Abstract
In order to characterize the potential transcriptional regulation of core components of abscisic acid (ABA) signal transduction in tomato fruit development and drought stress, eight SlPYL (ABA receptor), seven SlPP2C (type 2C protein phosphatase), and eight SlSnRK2 (subfamily 2 of SNF1-related kinases) full-length cDNA sequences were isolated from the tomato nucleotide database of NCBI GenBank. All SlPYL, SlPP2C, and SlSnRK2 genes obtained are homologous to Arabidopsis AtPYL, AtPP2C, and AtSnRK2 genes, respectively. Based on phylogenetic analysis, SlPYLs and SlSnRK2s were clustered into three subfamilies/subclasses, and all SlPP2Cs belonged to PP2C group A. Within the SlPYL gene family, SlPYL1, SlPYL2, SlPYL3, and SlPYL6 were the major genes involved in the regulation of fruit development. Among them, SlPYL1 and SlPYL2 were expressed at high levels throughout the process of fruit development and ripening; SlPYL3 was strongly expressed at the immature green (IM) and mature green (MG) stages, while SlPYL6 was expressed strongly at the IM and red ripe (RR) stages. Within the SlPP2C gene family, the expression of SlPP2C, SlPP2C3, and SlPP2C4 increased after the MG stage; SlPP2C1 and SlPP2C5 peaked at the B3 stage, while SlPP2C2 and SlPP2C6 changed little during fruit development. Within the SlSnRK2 gene family, the expression of SlSnRK2.2, SlSnRK2.3, SlSnRK2.4, and SlSnRK2C was higher than that of other members during fruit development. Additionally, most SlPYL genes were down-regulated, while most SlPP2C and SlSnRK2 genes were up-regulated by dehydration in tomato leaf.
Keywords: ABA signal transduction, drought, expression analysis, fruit development and ripening, SlPP2C, SlPYL, SlSnRK2, tomato
Introduction
Abscisic acid (ABA) plays important roles in various aspects of plant growth and development, such as adaptation to different biotic and abiotic stresses, seed maturation and germination, as well as fruit development and ripening. ABA has been shown to regulate tomato fruit set with other plant hormones, such as gibberellin and auxin (Vriezen et al., 2008; Nitsch et al., 2009). ABA-deficient tomato mutants with only 25% of wild-type ABA levels have a decreased total fruit weight and average fruit weight, but accumulate 30% more carotenoids in ripening fruit and release more ethylene when stored at room temperature (Galpaz et al., 2008). Increased ABA levels have also been observed during the onset of fruit ripening in many species, suggesting the involvement of ABA during this process (Vendrell and Buesa, 1989; Buta and Spaulding, 1994; Kojima, 1996; Kondo and Tomiyama, 1998; Zhang et al., 2009a, b; Sun et al., 2010). In cooperation with other phytohormones such as ethylene, ABA may promote fruit ripening in both climacteric (Jiang et al., 2000; Zhang et al., 2009a) and non-climacteric fruit (Sun et al., 2010). ABA can also enhance pigment accumulation and post-harvest qualities in both citrus fruit and grapes (Alferez et al., 2005; Cantin et al., 2007). Taken together, these findings indicate that the physiological functions of ABA are relevant throughout the whole process of fruit development and ripening.
With the advantage of molecular biotechnology, the functions of many genes involved in ABA biosynthesis and metabolism have been investigated in many species (Schwartz et al., 1997; Krochko et al., 1998; Chernys and Zeevaart, 2000; Rodrigo et al., 2006; Ren et al. 2010a), including tomato (Galpaz et al., 2008; Nitsch et al., 2009; Zhang et al., 2009a) which is considered a model system for studying climacteric fruit ripening (Barry and Giovannoni, 2007). However, the integrated ABA signal transduction pathway and its mechanism are not clearly defined. Although some ABA receptor-like proteins had been reported (Shen et al., 2006; Pandey et al., 2009), their functions in transducing ABA signals are still being debated (Muller and Hansson, 2009; Wu et al., 2009; Ren et al., 2010b).
