Abstract
Jacalin-related lectins (JRLs) are a subgroup of proteins with one or more jacalin-like lectin domains. Although JRLs are often associated with biotic or abiotic stimuli, their biological functions in plants, as well as their relationships to plant disease resistance, are poorly understood. A mannose-specific JRL (mJRL)-like gene (TaJRLL1) that is mainly expressed in stem and spike and encodes a protein with two jacalin-like lectin domains was identified in wheat. Pathogen infection and phytohormone treatments induced its expression; while application of the salicylic acid (SA) biosynthesis inhibitor paclobutrazol and the jasmonic acid (JA) biosynthesis inhibitor diethyldithiocarbamic acid, respectively, substantially inhibited its expression. Attenuating TaJRLL1 through virus-induced gene silencing increased susceptibility to the facultative fungal pathogen Fusarium graminearum and the biotrophic fungal pathogen Blumeria graminis. Arabidopsis thaliana transformed with TaJRLL1 displayed increased resistance to F. graminearum and Botrytis cinerea. JA and SA levels in transgenic Arabidopsis increased significantly. A loss or increase of disease resistance due to an alteration in TaJRLL1 function was correlated with attenuation or enhancement of the SA- and JA-dependent defence signalling pathways. These results suggest that TaJRLL1 could be a component of the SA- and JA-dependent defence signalling pathways.
Keywords: Defence signalling, disease resistance, jacalin-related lectin, jasmonic acid, salicylic acid, wheat (Triticum aestivum L.)
Introduction
Plants employ highly sophisticated defence systems against a broad range of pathogens with different lifestyles and infection strategies. In addition to an array of structural barriers and chemical compounds employed to ward off pathogens, plants arrest pathogens via a broad spectrum of inducible defence mechanisms. Jones and Dangl (2006) characterized two types of inducible plant defence responses regulated by R genes or defence-responsive genes (i.e. the gene for gene and basal defense response). Active inducible defence mechanisms involve signal recognition, multiple signalling transductions, and defence response activation, which lead to local resistance response and systematic induced resistance. Phytohormones, such as salicylic acid (SA) and jasmonic acid (JA), are associated with resistance and play pivotal roles in initiating defence responses by transmitting defence signals (Glazebrook, 2001). SA generally induces defence against biotrophic pathogens, while JA is required for defence against necrotrophic pathogens and herbivorous insects (Beckers and Spoel, 2006). Both SA- and JA-dependent signalling pathways possibly confer resistance to hemi-biotrophic pathogens (Glazebrook et al., 2003). To date, most studies addressing the molecular mechanisms of disease resistance focus on dicots as model plants. However, extensive characterization of genes involved in disease resistance in monocots might provide important new insights into plant resistance mechanisms.
Plant lectins are a complex and heterogeneous group of carbohydrate-binding proteins that specifically recognize and bind to carbohydrate molecules (Van Damme et al., 2008). Jacalin-related lectins (JRLs) are a subgroup of proteins that have one or more domains with sequences similar to the jacalin protein isolated from Artocarpus integrifolia (Bunn-Moreno and Campos-Neto, 1981). Genes encoding JRLs have been identified in a number of plants. The majority of JRL proteins are mannose-specific lectins (mJRLs) and the remainder are galactose-specific lectins (gJRLs) (Peumans et al., 2000).
Recently, JRLs have received more and more attention because of their association with stress response. SA and JA commonly regulate JRLs. In wheat (Triticum aestivum), the mJRL protein gene WCI-1 is induced by Blumeria graminis f. sp. tritici infection (Görlach et al., 1996); the mJRL protein HFR-1 affects food detection and ingestion of Hessian fly larvae, resulting in development delay and premature death (Subramanyam et al., 2008). Both WCI-1 and HFR-1 have an N-terminal dirigent domain (disease-responsive domain) and a C-terminal jacalin-like lectin domain. In transgenic tobacco plants, overexpression of wheat Ta-JA1, which encodes an mJRL structurally similar to WCI-1 and Hfr-1, increased resistance to bacterial, fungal, and viral pathogens (Ma et al., 2010). Arabidopsis RTM1 and rice (Oryza sativa) Orysata encode proteins with a single jacalin-like lectin domain. RTM1 plays a role in restricting tobacco etch virus extension (Chisholm et al., 2001) and Orysata is responsive to infection by the pathogenic fungus Magnaporthe grisea (Qin et al., 2003). Barley (Hordeum vulgare) LEM2 is an mJRL-like protein with two jacalin-like lectin domains and is involved in systemic acquired resistance (Abebe et al., 2005). Despite the apparent association of JRLs with plant defence, their functions remain elusive, particularly due to their structural diversity.
Fusarium graminearum is a hemi-biotrophic fungus that causes disease in many plants. In wheat spikes, its infection results in Fusarium head blight (FHB) or scab, which is often disastrous to wheat production. A LEM2-like protein responsive to F. graminearum Schwabe [teleomorph=Gibberella zeae (Schwein) Petch] infection was previously identified in hexaploid wheat (T. aestivum L.) through 2D electrophoresis and mass spectrometry (Wang et al., 2005). In this study, this gene was cloned and its function in plant defence was investigated. This LEM2-like gene is responsive to infections of F. graminearum and the biotrophic fungal pathogen B. graminis f. sp. tritici. Silencing it in einkorn wheat (T. monococcum L.) or hexaploid wheat rendered the plants more susceptible to F. graminearum and B. graminis; however, its overexpression in Arabidopsis improved resistance to F. graminearum and the necrotrophic fungus Botrytis cinerea. It was shown that this altered response to pathogen attacks is associated with activation or depression of the SA- and JA-mediated defence pathways.
Materials and methods
Plant materials and growth conditions
Plant materials used in this study included common wheat accessions Wangshuibai, Meh0106, and Mianyang 87-19, einkorn wheat accessions TA2027 and M389, and Arabidopsis thaliana eco-type Columbia-0 (Col-0). TA2027 was introduced from the Wheat Germplasm Resource Center at Kansas State University courtesy of Dr BS Gill. ‘Meh0106’ was obtained from the progeny of Wangshuibai treated with 0.35% ethyl methanesulphonate. Wangshuibai plants were grown in a field in the experimental station of Nanjing Agricultural University, Nanjing, China, unless otherwise indicated. Arabidopsis plants were grown in a controlled environment chamber (150 μmol photons m−2 s−1, 10 h light/14 h dark per day at 20±2 °C).
Bioinformatics analysis
The protein sequence of barley LEM2 was used as a query to search for expressed sequence tags (ESTs) in the wheat dbEST (580 000 ESTs, the 154th release of GenBank, 2006) by TBLASTN. The cloned fragment was used as a query to search for EST homologues in the wheat dbEST (1 050 000 ESTs, the 159th release) by BLASTN. The conserved domain was predicted using the SMART database (http://smart.embl-heidelberg.de). Sequence alignments and sequence analyses were performed with the Macvector 10.0 software (Accelrys, USA).
DNA and RNA extraction, cDNA synthesis, and RT-PCR
Genomic DNA was extracted according to Ma and Sorrells (1995). Total RNA was extracted using Trizol reagent (Invitrogen, USA) and then subjected to RNase-free DNase I (Promega, USA) digestion and purification. First-strand cDNA was synthesized using Moloney murine leukaemia virus reverse transcriptase (Promega, USA) and oligo (dT15) primers according to the standard protocol.
RT-PCR for full-length cDNA isolation was performed in a 25 μl mixture containing ∼5 ng of template, 5 pmol of each primer, 5 nmol of each dNTP, 37.3 nmol MgCl2, 0.5 U of rTaq DNA polymerase (Takara, Japan), and 1× PCR buffer. The thermal cycle profile included 94 °C for 3 min; 30 cycles of 94 °C for 20 s, 60 °C for 30 s, 72 °C for 1.2 min; and a final extension of 72 °C for 5 min. The sequence of the forward primer is 5′-CTCTAGCTAGTTGCATCTTGATC-3′, and that of the reverse primer is 5'-CCGGGCTTGCGTAGTACAATAGG-3′. Sequencing was carried out at Invitrogen Corporation, Shanghai, China.
