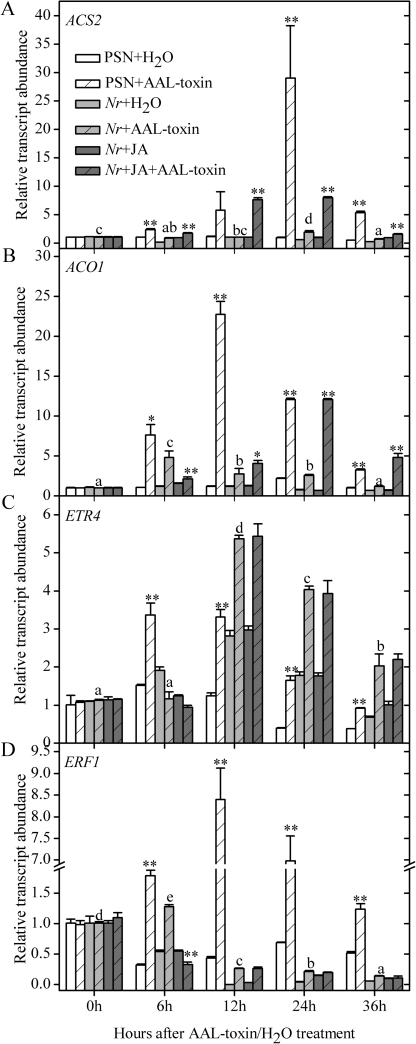
Fig. 7.
Transcription patterns of ET-biosynthetic genes (ACS2 and ACO1) and ET-regulated genes (ETR4 and ERF1) in the leaflets of PSN and Nr after AAL toxin application or co-treatment with AAL toxin and JA. Error bars indicate standard deviation. Letters indicate significant differences in gene expression of Nr treated with AAL toxin alone among different time points (P <0.05, Duncan's multiple range test); asterisks indicate significant differences in gene expression of AAL toxin treatment alone between PSN and Nr as well as in gene expression of Nr between treatment with AAL toxin alone and co-treatment with AAL toxin and JA at each time point (*P <0.05; **P <0.01; Student's t-test).
Lanahan et al. (1994) reported similar rates of ET production by Nr and cv. PSN following infiltration with ACC or the bacterial pathogen P. syringae, suggesting that Nr is not impaired in any step of ET biosynthesis. ACC treatment shortened the hypocotyls of dark-grown Nr mutant seedlings, suggesting that the Nr mutant retains a residual ET response (Barry et al., 2001). Here, the AAL toxin-induced expression levels of ACS2, ACO1, and ERF1 genes in Nr were all markedly lower than those in cv. PSN leaves (Fig. 7A, B, D). However, ETR4 transcript levels in Nr were significantly higher than in PSN leaves at 12, 24, and 36 h (Fig. 7C). This is consistent with existing findings that increased expression of the remaining receptor isoforms in receptor-deficient lines tend to compensate partially for the missing receptors at the level of mRNA expression (Tieman et al., 2000). Furthermore, the reduced relative conductivity triggered by AAL toxin in Nr (Fig. 3D) implied that the altered composition of receptor isoforms (NR and LeETR4) did not completely compensate the output of ET signalling, which was consistent with the findings reported by Tieman et al. (2000).
Compared with toxin treatment alone, co-treatment of Nr leaves with AAL toxin and exogenous JA restored the ACS2 and ACO1 mRNA contents (Fig. 7A, B). However, the toxin-induced transcript abundance of ET-responsive genes ETR4 and ERF1 in Nr leaves was not significantly influenced by exogenous JA application (Fig. 7C, D). Therefore, it is concluded that the JA pathway must act upstream of ET biosynthesis in the AAL toxin-induced PCD process.