Recently, two separate research teams reported a new type of ABA receptor named PYR/PYL/RCAR (Ma et al., 2009; Park et al., 2009). In this newly discovered ABA signal transduction pathway, there are three core components: ABA receptors PYR/PYL/RCARs (hereafter the ‘PYLs’), negative regulators named PP2Cs (type 2C protein phosphatases), and positive regulators termed SnRK2s (subfamily 2 of SNF1-related kinases). The PYLs, which belong to the START protein superfamily, can sense and bind to ABA by their ligand-binding pockets (Ma et al., 2009; Melcher et al., 2009; Nishimura et al., 2009; Park et al., 2009). After binding to ABA, PYL closes two highly conserved β-loops, which function as a ‘gate’ and ‘latch’, around the entry of the ligand-binding pocket and forms a ‘gate–latch’ interface, which in turn binds to the PP2C active site and inhibits PP2C from dephosphorylating SnRK2 (R Yoshida et al., 2006; Fujii et al., 2009; Melcher et al., 2009). Finally, SnRK2 is then activated and can phosphorylate downstream effectors, such as the basic leucine zipper transcription factors ABFs/AREBs, thus switching on the transcription of ABA-responsive genes (Kobayashi et al., 2005; Furihata et al., 2006).
Among the three ABA core components, 13 PYLs (Ma et al., 2009; Park et al., 2009), nine group A PP2Cs (Leung et al., 1994, 1997; Meyer et al., 1994; Rodriguez et al., 1998; Merlot et al., 2001; Saez et al., 2004; Kuhn et al., 2006; T Yoshida et al., 2006; Nishimura et al., 2007; Umezawa et al., 2009) and 10 SnRK2s (R Yoshida et al., 2002, 2006; Hrabak et al., 2003; Boudsocq et al., 2004; Belin et al., 2006; Fujita et al., 2009) have been identified in Arabidopsis. Many homologous genes belonging to the PYL, PP2C, and SnRK2 gene families in other species, such as Oryza sativa and Zea mays, have also been identified (Miyazaki et al., 1999; Gonzalez-Garcia et al., 2003; Huai et al., 2008; Umezawa et al., 2010). The functions of genes mentioned above in response to ABA treatment and abiotic stresses, such as drought, have been investigated at the protein and transcriptional levels (Miyazaki et al., 1999; Huai et al., 2008; Li et al., 2009; Park et al., 2009; Umezawa et al., 2009). However, due to the different aims of these studies, expression patterns of PYL, PP2C, and SnRK2 genes were not investigated systematically as a whole; meanwhile, little attention has been paid to their functions in fruit.
In this study, 23 genes belonging to the PYL, PP2C, and SnRK2 gene families were identified in tomato. Their expression patterns were also investigated during fruit development and under drought stress. From the results obtained in this work, it is hoped to characterize the potential transcriptional regulation of core components of ABA signal transduction in tomato fruit development and drought stress, and deepen the understanding of ABA's biological effects in those processes.
Materials and methods
In silico analysis
The PYL, PP2C, and SnRK2 full-length cDNA sequences of Arabidopsis, Z. mays, O. sativa, and tomato were obtained from the nucleotide database of NCBI (http://www.ncbi.nlm.nih.gov/nucleotide/) as reported in previous studies (Tao et al., 2004; Yuasa et al., 2007; Huai et al., 2008; Ma et al., 2009; Melcher et al., 2009; Park et al., 2009; Umezawa et al., 2010). To identify new homologues in tomato, the cDNA sequences of PYL, PP2C, and SnRK2 gene families in Arabidopsis, Z. mays, O. sativa, and tomato were subjected to blastn searches (http://blast.ncbi.nlm.nih.gov/) against the nucleotide collection database in tomato. The open reading frames (ORFs) were determined by using NCBI ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html).
Phylogenetic analysis
Deduced amino acid sequences of SlPYLs, SlPP2Cs, and SlSnRK2s were aligned with the homologous proteins in Arabidopsis, Z. mays, and/or O. sativa using ClustalX 2.0.12 software in the default setting. The alignment results were edited and marked using BOXSHADE 3.21 software (http://www.ch.embnet.org/software/BOX_form.html). The phylogenetic trees were constructed by using the Neighbor–Joining (N-J) method in MEGA 4.0.2 software with the bootstrap analysis setting at 1000 replicates for evaluating the reliability of different phylogenetic groups. Tree files were viewed and edited using MEGA 4.0.2 software.
