Abstract
Previous studies demonstrated that expression of the Arabidopsis phytochelatin (PC) biosynthetic gene AtPCS1 in Nicotiana tabacum plants increases the Cd tolerance in the presence of exogenous glutathione (GSH). In this paper, the Cd tolerance of Arabidopsis plants over-expressing AtPCS1 (AtPCSox lines) has been analysed and the differences between Arabidopsis and tobacco are shown. Based on the analysis of seedling fresh weight, primary root length, and alterations in root anatomy, evidence is provided that, at relatively low Cd concentrations, the Cd tolerance of AtPCSox lines is lower than the wild type, while AtPCS1 over-expressing tobacco is more tolerant to Cd than the wild type. At higher Cd concentrations, Arabidopsis AtPCSox seedlings are more tolerant to Cd than the wild type, while tobacco AtPCS1 seedlings are as sensitive as the wild type. Exogenous GSH, in contrast to what was observed in tobacco, did not increase the Cd tolerance of AtPCSox lines. The PC content in wild-type Arabidopsis at low Cd concentrations is more than three times higher than in tobacco and substantial differences were also found in the PC chain lengths. These data indicate that the differences in Cd tolerance and in its dependence on exogenous GSH between Arabidopsis and tobacco are due to species-specific differences in the endogenous content of PCs and GSH and may be in the relative abundance of PCs of different length.
Keywords: Arabidopsis, Cd tolerance, PCS1 over-expression, phytochelatins, seedlings, tobacco
Introduction
Phytochelatins (PCs) are small, heavy metal-binding peptides with the general structure (γ-Glu-Cys)n-Gly found in some fungi, in plants and in other organisms (Cobbett and Goldsbrough, 2002). PCs are synthesized, as a mixture of peptides of different length, from reduced glutathione (GSH) by phytochelatin synthase (PCS), which is activated by a range of heavy metals (Grill et al., 1989; Clemens and Peršoh, 2009). PCs form stable heavy metal complexes that are subsequently transported into the vacuoles. Longer chain lengths bind metals more tightly in vitro (Loeffler et al., 1989), but their cellular roles are unknown. PCs have a major role in Cd detoxification, as mutant lines of Arabidopsis thaliana, Schizosaccharomyces pombe, and Caenorhabditis elegans defective in PC synthesis are sensitive to Cd. PCs are induced in plants and cultured cells exposed to metal ions, Cd in particular. To increase the level of these metal-binding peptides and, consequently, to enhance heavy metal tolerance, PCS genes from Arabidopsis thaliana (Ha et al., 1999; Vatamaniuk et al., 1999) Triticum aestivum (Clemens et al., 1999), Caenorhabditis elegans, Schizosaccharomyces pombe (Ha et al., 1999; Clemens et al., 2001), Allium sativum (Zhang et al., 2005), and Brassica juncea (Heiss et al., 2003), have been over-expressed in different plant species, as well as in yeast and Escherichia coli. Over-expression of the Arabidopsis AtPCS1 gene in E. coli (Sauge-Merle et al., 2003) and in Saccharomyces cerevisiae (Vatamaniuk et al., 1999) resulted in enhanced Cd tolerance and accumulation. Analogously, over-expression of wheat TaPCS1 in Nicotiana glauca greatly enhanced Cd and Pb tolerance and Pb accumulation (Gisbert et al., 2003; Martinez et al., 2006). Nicotiana tabacum expressing AtPCS1 also displayed enhanced Cd tolerance and accumulation, mainly when the plants were cultivated in culture medium supplied with GSH (Pomponi et al., 2006), and expression of the same gene in Brassica juncea led to higher Cd and Zn tolerance, but a lower accumulation of these metals in both root and shoot tissues (Gasic and Korban, 2007). By contrast, over-expression of AtPCS1 in Arabidopsis led to hypersensitivity to Cd despite enhanced PC production (Lee et al., 2003a, b; Li et al., 2004). Similarly, it has been reported recently that Nicotiana tabacum lines over-expressing AtPCS1, but not those over-expressing the Caenorhabditis CePCS1 gene, were hypersensitive to Cd in the absence of exogenous GSH, whereas no changes in Cd accumulation were observed relative to controls (Wojas et al., 2008). On the other hand, simultaneous over-expression in Arabidopsis of Allium sativum PCS1 and Saccharomyces cerevisiae GSH1 (which encodes a GSH biosynthetic enzyme) increases the tolerance and accumulation of Cd (Guo et al., 2008). Thus, while in most species an increase in PC production results in an enhanced metal tolerance, apparently conflicting results were obtained for Nicotiana tabacum and Arabidopsis thaliana. However, these results were obtained by different authors under widely different experimental conditions.
In this work, the tolerance to Cd of Arabidopsis seedlings over-expressing AtPCS1 in relation to the levels of PCs is analysed in the presence and absence of GSH, in much the same way as the response of tobacco plants was previously analysed. The results obtained with Arabidopsis are compared with new and published data obtained on tobacco.
Materials and methods
Plant expression constructs, transformation, and selection
Agrobacterium tumefaciens strain GV3101 carrying the binary plasmid pCAMBIA::35S-PCS1 (Pomponi et al., 2006) was used to transform Arabidopsis thaliana wild-type plants (ecotype Columbia) by standard dip floral transformation (Clough and Bent, 1998). Transformed plants were analysed by PCR with the following primers: 35S 5′-ACGCACAATCCCACTATCCTTC-3′; PCS1rev: 5′-GAACTAATAGGCAGGAGCG-3′ and by real-time RT-PCR (see below). Homozygous T2 generations were obtained by self-fertilization of primary transformants and the seeds were grown as described below.
RNA extraction and real-time RT-PCR
Total RNA was extracted from seedlings grown for 5 d or 9 d in the presence of 30 μM CdSO4, using the TRIZOL method (Invitrogen) and treated with RNase-free Dnase (DNase I Amplification Grade, Carlsbad, CA, USA). RT reactions were performed by using SUPERSCRIPT™ II First-Strand Synthesis System (Invitrogen) according to the manufacturer's instructions.
A SYBR-Green based quantitative assay was performed as previously described by Cecchetti et al. (2004). The primers used for PCS1 were 5′-TGCGTGATGGGAATGAACAA-3′; 5'-TTTGCGTCGATGGCACTAAC-3′ and ACTIN2 gene 5′-CCGATCCAGACACTGTACTTCCTT-3′; 5′-CTTGCACCAAGCAGCATGAA-3′ and were designed by using Primer Express 2 (ABI PRISM).