Conclusions
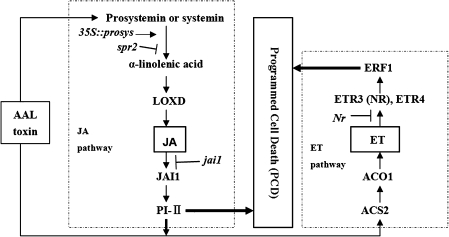
In this study, a pathogen-free model system was employed to investigate the function of JA and ET signalling as well as the interactions between the two hormones in regulating the AAL toxin-induced PCD process. Both JA and ET promote AAL toxin-induced cell death alone in detached tomato leaves. In addition, JAI1 receptor-dependent JA signalling promotes PCD through ET pathway action, at some point upstream of ET biosynthesis (Fig. 8). The expression of some JA- and ET-related genes was detected, but the possibility that other genes may also participate in this process could not be excluded. Interaction and balance between the hormone pathways may serve to fine-tune disease propagation or containment processes, resulting in different lesion sizes and formation dynamics.
Fig. 8.
A proposed model of synergistic interactions between ET and JA signalling pathways in the regulation of AAL toxin-induced PCD in detached tomato leaves. Both JA and ET pathways promote AAL toxin-induced PCD alone. Furthermore, JA signalling mediated through JAII seems to act upstream of ET biosynthesis in this PCD process. Simplified JA and ET pathways are included in two rectangles composed of dashed lines and dots, respectively. Mutant lines in which the components of ET or JA signalling alter were used in this study. Solid arrows represent positive interactions, whereas T-bars indicate inhibition.
The findings have extended our understanding of JA and ET interaction during the AAL toxin-induced PCD process, which differs from what is known in Arabidopsis. JA signalling promotes AAL toxin-induced tomato cell death, while JA marker genes are either not induced or down-regulated in Arabidopsis leaves after AAL toxin application (Gechev et al., 2004), which is consistent with the existing reports that the hormones led to increased or decreased disease severity depending on the plant–pathogen combination. The results are helpful to future effort in controlling plant cell death through the manipulation of disease-related signalling molecules.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Effects of MeJA on the sensitivity of tomato leaf discs to AAL toxin.
Figure S2. The plants of spr2, def1, 35S::prosys, and jai1, as well as their parental line cultivar Castlemart (CA) used in this study.
Figure S3. Treatment method of detached tomato leaflets.
Figure S4. The relative conductivity in leaflets of cv. CA, cv. PSN, spr2, jai1, 35S::prosys, and Nr was measured after exogenous JA, STS, or ACC treatment alone for 48 h.
Acknowledgments
We are grateful to the Tomato Genetics Resource Center (University of California, Davis, CA, USA) for providing epi and VFN8 tomato seeds, and to Dr Liangchen Du (University of Nebraska, Lincoln) for providing AAL toxin. We also thank Dr Sixue Chen (University of Florida, Gainesville) and Dr Zhixiang Chen (Purdue University, West Lafayette, Indiana) for critical reading of the manuscript. This work was supported by the National Basic Research Program of China (2009CB119000) and the National Science Foundation of China (NO. 30970244).