Plant materials
Tomatoes (Solanum lycopersicum L. cv. Hongyu) were grown under standard greenhouse conditions (25±5 °C and 70% humidity under a 14 h/10 h light/dark regime). Tomato seedlings, used for water stress treatments, were grown in plugs in a tissue culture room at 25±2 °C with a 16 h light/8 h dark cycle. Fruit ripening stages were divided according to days after flowering (DAF) and fruit colour (Fig. 3): immature green (IM), 15 DAF; mature green (MG), 33 DAF; breaker 1 (B1), 38 DAF; breaker 2 (B2), 39 DAF; breaker 3 (B3), 40 DAF; breaker 4 (B4), 41 DAF; turning 1 (T1), 44 DAF; turning 2 (T2), 48 DAF; and red ripe (RR), 54 DAF. Five fruit were harvested at each stage, and the root, stem, and leaf used for organ-specific expression analysis were sampled from 25-day-old seedlings which were grown under normal soil moisture. All fruit and samples were immediately frozen with liquid nitrogen, powdered, mixed, and stored at –80 °C until further use.
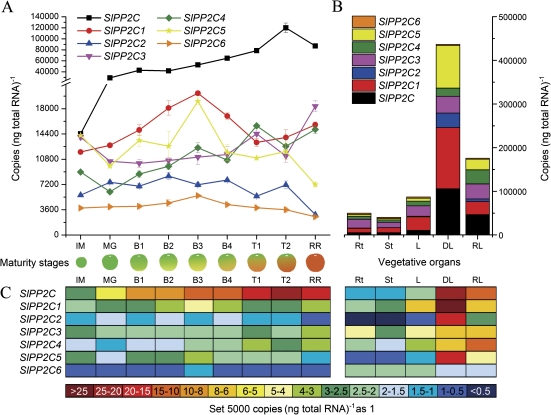
Fig. 3.
Expression of SlPP2C genes. (A) Expression of SlPP2C genes during fruit development. Fruit colour and relative size at each stage are shown below the graph. (B) Combined expression levels of SlPP2C genes in tomato vegetative organs and in response to dehydration. (C) Colour-coded expression levels of SlPP2C genes.
Water stress treatments
The 25-day-old tomato seedlings were divided into three groups with 10 seedlings in each. Leaves of seedlings in group I, growing under normal soil moisture, were sampled as controls. Seedlings in group II were grown without watering for 4 d, and then the leaves were sampled when wilting. Group III was first left unwatered for 4 d as described for group II, and then were watered and incubated for 1 h before sampling the leaves. Leaves sampled from each group were immediately frozen with liquid nitrogen, powdered, mixed, and stored at –80 °C for further use. The entire experiment was repeated twice. For testing the effects of treatment, expression of SlNCED1 and SlCYP707A2 which encode key enzymes in ABA biosynthesis and degradation, respectively, was investigated as shown in Supplementary Fig. S7 available at JXB online.
ABA treatment
Twenty fruit at each stage of IM, MG, B3, and T2 were harvested, divided into two groups (n=10 per group), and immediately soaked in a 100 μmol l−1 ABA (Sigma, A1049) water solution (group I) or distilled water (group II, control) for 10 min. The fruit were then placed in a tissue culture room at 25±2 °C and 95% relative humidity. Absorbent cotton was placed on each fruit sepal and stalk. A 3 ml aliquot of 100 μmol l−1 ABA water solution (group I) or 3 ml of distilled water (group II, control) was added to the absorbent cotton immediately and then every 6 h. After 12 h, all fruit were sampled, frozen with liquid nitrogen, powdered, mixed, and stored at –80 °C for further use. For testing the effect of this method, the expression of SlNCED1 and SlCYP707A2 which encode key enzymes in ABA biosynthesis and degradation, respectively, was investigated in the MG stage (Supplementary Fig. S7 at JXB online).
Determination of ABA content
For ABA extraction, 1 g of flesh or leaf was ground in a mortar and homogenized in extraction solution (80% methanol, v/v). Extracts were centrifuged at 10 000 g for 20 min. The supernatant liquid was eluted through a Sep-Pak C18 cartridge (Waters, Milford, MA, USA) to remove polar compounds, and then stored at –20 °C for enzyme-linked immunosorbent assay (ELISA). The ELISA procedures were conducted according to the instructions provided by the manufacturer (China Agricultural University, Beijing, China). ABA was determined by Thermo Electron (Labsystems) Multiskan MK3 (PIONEER Co., China).
