Abstract
Recent studies have shown that hypergravity enhances lignification through up-regulation of the expression of lignin biosynthesis-related genes, although its hormonal signalling mechanism is unknown. The effects of hypergravity on auxin dynamics were examined using Arabidopsis plants that were transformed with the auxin reporter gene construct DR5::GUS. Hypergravity treatment at 300 g significantly increased β-glucuronidase activity in inflorescence stems of DR5::GUS plants, indicating that endogenous auxin accumulation was enhanced by hypergravity treatment. The hypergravity-related increased expression levels of both DR5::GUS and lignin biosynthesis-related genes in inflorescence stems were suppressed after disbudding, indicating that the increased expression of lignin biosynthesis-related genes is dependent on an increase in auxin influx from the shoot apex.
Keywords: Arabidopsis, auxin, disbudding, DR5::GUS, hypergravity, lignin
Introduction
Land plants evolutionarily developed rigid cell walls to support their weight, and to grow and extend upward in the 1 g environment of the earth. Previous studies have demonstrated that prolonged hypergravity treatment significantly increased the content of acetylbromide-extractable lignins in Arabidopsis inflorescence stems (Tamaoki et al., 2006), promoted metaxylem development, and decreased extensibility of the secondary cell walls in Arabidopsis inflorescence stems (Nakabayashi et al., 2006). In addition, microarray analysis showed that hypergravity up-regulated genes responsible for the biosynthesis or modification of secondary cell wall components such as lignin (Tamaoki et al., 2009). These facts indicate that regulation of lignin formation in response to hypergravity is mediated through the transcriptional regulation of genes related to lignin formation (Karahara et al., 2009).
Mechanosensitive ion channels were thought to be involved in graviperception in plants (Soga et al., 2004; Nakabayashi et al., 2006; Tamaoki et al., 2006). Moreover, auxin was demonstrated to be involved in signalling of gravitropism (Salisbury et al., 1988; Young et al., 1990), peg formation in cucumber seedlings (Witztum and Gersani, 1975; Kamada et al., 2000), and the negative gravitropic response of pea epicotyls (Hoshino et al., 2006). It was reported previously that application of artificial weight induced secondary cell wall growth, as well as auxin transport in Arabidopsis inflorescence stem (Ko et al., 2004). Although little is known about the signal transduction events involved in these responses to hypergravity, microarray analysis in Arabidopsis callus culture demonstrated that a hypergravity stimulus (7 g) up-regulates the expression of genes related to plant hormones (Martzivanou and Hampp, 2003). In addition, microarray analysis showed that hypergravity significantly altered the expression of genes related to auxin biosynthesis and signalling (Tamaoki et al., 2009).
The purpose of this study is to test whether auxin is involved in the hypergravity-related induction of expression of genes related to lignin in Arabidopsis inflorescence stems, using plants transformed with the auxin reporter gene construct DR5::GUS. The synthetic promoter DR5, which consists of seven tandem repeats of 11 bp containing the auxin-responsive TGTCTC element (Ulmasov et al., 1997), increases auxin responsiveness and is widely used to monitor auxin responses in plants (Guilfoyle, 1999).
Materials and methods
Plant material and hypergravity treatment
Wild-type and DR5::GUS transgenic plants (Ulmasov et al., 1997) of Arabidopsis thaliana (L.) Heynh. ecotype Columbia-0 were used throughout the study. Seeds were treated with 99% (v/v) ethanol for 10 s for the purpose of sterilization, and planted on 4 ml of Murashige and Skoog medium (Wako Pure Chemical Industries Ltd, Tokyo, Japan) containing 2% (w/v) sucrose and 1.0% (w/v) agar in test tubes (15 mm×105 mm). After 3 d at 4 °C in the dark, plants were grown at 22 °C for 20–26 d under continuous white light with an intensity of 130 μmol m−2 s−1 at the plant level from fluorescent tubes (FL20SS·N/18; Toshiba Corp., Tokyo, Japan). Arabidopsis plants with 5 mm inflorescence stems (i.e. growth stage number 5; Boyes et al., 2001), were exposed to 300 g hypergravity in the shoot to root direction for 1–24 h at 25 °C in the dark using a centrifuge (SL-05A; Sakuma Seisakusho Ltd, Tokyo, Japan). For disbudding experiments, apical buds as well as lateral buds were removed 3 h before the hypergravity treatment. For the 1 g control, test tubes containing plants with 5 mm inflorescence stems were placed in the dark without centrifugation.