Glossary
Abbreviations
- AAL
Alternaria alternata f. sp. Lycopersici
- ASC
Alternaria Stem Canker
- ACC
1-aminocyclopropane-1-carboxylic acid
- CA
Castlemart
- ET
ethylene
- epi
epinastic
- JA
jasmonic acid
- JAI1
JASMONIC ACID INSENSITIVE1
- jai1
jasmonic acid insensitive1
- MeJA
methyl jasmonate
- Nr
Never ripe
- PCD
programmed cell death
- qPCR
real-time quantitative PCR
- 35S::prosys
35S::prosystemin
- STS
silver thiosulphate
- O2·−
superoxide anion
- spr2
suppressor of (pro)systemin-mediated responses2
References
- Abbas HK, Tanaka T, Duke SO. Pathogenicity of Alternaria alternata and Fusarium moniliforme and phytotoxicity of AAL-toxin and Fumonisin B1 on tomato cultivars. Journal of Phytopathology. 2008;143:329–334. [Google Scholar]
- Abbas HK, Tanaka T, Duke SO, Boyette CD. Susceptibility of various crop and weed species to AAL-toxin, a natural herbicide. Weed Technology. 1995;9:125–130. [Google Scholar]
- Abbas HK, Tanaka T, Duke SO, Porter JK, Wray EM, Hodges L, Sessions AE, Wang EAHM, Jr, Riley RT. Fumonisin- and AAL-toxin-induced disruption of sphingolipid metabolism with accumulation of free sphingoid bases. Plant Physiology. 1994;106:1085–1093. doi: 10.1104/pp.106.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazana K. Antagonistic interaction between abscisic acid and jasmonate–ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. The Plant Cell. 2004;16:3460–3479. doi: 10.1105/tpc.104.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T, Stone JM, Heard JE, Kovtun YP, Sheen J, Ausubel FM. Fumonisin B1-induced cell death in Arabidopsis protoplasts requires jasmonate-, ethylene-, and salicylate-dependent signaling pathways. The Plant Cell. 2000;12:1823–1836. doi: 10.1105/tpc.12.10.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry CS, Fox EA, Yen H, Lee S, Ying T, Grierson D, Giovannoni JJ. Analysis of the ethylene response in the epinastic mutant of tomato. Plant Physiology. 2001;127:58–66. doi: 10.1104/pp.127.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellés JM, Pérez-Amador MA, Carbonell J, Conejero V. Correlation between ornithine decarboxylase and putrescine in tomato plants infected by citrus exocortis viroid or treated with ethephon. Plant physiology. 1993;102:933–937. doi: 10.1104/pp.102.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrocal-Lobo M, Molina A. Ethylene response factor 1 mediates Arabidopsis resistance to the soilborne fungus Fusarium oxysporum. Molecular Plant-Microbe Interactions. 2004;17:763–770. doi: 10.1094/MPMI.2004.17.7.763. [DOI] [PubMed] [Google Scholar]
- Brandwagt BF, Kneppers TJA, Nijkamp HJJ, Hille J. Overexpression of the tomato Asc-1 gene mediates high insensitivity to AAL toxins and Fumonisin B1 in tomato hairy roots and confers resistance to Alternaria alternata f. sp. lycopersici in Nicotiana umbratica plants. Molecular Plant-Microbe Interactions. 2002;15:35–42. doi: 10.1094/MPMI.2002.15.1.35. [DOI] [PubMed] [Google Scholar]
- Brandwagt BF, Mesbah LA, Takken FLW, Laurent PL, Kneppers TJA, Hille J, Nijkamp HJJ. A longevity assurance gene homolog of tomato mediates resistance to Alternaria alternata f. sp. lycopersici toxins and Fumonisin B1. Proceedings of the National Academy of Sciences, USA. 2000;97:4961–4966. doi: 10.1073/pnas.97.9.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos ML, Almeida M, Rossi ML, Martinelli AP, Junior CGL, Figueira A, Rampelotti-Ferreira FT, Vendramim JD, Benedito VA, Peres LEP. Brassinosteroids interact negatively with jasmonates in the formation of anti-herbivory traits in tomato. Journal of Experimental Botany. 2009;60:4347–436. doi: 10.1093/jxb/erp270. [DOI] [PubMed] [Google Scholar]
