Abstract
Spartina densiflora is a C4 halophytic species that has proved to have a high invasive potential which derives from its physiological plasticity to environmental factors, such as salinity. It is found in coastal marshes of south-west Spain, growing over sediments with between 1 mmol l−1 and 70 mmol l−1 zinc. A glasshouse experiment was designed to investigate the synergic effect of zinc from 0 mmol l−1 to 60 mmol l−1 at 0, 1, and 3% NaCl on the growth and the photosynthetic apparatus of S. densiflora by measuring chlorophyll fluorescence parameters and gas exchange, and its recovery after removing zinc. Antioxidant enzyme activities and total zinc, sodium, calcium, iron, magnesium, manganese, phosphorus, potassium, and nitrogen concentrations were also determined. Spartina densiflora showed the highest growth at 1 mmol l−1 zinc and 1% NaCl after 90 d of treatment; this enhanced growth was supported by the measurements of net photosynthetic rate (A). Furthermore, there was a stimulatory effect of salinity on accumulation of zinc in tillers of this species. Zinc concentrations >1 mmol l−1 reduced growth of S. densiflora, regardless of salinity treatments. This declining growth may be attributed to a decrease in A caused by diffusional limitation of photosynthesis, owing to the modification of the potassium/calcium ratio. Also, zinc and salinity had a marked overall effect on the photochemical (photosystem II) apparatus, partially mediated by the accumulation of H2O2 and subsequent oxidative damage. However, salinity favoured the recovery of the photosynthetic apparatus to the toxic action of zinc, and enhanced the nutrient uptake.
Keywords: Antioxidant enzyme activities, chlorophyll fluorescence, cordgrass, gas exchange, growth, salinity, synergic effect, zinc
Introduction
The adaptation of plants to heavy metals under conditions of salinity is an increasingly important problem due to the pollution of salinized lands with heavy metals (Kholodova et al., 2010). Field surveys and greenhouse experiments have shown that salinity can influence metal uptake in salt-marsh plants (Fitzgerald et al., 2003; Kadukova and Kalogerakis, 2007). Mahon and Carman (2008) studied the effect of salinity on the concentration of metals in Spartina alterniflora tissues and excreted salts. However, the functioning of plant defensive systems under saline conditions remains poorly studied, despite previous attempts to study plant responses to heavy metals under saline conditions (Helal et al., 1998). In fact, so far no studies have assessed the physiological impact of both salinity and heavy metals.
The austral cordgrass, Spartina densiflora Brongn. (Poaceae), is a C4 halophytic species with a South American origin that is invading salt marshes in southern Europe, North Africa, and North America (Mateos Naranjo et al., 2008a). Its physiological and morphological versatility apparently allow S. densiflora to tolerate a wide range of salinity, of tidal submergence, and of drainage (Mateos-Naranjo et al., 2007, 2010). In the salt marshes of the joint estuary of the Tinto and Odiel rivers (south-west Spain) S. densiflora grows over sediments with between 1 mmol l−1 and 70 mmol l−1 zinc (Zn) (Nelson and Lamothe, 1993; Sáinz et al., 2002), and with the greatest mobility and bioavailability amongst the metals present (Morillo et al., 2004). Furthermore, this species has shown to be useful for bioremediation. Cambrollé et al. (2008) found that heavy metals (including Zn) accumulated at different rates in S. densiflora tissues and around its roots, concluding that these species could be used for phytoremediation and phytostabilization of estuarine sediments. Mateos-Naranjo et al. (2008a) studied the effect of Zn on the growth and photosynthetic apparatus of S. densiflora in a glasshouse experiment, but no attention was paid to the interaction with salt.
The specific aims of the present study were to: (i) investigate the growth of S. densiflora in experimental Zn treatments ranging from 0 mmol l−1 to 60 mmol l−1 Zn at 0, 1, and 3% NaCl and during recovery (after removing Zn); (ii) determine the extent of the effects of Zn and NaCl on photosystem II (PSII) photochemistry and photosynthetic gas exchange; (iii) examine the response of other foliar minerals to increasing external Zn in the presence or absence of salt stress; and (iv) investigate defensive responses (antioxidant enzyme activities) to the interaction of Zn and salinity. It is well known that several stress conditions including Zn contamination can unbalance the steady-state level of reactive oxygen species (ROS; O2–, H2O2, etc.; Foyer et al., 1997; Tewari et al., 2008). In fact, defensive mechanisms against oxidative damage have been specifically observed in S. densiflora under natural growing conditions (Martínez-Domínguez et al., 2008).
Materials and methods
Plant material and stress treatments
Seeds of S. densiflora were collected in November 2009 from Odiel Marshes (37º15'N, 6º58'W; soutn-west Spain), and subsequently stored at 4 °C (in darkness) for 2 months. After the storage period, seeds were surface-sterilized by vigorous shaking in sodium hypochlorite solution (5%, v/v) for 1 min and then washed with sterilized water. Then, 100 seeds per treatment were placed on filter paper in 9 cm Petri dishes and submerged in 3 ml solutions of 0, 1, 10, 30, and 60 mmol l−1 Zn and 0, 1, and 3% (w/v) NaCl. Zn treatments were established by combining distilled water and ZnSO4·7H2O of the appropriate concentration. Dishes were wrapped with parafilm and placed in a germinator (ASL Aparatos Científicos M-92004, Madrid, Spain), and subjected to an alternating diurnal regime of 16 h of light (photon flux rate, 400–700 nm, 35 μmol m−2 s−1) at 25 °C and 8 h of darkness at 12 °C, for a month. During this period the entire solutions were changed on a 5 d basis. Zn and NaCl concentrations were chosen to cover variations recorded by Mateos-Naranjo et al. (2008a and b, respectively) in the salt marshes of Odiel River where S. densiflora occurs.