Generation of transgenic Arabidopsis plants
To generate cauliflower mosaic virus (CaMV) 35S-driven constructs, the open reading frame (ORF) amplified using primers 5′-TAATCTAGAATGGCCGGCGCTGTGAAGATT-3′ and 5′-TAAGGATCCGTCATCCAGCGGCACGACATA-3′ was cloned into a modified pBI121 expression vector, provided courtesy of Dr Deyue Yu of Nanjing Agricultural University. The underlined sequences indicate the introduced XbaI and BamHI sites, respectively. The recombinant vector was then transformed into Agrobacterium tumefaciens strain LBA4404 by electroporation. Arabidopsis transformation was performed using the floral-dip method (Clough and Bent, 1998). Transgenic plants were examined by PCR amplification of the introduced wheat DNA fragment and the 35S-driven expression was checked by RT-PCR (Supplementary Fig. S1 available at JXB online). The homozygous transgenic lines were identified and propagated to produce T3 seeds.
Chemical treatments
Ten-day old Wangshuibai seedlings were grown in 9 cm plastic Petri dishes at 25 °C/18 °C (day/night) with a 15 h photoperiod at 150 μmol m−2 s−1 and were used for chemical treatments, unless otherwise indicated.
From 15 to 25 seedlings at 10 d old were sprayed with 1 mM SA (Sigma, USA) plus 0.1% (v/v) Tween-20, and 0.1 mM methyl jasmonate (MeJA; Bio Basic Inc, Toronto, Canada) plus 0.1% Tween-20, respectively. Tissues in the SA treatment were collected 3, 6, 12, 24, and 36 h after spraying, and tissues in the MeJA treatment were collected 12, 24, and 48 h after spraying.
Paclobutrazol (PAC) is the inhibitor of benzoic acid 2-hydroxylase (BA2H) that converts benzoic acid to SA (León et al., 1995), and diethyldithiocarbamic acid (DIECA) inhibits JA biosynthesis (Farmer et al., 1994). PAC and DIECA treatments were performed in the 10-day-old seedlings by spraying with 100 μM PAC (Sigma, USA) and 1 mM DIECA (Sigma, USA) plus 0.1% (v/v) Tween-20, respectively. Leaf tissues were collected 24 h after spraying. Approximately 1 g of leaf tissue was harvested from each treatment for RNA extraction. Tissues sprayed with water containing 0.1% Tween-20 were used as a control for all treatments.
Barley stripe mosaic virus (BSMV) vector construction, in vitro transcription, and inoculation
BSMV ND18 α, β, and γ, and BSMV:PDS4as were provided courtesy of Dr Scofield of Purdue University. A 204 bp PCR-amplified fragment of the cloned gene using primers 5′-CCTTCATCAGCGGCACCTAC-3′ and 5′-CATCCAGCGGCACGACATAG-3′ was inserted into the γ-subfragment (Scofield et al., 2005; Bhullar et al., 2009). The sequence identity between this fragment and the other identified wheat homologues was >95%, thus making it possible to silence the cloned gene and its homologues (Holzberg et al., 2002). The virus RNAs were prepared by in vitro transcription using T7 DNA-dependent RNA polymerase (mMessage mMachine T7 Kit; Ambion). The primary leaves of 5-day-old seedlings of Mianyang 87-19 and TA2027 were treated with a mixture of three in vitro transcripts in a ratio of 1:1:1. Inoculation of plants with FES buffer (Scofield et al., 2005) or a 1:1:1 mixture of the α, β, and γ RNAs was used as the mock control. Blumeria graminis f. sp. tritici inoculation of TA2027 and F. graminearum inoculation of Mianyang 87-19 were performed 8 d and 14 d, respectively, after treatments with the viral RNA.
Fungal culture and pathogen inoculation assays
Wheat spike inoculation with F. graminearum followed the procedures described in Lin et al. (2006). The inoculated wheat spikes were covered immediately with plastic bags and subsequently collected 3, 9, and 12 h after infection for RNA extraction. Spikes sprayed with water were used as the mock treatment (i.e. the control).
Blumeria graminis f. sp. tritici inoculation of TA2027 and M389 followed the procedures described in Yao et al. (2007). Approximately 1 g of leaf tissue was collected 6, 12, 36, and 72 h following the inoculation. Tissues from seedlings without inoculation were used as the control.
In vitro F. graminearum and B. cinerea infection assays on detached Arabidopsis leaves followed the procedures described in Chen et al. (2006) and Kidd et al. (2009), respectively. For F. graminearum inoculation, the inoculum comprised 5 μl of conidial suspension of F. graminearum containing 5×105 conidia ml−1 and 75 μM deoxynivalenol (DON). For B. cinerea inoculation, the inoculum was a 5 μl of conidial suspension with 5×104 conidia ml−1. Disease severity assessment and conidium counting in the F. graminearum infection assay were carried out 7 days after inoculation (DAI) as described by Chen et al. (2006). This experiment was repeated three times with 14 plants representing each line. Disease severity in the B. cinerea infection assay was assessed 5 DAI. Botrytis cinerea was provided courtesy of Dr Dingzhong Tang, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences.
In the in vitro F. graminearum infection assay on wheat, 5 cm long leaf sections were cut from the central portion of the second leaves detached from Mianyang 87-19 seedlings and wounded on the adaxial surface, and were then placed in glass plates using the method of Chen et al. (2009). A conidial suspension of F. graminearum (5 μl of 1×106 conidia ml−1) with 75 μM DON was applied to the fresh wound on the adaxial surface. After inoculation, the plates were sealed and placed in a growth chamber (150 μmol photons m−2 s−1, 14 h light/10 h dark, 22 °C (day)/18 °C (night)). Conidial spores were counted 7 DAI.
Expression assays
For semi-quantitative RT-PCR (sqRT-PCR), the template was calibrated through RT-PCR amplification of the wheat α-tubulin gene. The thermal cycle parameters were 94 °C for 3 min; 20 or 31 cycles of 94 °C for 15 s, 60 °C for 25 s, 72 °C for 30 s; and a final extension of 72 °C for 5 min. PCR products were resolved on 1.5% agarose gels and visualized under UV light after staining with ethidium bromide.
Quantitative real-time PCR (qRT-PCR) was performed using a SYBR-Green PCR Mastermix (Toyoba, Japan) on a Bio-Rad iCYCLER iQ5 (Bio-Rad, USA). Each sample was analysed in triplicate. The experiment was repeated twice. Data were normalized using the reference gene (Actin2 of Arabidopsis or α-Tubulin of wheat). Relative expression was estimated using the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Primer sets used for sqRT-PCR and qRT-PCR are listed in Supplementary Table S1 at JXB online. The primers for sqRT-PCR and qRT-PCR of the cloned gene after virus-induced gene silencing (VIGS) were in regions with 100% sequence identity between the homologues to allow examination of all homologues.
Fungal hyphae staining
Fungal hyphae were stained with trypan blue according to Hein et al. (2005) and observed with ×100 magnification under a Nikon Eclipse 80i light microscope (Nikon, Kingston, UK).