- Castagna A, Ederli L, Pasqualini S, Mensuali-Sodi A, Baldan B, Donnini S, Ranieri A. The tomato ethylene receptor LE-ETR3 (NR) is not involved in mediating ozone sensitivity: causal relationships among ethylene emission, oxidative burst and tissue damage. New Phytologist. 2007;174:342–356. doi: 10.1111/j.1469-8137.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Devadas SK, Enyedi A, Raina R. The Arabidopsis hrl1 mutation reveals novel overlapping roles for salicylic acid, jasmonic acid and ethylene signalling in cell death and defence against pathogens. The Plant Journal. 2002;30:467–480. doi: 10.1046/j.1365-313x.2002.01300.x. [DOI] [PubMed] [Google Scholar]
- Egusa M, Ozawa R, Takabayashi J, Otani H, Kodama M. The jasmonate signaling pathway in tomato regulates susceptibility to a toxin-dependent necrotrophic pathogen. Planta. 2009;229:965–976. doi: 10.1007/s00425-009-0890-x. [DOI] [PubMed] [Google Scholar]
- Feys B, Benedetti CE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. The Plant Cell. 1994;6:751–759. doi: 10.1105/tpc.6.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino DW, Burger DW, Yang SF, Bradford KJ. Characterization of an ethylene overproducing mutant of tomato (Lycopersicon esculentum Mill. Cultivar VFN8) Plant Physiology. 1988;88:774–779. doi: 10.1104/pp.88.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gechev TS, Gadjev IZ, Hille J. An extensive microarray analysis of AAL-toxin-induced cell death in Arabidopsis thaliana brings new insights into the complexity of programmed cell death in plants. Cellular and Molecular Life Sciences. 2004;61:1185–1197. doi: 10.1007/s00018-004-4067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA. An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. The Plant Cell. 1996;8:2067–2077. doi: 10.1105/tpc.8.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Ryan CA. Suppressors of systemin signaling identify genes in the tomato wound response pathway. Genetics. 1999;153:1411–1421. doi: 10.1093/genetics/153.3.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Zhang Z, Zhang X, Zhang H, Huang D, Huang R. Tomato TERF1 modulates ethylene response and enhances osmotic stress tolerance by activating expression of downstream genes. FEBS Letters. 2004;573:110–116. doi: 10.1016/j.febslet.2004.07.064. [DOI] [PubMed] [Google Scholar]
- Kim CY, Liu Y, Thorne ET, Yang H, Fukushige H, Gassmann W, Hildebrand D, Sharp RE, Zhang S. Activation of a stress-responsive mitogen-activated protein kinase cascade induces the biosynthesis of ethylene in plants. The Plant Cell. 2003;15:2707–2718. doi: 10.1105/tpc.011411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee HJ. Control of ethylene-mediated processes in tomato at the level of receptors. Journal of Experimental Botany. 2002;53:2057–2063. doi: 10.1093/jxb/erf062. [DOI] [PubMed] [Google Scholar]
- Klee HJ. Ethylene signal transduction. Moving beyond Arabidopsis. Plant Physiology. 2004;135:660–666. doi: 10.1104/pp.104.040998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanahan MB, Yen HC, Giovannoni J, Klee HJ. The Never Ripe mutation blocks ethylene perception in tomato. The Plant Cell. 1994;6:521–530. doi: 10.1105/tpc.6.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Liu G, Xu C, Lee GI, Bauer P, Ling HQ, Ganal MW, Howe GA. The tomato suppressor of prosystemin-mediated responses2 gene encodes a fatty acid desaturase required for the biosynthesis of jasmonic acid and the production of a systemic wound signal for defense gene expression. The Plant Cell. 2003;15:1646–1661. doi: 10.1105/tpc.012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhao Y, McCaig BC, Wingerd BA, Wang J, Whalon ME, Pichersky E, Howe GA. The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. The Plant Cell. 2004;16:126–143. doi: 10.1105/tpc.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔ CT method [J] Method. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Chico JM, Sanchez-Serrano JJ, Solano R. JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. The Plant Cell. 2004;16:1938–1950. doi: 10.1105/tpc.022319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Piqueras R, Sanchez-Serrano JJ, Solano R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. The Plant Cell. 2003;15:165–178. doi: 10.1105/tpc.007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Xu W, Xu H, Chen Y, He Z, Ma M. Nitric oxide modulates cadmium influx during cadmium-induced programmed cell death in tobacco BY-2 cells. Planta. 2010;232:325–335. doi: 10.1007/s00425-010-1177-y. [DOI] [PubMed] [Google Scholar]