Plant growth conditions and metal treatments
Wild-type and AtPCSox seedlings were germinated on half-strength MS basal agar medium (pH 5.8) (Murashige and Skoog, 1962) in a growth chamber in a 16/8 h light/dark cycle at 22 °C. After 7 d, 10 seedlings were transferred to a half-strength MS basal medium supplemented with sucrose at the indicated percentages, in the absence or at different concentrations of CdSO4 (15, 30, 60, 90, or 180 μM) on vertical plates. Seedlings were weighed and the root length was measured after 5 d or 9 d of further growth. Experiments were performed with or without 100 or 250 μM GSH. The experiments were performed in triplicate.
PC and GSH analysis
Seedlings were grown as described above, on half-strength MS basal medium supplemented with 0.5% sucrose, in the absence or presence of 30 or 90 μM CdSO4, with or without 250 μM GSH. After 3 d or 9 d of Cd exposure, seedlings were immediately frozen in liquid nitrogen and 200 mg of seedlings were used for HPLC analyses according to Pomponi et al. (2006). The experiments were performed in triplicate.
Statistical analysis
Student's t test was used to evaluate the statistical significance probability levels. All experiments were repeated at least three times.
Histological analysis
The morphological and histological analyses were carried out on wild-type and AtPCSox roots from seedlings grown for 9 d on half-strength MS basal medium supplemented with 0.5% sucrose, in the absence or presence of 30, 60 or 90 μM CdSO4. Roots were treated with chloral hydrate solution (chloral hydrate:distilled water:glycerol, 8:1:2 w/v/v) for observation with Nomarski optics applied to a DAS Leica DMRB microscope (Leica). The evaluation of the percentage of damaged roots and anomalous lateral branching was carried out on 30 plants for each genotype and treatment.
Alternatively, roots were fixed in 70% ethanol, dehydrated, embedded in Technovit 7100 (Heraeus Kulzer), sectioned at 4 μm with an automatic microtome (Microm HM 350 SV), stained with 0.05% (w/v) toluidine blue, and examined under the same microscope. All the histological and morphological images were acquired with a DC500 video camera applied to the DMRB microscope and then analysed with a personal computer (Opti-Xex GX 240 MT) using the Leica IM1000 image-analysis software (Leica).
Results
Production of Arabidopsis lines over-expressing AtPCS1
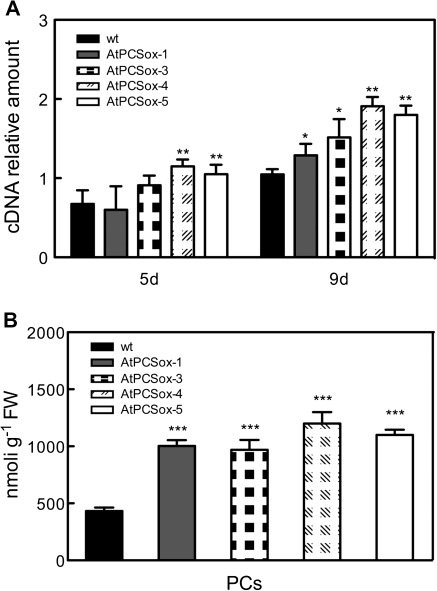
Arabidopsis plants were transformed with the construct pCAMBIA::35S-PCS1 harbouring the coding region of AtPCS1 under the control of the CaMV-35S promoter, previously used to transform tobacco plants (Pomponi et al., 2006): ten hygromycin-resistant plants were selected and the over-expression of AtPCS1 was determined in seedlings after 7 d of germination in the absence of Cd by real-time RT-PCR (not shown). Four homozygous lines AtPCSox-1, AtPCSox-3, AtPCSox-4, and AtPCSox-5 were analysed for transcript levels of AtPCS1 and PC content under experimental conditions used to test Cd sensitivity (see below). After 7 d of germination, seedlings from wild-type and AtPCSox lines were transferred to a medium containing 30 μM CdSO4 and after 5 d and 9 d of growth AtPCS1 transcript levels were measured by real-time RT-PCR. As shown in Fig. 1A, all over-expressing lines exhibited higher levels of AtPCS1 transcripts, compared with the wild type, after 9 d of growth. To determine the content of PCs in AtPCSox lines, seedlings grown for 9 d in the presence of 30 μM CdSO4 were collected and PC levels measured by HPLC. As shown in Fig. 1B, the PC content in AtPCSox-4 and AtPCSox-5 seedlings was 2.78 and 2.54 times higher, respectively, than in the wild-type seedlings. A slightly smaller increase was observed in AtPCSox-3 and AtPCSox-1 seedlings that have PC contents of 2.32 and 2.24 times higher, respectively, than in the wild-type seedlings. All four lines were used for subsequent analysis.
Fig. 1.
Quantitative analysis of AtPCS1 transcript and PC content in AtPCSox seedlings. Wild-type, AtPCSox-1, AtPCSox-3, AtPCSox-4, and AtPCSox-5 seedlings were grown in the presence of 30 μM Cd. (A) Real-time RT-PCR of mRNA extracted from wild-type and AtPCSox seedlings after 5 d and 9 d of growth. Data are expressed as a mean value (±SEM) of AtPCS1 cDNA levels relative to actin cDNA. (B) The content of total PCs was analysed, by means of HPLC, after 9 d of growth. Values correspond to means ±SE (n=3). Significant differences of AtPCSox from the wild type are indicated (*P <0.05, **P <0.01, ***P <0.001)
Cd tolerance of AtPCSox lines relative to the wild type varies with Cd concentration
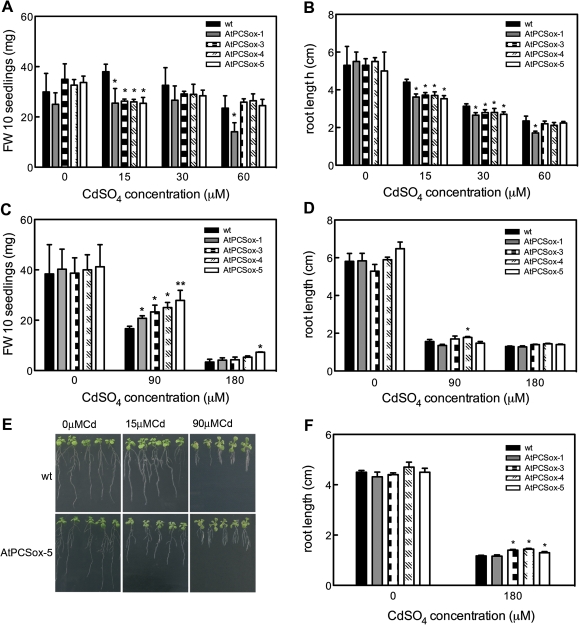
To test the Cd sensitivity, the growth of wild-type and AtPCSox seedlings was analysed through two different parameters: fresh weight and primary root length. After 7 d of germination, wild-type and AtPCSox seedlings were grown in the absence or presence of 15, 30, 60, 90, and 180 μM CdSO4. Fresh weight and root length were scored after 9 d. As shown in Fig. 2A–D, the growth of wild-type seedlings was unaffected at 15 μM CdSO4, slightly reduced at 30 and 60 μM CdSO4, and severely inhibited at 90 μM and 180 μM Cd CdSO4. The growth of AtPCSox seedlings was comparable with that of the wild type in the absence of Cd, whereas it was more inhibited than in the wild type from 15–30 μM CdSO4, mainly in terms of root length (Fig. 2A, B, E). At 60 μM CdSO4, the growth of AtPCSox seedlings was comparable with the wild type with the exception of AtPCSox-1 seedlings that showed a reduction in fresh weight and root length.