Endogenous hormone measurement
Rosette leaves from six 6-week-old Arabidopsis plants (500 mg) were harvested for SA and JA extraction. The harvested tissues were immediately ground to a fine powder in liquid N2, and then exposed to extraction buffer (1.0 ml of 80% methanol) at 4 °C overnight. The samples were centrifuged at 15 200 g for 10 min, and the residues were re-extracted with 1.0 ml of 80% methanol. The supernatants were vacuum dried to dryness at –60 °C, then dissolved in 200 μl of 0.1 M sodium phosphate buffer (pH7.8), and extracted with 400 μl of petroleum ether. The aqueous phase was purified using a Waters Sep-Pak C18 cartridge. The cartridge was washed with 800 μl of ddH2O and then eluted with 1.5 ml of 50% methanol. The eluate with 50% methanol was vacuum dried. The dried extracts was dissolved in 50 μl of 10% acetonitrile and used for LC/MS assay in a Waters Acquity SQD (UPLC/MS) system.
A 10 μl aliquot of the sample was injected onto a Waters Acquity UPLC BEH C18 column (2.1×100 mm, 1.7 μm) at 30 °C. The mobile phase comprised solvent A (10% aqueous acetonitrile) and solvent B (acetonitrile) used in a gradient mode [time/concentration of A/concentration of B (min/%/%) for 0/90/10, 0.2/90/10, 2.0/10/90, 3/10/90, and 4/90/10]. The eluant flow rate employed was 0.3 ml min−1. The mass spectrometer was set to collect data in selected ion recording (SIR) mode using electrospray ionization (ESI) in negative ion mode. The optimized conditions are as follows: capillary voltage 2.8 kV, source temperature 120 °C, desolation temperature 350 °C, desolation gas flow 600 l h−1, cone gas flow 50 l h−1, molecular ions m/z 137 (SA) and 209.2 (JA).
Statistical analysis
Statistical comparison between data sets was performed with the pairwise t-test module installed in the Microsoft Office Excel 2008 program.
Results
The LEM2-like protein in wheat showed the greatest similarity to mJRL-like proteins
Mining wheat dbESTs of the 154th release using the barley LEM2 sequence resulted in seven wheat ESTs that encode LEM2-like proteins. Based on the assembled contig, a 1016 bp cDNA (HQ317136) including the entire ORF was isolated from the spike tissues of the hexaploid wheat cultivar Wangshuibai inoculated with F. graminearum. In a garden blot including DNA of common wheat, barley, maize (Zea mays), rice, and Arabidopsis, signals were solely produced in wheat in the form of three bands of difference sizes when the cloned fragment was used as a probe at 65 °C (data not shown). Mining wheat dbESTs of the 159th release using the cloned fragment did not reveal new homologous ESTs although the database size has almost doubled. There was >98% sequence identity between the cloned fragment and the seven homologous ESTs. Since common wheat is a hexaploid with three homoeologous subgenomes, it was concluded that the cloned fragment represents a single-copy gene in wheat.
A BLASTN search against the NCBI EST database using the cloned fragment identified only two non-wheat ESTs (GR332743 and GR332744 from Avena barbata) showing considerable similarity (with 82% identity in 45% query coverage). However, a TBLASTN search against this database identified six non-wheat ESTs, including the two A. barbata ESTs, three ESTs from Festuca arundinacea, and one from Lolium multiflorum, encoding proteins with >70% similarity to the protein sequence encoded by the cloned genes. All these species are monocot grasses. These results implied that orthologues of the cloned wheat gene exist in some but not all grass species.
The cloned cDNA encodes a protein of 301 residues without known signal peptides. A search against the NCBI protein database revealed that this protein contains two jacalin-like lectin domains. Among the plant proteins reported in literatures with a similar structure, it is most closely related to the mJRL-like barley LEM2, but with only 48% sequence identity (Fig. 1). It also shows similarity to other mJRL proteins, such as CCA (Nakamura et al., 2002) and CRLL (Haraguchi et al., 2006), and mJRL-like proteins such as AtMBP (Takeda et al., 2008), AtNSP4 (Burow et al., 2009), and EcJRL1 (Liu et al., 2009) (Fig. 1). These proteins have eight invariant residues in the first jacalin-like lectin domain and 13 in the second domain (Fig. 1). Most of these invariant residues are non-polar amino acids, predominantly glycine, and the aromatic amino acid phenylalanine, which are critical for the integrity of the β-prism fold and for packing the three Greek-key motifs of mannose-binding JRLs (Bourne et al., 1999). In the N-terminus of each domain, a GXXXD motif is present that conditions carbohydrate recognition capability (Raval et al., 2004). A highly conserved region near the C-terminus of each domain corresponds to the additional mannose-binding site in the Heltuba protein (Bourne et al., 1999). Since the cloned cDNA encodes a protein with all the conserved structural features of mJRL proteins, it represents an mJRL-like gene and was thus designated as TaJRLL1.
Fig. 1.
Sequence alignments of the protein encoded by the cloned cDNA fragment (TaJRLL1) and mJRL proteins or mJRL-like proteins with two jacalin-like lectin domains. The sequences were: barley LEM2 (AAM18206), Castanea crenata CCA (AAG40322.1), Arabidopsis AtMBP (NP_001030711) and AtNSP4 (NP_188262) (residues 1–292), Cycas revoluta CRLL (BAE95375.1), and Eichhornia crassipes EcJRL1 (ACT79247.1, the 81 residues to the N-terminus were not included). Dark shading and asterisks indicate conserved and invariant residues, respectively. Black triangles indicate mannose-binding sites in the Heltuba protein (Bourne et al., 1999). Black lined boxes are the GXXXD motifs.
Expression of TaJRLL1 is inducible by biotic stresses and defence phytohormones
The expression of TaJRLL1 was investigated by examination of its tissue-wide expression pattern. This gene is expressed at very low levels in root and leaf tissues as well as in developing seeds, but at high levels in stem and spike (Fig. 2A, left panel). Of the individual organ components of the spike, TaJRLL1 is expressed at higher levels in floret bracts, rachises, and spike awns (Fig. 2A, right panel).
Fig. 2.
Expression of TaJRLL1 and its responses to pathogen infections and variations in SA and JA levels. (A) Expression in tissues collected 15 d after anthesis from field-grown plants examined by RT-PCR. PCR cycles are shown in parentheses. (B–E) Relative expression levels of TaJRLL1 in spikes at anthesis at different times after inoculation with F. graminearum (B), in seedlings after inoculation with B. graminis f. sp. tritici isolate Bgt19 (C), and in seedlings after treatments with 1 mM SA (D) and 0.1 mM MeJA (E). (F and G) Relative expression levels of TaJRLL1, Glu2, and PR2 in seedlings 24 h after treatments with 100 μM PAC and 1 mM DIECA, respectively. ‘0’ represents no treatment (B and C) or simulated treatment (D–F), and CK represents simulated treatment (G). Error bars represent the standard deviation from three replicated experiments.
TaJRLL1 was rapidly induced during F. graminearum infection in the FHB-resistant Wangshuibai and the transcripts peaked at 9 h after the infection. This induction also occurred in the FHB-susceptible Meh0106 mutant of Wangshuibai, but at a lower level (Fig. 2B). Similar inducible expression patterns were also observed in the seedling leaves of powdery mildew-resistant T. monococcum accession TA2027 and susceptible accession M389 after inoculation with the powdery mildew pathogen B. graminis f. sp. tritici (Fig. 2C). The strong induction of TaJRLL1 following pathogen infection in a resistant genotype suggests its involvement in disease resistance reactions.
Early plant defences against pathogen attack usually involve SA and JA signalling pathways. Therefore, TaJRLL1 expression in response to exogenous application of SA and JA was examined. It was found that TaJRLL1 expression was strongly induced in Wangshuibai seedlings after 1 mM SA or 0.1 mM MeJA spray treatment (Fig. 2D, E). Therefore, TaJRLL1 could be associated with both SA and JA defence pathways.