Morphometric analysis of the cross-sectional area of inflorescence stems
Wild-type Arabidopsis plants were grown at 22 °C for another 3 d in continuous light after the 24 h 300 g or 1 g (hyper)gravity treatment, which took place in the dark. Cross-sections of 50 μm thickness were cut at 1 mm above the base of the inflorescence stems using a vibrating microtome (Linear slicer Pro. 7; DSK, Kyoto, Japan), and observed under a light microscope (BX-50; Olympus, Tokyo, Japan). Photographs were taken with a digital camera (Coolsnap cf, Nippon Roper, Tokyo, Japan) fitted to the light microscope. Measurements of the cross-sections were carried out on the digital micrographs using Openlab Darkroom 3.0.2 (Improvision, Coventry, UK).
Histochemical staining of β-glucuronidase activity
For whole-mount histochemical observation of β-glucuronidase (GUS) activities in DR5::GUS plants, seedlings were incubated with a staining solution consisting of 100 mM Na2PO4 (pH 7.0), 10 mM ethylenediaminetetraacetic acid (EDTA), 3 mM K3Fe(CN)6, 0.1% (v/v) Triton X-100, and 40 mg ml−1 5-bromo-4-chloro-3-indolyl-β-D-glucuronide (Wako Pure Chemical Industries Ltd) at 37 °C, fixed in a mixed solution of ethanol and acetic acid, and cleared with a mixture of chloral hydrate, glycerol, and water (8:1:2, w/v/v). The cleared seedlings were mounted on slide glasses and observed under a dissecting microscope (SZH-10; Olympus, Tokyo, Japan). Photographs were taken with a digital camera (Digital sight DS-Fi1; Nikon, Tokyo, Japan) fitted to the dissecting microscope.
Because the inflorescence stem segments of 10 mm in length were cut from the base for the fluorometric assay of GUS activity, cross-sections of 50 μm thickness were taken at 5 mm above the base of the inflorescence stems (half-way up the 10 mm segments). Cross-sections were cut using a Linear Slicer Pro. 7 (DSK), and observed under a light microscope (BX-50; Olympus). Photographs were taken with a digital camera (Coolsnap cf; Nippon Roper) fitted to the light microscope.
Fluorometric assay of GUS activity
For quantitative measurements of GUS activity, the fluorometric GUS assay was performed using 4-methylumbelliferyl-β-d-glucuronide (4-MUG) as the substrate. This compound is converted by GUS into the fluorescent product 4-methyl umbelliferone (4-MU) (Jefferson et al., 1987). Ten millimetre inflorescence stem segments (as above) of DR5::GUS plants were homogenized with GUS extraction buffer [50 mM Na2PO4, pH 7.0, 10 mM EDTA, 0.1% (v/v) Triton X-100, and 0.1% sarcosyl] immediately after hypergravity treatment at 300 g, and centrifuged at 12 000 g for 5 min at 4 °C. A portion of the supernatant fraction was mixed with the GUS extraction buffer containing 1 mM 4-MUG (Wako Pure Chemical Industries Ltd). This reaction mixture was incubated at 37 °C. Aliquots of the reaction mixture were removed at 0 min and 60 min. The reaction was terminated by adding 0.2 M Na2CO3. Production of 4-MU was quantified by measuring the 4-MU fluorescence (365 nm excitation, 455 nm emission) using a Nanodrop-ND-3300 spectrometer (Nanodrop Technologies Inc., Wilmington, DE, USA). The amount of 4-MU was deduced from a standard curve. GUS activity was expressed as nmol 4-MU min−1 mg−1 protein. The protein concentration was determined using the Bradford method (Bradford, 1976), using the Quick Start protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA), with bovine serum albumin as standard.
Quantitative RT-PCR analysis
Basal 10 mm inflorescence stem segments were excised from plants that had been incubated at 300 g and 1 g for 24 h. Total RNA was isolated from the basal region using the Agilent Plant RNA Isolation Mini kit (Agilent Technologies Inc., Palo Alto, CA, USA). First-strand cDNA was synthesized from 500 ng of total RNA, in a 10 μl reaction mixture, using a PrimeScript RT reagent kit (Takara Bio Inc., Shiga, Japan). Quantitative RT-PCR amplifications and measurements were performed using an ABI PRISM 7000 sequence detection system (Applied Biosystems Japan Ltd, Tokyo, Japan) using SYBR Premix Ex Taq (Takara Bio Inc.). The PCR amplification had an initial denaturing step of 30 s at 95 °C, followed by 40 cycles of 95 °C for 5 s and 60 °C for 31 s. All data were normalized using 18S rRNA as an internal standard. The sequences of the gene-specific primers were as follows: C4H, 5′-CGTTATGAAACCAAGGAACTGTTAAA-3′ and 5′-GCAATCGTAGAACGAACCATTTAAA-3′ (Sibout et al., 2005); C3H1, 5′-AAGTCTAGTGGAGCGAAACAGCATT-3′ and 5′-TCCTCACTAAGATCATACTGATCCTTCA-3' (Abdulrazzak et al., 2006); ATPA2, 5'-CCTTGACTGGGAGTAATGGAGA-3′ and 5′-TCAACCAAAGCTTGCCAATG-3′ (Tamaoki et al., 2009); and 18S rRNA, 5′-CGGCTACCACATCCAAGGAA-3′ and 5′-GCTGGAATTACCGCGGCT-3′ (Tamaoki et al., 2009).