In February 2010, germinated seeds were transferred to individual plastic pots (square pots of 7 cm each side) filled with perlite and placed in a glasshouse with minimum–maximum temperatures of 21–25 °C, 40–60% relative humidity, and natural daylight (minimum and maximum light flux: 200 μmol m−2 s−1 and 1000 μmol m−2 s−1, respectively). Thereafter, two experiments were conducted for 90 d (when the plants appeared to have stable growth rates).
Effects of salinity and zinc on seedlings
Pots were allocated to the same NaCl and Zn concentrations as the seedlings (10 pots per tray, with one tray per NaCl and Zn treatment). In this case, Zn treatments were established by combining 20% Hoagland's solution (Hoagland and Arnon, 1938) and ZnSO4·7H2O of the appropriate concentration. The control, 0 mmol l−1 Zn treatment, had exactly 0.002 mmol l−1 of Zn, since Hoagland's solution contains a small amount of Zn as an essential trace nutrient.
Recovery experiment
Pots were allocated to three NaCl treatments in shallow trays (five pots per tray, with one tray per NaCl treatment): 0, 1, and 3% in Hoagland's solution.
At the beginning of the experiment 3.0 l of the appropriate solution were placed in each of the trays to a depth of 1 cm. During the experiment, the levels in the trays were monitored and they were topped up to the marked level with 20% Hoagland's solution (without additional ZnSO4·7H2O or NaCl) as a way to limit the change of Zn and NaCl concentrations due to water evaporation of the nutritive solution. In addition, the entire solution (including ZnSO4·7H2O and NaCl) was changed on a weekly basis.
Growth
At the end of the experiment, 10 and five plants (for the first and the second experiment, respectively) from each treatment were separated into tillers and roots, dried at 80 °C for 48 h, and then weighed. The height of all fully developed tillers was measured.
The leaf elongation rate (LER) was measured in random leaves (n=10, per treatment; two measurements per plant for the second experiment) by placing a marker of inert sealant at the base of the youngest accessible leaf. The distance between the marker and the leaf base was measured after 24 h (Ewing et al., 1995).
Chemical analysis of plant samples
Only plants grown at 0 mmol l−1 and 1 mmol l−1 Zn and 0, 1, and 3% NaCl were analysed because insufficient plant material was available for the other treatments. Following the protocols of Redondo-Gómez et al. (2007), at the end of the experiment, tiller and root samples were dried at 80 °C for 48 h and ground. Tillers and roots were carefully washed with distilled water before any further analysis. Then 0.5 g samples, taken from a mixture of the tillers or the roots belonging to the 10 plants used for each treatment, were digested with 6 ml of HNO3, 0.5 ml of HF, and 1 ml of H2O2. Calcium (Ca), iron (Fe), potassium (K), magnesium (Mg), manganese (Mn), sodium (Na), phosphorus (P), and Zn were measured by inductively coupled plasma (ICP-OES) spectroscopy (Thermo ICAP 6500 DUO, USA). Total nitrogen (N) and carbon (C) concentrations were determined for undigested dry samples with an elemental analyser (Leco TRUSPEC CN, Spain).
Leaf water content
Leaf water content (WC) was calculated as:
where FW is the fresh mass of the leaves, and DW is the dry mass after oven-drying at 80 °C for 48 h.
Chlorophyll fluorescence
Chlorophyll fluorescence was measured in random, fully developed penultimate leaves (n=10, per treatment; two measurements per plant for the recovery experiment) using a portable modulated fluorometer (Mini-PAM, Heinz Walz, Germany) after 90 d of treatment. Light- and dark-adapted fluorescence parameters were measured at midday (1600 μmol m−2 s−1) to investigate whether Zn and NaCl concentrations affected the sensitivity of plants to photoinhibition.
Plants were dark-adapted for 30 min, using leaf clips exclusively designed for this purpose. The minimal fluorescence level in the dark-adapted state (F0) was measured using a modulated pulse (<0.05 μmol m−2 s−1 for 1.8 μs) which was too small to induce significant physiological changes in the plant. The data stored were an average taken over a 1.6 s period. Maximal fluorescence in this state (Fm) was measured after applying a saturating actinic light pulse of 10 000 μmol m−2 s−1 for 0.8 s. The value of Fm was recorded as the highest average of two consecutive points. Values of the variable fluorescence (Fv=Fm–F0) and maximum quantum efficiency of PSII photochemistry (Fv/Fm) were calculated from F0 and Fm. This ratio of variable to maximal fluorescence correlates with the number of functional PSII reaction centres, and dark-adapted values of Fv/Fm can be used to quantify photoinhibition (Maxwell and Johnson, 2000).
Gas exchange
Gas exchange measurements were taken on random, fully expanded leaves (n=10 per treatment; two measurements per plant for the recovery experiment) using an infrared gas analyser in an open system (LCI-portable, ADC system, UK) after 90 d of treatment. Net photosynthetic rate (A), intercellular CO2 concentration (Ci), and stomatal conductance to CO2 (Gs) were determined at ambient CO2 concentration, a temperature of 20/25 °C, 50±5% relative humidity, and a photon flux density of 1000 μmol m−2 s−1. A, Ci, and Gs were calculated using standard formulae of Von Caemmerer and Farquhar (1981). Photosynthetic area was approximated as the area of a trapezium. The instantaneous water use efficiency (WUE) was calculated as the ratio between A and transpiration rate [mmol (CO2 assimilated) mol−1 (H2O transpired)].