Expression of TaJRLL1 requires both SA and JA
TaJRLL1 expression was investigated to determine if it is a component of the SA and JA signalling pathways. To accomplish this, 10-day-old wheat seedlings were treated with 100 μM PAC or 1 mM DIECA for 24 h. The treatments significantly reduced the endogenous SA and JA contents (Supplementary Fig. S2 at JXB online) and severely reduced the expression of the SA pathway marker genes Glu2 and PR2 and the JA pathway marker gene PR3, and remarkably nearly abolished the expression of TaJRLL1 (Fig. 2F, G). The results implied that the expression of TaJRLL1 requires both SA and JA.
Silencing TaJRLL1 in wheat seedlings weakened resistance to hemi-biotrophic F. graminearum and biotrophic B. graminis f. sp. tritici
To determine whether TaJRLL1 is involved in resistance to F. graminearum, VIGS was used to suppress its expression in Mianyang 87-19, a hexaploid wheat cultivar susceptible to F. graminearum. BSMV:PDS4as [with a barley phytoene desaturase 4 (PDS4) fragment inserted in the antisense orientation] was used to verify the feasibility of BSMV-mediated gene silencing in wheat. PDS is the essential component of the carotenoid pigment biosynthetic pathway and its silencing causes chlorophyll photolysis. As expected, photobleaching was observed in the second leaves 8 DAI (Fig. 3A), suggesting that the BSMV VIGS system used is functional.
Fig. 3.
VIGS-mediated TaJRLL1 silencing in Mianyang 87-19 led to increased susceptibility to F. graminearum infection. (A) Seedling leaf phenotypes 21 DAI with FES buffer and the BSMV:PDS construct. (B–E) Leaves were inoculated with the inocula indicated under the respective panels and then inoculated 14 d later at the wound sites with water (B) or F. graminearum (C–E). The photos were taken 7 d after pathogen inoculation. (F) Fungal growth on leaves of Mianyang 87-19 inoculated with BSMV:00 (upper panel) and BSMV:TaJRLL1 (lower panel), observed 7 d after pathogen infection using trypan blue staining. (G) Conidial production 7 d after pathogen inoculation on leaves inoculated with FES (Scofield et al., 2005) (F), BSMV:00 (B), or BSMV:TaJRLL1 (S). Error bars represent the standard deviation of 24 plants. Asterisks indicate significant differences at P=0.05. (H) TaJRLL1 qRT-PCR with RNA from leaves 8 DAI with FES (F), BSMV:00 (B-1 and B-2), and BSMV:TaJRLL1 (S-1, S-2, and S3). Error bars represent the standard deviation from three replicated experiments. Scale bars=0.5 cm.
When detached leaves from seedlings were challenged with F. graminearum 2 weeks after inoculation with water, FES buffer alone, RNA mixtures of α, β, and γ construct BSMV:00, or RNA mixtures of α, β, and γ construct BSMV:TaJRLL1, disease development at the pathogen inoculation points was noted for all treatments on the seventh day after inoculation (Fig. 3B–E); however, the symptoms on leaves from plants treated with BSMV:TaJRLL1 were more severe. Significantly more and faster hyphae growth and increased conidial yield was noted in the TaJRLL1-silenced leaves than in the control (Fig. 3F, G), together with a much lower level of TaJRLL1 expression (Fig. 3H). Therefore, silencing TaJRLL1 enhanced susceptibility to F. graminearum infection.
In addition, experiments were carried out to determine if TaJRLL1 was involved in wheat resistance to B. graminis f. sp. tritici infection. Similar virus RNA inoculations were applied to TA2027 primary leaves at the one-leaf stage, and powdery mildew isolate Bgt19 was used to inoculate plants 8 d later. The PDS4as-inoculated control again exhibited the expected photobleaching phenotype in the second leaves (Fig. 4A). The leaves of the susceptible control M389 were almost fully covered with fungal mycelia on the eighth day after pathogen inoculation (Fig. 4B), indicating successful pathogen inoculation and disease development. Compared with disease symptoms observed on TA2027 leaves inoculated with either FES buffer or BSMV:00 mixture, an increase in white powdery spots and hyphae was observed on leaves of BSMV:TaJRLL1-treated TA2027 plants (Fig. 4C–F). Furthermore, TaJRLL1 transcripts in BSMV:TaJRLL1-inoculated plants were substantially reduced relative to BSMV:00-treated plants (Fig. 4G). These results indicated that TaJRLL1 silencing reduced TA2027 resistance to B. graminis f. sp. tritici. However, TaJRLL1-silenced TA2027 remained more resistant than M389, suggesting that TaJRLL1 is not sufficient to overcome powdery mildew.
Fig. 4.
VIGS-mediated TaJRLL1 silencing in TA2027 led to reduced resistance to B. graminis. (A) Leaf phenotypes of TA2027 seedlings 10 DAI with FES (left) and BSMV:PDS4as (right). (B) Disease symptoms of M389 seedlings 8 DAI with B. graminis f. sp. tritici isolate Bgt19. (C–E) TA2027 disease symptoms 8 DAI of Bgt19 in leaves pre-inoculated with FES buffer, BSMV:00, and BSMV:TaJRLL1, respectively. (F) Hyphae growth on the leaves shown in D and E, observed after trypan blue staining. Scale bars=100 μm. (G) TaJRLL1 qRT-PCR with RNA from TA2027 seedlings 8 d after inoculation with FES buffer (F), BSMV:00 (B-9, B-11, plants inoculated with BSMV:00), and BSMV:TaJRLL1 (S-15, S-16, S-17, S-19, plants inoculated with BSMV:TaJRLL1). The experiment was repeated three times with n >12. Each repeat generated similar results.
Expression of TaJRLL1 in Arabidopsis enhanced resistance to F. graminearum and B. cinerea
The involvement of TaJRLL1 in plant defence against pathogen attack was further addressed by investigating Arabidopsis plants transformed with the 35S promoter-driven TaJRLL1 for resistance to infection by F. graminearum and the necrotrophic fungus B. cinerea. Rosette leaves were detached from 4-week-old plants and inoculated with F. graminearum. Subsequently, marked necrobiosis and fungal hyphae appeared, regardless of whether the leaves were from transgenic or from untransformed control plants (Fig. 5A). However, the transgenic plants showed significantly reduced severity (Fig. 5A, B), and had far fewer conidia on the leaves relative to the control (Fig. 5C). A similar result was obtained when leaves were inoculated with B. cinerea (Fig. 5D). The average lesion area estimated by lesion diameter was significantly smaller in the transgenic lines than in the control (Fig. 5E). Consequently, overexpressing TaJRLL1 in Arabidopsis enhanced resistance to both F. graminearum and B. cinerea.
Fig. 5.
Arabidopsis transformed with 35S:TaJRLL1 showed increased resistance to F. graminearum and B. cinerea. (A and B) Fusarium graminearum disease symptoms and severity developed on detached rosette leaves at 7 DAI. Col-0, wild-type; O-1 and O-5, transgenic plants. (C) Conidial production on leaves. (D and E) Botrytis disease symptoms and disease lesion size on detached rosette leaves at 5 DAI. Error bars represent the standard deviation from three (B and C) or four (E) replicated experiments. Asterisks indicate significant differences from the control at P=0.05.
TaJRLL1 regulates the expression of a set of defence response genes
Because TaJRLL1 expression is SA and JA dependent and related to disease resistance, the expression of a set of genes associated with SA and JA pathways was examined in the TaJRLL1-silenced wheat plants and TaJRLL1-transformed Arabidopsis.
The genes investigated for SA signalling included wheat Glu2 and Arabidopsis PR1 and PR2. Expression of NPR1 was investigated in both wheat and Arabidopsis. These genes are positively related to the SA defence pathway (Thomma et al., 1998; Kinkema et al., 2000; Lu et al., 2005). All genes examined in the TaJRLL1-silenced wheat plants were expressed at a substantially lower level (Fig. 6A, B), and all genes examined in transgenic Arabidopsis were expressed at a significantly higher level (Fig. 6G–I).