Results
Hypergravity induces DR5::GUS expression in inflorescence stems
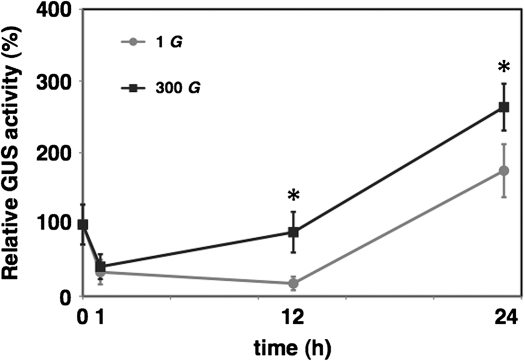
It was demonstrated previously that hypergravity up-regulated the expression of auxin signalling-related genes [auxin-inducible genes and auxin response factor (ARF) genes] in Arabidopsis inflorescence stems (Tamaoki et al., 2009), indicating that the endogenous auxin level increased upon exposure to hypergravity. To test this, the effects of hypergravity on expression of a synthetic auxin response element, DR5, were examined using DR5::GUS transgenic Arabidopsis plants. Histochemical data showed that GUS expression was up-regulated in inflorescence stems of DR5::GUS transgenic plants sampled at 12 h or 24 h after the start of the hypergravity treatment (Fig. 1A–C). This result indicates that the endogenous auxin level increased in the inflorescence stems upon hypergravity treatment. It also indicates that endogenous auxin already begins to accumulate before 12 h after the start of the hypergravity treatment, and that the area in which endogenous auxin accumulates expands. Quantitative fluorometric analysis of GUS activity showed that the GUS expression was significantly higher at both 12 h and 24 h after the start of hypergravity treatment in inflorescence stems of the DR5::GUS transgenic plants (Fig. 2). These results indicate that endogenous auxin levels increase in response to hypergravity treatment in Arabidopsis inflorescence stems.
Fig. 1.
Whole-mount, GUS-stained inflorescence stems of DR5::GUS transgenic Arabidopsis exposed to hypergravity for 1 h (A), 12 h (B), or 24 h (C, D). (A–C) Intact plants. (D) Transgenic plants disbudded before the hypergravity treatment. Bar=5 mm. (This figure is available in colour at JXB online.)
Fig. 2.
Fluorometric analysis of GUS activities in the inflorescence stems of hypergravity-treated DR5::GUS transgenic Arabidopsis plants. Values are the mean ±SE (n=5–12). The values were normalized to the mean value of samples at 0 h. *P <0.05 Mann-Whitney U-test, two-tailed.
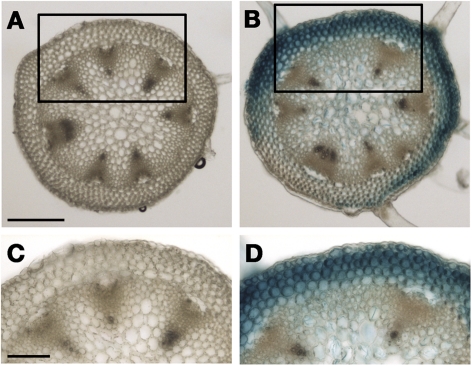
Cross-sections were taken from inflorescence stems of the DR5::GUS plants, and the tissue localization of the GUS activity was determined. High GUS activity was found in both the epidermis and the cortex, whereas the activity was weak in the stele (Fig. 3).
Fig. 3.
Histochemical analysis of GUS expression in cross-sections of inflorescence stems of DR5::GUS transgenic Arabidopsis in response to hypergravity. GUS staining was performed just after the hypergravity treatment. (A) The 1 g control. (B) 300 g. (C and D) Magnifications of areas shown in A and B, respectively. Bars: (A) 200 μm, (C) 100 μm. (This figure is available in colour at JXB online.)