Quantitative real-time PCR analysis
Total RNA was isolated from tomato samples using the hot borate method (Wan and Wilkins, 1994). Genomic DNA was eliminated using an RNase Free DNase I kit (Takara, Dalian, China) according to the manufacturer's recommendations. The quality and quantity of every RNA sample were assessed by agarose gel electrophoresis. The cDNA was synthesized from the total RNA using the PrimeScript™ RT reagent kit (Takara) according to the manufacturer's instructions. Primers used for real-time PCR, designed using Primer 5 software, are listed in Supplementary Table S1 at JXB online. The SAND gene (SGN-U573169) encoding the SAND protein was selected as an internal control gene according to Exposito-Rodriguez et al. (2008), and the stability of its expression was tested in preliminary studies shown in Supplementary Fig. S6. All primer pairs were tested by PCR. A single product of the correct size for each gene was confirmed by agarose gel electrophoresis and double-strand sequencing (Invitrogen, Beijing, China). The amplified fragment of each gene was subcloned into the pMD18-T vector (Takara) and used to generate standard curves by serial dilution. The real-time PCR was conducted using a Rotor-Gene 3000 system (Corbett Research, Australia) with SYBR Premix Ex Taq™ (Takara). Each 20 μl reaction contained 0.8 μl of primer mix (containing 4 μM of each forward and reverse primer), 1.5 μl of cDNA template, 10 μl of SYBR Premix Ex Taq™ (2×) mix, and 7.7 μl of water. Reactions were carried out under the following conditions: 95 °C/30 s (one cycle); 95 °C/15 s, 60 °C/20 s; 72 °C/15 s (40 cycles). Relative fold expression changes were calculated using the relative two standard curves method with Rotor-Gene 6.1.81 software.
Results
Gene isolation and analysis
The eight SlPYL, seven SlPP2C, and eight SlSnRK2 full-length cDNA sequences from tomato were isolated and designated as SlPYL1–SlPYL8; SlPP2C1–SlPP2C6 and SlPP2C; and SlSnRK2.1–SlSnRK2.7 and SlSnRK2C, respectively (Fig. 1). Among them, SlPYL1–SlPYL8, SlPP2C3–SlPP2C6 and SlSnRK2.1–SlSnRK2.7 were not previously reported.
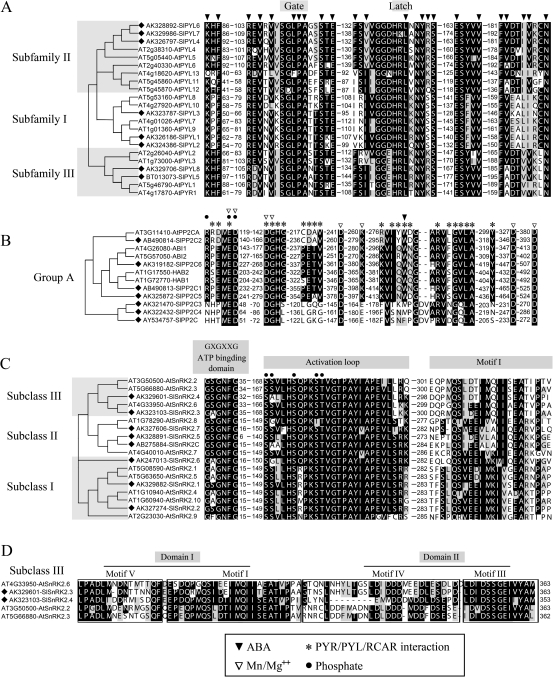
Fig. 1.