- Melotto M, Mecey C, Niu Y, et al. A critical role of two positively charged amino acids in the Jas motif of Arabidopsis JAZ proteins in mediating coronatine- and jasmonoyl isoleucine-dependent interactions with the COI1 F-box protein. The Plant Journal. 2008;55:979–988. doi: 10.1111/j.1365-313X.2008.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesbah LA, Weerden GMVD, Nijkamp HJJ, Hille J. Sensitivity among species of Solanaceae to AAL toxins produced by Alternaria alternata f.sp. lycopersici. Plant Pathology. 2000;49:734–741. [Google Scholar]
- Moore T, Martineau B, Bostock RM, Lincoln JE, Gilchrist DG. Molecular and genetic characterization of ethylene involvement in mycotoxin-induced plant cell death. Physiological and Molecular Plant Pathology. 1999;54:73–85. [Google Scholar]
- Morisseau C, Ward BL, Gilchrist DG, Hammock BD. Multiple epoxide hydrolases in Alternaria alternata f. sp. Lycopersici and their relationship to medium composition and host-specific toxin production. Applied and Environmental Microbiology. 1999;65:2388–2395. doi: 10.1128/aem.65.6.2388-2395.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussatos VV, Yang SF, Ward B, Gilchrist DG. AAL-toxin induced physiological changes in Lycopersicon esculentum Mill: roles for ethylene and pyrimidine intermediates in necrosis. Physiological and Molecular Plant Pathology. 1994;44:455–468. [Google Scholar]
- O'Donnell PJ, Jones JB, Antoine FR, Ciardi J, Klee HJ. Ethylene-dependent salicylic acid regulates an expanded cell death response to a plant pathogen. The Plant Journal. 2001;25:315–323. doi: 10.1046/j.1365-313x.2001.00968.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell PJ, Schmelz E, Block A, Miersch O, Wasternack C, Jones JB, Klee HJ. Multiple hormones act sequentially to mediate a susceptible tomato pathogen defense response. Plant Physiology. 2003;133:1181–1189. doi: 10.1104/pp.103.030379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onkokesung N, Gális I, Dahl CC, Matsuoka K, Saluz H-P, Baldwin IT. Jasmonic acid and ethylene modulate local responses to wounding and simulated herbivory in Nicotiana attenuata leaves. Plant Physiology. 2010;153:785–798. doi: 10.1104/pp.110.156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzaez D, Jong AJ, Woltering EJ. A tomato homologue of the human protein PIRIN is induced during programmed cell death. Plant Molecular Biology. 2001;46:459–468. doi: 10.1023/a:1010618515051. [DOI] [PubMed] [Google Scholar]
- Overmyer K, Tuomainen H, Kettunen R, Betz C, Langebartels C, Sandermann Jr, H, Kangasjärvi J. The ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signalling pathways in regulating superoxide-dependent cell death. The Plant Cell. 2000;12:1849–1862. doi: 10.1105/tpc.12.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Davis KR. The physiology of ozone induced cell death. Planta. 2001;213:682–690. doi: 10.1007/s004250100618. [DOI] [PubMed] [Google Scholar]
- Rao MV, Lee H, Creelman RA, Mullet JE, Davis KR. Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. The Plant Cell. 2000;12:1633–1646. doi: 10.1105/tpc.12.9.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottmann WH, Peter GF, Oeller PW, Keller JA, Shen NF, Nagy BP, Taylor LP, Campbell AD, Theologis A. 1-Aminocyclopropane-1-carboxylate synthase in tomato is encoded by a multigene family whose transcription is induced during fruit and floral senescence. Journal of Molecular Biology. 1991;222:937–961. doi: 10.1016/0022-2836(91)90587-v. [DOI] [PubMed] [Google Scholar]