Disbudding treatment suppresses the hypergravity-induced increase of DR5::GUS expression in inflorescence stems
Microarray analysis showed that the expression of auxin biosynthesis-related genes was up-regulated by hypergravity in Arabidopsis inflorescence stems (Tamaoki et al., 2009). Auxin is produced predominantly in shoot apical regions (Woodward and Bartel, 2005) and transported to the stem by polar transport (Lomax et al., 1995). To test whether the hypergravity-induced increase in endogenous auxin in the inflorescence stems also originated from the buds, the effect of disbudding on the hypergravity-induced increase of DR5::GUS expression in inflorescence stems was examined. It was observed that hypergravity did not result in increased GUS activity in inflorescence stems after the plants were disbudded (Fig. 1D). The effect of disbudding on the GUS activity after hypergravity treatment was quantified using fluorometric analysis of inflorescence stems of DR5::GUS plants. The GUS activity values of hypergravity-treated plants were normalized to those of the 1 g samples. The GUS activity in the disbudded, hypergravity-treated samples was 98.7±27.3% (n=8) of the 1 g samples, while that in the control (non-disbudded, hypergravity treated) was 150.6±18.6% (n=12) that of the 1 g samples. These results indicate that the hypergravity-induced increase in endogenous auxin levels in inflorescence stems resulted from either enhanced production of auxin in buds or enhanced auxin transport from the buds.
Disbudding suppresses hypergravity-related induction of lignin biosynthesis genes in inflorescence stems
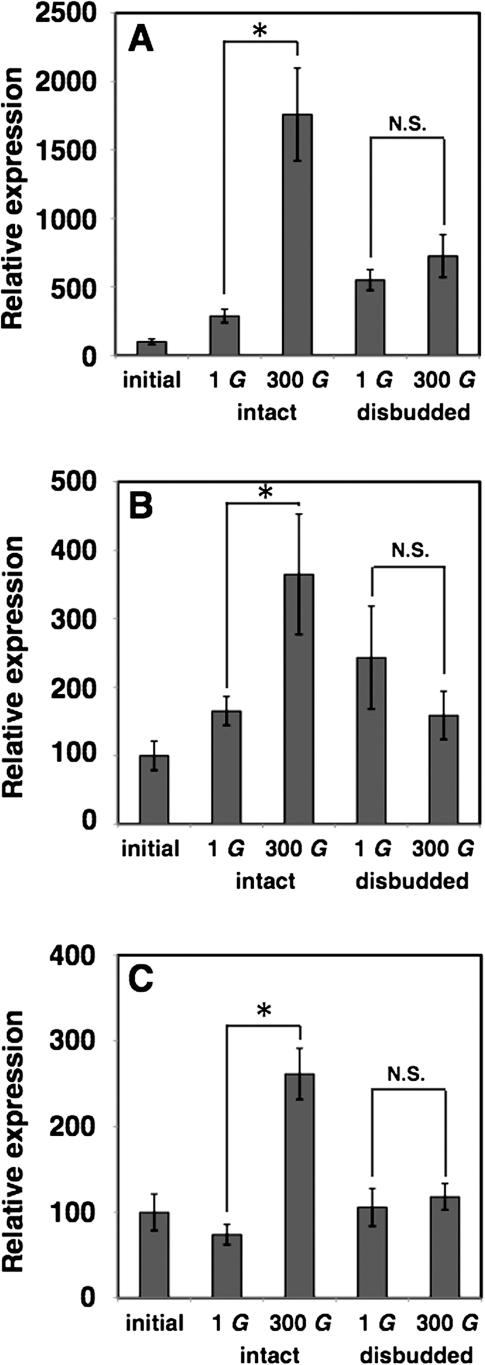
Previously, it was demonstrated that hypergravity treatment up-regulated the expression of lignin biosynthesis or lignin-related genes (Tamaoki et al., 2009). It also increased the content of lignin in the secondary cell wall (Tamaoki et al., 2006). Auxin was reported to be involved in vascular bundle formation (Mattsson et al., 1999, 2003) and tracheary element differentiation (Yoshida et al., 2005). To test whether hypergravity-induced endogenous auxin accumulation plays a role in the induction of lignin-related genes, the effects of disbudding on the expression of the lignin-related genes, ATPA2 PEROXIDASE (ATPA2), CINNAMATE 4-HYDROXYLASE (C4H), and COUMARATE 3-HYDROXYLASE1 (C3H1), were examined in inflorescence stems of hypergravity-treated and of 1 g control wild-type Arabidopsis plants. The expression levels of these three genes in inflorescence stems of plants with buds were enhanced significantly by hypergravity, while they remained unchanged in inflorescence stems of disbudded plants (Fig. 4). This raises the possibility that the hypergravity-induced auxin accumulation causes the expression levels of lignin biosynthesis-related genes to increase.
Fig. 4.