Fig. 2.
Cd tolerance of wild-type and AtPCSox seedlings. Wild-type, AtPCSox-1, AtPCSox-3, AtPCSox-4, and AtPCSox-5 seedlings were incubated on medium containing 0, 15, 30 or 60 μM CdSO4 (A, B) or 0, 90 or 180 μM CdSO4 (C, D), or 0 or 180 μM CdSO4 (F). Fresh weight was measured after 9 d (A, C). Root length was measured after 9 d (B, D) or 5 d (F). (E) Wild-type and AtPCSox-5 seedlings at 0 μM CdSO4 (left), 15 μM CdSO4 (middle), 90 μM CdSO4 (right). Wild-type seedlings show no root growth inhibition at 15 μM CdSO4, whereas AtPCSox-5 root growth is inhibited. Wild-type seedlings show severe root growth inhibition and foliar chlorosis at 90 μM CdSO4. AtPCSox-5 root growth inhibition is comparable to the wild type, but foliar chlorosis is not observed. (F) AtPCSox-3, AtPCSox-4, and AtPCSox-5 root growth is less inhibited than the wild type after 5 d at 180 μM CdSO4. Fresh weight values correspond to means ±SE (n=6). Root length values correspond to means ±SE (n=6). Significant differences from wild type are indicated (*P <0.05, **P <0.01).
By contrast, at 90 μM CdSO4 the fresh weight of AtPCSox seedlings was significantly higher than that of the wild type, and foliar chlorosis and necrosis were less severe (Fig. 2C, E), while root growth was comparable with that of wild-type seedlings, with the exception of a slight enhancement in the case of AtPCSox-4 at 90 μM Cd (Fig. 2D). After 9 d at 180 μM CdSO4, AtPCSox and wild-type seedling growth was seriously affected, with the exception of a slight increase in fresh weight of AtPCSox-5 seedlings. Interestingly, a slight but significantly enhanced root growth was observed at 180 μM Cd in the case of AtPCSox-5, AtPCSox-3, and AtPCSox-4 seedlings after 5 d of culture (Fig. 2F).
Since tobacco plants over-expressing AtPCS1 had a much higher Cd tolerance when GSH was added to the growth medium (Pomponi et al., 2006), the effects of GSH on the sensitivity to Cd of Arabidopsis AtPCSox seedlings was assessed.
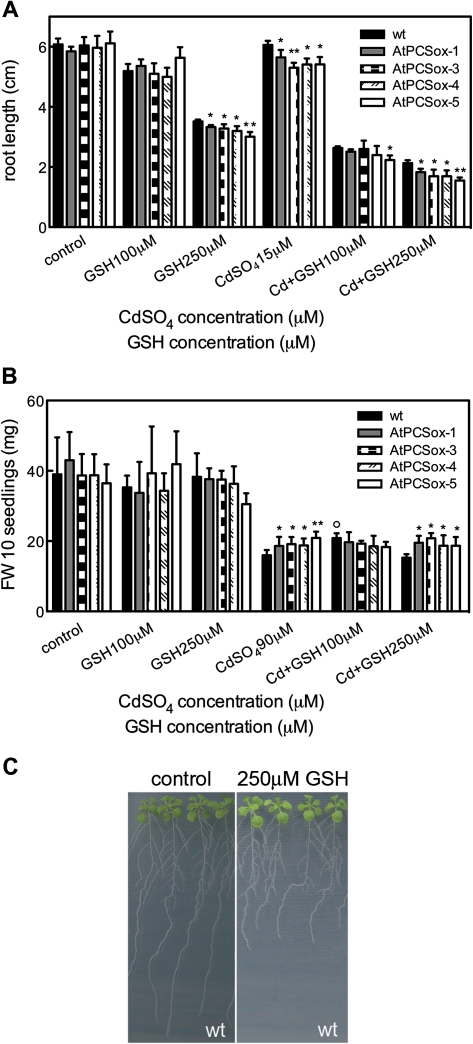
At low Cd concentrations, root growth was mainly affected and so the primary root length assay was used to analyse the effect of different GSH concentrations (100 and 250 μM) on seedlings exposed to 0, 15, and 30 μM CdSO4. As shown in Fig. 3A, in the absence of Cd, 100 μM of GSH resulted in a slight root growth inhibition both in wild-type and AtPCSox seedlings, while 250 μM GSH caused a strong inhibition of growth in all types of roots (Fig. 3A, C), that was more severe in AtPCSox roots. At both 15 and 30 μM CdSO4, 100 μM GSH caused a considerable decrease in the growth of both wild-type and AtPCSox roots, with the exception of AtPCSox-5 roots whose growth was more inhibited than in the wild type (Fig. 3A, only results with 15 μM CdSO4 are reported) whereas at 250 μM GSH root growth was more inhibited in all AtPCSox seedlings compared with the wild-type seedlings. To assess whether exogenous GSH has an inhibitory effect on the expression of GSH biosynthetic genes that are responsible for the reduction in growth of wild-type and AtPCSox seedlings, the transcript levels of AtGSH1, the controlling gene in GSH synthesis, were analysed. Wild-type and AtPCSox seedlings were grown in the presence or absence of exogenous GSH and, after 9 d, seedlings were collected and AtGSH1 mRNA levels were analysed by real time RT-PCR. As shown in Supplementary Fig. S1 at JXB online, the transcript levels of AtGSH1 in wild-type and AtPCSox seedlings, grown in the presence or absence of exogenous GSH, was comparable in all genotypes.