Fig. 6.
TaJRLL1 positively regulated components of the SA- and JA-dependent signalling pathways. Error bars represent the standard deviation from three replicated experiments. (A–F) Expression of Glu2, NPR1, COI1, ERF1, PR3, and EIN3 in TaJRLL1-silenced seedlings. B-9, plants inoculated with BSMV:00; S-15 and S-16, plants inoculated with BSMV:TaJRLL1. (G–N) Expression of PR1, PR2, NPR1, COI1, ERF1, PR3, PDF1.2, and EIN2 in Arabidopsis plants transformed with TaJRLL1. Col-0, wild-type; O-1 and O-5, transgenic plants.
The following genes were investigated for JA/ethylene (ET) signalling: COI1, ERF1, PR3 in both wheat and Arabidopsis, EIN3 in wheat, and EIN2 and PDF1.2 in Arabidopsis. A substantial reduction in expression of COI1, ERF1, and PR3 was noted in TaJRLL1-silenced wheat plants (Fig. 6C–E), and a substantial increase in expression of PDF1.2 in addition to COI1, ERF1, and PR3 was seen in transgenic Arabidopsis (Fig. 6J–M). These genes are positively related to the JA defence pathway (Thomma et al., 1998; Lorenzo et al., 2003).
EIN3 expression in the TaJRLL1-silenced wheat plants and EIN2 expression in the TaJRLL1-transformed Arabidopsis plants were not affected (Fig. 6F, N). EIN2 and EIN3 are key components of the ET signalling pathway (Thomma et al., 1999; Guo et al., 2003). These results imply that the functions of TaJRLL1 are pathway dependent.
TaJRLL1 affects endogenous SA and JA accumulation
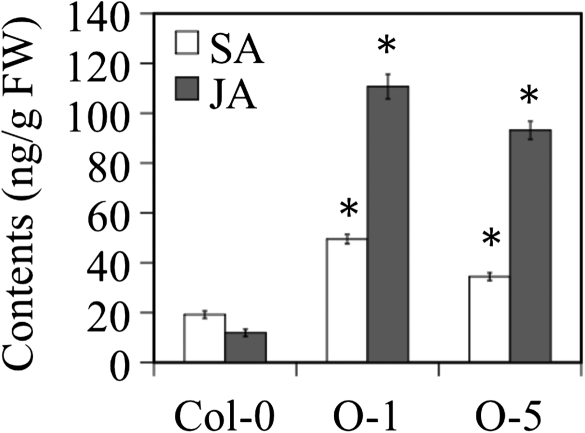
To test if TaJRLL1 affects the SA and JA levels, their endogenous contents in the leaves of TaJRLL1-transformed Arabidopsis were examined. The obtained data showed that the contents of free SA and JA in the transgenic plants were significantly higher than in the untransformed plants at P=0.01 (Fig. 7). This change was probably associated with elevated resistance. Application of both SA and JA enhanced Arabidopsis resistance to B. cinerea (Supplementary Fig. S3 at JXB online). Application of SA also resulted in elevated resistance to powdery mildew in the disease-susceptible T. monococcum accession M389 (Supplementary Fig. S3).
Fig. 7.
Overexpression of TaJRLL1 in Arabidopsis elevated free SA and JA levels. FW, fresh weight; O-1 and O-5, transgenic plants. Error bars represent the standard deviation from three replicated experiments. Asterisks indicate significant difference from the control at P=0.01.
Discussion
TaJRLL1 is a defence-related mJRL-like gene
In this study, the pathogen-induced wheat TaJRLL1 gene was characterized. TaJRLL1 encodes a protein with two jacalin-like lectin domains without a signal peptide, and structurally it has the necessary features of an mJRL protein. The mJRL-like proteins have been characterized in many monocot and dicot plant species (Lannoo and van Damme, 2010), but TaJRLL1 does not have a high level of sequence homology to them.
Many JRLs have multiple jacalin-like lectin domains in a tandem arrangement. Some JRLs also have other structural domains, for instance the dirigent and Kelch domains. Different JRLs vary substantially at the sequence level. This structural diversity contributes to the functional diversity of the JRL proteins. Currently, there are only a few characterized mJRL proteins with two tandem arranged jacalin-like lectin domains. LEM2 is so far the only one from a monocot species reported in the literatures (Abebe et al., 2005). OsJAC1, wheat WCI-1, Hfr-1, VER2, and Ta-JA1 all have an N-terminal dirigent domain and a C-terminal jacalin-like lectin domain (Görlach et al., 1996; Esen and Blanchard, 2000; Williams et al., 2002; Yong et al., 2003; Wang and Ma, 2005; Jiang et al., 2006). Ipomoelin of sweet potato, Helianthus tuberosus HTA1, Arabidopsis RTM1, and rice Orysata have only one jacalin-like lectin domain (Imanishi et al., 1997; Nakagawa et al., 2000; Chisholm et al., 2001; Qin et al., 2003). Arabidopsis JAL23 and JAL35 have three jacalin-like lectin domains (Nagano et al., 2008); AtNSP4 is comprised of two jacalin-like lectin domains and five Kelch domains (Burow et al., 2009).
Lem2 and TaJRLL1 were observed to have both similarity and incongruity at the gene expression level, depending on the tissues investigated. Both genes are expressed in spike tissues, such as floret bracts, rachises, and awns, and respond to SA treatment. TaJRLL1 is also expressed in stems in considerable abundance and is up-regulated by applying MeJA; however, Lem2 exhibits the absence of expression in stems, and is not responsive to MeJA treatment (Abebe et al., 2005). Meanwhile, TaJRLL1 is inducible by fungal pathogen. It would be interesting to find out if the homologues of TaJRLL1 in A. barbata, F. arundinacea, and L. multiflorum have a similar expression pattern.
The induction of expression of JRL-like genes by SA (or its analogues) or JA (or its analogues) or both has been well documented. HTA1, Ipomoelin, barley Hv-JA1, OsJAC1, Orysata, and VER2 are responsive to MeJA treatment (Lee et al., 1996; Imanishi et al., 1997; Garcia et al., 1998; Nakagawa et al., 2000; Yong et al., 2003; Jiang et al., 2006). Both Hfr-1 and WCI-1 are up-regulated by SA treatment and the latter is also responsive to MeJA treatment (Subramanyam et al., 2006). In addition to defence hormone inducibility, mJRL-like genes including AtNSP1, Hfr-1, creeping bentgrass Crs1, WCI-1, and Orysata have been associated with defence due to their induction by pathogen infection or pest attack (Williams et al., 2002; Qin et al., 2003; Li et al., 2005; Subramanyam et al., 2006; Burow et al., 2009).
TaJRLL1 participates in basal resistance through SA- and JA-dependent signalling pathways
Several lines of evidence have been provided to support the participation of TaJRLL1 in defence against pathogens. First, the expression of TaJRLL1 is inducible by both hemi-biotrophic F. graminearum and biotrophic B. graminis infections and treatments by defence hormones, such as SA and MeJA. Secondly, TaJRLL1 transcripts showed more rapid accumulation in disease-resistant genotypes than in disease-susceptible genotypes following pathogen inoculations. Tomato Rcr3 has an expression profile similar to that of TaJRLL1; Krüger et al. (2002) demonstrated that Rcr3 is required for Cf-2-dependent disease resistance and suppression of autonecrosis. Thirdly, VIGS-mediated TaJRLL1 silencing resulted in weakened resistance to F. graminearum and B. graminis, while expression of TaJRLL1 in Arabidopsis enhanced the resistance to F. graminearum and B. cinerea. Although Arabidopsis genes that encode proteins with jacalin-like lectin domains could be induced by pathogen infections and subsequently lead to resistance, they are unlikely to have caused the disease symptom difference between the transgenic plants and the recipient parent. However, it was noted that resistance to B. graminis was not completely lost in TaJRLL1-silenced plants. This might be due to insufficient silencing or other pathways that contribute to resistance. It is also likely that TaJRLL1 takes part in defence by potentiating resistance.