Effects of disbudding on the enhanced expression levels of lignin biosynthesis-related genes in Arabidopsis inflorescence stems under hypergravity. The expression of (A) ATPA2, (B) C4H, and (C) C3H1 genes was determined by quantitative RT-PCR. The values of gene expression were normalized to the mean value of those before the treatment (initial). Values are mean ±SE (n=3). *P <0.05 Mann–Whitney U-test, two-tailed. N.S., not significant
Disbudding does not suppress the hypergravity-related increase in the cross-sectional area of inflorescence stems
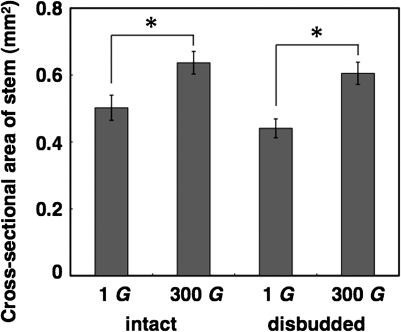
Thickening of the stem, as well as lignin deposition, is important in supporting the plant shoots during growth against the gravitational force. It has been demonstrated that hypergravity increased the cross-sectional area of Arabidopsis inflorescence stems (Nakabayashi et al., 2006). Therefore, the effect of disbudding on the hypergravity-induced increase in the cross-sectional area of inflorescence stems was examined. In disbudded and intact plants, equally, increases in the cross-sectional area of inflorescence stems were observed in response to hypergravity [Mann–Whitney U–test, two-tailed, z= –2.562, P=0.0104 (disbudded plants); z= –2.397, P=0.0165 (intact plants)] (Fig. 5). This indicates that the increase in endogenous auxin does not play a role in the increase in the cross-sectional area of inflorescence stems upon hypergravity treatment.
Fig. 5.
Effects of disbudding on the increase in cross-sectional area of wild-type Arabidopsis inflorescence stems at the base by hypergravity treatment. Values are mean ±SE (n=7–8). *P <0.05, Mann–Whitney U-test, two-tailed.
Discussion
Growth parameters such as elongation of Arabidopsis hypocotyls varied in proportion to the logarithm of the magnitude of gravity in the range from microgravity to hypergravity (Soga et al., 2001). Such a linear relationship also existed between the logarithm of the magnitude of gravitational acceleration and the inhibition of root elongation, at least up to 300 g in azuki bean roots (Soga et al., 2005). In addition, the change in cell wall extensibility as a result of hypergravity at 300 g was shown to be reversible within a few hours in azuki bean epicotyls, and in maize coleoptiles and mesocotyls (Soga et al., 2003). These results clearly indicate that such hypergravity treatment at 300 g is not excessive and does not cause irreversible damage. Further, it was demonstrated that hypergravity treatment at 300 g for 24 h increased the lignin content, as well as the expression of genes related to lignin synthesis in Arabidopsis inflorescence stems (Tamaoki et al., 2006, 2009). The aim of the present study was to test whether auxin is involved in hypergravity-related induction of expression of genes related to lignin synthesis. Therefore, it was decided to use gravitational acceleration at 300 g as the most suitable hypergravity treatment.
Involvement of auxin in signal transductions of gravitropism has been well documented in plants (Salisbury et al., 1988; Young et al., 1990). On the other hand, only a few studies have indicated an involvement of auxin signalling in responses to changes in gravitational acceleration. Microarray analysis indicated a possible involvement of auxin signalling in the response of Arabidopsis callus culture to hypergravity (Marzivanou and Hampp, 2003). In addition, microarray analysis showed that hypergravity up-regulated the expression of auxin signalling-related genes in Arabidopsis inflorescence stems (Tamaoki et al., 2009). More specifically, up-regulation of auxin-responsive genes, such as IAA28, by hypergravity implies that hypergravity increases the endogenous level of auxin in Arabidopsis inflorescence stems (Tamaoki et al., 2009). In addition, the present study showed that DR5::GUS expression significantly increased under hypergravity in the Arabidopsis inflorescence stems (Figs 1, 2), indicating that hypergravity increased the level of endogenous auxin in Arabidopsis inflorescence stems.
Microarray analysis showed that the expression of several auxin biosynthesis-related genes was up-regulated >2-fold upon hypergravity in Arabidopsis inflorescence stems, raising the possibility that hypergravity enhanced auxin biosynthesis in the stems (Tamaoki et al., 2009). The results obtained in the disbudding experiment (Fig. 1) clearly indicated that the accumulated endogenous auxin in inflorescence stems upon hypergravity treatment, which was important for the hypergravity-enhanced expression of genes related to lignin biosynthesis, mainly originated from the buds. However, disbudding treatment may partially affect the auxin biosynthesis in inflorescence stems under hypergravity.