Fig. 3.
Cd tolerance of wild-type and AtPCSox seedlings grown in the presence of different concentrations of GSH. Wild-type, AtPCSox-1, AtPCSox-3, AtPCSox-4, and AtPCSox-5 seedlings were incubated on medium containing 15 μM CdSO4 in the presence of 0, 100 or 250 μM GSH (A) or containing 90 μM CdSO4 in the presence of 0, 100 or 250 μM GSH (B). Root length (A) and fresh weight (B) were measured after 9 d. (C) Wild-type seedlings at 0 μM CdSO4, 0 μM GSH (left), 0 CdSO4, 250 μM GSH (right). Wild-type seedlings show root growth inhibition and a slight increase in the growth of the aerial part, at 250 μM GSH. Fresh weight values correspond to means ±SE (n=6). Root length values correspond to means ±SE (n=30). Significant differences of AtPCSox seedlings from the wild type are indicated (*P <0.05, **P <0.01). Significant differences of wild-type seedlings at different GSH and Cd concentrations are indicated (°P <0.01)
The effect of 100 and 250 μM GSH on the fresh weight of seedlings growth was analysed in the presence of 0, 90 or 180 μM CdSO4, as, at high Cd concentrations, this growth parameter is mainly affected (see above). In the absence of Cd, both 100 and 250 μM GSH had no effect on wild-type and AtPCSox seedlings (Fig. 3B). At 90 or 180 μM CdSO4, 100 μM GSH resulted in a slight increase in Cd tolerance of wild-type, but not of AtPCSox, seedlings and 250 μM GSH had no appreciable effect on both types of plants (Fig. 3B, only the results with 90 μM CdSO4 are reported). In summary, AtPCSox plants are more sensitive than the wild type to low Cd concentrations, while they show a higher tolerance than the wild type to higher Cd concentrations. In addition, in contrast with what observed in tobacco, exogenous GSH does not affect the Cd tolerance of Arabidopsis seedlings over-expressing AtPCS1. These four lines all behaved similarly when exposed to different Cd concentrations in the presence and in the absence of GSH. Thus AtPCSox-1 and AtPCSox-5 lines were used for subsequent analysis.
Cd induces histo-anatomical alterations in roots
To assess the possible damage to the root system of plants exposed to Cd, a morphological and histological analysis of primary and lateral roots of wild-type and AtPCSox seedlings was performed after 9 d of growth under the conditions described above.
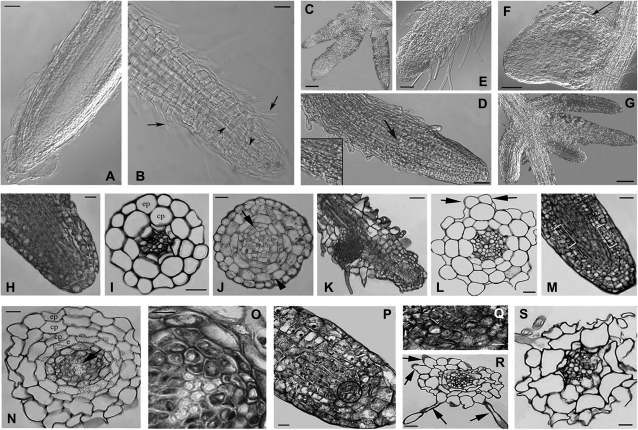
As shown in Fig. 4A, no morphological alterations were observed in wild-type roots up to 30 μM CdSO4. At 60 μM CdSO4, about 40% of roots showed alterations in the elongation zone characterized by the anomalous presence of root hairs and premature xylem differentiation (Fig. 4B). By contrast, the apex of the elongated primary root, as well as of lateral roots, was regular in shape and size and so was the apical dome of the lateral primordia (not shown). At 90 μM CdSO4, about 75% of roots in wild-type seedlings were very hairy and stunted, and 16% showed irregular lateral branching (Fig. 4C).
Fig. 4.
The effects of Cd treatments on root anatomy of wild-type, AtPCSox-5, and AtPCSox-1 seedlings. Nomarski DIC images (A–G), and histological images (H–S) of wild-type and AtPCSox roots from seedlings treated for 9 d with different concentrations of CdSO4. (A) Regular morphology of wild-type roots in seedlings treated with 30 μM CdSO4. (B) Root showing precocious xylem differentiation in the elongation zone (arrowheads) and abundant hair formation (arrows) in wild-type seedlings treated with 60 μM CdSO4. (C) Irregular lateral root branching in wild-type seedlings treated with 90 μM CdSO4. (D) Abundant hair formation and xylem differentiation up to the apex (arrow and inset) in the root of AtPCSox-1 seedlings treated with 30 μM CdSO4. (E) Very high production of root hairs up to the apex, and a reduction in cap extension in AtPCSox-1 seedlings treated with 60 μM CdSO4. (F) Anomalous shape of a lateral root primordium due to irregular proliferation of the cortical cells (arrow) in AtPCSox-5 seedlings treated with 60 μM CdSO4. (G) Irregular lateral root branching of AtPCSox-5 seedlings treated with 90 μM CdSO4. (H) Normal histological structure of a wild-type root apex (longitudinal section, 30 μM CdSO4 treatment). (I) Normal histological structure of a wild-type root apex (transection, 30 μM CdSO4 treatment). (J) Hypertrophy in the cortical parenchyma (arrowhead) and perycicle proliferation (arrow) of a wild-type root near the apex (transection, 60 μM CdSO4 treatment). (K) Anomalous position of a lateral root primordium in a wild-type primary root (longitudinal section, 60 μM CdSO4 treatment). (L) Anomalous trichoblast differentiation (arrows) in a wild-type root (transection, 60 μM CdSO4 treatment). (M) Anomalous proliferation and expansion of cells committed to differentiate cortical parenchyma, endodermis, and pericycle in an AtPCSox-1 root apical meristem (rectangles) (longitudinal section, 30 μM CdSO4 treatment). (N) Doubling of the cortical parenchyma and aboundant xylem differentiation (arrow) in an AtPCSox-1 root (transection, 60 μM CdSO4 treatment). (O) Detail of the stelar region in a primary root, showing xylem overproduction at higher magnification. (P) AtPCSox-5 primary root apex showing the proliferation of initial cells around the quiescent centre (circle) (longitudinal section, 60 μM CdSO4 treatment). (Q) The proliferating zone at higher magnification. (R) Anomalous root hairs (arrows) differentiated in an AtPCSox-5 root (transection, 60 μM CdSO4 treatment). (S) Epidermal and cortical cells anomalous in shape in an AtPCSox-5 root (transection, 90 μM CdSO4 treatment). Sections from (H) to (S) were stained with toluidine blue. Bars: 10 μm (I, J, L, N–Q), 20 μm (H, R, S), 25 μm (M), 50 μm (A, B, E, F, D, K), 100 μm (C, G); e, endodermis; cp, cortical parenchyma; ep, epidermis.