Antioxidant enzyme activities
Only plants grown at 0 mmol l−1 and 1 mmol l−1 Zn and 0, 1, and 3% NaCl, and those at 10 mmol l−1 Zn and 1% NaCl were analysed because insufficient plant material had grown for the rest of the treatments. Enzyme extraction was done as described before by Aroca et al. (2001). About 0.25 g of fresh tiller tissue (n=4–6, per treatment) was homogenized in a cold mortar with 5 ml of 100 mM phosphate buffer (pH 7.0) containing 0.1 mM DTPA (diethylenetriamine pentaacetic acid; a metal-chelating agent) and 50 mg of PVPP (polyvinylpolypyrrolidone) which removes phenolics and alkaloids from plant extracts, avoiding interference with spectrophotometric measurements and enhancing enzyme stability. The homogenate was filtered and centrifuged at 38 000 g for 10 min. The supernatant was used to determine antioxidant enzyme activities. Ascorbate peroxidase (APX; EC 1.11.1.11), glutathione reductase (GR; EC 1.6.4.2), and superoxide dismutase (SOD; EC 1.15.1.1) activities were measured as described previously by Aroca et al. (2001). Catalase (CAT; EC 1.11.1.6) activity was measured as described by Aebi (1984). Consumption of H2O2 (extinction coefficient of 39.6 mM−1 cm−1) at 240 nm for 1 min was monitored. The reaction mixture consisted of 50 mM phosphate buffer (pH 7.0) containing 10 mM H2O2 and 100 μl of enzyme extract in a 2 ml volume.
Statistical analysis
Statistical analysis was carried out using Statistica v. 6.0 (Statsoft Inc.). Pearson coefficients were calculated to assess correlation between different variables. Data were analysed using one- and two-way analysis of variance (ANOVA; F-test). Data were first tested for normality with the Kolmogorov–Smirnov test and for homogeneity of variance with the Brown–Forsythe test. Significant test results were followed by LSD test for identification of pair-wise contrasts. Differences between root and tiller Zn concentrations were compared by the Student test (t-test).
Results
Growth
Plants grown at 60 mmol l−1 Zn and 0% and 3% NaCl died a week after the beginning of the experiment, thus data on growth and physiological responses were not available for either of these treatments.
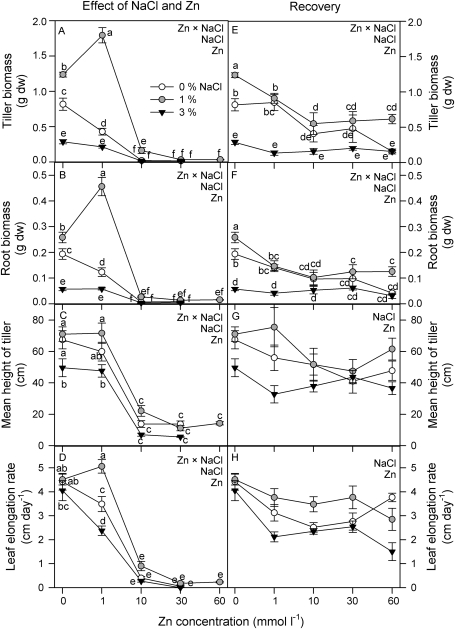
The effects of salinity and Zn concentration on final biomass were highly significant after 90 d of treatment (two-way ANOVA, Zn×salinity: P <0.0001 for both tiller and root dry biomass). The highest tiller and root dry biomass was recorded at 1 mmol l−1 Zn and 1% NaCl (Fig. 1A, B). Plants grown at 3% NaCl showed lower tiller and root biomass in all Zn treatments. The highest salinity decreased the tiller biomass of S. densiflora by ∼65% and 50% (with respect to plants grown without NaCl) at 0 mmol l−1 and 1 mmol l−1 Zn, respectively, and root biomass by ∼70% and 53% at the same Zn concentrations. Moreover, the mean height of tillers and LER were substantially reduced at Zn concentrations >1 mmol l−1, as well as at the highest salinity (two-way ANOVA, Zn×salinity: P <0.0001 for both parameters; Fig. 1C, D). Both parameters were directly correlated with tiller biomass at 1% and 3% NaCl (height of tiller, r=0.97, P <0.01 and r=0.98, P <0.05; LER, r=0.99, P <0.01 and r=0.99, P <0.05, for 1% and 3% NaCl, respectively); LER was also correlated with tiller biomass in the absence of salt (r=0.97, P <0.05). Otherwise, plants grown at 0 mmol l−1 and 1 mmol l−1 Zn showed higher number of tillers at 1% NaCl (four tillers) than the rest of the salinity treatments (1–2 tillers).
Fig. 1.
Growth analysis of Spartina densiflora seedlings in response to treatment (A–D) or pre-treatment with a range of Zn concentrations (recovery; E–H) at 0, 1, and 3% NaCl over 90 d. Tiller biomass (A, E), root biomass (B, F), mean height of tiller (C, G), and leaf elongation rate (D, H). Values represent the mean ±SE, n=10 (n=5 for the recovery experiment). Different letters indicate means that are significantly different from each other (two-way ANOVA, Zn×salinity; LSD test, P <0.05). Zn, NaCl, or Zn×NaCl in the corner of the panels indicate when the main effects and interaction term are significant.
The effects of salinity and Zn pre-treatment on final biomass were also highly significant (two-way ANOVA, Zn×salinity: P <0.001 and P <0.01 for tiller and root dry biomass, respectively). Plants grown at 1% NaCl showed higher tiller and root biomass for all Zn pre-treatments, although the highest growth was recorded in plants that were not pre-treated with Zn (Fig. 1E, F). Moreover, in the absence of salt, plants exposed to 10–60 mmol l−1 Zn showed lower tiller biomass than those at 0 mmol l−1 and 1 mmol l−1 Zn. In contrast, Zn pre-treatment does not affect plants grown at 3% NaCl. Finally, there was not a synergic effect of salinity and Zn pre-treatment on height of tiller and LER (P >0.05; Fig. 1G, H).
Chemical analysis of plant samples
The Zn concentration in tillers increased with increasing external salinity in plants treated with 1 mmol l−1 Zn (Table 1). Similarly, the Na concentration of both tillers and roots increased with external Na concentration. In contrast, tiller and root Ca, Mg, P, and K concentrations diminished with increasing Na concentration. Tiller Fe and Mg, and tiller and root Ca, Mn, and P concentrations were lower at 1 mmol l−1 Zn in the absence of salt; while root Fe and Mg concentrations were higher. Finally, N concentration in roots diminished with increasing Na concentration in the absence of external Zn concentration.