Variation in resistance levels, as demonstrated by changes in TaJRLL1 expression, was associated with variation in SA and JA levels and changes in expression of other known SA and JA pathway genes. The changes in expression of marker genes for SA and JA defence signalling pathways and of TaJRLL1 in seedlings treated with SA and JA biosynthesis inhibitors (Fig. 2F, G) implied that both hormones might be required for appropriate TaJRLL1 expression. In support of TaJRLL1 participation in SA- and JA-mediated defence pathways, expression of genes involved in the two pathways was either enhanced in TaJRLL1 transgenic plants or reduced in TaJRLL1-silenced plants (Fig. 6). It could be deduced from these results that TaJRLL1 participates in resistance through SA- and JA-dependent defence pathways. TaJRLL1 affects ET signalling in association with ERF1 (Fig. 6), the convergent point of the JA and ET defence pathways.
It is well documented that the SA-mediated defence and JA-mediated defence are usually mutually antagonistic and are responsible for resistance to biotrophic and necrotrophic pathogens, respectively (Glazebrook, 2005). Therefore, TaJRLL1 activation requiring both SA and JA suggests a positive cross-talk of these two defence pathways. Interestingly, TaJRLL1 is responsive to all trophic types of pathogenic fungi. Previous reports indicate that SA- and JA-dependent defence pathways respond to infection by F. graminearum (Pritsch et al., 2000, 2001; Chen et al., 2006; Makandar et al., 2010). Zimmerli et al. (2001) also demonstrated that SA- and JA-dependent signalling both contribute resistance to the necrotrophic fungal pathogen B. cinerea. These findings clearly show that SA- and JA-mediated defences are required for resistance to some types of pathogens, and genes such as TaJRLL1 that function harmoniously might play a unique role. TaJRLL1 is not alone in this type of function. Recently, a few genes were reported to regulate synergistic interactions of SA- and JA-dependent defences, for instance Arabidopsis FAAH and PFT1 (Kang et al., 2008; Kidd et al., 2009) and rice OsWRKY45-1 (Tao et al., 2009). However, unlike TaJRLL1, both AtFAAH and OsWRKY45-1 are negative regulators.
TaJRLL1 might affect the biosynthesis of SA and JA
It was shown that the endogenous SA levels in TaJRLL1-transgenic plants were significantly higher than in untransformed plants, and the expression of NPR1, which is inducible by SA, was also expressed at a significantly higher level. Thus, it is possible that TaJRLL1, directly or indirectly, affects SA biosynthesis, and alterations in the SA levels subsequently cause variation in NPR1 expression. In accordance with these results, Hwang and Hwang (2011) demonstrated that CaMBL1 of pepper, a mannose-binding lectin localized to the plasma membrane, induces accumulation of SA, activation of defence-related genes, and cell death phenotype in pepper. Induction of NPR1 genes by SA or its analogues has been reported in various plant species (Yuan et al., 2007; Endah et al., 2008). It is interesting to note that, similarly to SA content, the JA content in TaJRLL1 transgenic plants was also significantly increased. This result implies that TaJRLL1 could also affect JA biosynthesis. It is therefore not surprising that as a consequence of TaJRLL1 regulation, the JA pathway downstream genes, such as COI1 and ERF1, were all up-regulated in TaJRLL1 transgenic plants, and down-regulated in TaJRLL1-silenced plants.
Based on the present results, it is hypothesized that when the wheat plants are infected by pathogens, the activation of SA or JA accumulation (Fan et al., 2009; Makandar et al., 2010) induces expression of TaJRLL1 and other downstream genes in SA or JA defence pathways. The synthesis of TaJRLL1 in turn causes more SA and JA accumulation by regulating expression of their biosynthetic genes, and thus amplification of the related defence signalling pathways. This positive feedback regulation of TaJRLL1 might explain why TaJRLL1 contributes to resistance against fungi of different trophic types. Even though TaJRLL1 does not have an apparent homologue in Arabidopsis, it might have enhanced resistance in the TaJRLL1-transformed Arabidopsis plants. Similarly, Xiao et al. (2003) reported that the Arabidopsis-specific RPW8 conferred powdery mildew resistance in transgenic tobacco plants.
Carbohydrate-binding proteins such as mJRLs are a group of substances critical for cellular activities. Even though information on sugar signalling in plant defence is just emerging, its functional mechanisms and cross-talk with other defence pathways remain to be elucidated. Therefore, investigations of the carbohydrate binding specificity of TaJRLL1 and of how TaJRLL1 functions in SA and JA signalling and/or biosynthesis are important to clarify the molecular mechanisms of its involvement in disease resistance.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Detection and expression analysis of TaJRLL1 in transgenic Arabidopsis plants.
Figure S2. Free SA and JA levels in wheat seedlings 24 h after treatments with 100 μM PAC and 1 mM DIECA, respectively.
Figure S3. The effects of the application of SA and JA on resistance.
Table S1. Primers used for sqRT-PCR and qRT-PCR.
Acknowledgments
This study was partially supported by the NSFC program (30721140555, 31030054, and 30430440), the GCP Project (SP2-1), the Special Transgenic Plant Fund 2009ZX08009-049B, the ‘863’ program (2006AA10A104), the ‘111’ project (B08025), the PAPD project of Jiangsu Higher Education Institutions, and the Open Funds of State Key Laboratory of Crop Genetics & Germplasm Enhancement (ZW2007006). We are grateful to Mark E. Sorrells of Cornell University for reviewing the manuscript.