Enhanced expression of DR5::GUS was observed in the epidermis, cortex, and stele cells, but not in the xylem region of the inflorescence stem (Fig. 3). Polar auxin transport (Lomax et al., 1995) is mediated by auxin efflux carriers, such as PIN-FORMED protein (PIN) and P-glycoprotein (PGP), and auxin influx carriers, such as AUXIN/LIKE-AUXIN (AUX/LAX) families. PINs and PGPs control the direction of auxin transport because of their subcellular localization (Blakeslee et al., 2007). The localization of auxin uptake and efflux proteins was investigated in detail in Arabidopsis roots and embryos, thereby providing a model of auxin dynamics (Petrášek and Friml, 2009). Although auxin dynamics in Arabidopsis hypocotyls and inflorescence stems was poorly understood, several reports showed localization and expression of auxin uptake and efflux proteins in the inflorescence stem. PIN1 localized basally in xylem parenchyma cells (Galweiler et al, 1998). In dark-grown seedlings, PIN1 localized in the vascular parenchyma, in the epidermis of the shoot apical hook, in the vascular parenchyma, and in adjacent cortical cells in the mid-hypocotyl (Blakeslee et al., 2007). A change in the localization and/or expression of such proteins might affect auxin transport in the inflorescence stem upon hypergravity treatment.
Disbudding suppressed the hypergravity-induced increases in both DR5::GUS expression and the expression of lignin biosynthesis genes, such as C4H, C3H1, and ATPA2 (Fig. 4), in inflorescence stems. This indicates that the hypergravity-related induction of the expression of lignin biosynthesis-related genes is mediated by the hypergravity-related increase in endogenous auxin. C4H and C3H1 are involved in monolignol synthesis (Ruegger and Chapple, 2001; Goujon et al., 2003). C4H encodes cinnamate 4-hydoxylase, which is the first cytochrome P450-dependent monooxygenase of the phenylpropanoid pathway (Bell-Lelong et al., 1997), and controls the conversion of cinnamate into p-coumarate (Raes et al., 2003). C3H1 encodes p-coumarate 3-hydroxylase, which converts the shikimate and quinate esters of p-coumaric acid into the corresponding caffeic acid conjugates (Raes et al., 2003). ATPA2 encodes an extracellular anionic peroxidase, which has the ability to oxidize monolignols and is involved in lignin polymerization (Østergaard et al., 2000; Nielsen et al., 2001). The increase in ATPA2 expression by hypergravity is more prominent than the C4H and C3H1 expression, which is consistent with a previous report (Tamaoki et al., 2009). This indicates that hypergravity enhances lignin polymerization more than monolignol synthesis. Disbudding suppressed the hypergravity-related increases in expression of all these genes, indicating that the processes of lignin biosynthesis are regulated in response to a change in gravity and are dependent on the level of auxin. A basipetal gradient of lignification exists in Arabidopsis inflorescence stems (Roger and Campbell, 2004). This gradient has been suggested to be necessary to support the weight of the plant. It was reported that development of the secondary cell wall was promoted by application of artificial weight on the shoot (Ko et al., 2004). In addition, the level of lignin, a characteristic component of the secondary wall, increased upon hypergravity in Arabidopsis inflorescence stems (Tamaoki et al., 2006). Therefore, body weight of Arabidopsis plants is thought to be an important factor in the regulation of secondary cell wall formation in inflorescence stems. Ko et al. (2004) also reported that auxin transport and signalling were promoted by artificial application of a weight on Arabidopsis inflorescence stems. In this study, it was demonstrated that disbudding suppressed the hypergravity-enhanced expression of genes related to lignin biosynthesis (Fig. 4). These facts indicate that auxin signalling is involved in the up-regulation of lignin biosynthesis under hypergravity, thereby contributing to supporting the inflorescence stem mechanically.
A previous study demonstrated that auxin transport inhibitors prevented tracheary element differentiation in a Zinnia elegans cell culture system (Yoshida et al., 2005), indicating that auxin signalling plays an important role in vascular formation. It is likely that hypergravity-related up-regulation of auxin signalling in the inflorescence stem also promoted xylem formation. This idea is supported by a previous study demonstrating that the number of metaxylem elements in the basal region of inflorescence stems increased in response to hypergravity (Nakabayashi et al., 2006).
On the other hand, disbudding did not affect the increase in cross-sectional area of inflorescence stems by hypergravity (Fig. 5), indicating that the hypergravity-induced lateral growth of the inflorescence stem is not mediated by auxin signalling. Microarray analysis showed that hypergravity increased the expression of genes that are related to plant hormones such as ethylene and cytokinin, as well as auxin, in Arabidopsis inflorescence stems (Tamaoki et al., 2009). Therefore, it is possible that plant hormones other than auxin might be involved in the hypergravity-induced lateral growth of the inflorescence stem.
The findings of this study indicated for the first time that auxin signalling plays a key role in the responses of plants to hypergravity, particularly in the enhancement of expression of genes related to lignin synthesis. As a next step, these findings will be tested using Arabidopsis mutants with defects in auxin transport.