Table 1.
Total zinc, sodium, calcium, iron, magnesium, manganese, phosphorus, potassium, and nitrogen concentrations for tillers and roots of Spartina densiflora in response to a treatment with a range of salinity concentrations at 0 mmol l−1 and 1 mmol l−1mmol l−1 Zn over 90 d
| Treatment | Concentration | |||||||||
| [Zn] (mmol l−1) | Salinity (%) | Zn (mg kg−1) | Na (mg g−1) | Ca (mg g−1) | Fe (mg kg−1) | Mg (mg g−1) | Mn (mg kg−1) | P (mg g−1) | K (mg g−1) | N (%) |
| Tillers | ||||||||||
| 0 | 0 | 56 a | 5.6 c | 5.6 a | 1542 a | 6.9 a | 70.5 b | 5.8 a | 42.3 a | 2.84 a |
| 0 | 1 | 38 b | 17.6 b | 4.9 b | 399 c | 5.6 b | 46.3 c | 4.6 b | 29.8 b | 2.68 a |
| 0 | 3 | 57 a | 29.0 a | 3.1 c | 984 b | 2.9 c | 91.0 a | 4.1 c | 24.9 c | 2.75 a |
| 1 | 0 | 110 c | 3.2 c | 4.2 b | 820 a | 5.6 a | 50.3 b | 5.3 a | 47.0 a | 2.81 b |
| 1 | 1 | 128 b | 18.2 b | 5.0 a | 406 c | 5.9 a | 36.8 c | 4.7 b | 33.3 b | 2.68 b |
| 1 | 3 | 198 a | 23.1 a | 3.0 c | 610 b | 2.7 b | 110.5 a | 4.1 c | 19.7 c | 3.17 a |
| Roots | ||||||||||
| 0 | 0 | 113 b | 5.1 c | 6.6 a | 1677 c | 3.9 a | 61.8 b | 5.6 a | 27.0 b | 3.14 a |
| 0 | 1 | 103 c | 14.1 b | 4.4 b | 2313 a | 3.8 a | 54.1 c | 5.5 a | 30.6 a | 2.45 b |
| 0 | 3 | 227 a | 19.2 a | 2.0 c | 2008 b | 2.2 b | 96.2 a | 3.2 b | 21.4 c | 1.70 c |
| 1 | 0 | 1275 b | 5.0 c | 4.5 a | 3024 b | 6.3 a | 57.2 b | 4.7 a | 27.3 a | 3.05 a |
| 1 | 1 | 754 c | 12.6 b | 3.6 b | 573 c | 3.7 b | 22.2 c | 4.1 b | 28.3 a | 2.26 c |
| 1 | 3 | 2908 a | 18.6 a | 3.1 b | 9068 a | 4.1 b | 215.8 a | 4.7 a | 19.8 b | 2.59 b |
Means within a salinity treatment that have different letter are significantly different from each other (LSD test, P < 0.05).
Leaf water content
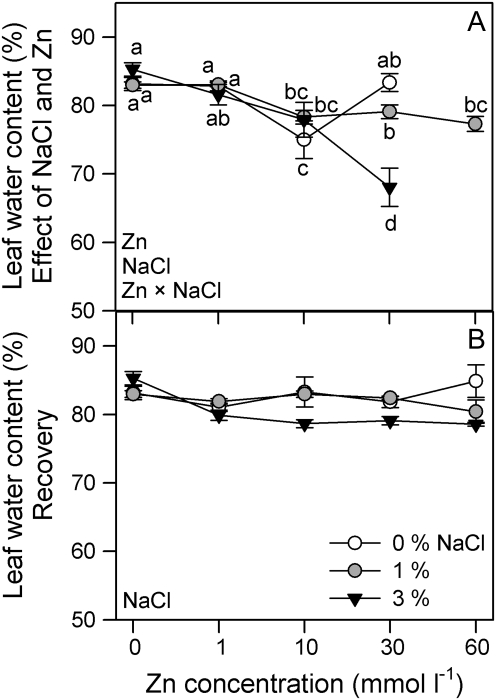
Leaf WC was affected by salinity and Zn concentration (two-way ANOVA, Zn×salinity: P <0.0001); thus, the lowest WC value was recorded at 30 mmol l−1 Zn and 3% NaCl (Fig. 2A). Overall, NaCl increasing Zn caused a corresponding decrease in WC at 3%. There were not synergic effects of salinity and Zn pre-treatment on WC during recovery (Fig. 2B).
Fig. 2.
Leaf water content in randomly selected, fully expanded penultimate leaves of Spartina densiflora in response to treatment (A) or pre-treatment with a range of Zn concentrations (B) at 0, 1, and 3% NaCl over 90 d. Values represent the mean ±SE, n=10. Different letters indicate means that are significantly different from each other (two-way ANOVA, Zn×salinity; LSD test, P <0.05). Zn, NaCl, or Zn×NaCl in the corner of the panels indicate when the main effects and interaction term are significant.
Chlorophyll fluorescence
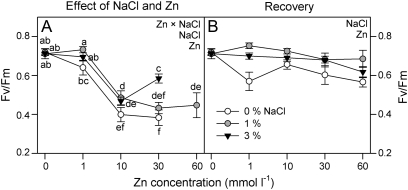
There was a synergic effect of salinity and Zn concentration on Fv/Fm values at midday (two-way ANOVA, Zn×salinity: P <0.01). Fv/Fm diminished at Zn concentrations >1 mmol l−1, and this reduction was higher in the absence of NaCl (Fig. 3A). Nevertheless, there was not a synergic effect of salinity and Zn pre-treatment on Fv/Fm (P >0.05; Fig. 3B). Plants grown in the absence of salt showed lower Fv/Fm values for all Zn pre-treatments (P <0.0001).