References
- Abebe T, Skadsen RW, Kaeppler HF. A proximal upstream sequence controls tissue-specific expression of Lem2, a salicylate-incucible barley lectin-like gene. Planta. 2005;221:170–183. doi: 10.1007/s00425-004-1429-9. [DOI] [PubMed] [Google Scholar]
- Beckers GJM, Spoel SH. Fine-tuning plant defense signaling: salicylate versus jasmonate. Plant Biology. 2006;8:1–10. doi: 10.1055/s-2005-872705. [DOI] [PubMed] [Google Scholar]
- Bhullar NK, Street K, Mackay M, Yahiaoui N, Keller B. Unlocking wheat genetic resources for the molecular identification of previously undescribed functional alleles at the Pm3 resistance locus. Proceedings of the National Academy of Sciences, USA. 2009;106:9519–9524. doi: 10.1073/pnas.0904152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne Y, Zamboni V, Barre A, Peumans WJ, Van Damme EJM, Rougé P. Helianthus tuberosus lectin reveals a widespread scaffold for mannose-binding lectins. Structure. 1999;7:1473–1482. doi: 10.1016/s0969-2126(00)88338-0. [DOI] [PubMed] [Google Scholar]
- Bunn-Moreno MM, Campos-Neto A. Lectin(s) extracted from seeds of Artocarpus integrifolia (jackfruit): potent and selective stimulator(s) of distinct human T and B cell function. Journal of Immunology. 1981;127:427–429. [PubMed] [Google Scholar]
- Burow M, Losansky A, Müller R, Plock A, Kliebenstein DJ, Wittstock U. The genetic basis of constitutive and herbivore-induced ESP-independent nitrile formation in Arabidopsis. Plant Physiology. 2009;149:561–574. doi: 10.1104/pp.108.130732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Steed A, Harden C, Nicholson P. Characterization of Arabidopsis thaliana–Fusarium graminearum interactions and identification of variation in resistance among ecotypes. Molecular Plant Pathology. 2006;7:391–403. doi: 10.1111/j.1364-3703.2006.00349.x. [DOI] [PubMed] [Google Scholar]
- Chen X, Steed A, Travella S, Keller B, Nicholson P. Fusarium graminearum exploits ethylene signaling to colonize dicotyledonous and monocotyledonous plants. New Phytologist. 2009;182:975–983. doi: 10.1111/j.1469-8137.2009.02821.x. [DOI] [PubMed] [Google Scholar]
- Chisholm ST, Parra MA, Anderberg RJ, Carrington JC. Arabidopsis RTM1 and RTM2 genes function in phloem to restrict long-distance movement of tobacco etch virus. Plant Physiology. 2001;127:1667–1675. [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Endah R, Beyene G, Kiggundu A, van den Berg N, Schlüter U, Kunert K, Chikwamba R. Elicitor and Fusarium-induced expression of NPR1-like genes in banana. Plant Physiology and Biochemistry. 2008;46:1007–1014. doi: 10.1016/j.plaphy.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Esen A, Blanchard DJ. A specific β-glucosidase-aggregating factor is responsible for the β-glucosidase null phenotype in maize. Plant Physiology. 2000;122:563–572. doi: 10.1104/pp.122.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Hill L, Crooks C, Doerner P, Lamb C. Abscisic acid has a key role in modulating diverse plant–pathogen interactions. Plant Physiology. 2009;150:1750–1761. doi: 10.1104/pp.109.137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Caldelari D, Pearce D, Walker-Simmons MK, Ryan CA. Diethyldithiocarbamic acid inhibits the octadecanoid signaling pathway for the wound induction of proteinase inhibitors in tomato leaves. Plant Physiology. 1994;106:337–342. [Google Scholar]
- Garcia AB, Engler Jde A, Claes B, Villarroel R, Van Montagu M, Gerats T, Caplan A. The expression of the salt-responsive gene salT from rice is regulated by hormonal and developmental cues. Planta. 1998;207:172–180. doi: 10.1007/s004250050470. [DOI] [PubMed] [Google Scholar]
- Glazebrook J. Genes controlling expression of defense responses in Arabidopsis—2001 status. Current Opinion Plant Biology. 2001;4:301–308. doi: 10.1016/s1369-5266(00)00177-1. [DOI] [PubMed] [Google Scholar]
- Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annual Review of Phytopathology. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Chen W, Estes B, Chang HS, Nawrath C, Métraux JP, Zhu T, Katagiri F. Topology of the network integrating salicylate and jasmonate signal transduction derived from global expression phenotyping. The Plant Journal. 2003;34:217–228. doi: 10.1046/j.1365-313x.2003.01717.x. [DOI] [PubMed] [Google Scholar]
- Görlach J, Volrath S, Knauf-Beiter G, et al. Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. The Plant Cell. 1996;8:629–643. doi: 10.1105/tpc.8.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Ecker JR. Plant responses to ethylene gas are mediated by SCF(EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor. Cell. 2003;115:667–677. doi: 10.1016/s0092-8674(03)00969-3. [DOI] [PubMed] [Google Scholar]
- Haraguchi T, Nomura K, Yagi F. Cloning and expression of a mannose-binding jacalin-related lectin from leaves of Japanese cycad (Cycas revoluta Thunb.) Bioscience, Biotechnology, and Biochemistry. 2006;70:2222–2229. doi: 10.1271/bbb.60156. [DOI] [PubMed] [Google Scholar]
- Hein I, Barciszewska-Pacak M, Hrubikova K, Williamson S, Dinesen M, Soenderby IE, Sundar S, Jarmolowski A, Shirasu K, Lacomme C. Virus-induced gene silencing-based functional characterization of genes associated with powdery mildew resistance in barley. Plant Physiology. 2005;138:2155–2164. doi: 10.1104/pp.105.062810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzberg S, Brosio P, Gross C, Pogue G. Barley stripe mosaic virus induced gene silencing in a monocot plant. The Plant Journal. 2002;30:315–327. doi: 10.1046/j.1365-313x.2002.01291.x. [DOI] [PubMed] [Google Scholar]
- Hwang IS, Hwang BK. The pepper mannose-binding lectin gene CaMBL1 is required to regulate cell death and defense responses to microbial pathogens. Plant Physiology. 2011;155:447–463. doi: 10.1104/pp.110.164848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi S, Kito-Nakamura K, Matsuoka K, Morikami A, Nakamura K. A major jasmonate-inducible protein of sweet potato, ipomoelin, is an ABA-independent wound-inducible protein. Plant and Cell Physiology. 1997;38:643–652. doi: 10.1093/oxfordjournals.pcp.a029216. [DOI] [PubMed] [Google Scholar]
- Jiang JF, Han Y, Xing LJ, Xu YY, Xu ZH, Chong K. Cloning and expression of a novel cDNA encoding a mannose-specific jacalin-related lectin from. Oryza sativa. Toxicon. 2006;47:133–139. doi: 10.1016/j.toxicon.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Kidd BN, Edgar CI, Kumar KK, Aitken EA, Schenk PM, Manners JM, Kazan K. The mediator complex subunit PFT1 is a key regulator of jasmonate-dependent defense in. Arabidopsis. The Plant Cell. 2009;21:2237–2252. doi: 10.1105/tpc.109.066910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Wang YS, Uppalapati SR, et al. Overexpression of a fatty acid amide hydrolase compromises innate immunity in Arabidopsis. The Plant Journal. 2008;56:336–349. doi: 10.1111/j.1365-313X.2008.03603.x. [DOI] [PubMed] [Google Scholar]
- Kinkema M, Fan W, Dong X. Nuclear localization of NPR1 is required for activation of PR gene expression. The Plant Cell. 2000;12:2339–2350. doi: 10.1105/tpc.12.12.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger J, Thomas CM, Golstein C, Dixon MS, Smoker M, Tang SJ, Mulder L, Jones JDG. A tomato cysteine protease required for Cf-2-dependent disease resistance and suppression of autonecrosis. Science. 2002;296:744–747. doi: 10.1126/science.1069288. [DOI] [PubMed] [Google Scholar]
- Lannoo N, Van Damme EJ. Nucleocytoplasmic plant lectins. Biochimica et Biophysica Acta. 2010;1800:190–201. doi: 10.1016/j.bbagen.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Lee J, Parthier B, Löbler M. Jasmonate signaling can be uncoupled from abscisic acid signaling in barley: identification of jasmonate-regulated transcripts, which are not induced by abscisic acid. Planta. 1996;199:625–632. doi: 10.1007/BF00195196. [DOI] [PubMed] [Google Scholar]