In AtPCSox seedlings, no morphological alterations were observed in the absence of Cd, whereas at 30 μM CdSO4, 4.0% and 4.5% of AtPCSox-1 and AtPCSox-5 seedlings, respectively, exhibited damaged roots showing hair formation and xylem differentiation in the elongation zone in primary roots (Fig. 4D). At 60 μM CdSO4, these alterations were more severe compared with the wild type in AtPCSox-1 seedlings (Fig. 4E), showing a significantly higher percentage of altered roots compared with the wild type (54%, P <0.05), whereas in AtPCSox-5 seedlings, the percentage was slightly but not significantly higher than the wild type (47%). The apical dome of the lateral primordial in AtPCSox lines showed irregular expansion in the cortex (Fig. 4F). At 90 μM CdSO4, the damage to the roots was macroscopically comparable with the wild type (about 75%) and the percentage of lateral branching was significantly higher for AtPCSox-5 (32%, P <0.01) (Fig. 4G) and only slightly, but not significantly, higher in AtPCSox-1 (22.5%). At 180 μM CdSO4, the damage was less severe compared with wild-type roots after 5 d of exposure to Cd, but comparable to the wild type after 9 d (data not shown).
Histological analysis confirmed the absence of anomalies in the root apex (Fig. 4H), elongation zone, and primary structure zone (Fig. 4I) of wild-type seedlings at 30 μM CdSO4. At 60 μM CdSO4, the proliferation of randomly located cells of the pericycle, and the expansion of the cortical parenchyma cells were observed in wild-type primary roots (Fig. 4J); lateral root primordia very near the apex of the primary root (Fig. 4K) and the anomalous position of root hairs in the epidermis in primary roots were also observed (Fig. 4L).
The same anomalies were observed in AtPCSox roots at 30 μM CdSO4. In addition, in AtPCSox roots, an anomalous proliferation and expansion of the derivative cells committed to differentiate cortical parenchyma, endodermis, and perycicle occurred in the root apical meristem (Fig. 4M, rectangles). At 60 μM CdSO4 in AtPCSox roots, together with xylem overproduction, quiescent centre cell proliferation and cortex doubling were also observed (Fig. 4N, O, P, Q compared with Fig. 4L), anomalies, which were not observed in wild-type roots at any Cd concentration. At 90 μM CdSO4, most of the root cells were collapsed in both wild-type and AtPCSox seedlings (Fig. 4S).
Sucrose affects Cd sensitivity of AtPCSox seedlings
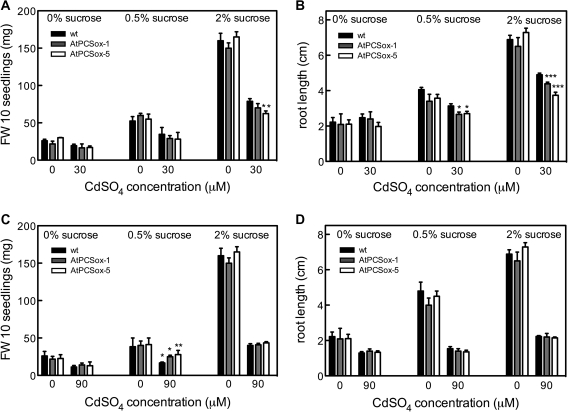
Cd tolerance of Arabidopsis seedlings has been analysed at different sucrose concentrations by different authors (Lee et al., 2003a, b; Li et al., 2004; Guo et al., 2008), while the possible effects of sucrose on Cd tolerance have not yet been verified. To assess whether sucrose affects the Cd tolerance of AtPCSox lines at low CdSO4 (15–30 μM), the fresh weight and the primary root length of wild-type AtPCSox-5 and AtPCSox-1 seedlings were measured after 9 d of culture in the absence and in the presence of CdSO4, and in the absence and in the presence of 0.5% and 2% sucrose. In the absence of Cd (Fig. 5A, B), fresh weight and primary root length increased with sucrose concentration in both wild-type and AtPCSox seedlings. At 30 μM CdSO4, the root growth of AtPCSox and the fresh weight of AtPCSox-5 seedlings were more inhibited at 2% sucrose than at 0.5% or in the absence of sucrose. At 90 μM CdSO4 (Fig. 5C, D) the fresh weight, but not root growth, of AtPCSox seedlings was significantly higher than that of the wild type but only in the presence of 0.5% sucrose.
Fig. 5.
Cd tolerance of wild-type, AtPCSox-1, and AtPCSox-5 seedlings grown at difference sucrose percentages. Wild-type, AtPCSox-1, and AtPCSox-5 seedlings were incubated on medium containing 0 or 30 μM CdSO4 at 0, 0.5 or 2% sucrose (A, B) or 0 or 90 μM CdSO4 at 0, 0.5 or 2% sucrose (C). Fresh weight (A, B) and root length (C, D) were measured after 9 d. Fresh weight values correspond to means ±SE (n=6). Root length values correspond to means ±SE (n=30). Significant differences from the wild type are indicated (*P <0.05, **P <0.01, ***P <0.001).
PC and GSH levels in wild-type and AtPCSox plants are related to Cd concentrations
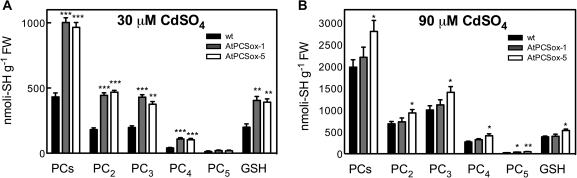
To establish a correlation between Cd tolerance, PC content, and GSH level, the concentration of PC and GSH was measured in wild-type and AtPCSox seedlings cultured in the presence of different concentrations of Cd. At 30 μM CdSO4 (Fig. 6A), PC and GSH levels in AtPCSox seedling were about the double that in the wild type. Differences in PC chain length between the wild type and AtPCSox were modest, PC2 and PC3 being the most abundant oligomers in both types of seedlings.
Fig. 6.