Acknowledgments
We acknowledge Dr Thomas J. Guilfoyle of University of Missouri (Columbia, MO) for providing the DR5::GUS transgenic line. This work was supported fully by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists (to DT), by a Grant-in-aid for Scientific Research nos 21570064 and 21657011 from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to IK), and ‘Ground-Based Research Announcement for Space Utilization’ funded by the Japan Space Forum (to SK and IK).
References
- Abdulrazzak N, Pollet B, Ehlting J, et al. A coumaroyl-ester-3-hydroxylase insertion mutant reveals the existence of nonredundant meta-hydroxylation pathways and essential roles for phenolic precursors in cell expansion and plant growth. Plant Physiology. 2006;140:30–48. doi: 10.1104/pp.105.069690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Lelong DA, Cusumano JC, Meyer K, Chapple C. Cinnamate-4-hydroxylase expression in Arabidopsis. Regulation in response to development and the environment. Plant Physiology. 1997;113:729–738. doi: 10.1104/pp.113.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeslee JJ, Bandyopadhyay A, Lee OR, et al. Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. The Plant Cell. 2007;19:131–147. doi: 10.1105/tpc.106.040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Gorlach J. Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. The Plant Cell. 2001;13:1499–1510. doi: 10.1105/TPC.010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Galweiler L, Guan C, Muller A, Wisman E, Mendgen K, Yephremov A, Palme K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282:2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- Goujon T, Sibout R, Eudes A, MacKay J, Jouanin L. Genes involved in the biosynthesis of lignin precursors in. Arabidopsis thaliana. Plant Physiology and Biochemistry. 2003;41:677–687. [Google Scholar]
- Guilfoyle TJ. Auxin-regulated genes and promoters. In: Hooykaas PPJ, Hall MA, Libbenga KR, editors. Biochemistry and molecular biology of plant hormones. New York: Elsevier; 1999. pp. 423–459. [Google Scholar]
- Hoshino T, Miyamoto K, Ueda J. Requirement for the gravity-controlled transport of auxin for a negative gravitropic response of epicotyls in the early growth stage of etiolated pea seedlings. Plant and Cell Physiology. 2006;47:1496–1508. doi: 10.1093/pcp/pcl015. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada M, Fujii N, Aizawa S, Kamigaichi S, Mukai C, Shimazu T, Takahashi H. Control of gravimorphogenesis by auxin: accumulation pattern of CS-IAA1 mRNA in cucumber seedlings grown in space and on the ground. Planta. 2000;211:493–501. doi: 10.1007/s004250000321. [DOI] [PubMed] [Google Scholar]
- Karahara I, Tamaoki D, Nishiuchi T, Schreiber L, Kamisaka S. Effects of altered gravity conditions on lignin and secondary wall formation in herbaceous dicots and woody plants. Biological Sciences in Space. 2009;23:177–182. [Google Scholar]
- Ko JH, Han KH, Park S, Yang J. Plant body weight-induced secondary growth in Arabidopsis and its transcription phenotype revealed by whole-transcriptome profiling. Plant Physiology. 2004;135:1069–1083. doi: 10.1104/pp.104.038844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax TL, Muday GK, Rubery PH. Auxin transport. In: Davies PJ, editor. Plant hormones. Physiology, biochemistry and molecular biology. Dordrecht: Kluwer Academic Publishers; 1995. pp. 509–530. [Google Scholar]
- Martzivanou M, Hampp R. Hyper-gravity effects on the Arabidopsis transcriptome. Physiologia Plantarum. 2003;118:221–231. doi: 10.1034/j.1399-3054.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- Mattsson J, Ckurshumova W, Berleth T. Auxin signaling in Arabidopsis leaf vascular development. Plant Physiology. 2003;131:1327–1339. doi: 10.1104/pp.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson J, Sung ZR, Berleth T. Responses of plant vascular systems to auxin transport inhibition. Development. 1999;126:2979–2991. doi: 10.1242/dev.126.13.2979. [DOI] [PubMed] [Google Scholar]
- Nakabayashi I, Karahara I, Tamaoki D, Masuda K, Wakasugi T, Yamada K, Soga K, Hoson T, Kamisaka S. Hypergravity stimulus enhances primary xylem development and decreases mechanical properties of secondary cell walls in inflorescence stems of Arabidopsis thaliana. Annals of Botany. 2006;97:1083–1090. doi: 10.1093/aob/mcl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen KL, Indiani C, Henriksen A, Feis A, Becucci M, Gajhede M, Smulevich G, Welinder KG. Differential activity and structure of highly similar peroxidases. Spectroscopic, crystallographic and enzymatic analyses of lignifying Arabidopsis thaliana peroxidase A2 and horseradish peroxidase A2. Biochemica. 2001;40:11013–11021. doi: 10.1021/bi010661o. [DOI] [PubMed] [Google Scholar]