- León J, Shulaev V, Yalpani N, Lawton MA, Raskin I. Benzoic acid 2-hydroxylase, a soluble oxygenase from tobacco, catalyzes salicylic acid biosynthesis. Proceedings of the National Academy of Sciences, USA. 1995;92:10413–10417. doi: 10.1073/pnas.92.22.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HM, Rotter D, Bonos SA, Meyer WA, Belanger FC. Identification of a gene in the process of being lost from the genus. Agrostis. Plant Physiology. 2005;138:2386–2395. doi: 10.1104/pp.105.063297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Xue SL, Zhang ZZ, et al. Mapping QTL associated with resistance to Fusarium head blight in the Nanda2419×Wangshuibai population II. Type I resistance. Theoretical and Applied Genetics. 2006;112:528–535. doi: 10.1007/s00122-005-0156-3. [DOI] [PubMed] [Google Scholar]
- Liu X, Chen X, Oliver DJ, Xiang CB. Isolation of a low-sulfur tolerance gene from Eichhornia crassipes using a functional gene-mining approach. Planta. 2009;231:211–219. doi: 10.1007/s00425-009-1045-9. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Piqueras R, Sánchez-Serrano JJ, Solano R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. The Plant Cell. 2003;15:165–178. doi: 10.1105/tpc.007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZX, Gaudet D, Puchalski B, Despins T, Frick M, Laroche A. Inducers of resistance reduce common bunt infection in wheat seedlings while differentially regulating defense-gene expression. Physiological and Molecular Plant Pathology. 2005;67:138–148. [Google Scholar]
- Ma QH, Tian B, Li YL. Overexpression of a wheat jasmonate-regulated lectin increases pathogen resistance. Biochimie. 2010;92:187–193. doi: 10.1016/j.biochi.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ZQ, Sorrells ME. Genetic analysis of fertility restoration in wheat using restriction fragment length polymorphisms. Crop Science. 1995;35:1137–1143. [Google Scholar]
- Makandar R, Nalam V, Chaturvedi R, Jeannotte R, Sparks AA, Shah J. Involvement of salicylate and jasmonate signaling pathways in Arabidopsis interaction with Fusarium graminearum. Molecular Plant-Microbe Interactions. 2010;23:861–870. doi: 10.1094/MPMI-23-7-0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano AJ, Fukao Y, Fujiwara M, Nishimura M, Hara-Nishimura I. Antagonistic jacalin-related lectins regulate the size of ER body-type β-glucosidase complexes in. Arabidopsis thaliana. Plant and Cell Physiology. 2008;49:969–980. doi: 10.1093/pcp/pcn075. [DOI] [PubMed] [Google Scholar]
- Nakagawa R, Yasokawa D, Okumura Y, Nagashima K. Cloning and sequence analysis of cDNA coding for a lectin from Helianthus tuberosus callus and its jasmonate-induced expression. Bioscience, Biotechnology, and Biochemistry. 2000;64:1247–1254. doi: 10.1271/bbb.64.1247. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Ikegami A, Matsumura Y, Nakanishi T, Nomura K. Molecular cloning and expression of the mannose/glucose specific lectin from Castanea crenata cotyledons. Journal of Biochemistry. 2002;131:241–246. doi: 10.1093/oxfordjournals.jbchem.a003094. [DOI] [PubMed] [Google Scholar]
- Peumans WJ, Hause B, Van Damme EJ. The galactose-binding and mannose-binding jacalin-related lectins are located in different subcellular compartments. FEBS Letters. 2000;477:186–192. doi: 10.1016/s0014-5793(00)01801-9. [DOI] [PubMed] [Google Scholar]
- Pritsch C, Muehlbauer GJ, Bushnell WR, Somers DA, Vance CP. Fungal development and induction of defense response genes during early infection of wheat spikes by Fusarium graminearum. Molecular Plant-Microbe Interactions. 2000;13:159–169. doi: 10.1094/MPMI.2000.13.2.159. [DOI] [PubMed] [Google Scholar]
- Pritsch C, Vance CP, Bushnell WR, Somers DA, Hohn TM, Muehlbauer GJ. Systemic expression of defense response genes in wheat spikes as a response to Fusarium graminearum infection. Physiological and Molecular Plant Pathology. 2001;58:1–12. [Google Scholar]
- Qin QM, Zhang Q, Zhao WS, Wang YY, Peng YL. Identification of a lectin gene induced in rice in response to Magnaporthe grisea infection. Acta Botanica Sinica. 2003;45:76–81. [Google Scholar]
- Raval S, Gowda SB, Singh DD, Chandra NR. A database analysis of jacalin-like lectins: sequence–structure–function relationships. Glycobiology. 2004;14:1247–1263. doi: 10.1093/glycob/cwh140. [DOI] [PubMed] [Google Scholar]
- Scofield SR, Huang L, Brandt AS, Gill BS. Development of a virus-induced gene-silencing system for hexaploid wheat and its use in functional analysis of the Lr21 mediated leaf rust resistance pathway. Plant Physiology. 2005;138:2165–2173. doi: 10.1104/pp.105.061861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanyam S, Sardesai N, Puthoff DP, Meyer JM, Nemacheck JA, Gonzalo M, Williams CE. Expression of two wheat defense-response genes, Hfr-1 and WCI-1, under biotic and abiotic stresses. Plant Science. 2006;170:90–103. [Google Scholar]
- Subramanyam S, Smith DF, Clemens JC, Webb MA, Sardesai N, Williams CE. Functional characterization of HFR-1, a high mannose N-glycan-specific wheat lectin induced by Hessian fly larvae. Plant Physiology. 2008;147:1412–1426. doi: 10.1104/pp.108.116145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Sugimori N, Torizawa T, et al. Structure of the putative 32 kDa myrosinase-binding protein from Arabidopsis (At3g16450.1) determined by SAIL-NMR. FEBS Journal. 2008;275:5873–5884. doi: 10.1111/j.1742-4658.2008.06717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z, Liu H, Qiu D, Zhou Y, Li X, Xu C, Wang S. A pair of allelic WRKY genes play opposite roles in rice–bacteria interactions. Plant Physiology. 2009;151:936–948. doi: 10.1104/pp.109.145623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BP, Eggermont K, Penninckx IA, Mauch-Mani B, Vogelsang R, Cammue BP, Broekaert WF. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proceedings of the National Academy of Sciences, USA. 1998;95:15107–15111. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BP, Eggermont K, Tierens KF, Broekaert WF. Requirement of functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by. Botrytis cinerea. Plant Physiology. 1999;121:1093–1102. doi: 10.1104/pp.121.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme EJ, Lannoo N, Peumans WJ. Plant lectins. Advances in Botanical Research. 2008;48:107–209. [Google Scholar]
- Wang XM, Ma QH. Characterization of a jasmonate regulated wheat protein related to a beta-glucosidase-aggregating factor. Plant Physiology and Biochemistry. 2005;43:185–192. doi: 10.1016/j.plaphy.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yang LM, Xu HB, Li QF, Ma ZQ, Chu CG. Differential proteomic analysis of proteins in wheat spikes induced by. Fusarium graminearum. Proteomics. 2005;5:4496–4503. doi: 10.1002/pmic.200401317. [DOI] [PubMed] [Google Scholar]
- Williams CE, Collier CC, Nemacheck JA, Liang C, Cambron SE. A lectin-like wheat gene responds systemically to attempted feeding by avirulent first-instar Hessian fly larvae. Journal of Chemical Ecology. 2002;28:1411–1428. doi: 10.1023/a:1016200619766. [DOI] [PubMed] [Google Scholar]
- Xiao S, Charoenwattana P, Holcombe L, Turner JG. The Arabidopsis genes RPW8.1 and RPW8.2 confer induced resistance to powdery mildew diseases in tobacco. Molecular Plant-Microbe Interactions. 2003;16:289–294. doi: 10.1094/MPMI.2003.16.4.289. [DOI] [PubMed] [Google Scholar]
- Yao GQ, Zhang JL, Yang LL, Xu HB, Jiang YM, Xiong L, Zhang CQ, Zhang ZZ, Ma ZQ, Sorrells ME. Genetic mapping of two powdery mildew resistance genes in einkorn (Triticum monococcum L.) accessions. Theoretical and Applied Genetics. 2007;114:351–358. doi: 10.1007/s00122-006-0438-4. [DOI] [PubMed] [Google Scholar]
- Yong WD, Xu YY, Xu WZ, et al. Vernalization induced flowering in wheat is mediated by a lectin-like gene. VER2. Planta. 2003;217:261–270. doi: 10.1007/s00425-003-0994-7. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zhong S, Li Q, et al. Functional analysis of rice NPR1-like genes reveals that OsNPR1/NH1 is the rice orthologue conferring disease resistance with enhanced herbivore susceptibility. Plant Biotechnology Journal. 2007;5:313–324. doi: 10.1111/j.1467-7652.2007.00243.x. [DOI] [PubMed] [Google Scholar]
- Zimmerli L, Métraux JP, Mauch-Mani B. β-Aminobutyric acid-induced protection of Arabidopsis against the necrotrophic fungus Botrytis cinerea. Plant Physiology. 2001;126:517–523. doi: 10.1104/pp.126.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.