Fig. 3.
Maximum quantum efficiency of PSII photochemistry (Fv/Fm) at mid-day in randomly selected, fully expanded penultimate leaves of Spartina densiflora in response to treatment (A) or pre-treatment with a range of Zn concentrations (recovery; B) at 0, 1, and 3% NaCl over 90 d. Values represent the mean ±SE, n=10. Different letters indicate means that are significantly different from each other (two-way ANOVA, Zn×salinity; LSD test, P <0.05). Zn, NaCl, or Zn×NaCl in the corner of the panels indicate when the main effects and interaction term are significant.
Gas exchange
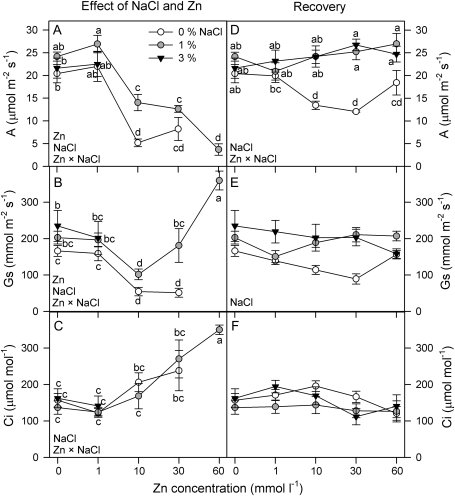
Plants grown at 3% NaCl and 10 mmol l−1 and 30 mmol l−1 Zn showed photosynthetic parameters outside the detection range of the infrared gas analyser, and therefore they have not been represented in Fig. 4A–C.
Fig. 4.
Net photosynthetic rate, A (A, D), stomatal conductance, Gs (B, E), and intercellular CO2 concentration, Ci (C, F),in randomly selected, fully expanded penultimate leaves of Spartina densiflora in response to treatment (A–C) or pre-treatment with a range of Zn concentrations (recovery; D–F) at 0, 1, and 3% NaCl over 90 d. Values represent the mean ±SE, n=10. Different letters indicate means that are significantly different from each other (two-way ANOVA, Zn×salinity; LSD test, P <0.05). Zn, NaCl, or Zn×NaCl in the corner of the panels indicate when the main effects and interaction term are significant.
The effects of salinity and Zn concentration A were significant after 90 d of treatment (two-way ANOVA, Zn×salinity: P <0.05). Values of A declined at Zn concentrations >1 mmol l−1 for all salinity treatments, and, overall, plants grown at 1% NaCl showed higher A in all Zn treatments (Fig. 4A). Gs was lower in the absence of salt and at Zn concentrations >1 mmol l−1 (two-way ANOVA, Zn×salinity: P <0.05). Plants treated with 60 mmol l−1 Zn and 1% NaCl recorded the highest Gs value (Fig. 4B). The Ci increased with Zn concentration at 0% and 1% NaCl (two-way ANOVA, Zn×salinity: P <0.0001; Fig. 4C).
Plants grown in the absence of NaCl and pre-treated with Zn concentrations >1 mmol l−1 showed the lowest values of A (two-way ANOVA, Zn×salinity: P <0.01; Fig. 4D). The lower Gs values were also recorded at 0% salinity (P <0.0001; Fig. 4E). Finally, Ci did not show any relationship with Zn pre-treatment and salinity concentration (Fig. 4F).
Antioxidant enzyme activities
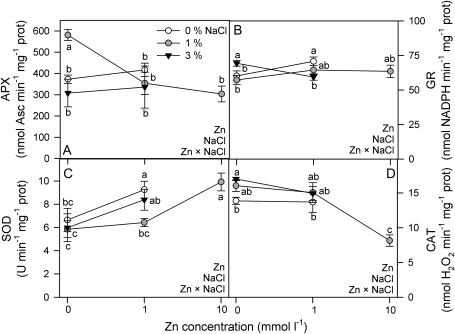
All antioxidant enzyme activities measured were affected by salinity and Zn concentration (two-way ANOVA, Zn×salinity: P <0.01, P <0.0001, P <0.0001, and P <0.0001 for APX, GR, SOD, and CAT, respectively; Fig. 5). APX was higher at 1% salinity in the absence of Zn (Fig. 5A). GR was higher at the highest salinity in the absence of Zn, but diminished with increasing salinity in the presence of Zn (1 mmol l−1 Zn; Fig. 5B). Overall, SOD increased with increasing Zn concentration (Fig. 5C), while CAT was lower at 10 mmol l−1 Zn and 1% salinity (Fig. 5D).
Fig. 5.
Ascorbate peroxidase (APX; A), glutathione reductase (GR; B), superoxide dismutase (SOD; C), and catalase (CAT; D) activities in randomly selected, fully expanded penultimate leaves of Spartina densiflora in response to teatment with 0 mmol l−1 and 1 mmol l−1 Zn at 0, 1, and 3% NaCl over 90 d. Values represent the mean ±SE, n=4–6. Different letters indicate means that are significantly different from each other (two-way ANOVA, Zn×salinity; LSD test, P <0.05). Zn, NaCl, or Zn×NaCl in the corner of the panels indicate when the main effects and interaction term are significant.