- Østergaard L, Teilum K, Mirza O, Mattsson O, Petersen M, Welinder KG, Mundy J, Gajhede M, Henriksen A. Arabidopsis ATP A2 peroxidase. Expression and high-resolution structure of a plant peroxidase with implications for lignification. Plant Molecular Biology. 2000;44:231–243. doi: 10.1023/a:1006442618860. [DOI] [PubMed] [Google Scholar]
- Petrášek J, Friml J. Auxin transport routes in plant development. Development. 2009;136:2675–2688. doi: 10.1242/dev.030353. [DOI] [PubMed] [Google Scholar]
- Raes J, Rohde A, Christensen JH, Van de Peer Y, Boerjan W. Genome-wide characterization of the lignification toolbox in Arabidopsis. Plant Physiology. 2003;133:1051–1071. doi: 10.1104/pp.103.026484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger LA, Campbell MM. The genetic control of lignin deposition during plant growth and development. New Phytologist. 2004;164:17–30. doi: 10.1111/j.1469-8137.2004.01143.x. [DOI] [PubMed] [Google Scholar]
- Ruegger M, Chapple C. Mutations that reduce sinapoylmalate accumulation in Arabidopsis thaliana define loci with diverse roles in phenylpropanoid metabolism. Genetics. 2001;159:1741–1749. doi: 10.1093/genetics/159.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury FB, Gillespie L, Rorabaugh P. Gravitropism in higher plant shoots. V. Changing sensitivity to auxin. Plant Physiology. 1988;88:1186–1194. doi: 10.1104/pp.88.4.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibout R, Eudes A, Mouille G, Pollet B, Lapierre C, Jouanin L, Seguin A. CINNAMYL ALCOHOL DEHYDROGENASE-C and -D are the primary genes involved in lignin biosynthesis in the floral stem of Arabidopsis. The Plant Cell. 2005;17:2059–2076. doi: 10.1105/tpc.105.030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soga K, Wakabayashi K, Hoson T, Kamisaka S. Gravitational force regulates elongation growth of Arabidopsis hypocotyls by modifying xyloglucan metabolism. Advances in Space Research. 2001;27:1011–1016. doi: 10.1016/s0273-1177(01)00176-4. [DOI] [PubMed] [Google Scholar]
- Soga K, Wakabayashi K, Kamisaka S, Hoson T. Growth restoration in Azuki bean and maize seedlings by removal of hypergravity stimuli. Advances in Space Research. 2003;31:2269–2274. doi: 10.1016/s0273-1177(03)00254-0. [DOI] [PubMed] [Google Scholar]
- Soga K, Wakabayashi K, Kamisaka S, Hoson T. Graviperception in growth inhibition of plant shoots under hypergravity conditions produced by centrifugation is independent of that in gravitropism and may involve mechanoreceptors. Planta. 2004;218:1054–1061. doi: 10.1007/s00425-003-1187-0. [DOI] [PubMed] [Google Scholar]
- Soga K, Wakabayashi K, Kamisaka S, Hoson T. Mechanoreceptors rather than sedimentable amyloplasts perceive the gravity signal in hypergravity-induced inhibition of root growth in azuki bean. Functional Plant Biology. 2005;32:175–179. doi: 10.1071/fp04145. [DOI] [PubMed] [Google Scholar]
- Tamaoki D, Karahara I, Nishiuchi T, Oliveira SD, Schreiber L, Wakasugi T, Yamada K, Yamaguchi K, Kamisaka S. Transcriptome profiling in Arabidopsis inflorescence stems grown under hypergravity in terms of cell walls and plant hormones. Advances in Space Research. 2009;44:245–253. [Google Scholar]
- Tamaoki D, Karahara I, Schreiber L, Wakasugi T, Yamada K, Kamisaka S. Effects of hypergravity conditions on elongation growth and lignin formation in the inflorescence stem of Arabidopsis thaliana. Journal of Plant Research. 2006;119:79–84. doi: 10.1007/s10265-005-0243-1. [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. The Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witztum A, Gersani M. The role of IAA in the development of the peg in Cucumis sativus L. Botanical Gazette. 1975;136:5–16. [Google Scholar]
- Woodward AW, Bartel B. Auxin: regulation, action, and interaction. Annals of Botany. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Kuriyama H, Fukuda H. Inhibition of transdifferentiation into tracheary elements by polar auxin transport inhibitors through intracellular auxin depletion. Plant and Cell Physiology. 2005;46:2019–2028. doi: 10.1093/pcp/pci217. [DOI] [PubMed] [Google Scholar]
- Young LM, Evans ML, Hertel R. Correlations between gravitropic curvature and auxin movement across gravistimulated roots of Zea mays. Plant Physiology. 1990;92:792–796. doi: 10.1104/pp.92.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]