Sequence alignments and phylogenetic trees of the PYL, PP2C, and SnRK2 gene families. Genes studied are marked with black diamonds. Amino acid sequences are shown only for functional residues and domains. Residue positions are marked with the numbers nearby. Conserved residues are marked with black or grey shading. Phylogenetic trees are shown only with their topological structures. (A) Sequence alignment and phylogenetic tree of the PYL family. Residues forming the ligand-binding pocket are marked with black triangles. The gate and latch domains are indicated. Functional residues and domains are based on reports by Melcher et al. (2009) and Santiago et al. (2009). (B) Sequence alignment and phylogenetic tree of the PP2C family. Residues interacting with ABA, PYLs, and Mn/Mg ions are marked with black triangles, asterisks, and white triangles, respectively. Phosphatase sites are marked with black circles. Functional residues and domains are based on studies by Melcher et al. (2009) and Santiago et al. (2010). (C) Sequence alignment and phylogenetic tree of the SnRK2 family. Potential phosphatase sites are marked with black circles according to Umezawa et al. (2009). Functional residues and domains are noted according to Yoshida et al. (2006) and Yuasa et al. (2007). (D) Sequence alignment of C-terminal regions of subclass III SnRK2s. Functional domains are noted according to Yoshida et al. (2006) and Huai et al. (2008).
The deduced amino acid sequence lengths of SlPYLs, SlPP2Cs, and SlSnRK2s were 181–231, 281–546, and 336–362, respectively (Fig. 1; Supplementary Figs S1–S3 at JXB online). The similarities of the deduced amino acid sequences within each gene family were 35.06–84.13% for SlPYLs (Supplementary Table S2), 15.41–79.80% for SlPP2Cs (Supplementary Table S3), and 60.75–82.13% for SlSnRK2s (Supplementary Table S4). Based on multiple alignments of these protein sequences (Fig. 1; Supplementary Figs S1–S3), most of the functional residues or domains were observed to be well conserved within each gene family. Based on the phylogenetic analysis (Fig. 1; Supplementary Fig. S5), the SlPYL and SlSnRK2 protein families each clustered into three subfamilies/subclasses: PYL subfamily I, SlPYL1, SlPYL2, and SlPYL3; PYL subfamily II, SlPYL4, SlPYL6, and SlPYL7; PYL subfamily III, SlPYL5 and SlPYL8 (Fig. 1A); SnRK2 subclass I, SlSnRK2.1, SlSnRK2.2, and SlSnRK2.6; SnRK2 subclass II, SlSnRK2.5, SlSnRK2.7, and SlSnRK2C; and SnRK2 subclass III, SlSnRK2.3 and SlSnRK2.4 (Fig. 1C; Supplementary Fig. S5B). Subclasses/subfamilies were named according to Ma et al. (2009) and Umezawa et al. (2010). All SlPP2Cs belonged to PP2C group A (Fig. 1B; Supplementary Fig. S5A).
Expression of SlPYL, SlPP2C, and SlSnRK2 genes in tomato during fruit development and ripening
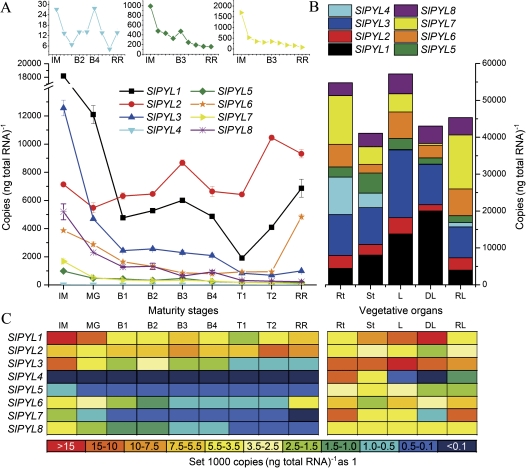
Compared with other genes of the SlPYL gene family (Fig. 2A), SlPYL1 and SlPYL2 were expressed at high levels throughout the process of fruit development and ripening, and their expression patterns could be clearly divided into three periods: early development, breaker, and ripening. Both of these genes peaked at the B3 stage. Other genes, such as SlPYL3, SlPYL5, SlPYL7, and SlPYL8, generally followed a decreasing expression pattern; among them, SlPYL3 was strongly expressed at the IM and MG stages. SlPYL6 was expressed strongly at the IM and RR stages, while SlPYL4 was consistently expressed at extremely low levels at all maturity stages.
Fig. 2.
Expression of SlPYL genes. (A) Expression of SlPYL genes during fruit development. Genes with low expression are shown at the top. Maturity stages: immature green (IM), mature green (MG), breaker 1 (B1), breaker 2 (B2), breaker 3 (B3), breaker 4 (B4), turning 1 (T1), turning 2 (T2), red ripe (RR). (B) Combined expression levels of SlPYL genes in tomato vegetative organs and in response to dehydration. Vegetative organs: root (Rt), stem (St), leaf (L), dehydrated leaf (DL), rehydrated leaf (RL). (C) Colour-coded expression levels of SlPYL genes.