- Ryan CA. The systemin signaling pathway: differential activation of plant defensive genes. Biochimica et Biophysica Acta. 2000;1477:112–121. doi: 10.1016/s0167-4838(99)00269-1. [DOI] [PubMed] [Google Scholar]
- Spassieva SD, Markham JE, Hille J. The plant disease resistance gene Asc-1 prevents disruption of sphingolipid metabolism during AAL-toxin-induced programmed cell death. The Plant Journal. 2002;32:561–572. doi: 10.1046/j.1365-313x.2002.01444.x. [DOI] [PubMed] [Google Scholar]
- Thatcher LF, Manners JM, Kazan K. Fusarium oxysporum hijacks COI1-mediated jasmonate signaling to promote disease development in Arabidopsis. The Plant Journal. 2009;58:927–939. doi: 10.1111/j.1365-313X.2009.03831.x. [DOI] [PubMed] [Google Scholar]
- Tieman DM, Taylor MG, Ciardi JA, Klee HJ. The tomato ethylene receptors NR and LeETR4 are negative regulators of ethylene response and exhibit functional compensation within a multigene family. Proceedings of the National Academy of Sciences, USA. 2000;97:5663–8. doi: 10.1073/pnas.090550597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen H, Overmyer K, Keinänen M, Kollist H, Kangasjärvi J. Mutual antagonism of ethylene and jasmonic acid regulates ozone-induced spreading cell death in Arabidopsis. The Plant Journal. 2004;39:59–69. doi: 10.1111/j.1365-313X.2004.02107.x. [DOI] [PubMed] [Google Scholar]
- Underwood W, Zhang S, He SY. The Pseudomonas syringae type III effector tyrosine phosphatase HopAO1 suppresses innate immunity in Arabidopsis thaliana. The Plant Journal. 2007;52:658–672. doi: 10.1111/j.1365-313X.2007.03262.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Li J, Bostock RM, Gilchrist DG. Apoptosis: a functional paradigm for programmed plant cell death induced by a host-selective phytotoxin and invoked during development. The Plant Cell. 1996;8:375–391. doi: 10.1105/tpc.8.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhuizen L, Shephard GS, Snyman SD, Abel S, Swanevelder S, Gelderblom WCA. Inhibition of sphingolipid biosynthesis in rat primary hepatocyte cultures by Fumonisin B1 and other structurally related compounds. Food and Chemical Toxicology. 1998;36:497–503. doi: 10.1016/s0278-6915(98)00012-x. [DOI] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ. An ethylene-inducible component of signal transduction encoded by never-ripe. Science. 1995;270:1807–1809. doi: 10.1126/science.270.5243.1807. [DOI] [PubMed] [Google Scholar]
- Yamagishi D, Akamatsu H, Otani H, Kodama M. Pathological evaluation of host-specific AAL-toxins and fumonisin mycotoxins produced by Alternaria and Fusarium species. Journal of General Plant Pathology. 2006;72:323–327. [Google Scholar]
- Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annual Review of Plant Physiology. 1984;35:155–189. [Google Scholar]
- Yu JQ, Komada H. Hinoki (Chamaecyparis obtusa) bark, a substrate with anti-pathogen properties that suppress some root diseases of tomato. Scientia Horticulturae. 1999;81:13–24. [Google Scholar]
- Zélicourt A, Montiel G, Pouvreau JB, Thoiron S, Delgrange S, Simier P, Delavault P. Susceptibility of Phelipanche and Orobanche species to AAL-toxin. Planta. 2009;230:1047–1055. doi: 10.1007/s00425-009-1008-1. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Thilmony R, Bender CL, Schaller A, He SY, Howe GA. Virulence systems of Pseudomonas syringae pv. tomato promote bacterial speck disease in tomato by targeting the jasmonate signaling pathway. The Plant Journal. 2003;36:485–499. doi: 10.1046/j.1365-313x.2003.01895.x. [DOI] [PubMed] [Google Scholar]
- Zhou YH, Zhang YY, Zhao X, Yu HJ, Shi K, Yu JQ. Impact of light variation on development of photoprotection, antioxidants, and nutritional value in Lactuca sativa L. Journal of Agricultural and Food Chemistry. 2009;57:5494–5500. doi: 10.1021/jf8040325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.