Discussion
In spite of a salinity-induced shift of the K/Na ratio towards Na, the total optimization of the physiological state of the plant upon combined action of both stressors, Zn and NaCl, was evident. This manifested itself, in particular, on final biomass of S. densiflora seedlings, since optimal growth was recorded at 1 mmol l−1 Zn and 1% NaCl; in fact, this positive effect disappeared when Zn was removed (recovery experiment). This response was apparent in the tiller and root biomass and the number of tillers, which appeared to be more sensitive to the synergic and positive effect of Zn and salinity than the mean height of tillers or LER. Additionally, enhanced growth of S. densiflora at 1 mmol l−1 Zn and 1% salinity was supported by the direct measurements of A; there was a general stimulation of A at 1% NaCl. Mateos-Naranjo et al. (2008a) also studied the growth response to Zn stress of S. densiflora, but in the absence of salt. They found the maximum value of the relative growth rate at the control level (0 mmol l−1 Zn) and concluded that Zn inhibited growth of this species. Therefore, neutralization of Zn toxicity and improvement of growth of S. densiflora were recorded with salt addition. Likewise, Kholodova et al. (2005) showed that 400 mM NaCl applied together with 0.8 mM Zn neutralized the damaging actions of Zn on biomass accumulation of Mesembryanthemum crystallinum. At the same time, under the combined action of salinity and Zn, increased biomass accumulation of S. densiflora coincided with an increase in tiller Zn concentration; thus, the total Zn accumulation per plant increased by 85% (with respect to the control, 1 mmol l−1 Zn and 0% NaCl). According with Weis and Weis (2004), the higher electric conductivity may cause this increase in metal uptake. A stimulatory effect of salinity on accumulation of heavy metals in other salt-marsh plants, primarily dicots, and their transport to above-ground organs has been previously reported in laboratory and field experiments (Mahon and Carman, 2008). In contrast, Drifmeyer and Redd (1981) found no correlation between salinity and metal content of S. alterniflora.
However, Zn concentrations >1 mmol l−1 reduced final biomass, the mean height of tillers, and LER of S. densiflora, regardless of salinity treatments. The inhibition of growth and the biomass reduction are general responses of higher plants to Zn excess (Vaillant et al., 2005). Mateos-Naranjo et al. (2008a) described higher tolerance of S. densiflora to Zn stress, since their plants survived even at 60 mmol l−1 and 100 mmol l−1 Zn and in the present experiment plants grown at 60 mmol l−1 Zn and 0% and 3% NaCl died. This discrepancy may be ascribed to the difference in seedling age; newly germinated seedlings (germinated under the same NaCl and Zn concentrations) were used here whereas Mateos-Naranjo et al. (2008a) used 1-month-old seedlings (germinated in distilled water and grown with Hoagland's solution). So a more severe stress was imposed in the present study.
The declining growth of S. densiflora at Zn concentrations >1 mmol l−1 may be attributed to a decrease in A. In the above-ground tissues, Zn has been reported to be concentrated in chloroplasts, especially in those of certain plants (e.g. spinach; Tinker, 1981). Zn interacts with the donor side of PSII, inhibiting photosynthetic CO2 fixation and the Hill reaction (Prasad and Strzalka, 1999). Decreased A, in turn, may lead to over-reduction of the reaction centres of PSII and excess energy absorption by oxygen (producing ROS), if the plant is unable to dissipate excess energy, and hence cause damage to the photosynthetic apparatus. On the other hand, the electron flow from water in PSII to O2 reduction in PSI without a net change of O2 has been proposed as an effective mechanism to dissipate the excess excitation energy under environmental stress. This cycle is also composed of some antioxidant enzymes including SOD, APX, and GR (Asada, 1999). SOD dismutates O2– to O2 and H2O2, APX reduces H2O2 formed by SOD to H2O, and GR reduces oxidized glutathione implicated in the regeneration of ascorbate (Alscher et al., 1997). However, an increase in SOD activity with Zn was recorded here, not coordinated with an increase in APX or GR activities. Such behaviour may cause accumulation of H2O2 and subsequent oxidative damage (Aroca et al., 2001). In contrast, Martínez-Domínguez et al. (2008) concluded that S. densiflora undergoes oxidative stress in its natural environment and is able to modulate its antioxidative system, based on the degree of metal pollution, in order to acclimatize successfully to its fluctuating environment; they recorded a large and rapid increase in CAT activity after transplanting plants to a polluted medium. However, lower CAT activity at 10 mmol l−1 Zn was found in the present study. Thus, the data indicate that S. densiflora did not enhance the antioxidative system in response to Zn or Zn and salinity; although it did in response to salinity.
When Zn concentrations >1 mmol l−1 were removed from the growth solutions (recovery experiment), values of A and Fv/Fm for plants treated with salt recovered to levels similar to the optimal treatment (1 mmol l−1 Zn and 1% NaCl), but plants without salt did not. In the absence of salt, the lower A can be accounted for by lower Fv/Fm. The present results agree with those of Padinha et al. (2000) and Mateos-Naranjo et al. (2008a), who found that PSII photochemistry of Spartina maritima and S. densiflora, respectively, were affected by Zn stress, indicating that Zn excess enhances photoinhibition induced by light stress. On the other hand, Hormaetxe et al. (2006) suggested that photoinhibition would play a photoprotective role, reducing the efficiency of light energy capture.
In addition, the lower A values of S. densiflora in the absence of salt for plants treated and pre-treated with Zn concentrations >1 mmol l−1 can be partially accounted for by the decreased effect of Gs, which led to an increase in Ci. The accumulation of CO2 in the intercellular spaces could indicate that the photosynthetic decline was caused by diffusional limitation of photosynthesis, rather than any effect on carboxylation capacity (Centritto et al., 2003). According to Redondo-Gómez et al. (2007), the decline in Gs was not accompanied by a loss of leaf WC in S. densiflora and therefore is likely to be the result of a signalling process rather than a general loss of turgor. In agreement with Mateos-Naranjo et al. (2008a), the lower Gs may be related to an alteration in the K/Ca ratio in the guard cells. In this way, lower Ca concentrations were recorded with Zn in the absence of salt.