Within the SlPP2C gene family (Fig. 3A), SlPP2C, SlPP2C3, and SlPP2C4 generally followed an increasing expression pattern after the MG stage; among them, SlPP2C was expressed at a much higher level than the other genes. SlPP2C1 and SlPP2C5 were both strongly expressed during the breaker stages and peaked at stage B3. Compared with the other genes, SlPP2C2 and SlPP2C6 were expressed at low levels and changed little during the whole process of fruit development and ripening.
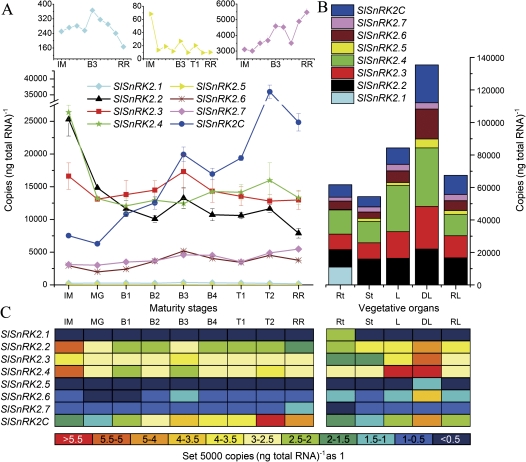
The genes within the SlSnRK2 family (Fig. 4A) could be clearly divided into three groups according to their expression levels. SlSnRK2.2, SlSnRK2.3, SlSnRK2.4, and SlSnRK2C belonged to the first group with high expression levels at all maturity stages; among them, SlSnRK2C expression continued to increase after the MG stage and had two peaks at the B3 and T2 stages. Other genes followed a decreasing expression pattern from the IM to MG stages, after which they were expressed differently, with SlSnRK2.3 and SlSnRK2.4 peaking at the B3 stage. Moreover, SlSnRk2.6 and SlSnRK2.7 were moderately expressed, while SlSnRK2.1 and SlSnRK2.5 were expressed at a low level.
Fig. 4.
Expression of SlSnRK2 genes. (A) Expression of SlSnRK2 genes during fruit development. Genes with low expression levels are shown at the top. (B) Combined expression levels of SlSnRK2 genes in tomato vegetative organs and in response to dehydration. (C) Colour-coded expression of SlSnRK2 genes.
Expression of SlPYL, SlPP2C, and SlSnRK2 genes in response to exogenous ABA treatment in tomato fruit
All tested genes within the SlPYL family (Fig. 5A) were up-regulated by exogenous ABA at the IM stage, but the influence was slight at the MG stage. At the B and T stages, different SlPYL genes showed different responses to ABA treatment. For example, SlPYL2 was consistently up-regulated, while SlPYL8 was consistently down-regulated by ABA throughout development.
Fig. 5.
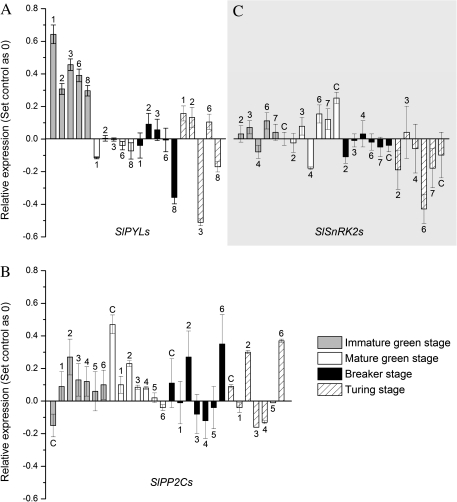
Gene expression in ABA-treated fruit. (A) Changes in expression of SlPYL genes in response to ABA in tomato fruit. (B) Changes in expression of SlPP2C genes in response to ABA in tomato fruit. (C) Changes in expression of SlSnRK2 genes in response to ABA in tomato fruit. Bars in the positive quadrant indicate up-regulated gene expression, while those in the negative quadrant indicate down-regulated gene expression. Numbers/letters above/below the bars correspond to the last number/letter of the indicated gene family members.