PC and GSH content of wild-type, AtPCSox-1, and AtPCSox-5 seedlings. Wild-type, AtPCSox-1, and AtPCSox-5 seedlings were incubated on medium containing 30 μM CdSO4 (A) or 90 CdSO4 (B). After 9 d, the content of GSH, total PCs, PC2, PC3, PC4, and PC5 fractions was analysed by means of HPLC. Values correspond to means ±SE (n=3). Significant differences from the wild type are indicated (*P <0.05, **P <0.01, ***P <0.001).
At 90 μM CdSO4, the PC level increased 4.6-, 2.2-, and 3-fold, respectively, in wild-type, AtPCSox-1, and AtPCSox-5 seedlings compared with 30 μM CdSO4, thus being significantly higher in AtPCSox-5, but not in AtPCSox-1, compared with the wild type (Fig. 6B). At this Cd concentration, PC3 but not PC2 is the most abundant oligomer in wild-type and AtPCSox lines (Fig. 6B), suggesting that quantitative as well as qualitative changes in PC content may account for the different sensitivity of over-expressers at different Cd concentrations. As of GSH, at 90 μM CdSO4, a 1.9-, 1.1-, and 1.35-fold increase was observed, respectively, in wild-type, AtPCSox-5, and AtPCSox-1 seedlings compared with 30 μM CdSO4.
PC and GSH levels are considerably higher in Arabidopsis than in tobacco
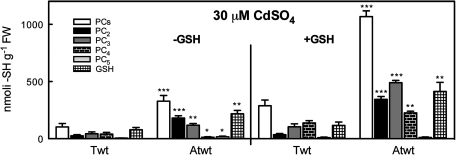
To assess whether differences in the content of PCs (and GSH) between Arabidopsis and tobacco seedlings would account for the different effects of AtPCS1 over-expression on Cd tolerance observed in the two species, PC and GSH content was measured in wild-type seedlings grown for 3 d at 30 μM CdSO4, in the presence or absence of 250 μM GSH—the optimal GSH concentration for tobacco (Pomponi et al., 2006).
In the absence of exogenous GSH (Fig. 7), PC and GSH contents were 3.2 and 2.77 higher, respectively, in untransformed Arabidopsis than in tobacco. In addition, PC2 was the most abundant oligomer in Arabidopsis while PC3 and PC4 were the more abundant oligomers in tobacco.
Fig. 7.
Comparison of PC and GSH content between tobacco wild-type and Arabidopsis wild-type seedlings. Tobacco wild-type and Arabidopsis wild-type seedlings were incubated on medium containing 30 μM CdSO4 in the presence or absence of 250 μM GSH. After 3 d, the content of GSH, total PCs, PC2, PC3, PC4, and PC5 fractions was analysed by means of HPLC. Values correspond to means ±SE (n=3). Significant differences from wild type are indicated (*P <0.05, **P <0.01, ***P <0.001).
In the presence of exogenous GSH, PC (and GSH) content increased in both Arabidopsis and tobacco seedlings, and was about four times higher in the former than in the latter. PC3 was the most abundant oligomer in Arabidopsis, followed by PC4, whereas, in tobacco, the PC4 content was slightly higher than PC3.
Discussion
Conflicting results have been reported on the Cd tolerance of Arabidopsis thaliana and Nicotiana tabacum over-expressing the PC biosynthetic gene (Lee et al., 2003a, b; Li et al., 2004; Pomponi et al., 2006; Wojas et al., 2008; Guo et al., 2008), possibly due to different experimental conditions and the use of different constructs. In this paper, the effects of over-expression of AtPCS1 in Arabidopsis seedlings were analysed under the experimental conditions used previously for tobacco. AtPCSox lines were obtained by transforming Arabidopsis with the same construct used for tobacco, containing the AtPCS1 coding region driven by the CaMV-35S promoter (Pomponi et al., 2006). To assess whether growth inhibition was due to defects in root elongation or in the overall growth of the aerial part of the seedlings, Cd tolerance was assayed using two different parameters, primary root length (as previously used for tobacco) and seedling fresh weight. Other authors have used these parameters to evaluate the Cd tolerance of Arabidopsis seedlings (Lee et al., 2003a, b; Li et al., 2004). Histological analysis of roots was also performed to compare the Cd damage of wild-type and over-expressing seedlings.
This work demonstrated that, at CdSO4 concentrations up to 60 μM that have no or only slightly toxic effects on the growth of wild-type Arabidopsis seedlings, AtPCS1 over-expression results in a decrease in Cd tolerance compared with the wild type, as mainly revealed by a reduced root growth. By contrast, at higher Cd concentrations (90–180 μM CdSO4)—toxic to wild-type seedlings as manifested by a significant decrease in fresh weight and root growth as well as by foliar chlorosis—AtPCS1 over-expression conferred an increase in Cd tolerance: AtPCSox seedlings showed a significantly less severe reduction of fresh weight and a less pronounced foliar chlorosis than the wild type. Root growth was slightly enhanced in AtPCSox seedlings. This effect was observed after 9 d at 90 μM CdSO4 only in the AtPCSox-4 line (showing the highest level of PCs) and in most of the other lines after 5 d of exposure to 180 μM CdSO4, suggesting a transient effect on root growth due to the increased PC content (see below).
Cd tolerance was correlated with the level of PCs by comparing PC content in wild-type and AtPCSox lines at different Cd concentrations. By means of HPLC analysis, evidence was obtained that the PC level increases in both types of plants with the increase of Cd in the medium, and that it is significantly higher in AtPCSox than in the wild type. At low Cd concentrations (30 μM CdSO4), the PC level of AtPCSox seedlings is approximately 2.5 times higher than that of the wild type, while it is approximately 1.5 times higher at high Cd (90 μM CdSO4). In addition, differences in the content of individual PC oligomers at low and high Cd concentrations were observed in both the wild type and over-expressers: the PC2 and PC3 oligomers are highest at low Cd, whereas PC3 is the most abundant oligomer at high Cd.
These data suggest that PC overproduction may be advantageous for Arabidopsis in tolerating high Cd concentrations when the initial level of PCs may possibly be below the concentration necessary to bind all the Cd. The reduced tolerance of AtPCSox lines to low Cd may thus be due to a supraoptimal PC content. In addition, since the the stability of PC–Cd complexes increases with the number of γ-Glu-Cys dipeptide repeats (Satofuka et al., 2000), the observed selective increase of PC3 oligomers in AtPCSox lines at high Cd may contribute to the increased tolerance to the metal as PC3 are able to displace PC2 in the complexes with Cd (Gusmão et al., 2010).