Accordingly, it was thus evident that salinity favoured the tolerance and recovery of the photosynthetic apparatus of S. densiflora to the toxic action of Zn, perhaps triggering mechanisms that are specific to halophytes, such as excreting metals in salt crystals released through salt glands. It has been shown that metals can be excreted with salts on the leaf surface of S. alterniflora (Krauss, 1988), and it has been hypothesized that increasing salinity would lead to increased excretion of metals (Weis and Weis, 2004). Nevertheless, Mahon and Carman (2008) reported for S. alterniflora that the concentrations of lead and Zn in excreted salts decreased as salinity increased.
On the other hand, salinity favoured the uptake of most nutrients in the presence of Zn, since tissue Ca, Mn, and P concentrations were lower at 1 mmol l−1 Zn in the absence of salt. Mateos-Naranjo et al. (2008a) recorded that Zn affected the tissue Ca, Mg, N, and P concentrations of S. densiflora. In addition, it was found that tiller Fe concentrations were affected by the presence of Zn (without NaCl). However, root Fe and Mg concentrations were enhanced at 1 mmol l−1 Zn in the absence of salt, which may indicate that Zn interfered more with the translocation of these elements. This Zn–Fe antagonism has been previously reported by Kabata-Pendias and Pendias (2001). Finally, root N concentration was only affected by salinity. Decreases in nutrient concentrations with the progressive accumulation of Na in roots and shoots have been found previously in other halophytes (Redondo-Gómez et al., 2007, 2010).
In conclusion, the results of this study indicate that moderate Zn concentration (1 mmol l−1) and salinity (1% NaCl) had a synergic and positive effect on the final biomass of S. densiflora seedlings. At the same time, there was a stimulatory effect of salinity on accumulation of Zn in tillers of this species, which is interesting for phytoremediation. However, Zn concentrations >1 mmol l−1 inhibited growth of S. densiflora, regardless of salinity treatments. Differences in growth rate over the range of Zn concentrations studied can be accounted for largely by effects on net photosynthesis; Zn has a marked overall effect on the photochemical (PSII) apparatus. It might be partially mediated by the accumulation of H2O2 and subsequent oxidative damage. The greatest impact of Zn on photosynthesis in the absence of salt appears to be via the regulation of Gs, owing to an alteration in the K/Ca ratio in the guard cells. However, salinity favoured the recovery of the photosynthetic apparatus of S. densiflora to the toxic action of Zn, and enhanced the nutrient uptake in the presence of Zn.
Acknowledgments
We are grateful to Mr F. Fernández-Muñoz for technical assistance. We also thank the Spanish Science and Technology Ministry for its support (project CTM2008-04453), and Seville University Glasshouse General Service for collaboration.
Glossary
Abbreviations
- A
net photosynthetic rate
- APX
ascorbate peroxidase
- CAT
catalase
- Ci
intercellular CO2 concentration
- F0
minimal fluorescence level in the dark-adapted state
- Fm
maximal fluorescence level in the dark-adapted state
- Fv
variable fluorescence level in the dark-adapted state
- Fv/Fm
maximum quantum efficiency of photosystem II photochemistry
- GR
glutathione reductase
- Gs
stomatal conductance
- LER
leaf elongation rate
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- WC
water content
- WUE
water use efficiency
References
- Aebi H. Catalase in vitro. Methods in Enzymology. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Alscher RG, Donahue JL, Cramer CL. Reactive oxygen species and antioxidants: relationship in green cells. Physiologia Plantarum. 1997;100:224–233. [Google Scholar]
- Aroca R, Irigoyen JJ, Sánchez-Díaz M. Photosynthetic characteristics and protective mechanisms against oxidative stress during chilling and subsequent recovery in two maize varieties differing in chilling sensitivity. Plant Science. 2001;161:719–726. [Google Scholar]
- Asada K. The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- Cambrollé J, Redondo-Gómez S, Mateos Naranjo E, Figueroa ME. Comparison of the role of two Spartina species in terms of phytostabilization and bioaccumulation of metals in the estuarine sediment. Marine Pollution Bulletin. 2008;56:2037–2042. doi: 10.1016/j.marpolbul.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Centritto M, Loreto F, Chartzoulakis K. The use of low [CO2] to estimate diffusional and non-diffusional limitations of photosynthetic capacity of salt-stressed olive saplings. Plant, Cell and Environment. 2003;26:585–594. [Google Scholar]
- Drifmeyer JE, Redd B. Geographic variability in trace element levels in Spartina alterniflora. Estuarine, Coastal and Shelf Science. 1981;13:709–716. [Google Scholar]
- Ewing K, McKee K, Mendelssohn I, Hester M. A comparison of indicators of sublethal salinity stress in the salt marsh grass, Spartina patens (Ait.) Muhl. Aquatic Botany. 1995;52:59–74. [Google Scholar]
- Fitzgerald FJ, Caffrey JM, Nesaratnam ST, McLoughlin P. Copper and lead concentrations in salt marsh plants on the Suir Estuary, Ireland. Environmental Pollution. 2003;123:67–74. doi: 10.1016/s0269-7491(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Foyer CH, López-Delgado H, Dat JF, Scout IM. Hydrogen peroxide and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiologia Plantarum. 1997;100:241–254. [Google Scholar]
- Helal HM, Baibagyshew E, Saber S. Uptake of Cd and Ni by spinach, Spinacea oleracea (L.) from polluted soil under field conditions as affected by salt water irrigation. Agronomie. 1998;18:443–448. [Google Scholar]
- Hoagland D, Arnon DI. The water culture method for growing plants without soil. California Agricultural Experiment Station Bulletin. 1938;347:1–39. [Google Scholar]
- Hormaetxe K, Becerril JM, Hernández A, Esteban R, García-Plazaola JI. Plasticity of photoprotective mechanisms of Buxus sempervirens L. leaves in response to extreme temperatures. Plant Biology. 2006;9:59–68. doi: 10.1055/s-2006-924456. [DOI] [PubMed] [Google Scholar]
- Kabata-Pendias A, Pendias H. Trace elements in soils and plants. Boca Ratón, FL: CRC Press; 2001. 131–142. [Google Scholar]
- Kadukova J, Kalogerakis N. Lead accumulation from non-saline and saline environments by Tamarix smyrnensis Bunge. European Journal of Soil Biology. 2007;43:216–223. [Google Scholar]
- Kholodova VP, Volkov KS, Kuznetson KLK. Adaptation of the common ice plant to high copper and zinc concentrations and their potential using for phytorremediation. Russian Journal of Plant Physiology. 2005;52:848–858. [Google Scholar]
- Kholodova V, Volkov K, Kuznetsov V. Plants under heavy metal stress in saline environments. In: Sherameti I, Varma A, editors. Soil heavy metals, soil biology. vol. 19. Heidelberg: Springer-Verlag; 2010. pp. 163–183. [Google Scholar]
- Kraus ML. Accumulation and excretion of five heavy metals by the salt marsh grass Spartina alterniflora. Bulletin of the New Jersey Academy of Science. 1988;33:39–43. [Google Scholar]
- Mahon S, Carman KR. The influence of salinity on the uptake, distribution, and excretion of metals by the smooth cordgrass, Spartina alterniflora (Loisel.), grown in sediment contaminated by multiple metals. Estuaries and Coasts. 2008;31:1089–1097. [Google Scholar]
- Martínez-Domínguez D, de las Heras MA, Navarro F, Torronteras R, Córdoba F. Efficiency of antioxidant response in Spartina densiflora: an adaptative success in a polluted environment. Environmental and Experimental Botany. 2008;62:69–77. [Google Scholar]
- Mateos-Naranjo E, Redondo-Gómez S, Álvarez R, Cambrollé J, Gandullo J, Figueroa ME. Synergic effect of salinity and CO2 enrichment on growth and photosynthetic responses of the invasive cordgrass Spartina densiflora. Journal of Experimental Botany. 2010;61:1643–1654. doi: 10.1093/jxb/erq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos-Naranjo E, Redondo-Gómez S, Cambrollé J, Luque T, Figueroa ME. Growth and photosynthetic responses to zinc stress of an invasive cordgrass Spartina densiflora. Plant Biology. 2008 a;10:754–762. doi: 10.1111/j.1438-8677.2008.00098.x. [DOI] [PubMed] [Google Scholar]
- Mateos-Naranjo E, Redondo-Gómez S, Luque CJ, Castellanos EM, Davy AJ, Figueroa ME. Environmental limitations on recruitment from seed in invasive Spartina densiflora on a southern European salt marsh. Estuarine, Coastal and Shelf Science. 2008 b;79:727–732. [Google Scholar]
- Mateos-Naranjo E, Redondo-Gómez S, Silva J, Santos R, Figueroa ME. Effect of prolonged flooding on the invader Spartina densiflora Brong. Journal of Aquatic Plant Management. 2007;45:121–123. [Google Scholar]
- Maxwell K, Johnson GN. Chorophyll fluorescence—a practical guide. Journal of Experimental Botany. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- Morillo J, Usero J, Gracia I. Heavy metal distribution in marine sediments from the southwest coast of Spain. Chemosphere. 2004;55:431–442. doi: 10.1016/j.chemosphere.2003.10.047. [DOI] [PubMed] [Google Scholar]
- Nelson CH, Lamothe PJ. Heavy metal anomalies in the Tinto and Odiel River and estuary system, Spain. Estuaries. 1993;16:496–511. [Google Scholar]
- Padinha C, Santos R, Brown MT. Evaluating environmental contamination in Ria Formosa (Portugal) using stress indexes of Spartina maritima. Marine Environmental Research. 2000;49:67–78. doi: 10.1016/s0141-1136(99)00049-5. [DOI] [PubMed] [Google Scholar]
- Prasad MNV, Strzalka K. Impact of heavy metals on photosynthesis. In: Prasad MNV, Hagemeyer J, editors. Heavy metals stress in plants: from molecules to ecosystems. Berlin: Springer; 1999. pp. 117–138. [Google Scholar]
- Redondo-Gómez S, Mateos-Naranjo E, Davy AJ, Fernández-Muñoz F, Castellanos E, Luque T, Figueroa ME. Growth and photosynthetic responses to salinity of the salt-marsh shrub Atriplex portulacoides. Annals of Botany. 2007;100:555–563. doi: 10.1093/aob/mcm119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo-Gómez S, Mateos-Naranjo E, Figueroa ME, Davy AJ. Salt stimulation of growth and photosynthesis in an extreme halophyte Arthrocnemum macrostachyum. Plant Biology. 2010;12:79–87. doi: 10.1111/j.1438-8677.2009.00207.x. [DOI] [PubMed] [Google Scholar]
- Sáinz A, Grande JA, De la Torre ML, Sánchez-Rodas D. Characterisation of sequential leachate discharge of mining waste rock dumps in the Tinto and Odiel rivers. Journal of Environmental Management. 2002;64:345–353. doi: 10.1006/jema.2001.0497. [DOI] [PubMed] [Google Scholar]
- Tinker PB. Levels, distribution and chemical forms of trace elements in food plants. Philosophical Transactions of the Royal Society B: Biological Sciences. 1981;294:41–55. doi: 10.1098/rstb.1981.0088. [DOI] [PubMed] [Google Scholar]
- Tewari RK, Kumar P, Sharma PN. Morphology and physiology of zinc-stressed mulberry plants. Journal of Plant Nutrition and Soil Science. 2008;171:286–294. [Google Scholar]
- Vaillant N, Monnet F, Hitmi A, Sallanon H, Coudret A. Comparative study of responses in four Datura species to zinc stress. Chemosphere. 2005;59:1005–1013. doi: 10.1016/j.chemosphere.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:377–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Weis JS, Weis P. Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Environment International. 2004;30:687–700. doi: 10.1016/j.envint.2003.11.002. [DOI] [PubMed] [Google Scholar]