A supraoptimal PC content may also explain the Cd hypersensitivity previously reported for Arabidopsis lines over-expressing AtPCS1 under the control of the constitutive A2 actin promoter (Li et al., 2004) and of the AtPCS1 promoter (Lee et al., 2003b). These authors did not report any positive effect on Cd tolerance of over-expressers at high Cd concentration. This discrepancy with our results could be a consequence of the different promoters utilized and of the different increases of PC content achieved in over-expressers compared with the wild type. In the case of the A2 promoter, Li et al. (2004) utilized for their analysis over-expressing lines with very high relative levels of PCs (about 6 times those of the wild type) compared with the ones reported here for AtPCS1ox lines. In the case of the PCS1 promoter, the overall PC level in over-expressers (1.6–2 times higher than in the wild type) was comparable with that of our AtPCSox lines. However, as the PCS1 promoter, in contrast to the 35S promoter utilized in the present work, is not expressed in all the tissues (Blum et al., 2010; P Brunetti et al., unpublished data), the levels of PCs in the specific cells over-expressing PCS1 can be much higher. In agreement with this hypothesis, Lee et al. (2003a) showed that Cd hypersensitivity was exhibited only by lines with the highest levels of PC production, and suggest that is a consequence of a supraoptimal PC concentration. Furthermore, in this work, it has been demonstrated that Cd tolerance of Arabidopsis seedlings also depends on sucrose, as the increase in Cd tolerance of AtPCSox seedlings is not observed at the high sucrose (2%) concentration such as those used by Li et al. (2004). This agrees with the observed stimulation of growth of Arabidopsis roots by 2% sucrose (Carrie et al., 2009).
The morphological and histological damage caused by Cd was analysed in the roots of Arabidopsis wild-type and AtPCSox seedlings. This analysis confirmed that, in wild-type seedlings at non-toxic CdSO4 concentrations (30 μM), there is no anatomical damage in the root, whereas Cd-sensitive AtPCS1 over-expressing seedlings show alterations. Part of these, such as root hair and abnormal xylem formation—pointing to premature maturation causing a reduction in root length—have already been described in pine (Schützendübel et al., 2001) and barley (Ďurčeková et al., 2007), and were also observed in wild-type roots at higher CdSO4 concentrations (60–90 μM). AtPCSox roots also showed quiescent centre cell proliferation and cortex doubling, which may be associated with repairing of the root apical meristems. This effect may be interpreted as a developmental defence reaction to limit the stress effect and damage induced by toxic Cd on root growth.
This work shows that significant differences exist between Arabidopsis and tobacco with respect to Cd tolerance. In a previous paper it was observed that at low Cd (30 μM CdSO4) root growth (elongation) of wild-type tobacco seedlings was severely inhibited, while AtPCS1 over-expressers were more tolerant to Cd than the wild type (Pomponi et al., 2006), in particular upon the addition of GSH. The present work shows that at 30 μM CdSO4, Arabidopsis AtPCSox lines are less tolerant to Cd than the wild type, and that their tolerance is not enhanced by exogenous GSH. It is also shown that Arabidopsis seedlings have PC and GSH contents approximately 3.5 times and 3 times higher, respectively, than in tobacco and that the abundance of individual PC oligomers of the two plants is different: PC3 and PC4 being the most abundant oligomers (the PC2 level is negligible) in wild-type tobacco, whereas PC2 and PC3 are the most abundant in Arabidopsis. These differences in GSH and PC levels (and PC chain length) between Arabidopsis and tobacco may account for the observed differences in Cd tolerance and GSH dependence. Our data suggest that Cd tolerance is mainly related to PC content. Accordingly, Wojas et al. (2010) found that AtPCS1-expressing tobacco plants, showing Cd hypersensitivity, had a significant decrease in both the cytosolic and vacuolar pool of PCs, whereas enhanced Cd tolerance of CePCS plants was accompanied by an increased cytosolic and vacuolar PC/Cd ratio.
Our data, although obtained in part from the comparison with previous experiments on tobacco AtPCS1 over-expressing plants, indicate that Cd tolerance is related to PC content and not on any particular characteristic of the species. This allows us to hypothesize a model on the effects of PCs on Cd tolerance.
In summary, it is proposed that PC overproduction increases Cd tolerance only when, in a given plant, overproduction results in an optimal ratio between PC level (and/or PC chain length) and Cd, which depends not only on PC overproduction but also on the endogenous levels of PCs.
Supplementary data
Supplementary data can be found at JXB online.
Supplementatry Fig. S1. Quantitative analysis of AtGSH1 transcript in AtPCSox seedlings.
Acknowledgments
This work was supported by a research grant from CNR (RSTL) to MC, a research grant from MIUR (PRIN 2007) to GF and MC, a research grant from Università La Sapienza (Progetto di Ateneo) to MMA and PC, and by Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR) Progetti di Ricerca di Interesse Nazionale, and MIUR Fondo per gli Investimenti della Ricerca di Base European Research Area-Plant Genomics to PC.
References
- Blum R, Meyer KC, Wünschmann J, Lendzian KJ, Grill E. Cytosolic action of phytochelatin synthase. Plant Physiology. 2010;153:159–169. doi: 10.1104/pp.109.149922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrie CS, Harrington GN. The impact of supplemental carbon sources on Arabidopsis thaliana growth, chlorophyll content and anthocyanin accumulation. Plant Growth Regulation. 2009;59:255–271. [Google Scholar]
- Cecchetti V, Pomponi M, Altamura MM, Pezzotti M, Marsilio S, D'Angeli S, Tornielli GB, Costantino P, Cardarelli M. Expression of rolB in tobacco flowers affects the coordinated processes of anther dehiscence and style elongation. The Plant Journal. 2004;38:512–525. doi: 10.1111/j.0960-7412.2004.02064.x. [DOI] [PubMed] [Google Scholar]
- Clemens S, Peršoh S. Multi-tasking phytochelatin synthases. Plant Science. 2009;177:266–271. [Google Scholar]
- Clemens S, Kim EJ, Neumann D, Schroeder JI. Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO Journal. 1999;18:3325–3333. doi: 10.1093/emboj/18.12.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Schroeder JI, Degenkolb T. Caenorhabditis elegans expresses a functional phytochelatin synthase. European Journal of Biochemistry. 2001;268:3640–3643. doi: 10.1046/j.1432-1327.2001.02293.x. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Cobbett C, Goldsbrough P. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annual Review of Plant Biology. 2002;53:159–182. doi: 10.1146/annurev.arplant.53.100301.135154. [DOI] [PubMed] [Google Scholar]
- Ďurčeková K, Huttová J, Mistrík I, Ollé M, Tamás L. Cadmium induces premature xylogenesis in barley roots. Plant and Soil. 2007;290:61–68. [Google Scholar]
- Gasic K, Korban SS. Transgenic Indian mustard (Brassica juncea) plants expressing an Arabidopsis phytochelatin synthase (AtPCS1) exhibit enhanced As and Cd tolerance. Plant Molecular Biology. 2007;64:361–369. doi: 10.1007/s11103-007-9158-7. [DOI] [PubMed] [Google Scholar]
- Gisbert C, Ros R, De Haro A, Walker DJ, Bernal MP, Serrano R, Navarro-Avinó J. A plant genetically modified that accumulates Pb is especially promising for phytoremediation. Biochemical and Biophysical Research Communications. 2003;303:440–445. doi: 10.1016/s0006-291x(03)00349-8. [DOI] [PubMed] [Google Scholar]
- Grill E, Loffler S, Winnacker EL, Zenk MH. Phytochelatins, the heavy metal-binding peptides of plants, are synthesised from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase) Proceedings of the National Academy of Sciences, USA. 1989;86:6838–6842. doi: 10.1073/pnas.86.18.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Dai X, Xu W, Ma M. Overexpressing GSH1 and AsPCS1 simultaneously increases the tolerance and accumulation of cadmium and arsenic in Arabidopsis thaliana. Chemosphere. 2008;72:1020–1026. doi: 10.1016/j.chemosphere.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Gusmão R, Ariño C, Díaz-Cruz JM, Esteban M. Electrochemical survey of the chain length influence in phytochelatins competitive binding by cadmium. Analytical Biochemistry. 2010;406:61–69. doi: 10.1016/j.ab.2010.06.034. [DOI] [PubMed] [Google Scholar]
- Ha SB, Smith AP, Howden R, Dietrich WM, Bugg S, O'Connell MJ, Goldsbrough PB, Cobbett CS. Phytochelatin synthase genes from Arabidopsis and the yeast. Schizosaccharomyces pombe. The Plant Cell. 1999;11:1153–1164. doi: 10.1105/tpc.11.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss S, Wachter A, Bogs J, Cobbett C, Rausch T. Phytochelatin synthase (PCS) protein is induced in Brassica juncea leaves after prolonged Cd exposure. Journal of Experimental Botany. 2003;54:1833–1839. doi: 10.1093/jxb/erg205. [DOI] [PubMed] [Google Scholar]
- Lee S, Moon JS, Ko TS, Petros D, Goldsbrough PB, Korban SS. Overexpression of Arabidopsis phytochelatin synthase paradoxically leads to hypersensitivity to cadmium stress. Plant Physiology. 2003b;131:656–663. doi: 10.1104/pp.014118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Petros D, Moon JS, Ko TS, Goldsbrough PB, Korban SS. Higher levels of ectopic expression of Arabidopsis phytochelatin synthase do not lead to increased cadmium tolerance and accumulation. Plant Physiology and Biochemistry. 2003a;41:903–910. [Google Scholar]
- Li Y, Dhankher OM, Carreira L, Lee D, Chen A, Schroeder JI, Balish RS, Meagher RB. Overexpression of phytochelatin synthase in Arabidopsis leads to enhanced arsenic tolerance and cadmium hypersensitivity. Plant and Cell Physiology. 2004;45:1787–1797. doi: 10.1093/pcp/pch202. [DOI] [PubMed] [Google Scholar]
- Loeffler S, Hochberger A, Grill E, Winnacker EL, Zenk MH. Termination of the phytochelatin synthase reaction through sequestration of heavy metals by the reaction product. FEBS Letters. 1989;258:42–46. [Google Scholar]
- Martínez M, Bernal P, Almela C, Vélez D, García-Agustín P, Serrano R, Navarro-Aviñó J. An engineered plant that accumulates higher levels of heavy metals than Thlaspi caerulescens, with yields of 100 times more biomass in mine soils. Chemosphere. 2006;64:478–85. doi: 10.1016/j.chemosphere.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for raoid growth and bioassay with tobacco cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Pomponi M, Censi V, Di Girolamo V, De Paolis A, di Toppi LS, Aromolo R, Costantino P, Cardarelli M. Overexpression of Arabidopsis phytochelatin synthase in tobacco plants enhances Cd2+ tolerance and accumulation but not translocation to the shoot. Planta. 2006;223:180–190. doi: 10.1007/s00425-005-0073-3. [DOI] [PubMed] [Google Scholar]
- Satofuka H, Amano S, Fukui T, Atomi H, Takagi M, Imanaka T. Anti-phytochelatin monoclonal antibody. Biotechnology Journal. 2000;22:1423–1428. [Google Scholar]
- Sauge-Merle S, Cuiné S, Carrier P, Lecomte-Pradines C, Luu DT, Peltier G. Enhanced toxic metal accumulation in engineered bacterial cells expressing Arabidopsis thaliana phytochelatin synthase. Applied and Environmental Microbiology. 2003;69:490–494. doi: 10.1128/AEM.69.1.490-494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schützendübel A, Schwanz P, Teichmann T, Gross K, Langenfeld-Heyser R, Godbold DL, Polle A. Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiology. 2001;127:887–888. doi: 10.1104/pp.010318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatamaniuk OK, Mari S, Lu YP, Rea PA. AtPCS1, a phytochelatin synthase from Arabidopsis: isolation and in vitro reconstitution. Proceedings of the National Academy of Sciences, USA. 1999;96:7110–7115. doi: 10.1073/pnas.96.12.7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojas S, Clemens S, Hennig J, Sklodowska A, Kopera E, Schat H, Bal W, Antosiewicz DM. Over-expression of phytochelatin synthase in tobacco: distinctive effects of AtPCS1 and CePCS genes on plant response to cadmium. Journal of Experimental Botany. 2008;59:2205–2219. doi: 10.1093/jxb/ern092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojas S, Ruszczyńska A, Bulska E, Clemens S, Antosiewicz DM. The role of subcellular distribution of cadmium and phytochelatins in the generation of distinct phenotypes of AtPCS1- and CePCS3-expressing tobacco. Journal of Plant Physiology. 2010;167:981–988. doi: 10.1016/j.jplph.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Zhang H, Xu W, Guo J, He Z, Ma M. Coordinated responses of phytochelatins and metallothioneins to heavy metals in garlic seedlings. Plant Science. 2005;169:1059–1065. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.