Almost all SlPP2C genes were up-regulated by exogenous ABA at the IM and MG stages (Fig. 5B). At the B and T stages, SlPP2C genes showed variable responses to ABA treatment. Generally, SlPP2C, SlPP2C2, and SlPP2C6 were up-regulated, while SlPP2C3 and SlPP2C4 were down-regulated. SlPP2C1 and SlPP2C5 showed almost no response to ABA treatment.
Within the SlSnRK2 gene family (Fig. 5C), all tested genes were up-regulated except for SlSnRK2.4 at the IM and MG stages. At the B stage, most of the SlSnRK2 genes showed a slight response to exogenous ABA, and all SlSnRK2 genes were down-regulated at the T stage, except for SlSnRK2.3 and SlSnRK2.4 which showed no significant changes.
Expression of SlPYL, SlPP2C, and SlSnRK2 genes in vegetative organs
All SlPYL genes were found to be expressed in all vegetative organs, except for SlPYL4 which was very weakly expressed in leaf (Fig. 2B). The highest combined expression of SlPYL genes is in the leaf, followed by the root and then the stem (Fig. 2B).
All SlPP2C genes were also found to be expressed in all vegetative organs, except for SlPP2C2 which was consistently expressed at low levels in all vegetative organs (Fig. 3B). The highest combined expression of SlPP2C genes is in the leaf, followed by the root and then the stem (Fig. 3B).
Within the SlSnRK2 gene family (Fig. 4B), SlSnRK2.1 was only expressed in the root, while all the other SlSnRK2 genes were expressed in all vegetative organs. The highest combined expression of SlSnRK2 genes is in the leaf, followed by the root and then the stem (Fig. 4B).
Expression of SlPYL, SlPP2C, and SlSnRK2 genes in response to dehydration in tomato leaf
Within the SlPYL gene family (Fig. 2B), all genes except for SlPYL1 and SlPYL8 were down-regulated by dehydration. SlPYL1 was up-regulated by dehydration, while SlPYL8 showed no response. After rehydration for 1 h, most of the SlPYL genes returned to their normal expression levels.
All genes within the SlPP2C gene family (Fig. 3B) were significantly up-regulated by dehydration, except for SlPP2C6 which showed no response. After rehydration for 1 h, expression of all SlPP2C genes decreased but was still higher than their normal expression levels.
Within the SlSnRK2 gene family (Fig. 4B), all genes were up-regulated by dehydration, except for SlSnRK2.7 which showed nearly no response. After rehydration for 1 h, all SlSnRK2 genes decreased to levels at or lower than their normal expression levels.
Variations in ABA content during tomato fruit development and ripening and in response to dehydration in leaf
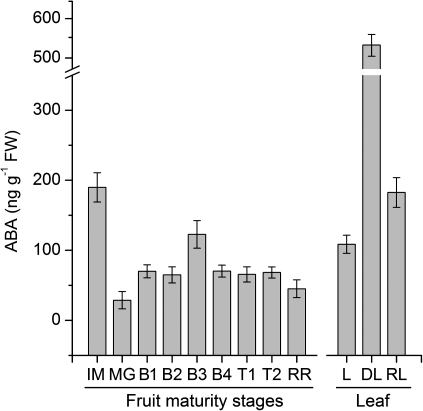
ABA content was determined during fruit development and ripening. As shown in Fig. 6, ABA was at its highest level at the IM stage and decreased to the lowest level at the MG stage. It increased and peaked again at the B3 stage, then decreased thereafter toward the RR stage. The ABA content was also determined in response to dehydration in tomato leaf. As shown in Fig. 6, the ABA content in dehydrated leaf increased to ∼5 times that of the control leaf. After rehydration for 1 h, the ABA content of the leaf sharply decreased to 40% of that of the dehydrated leaf, but it was still higher than that of the control leaf.
Fig. 6.
ABA content at different maturity stages of tomato fruit and in leaves in dehydration conditions. Maturity stages: immature green (IM), mature green (MG), breaker 1 (B1), breaker 2 (B2), breaker 3 (B3), breaker 4 (B4), turning 1 (T1), turning 2 (T2), red ripe (RR). Leaves: non-treated leaf (L), dehydrated leaf (DL), rehydrated leaf (RL).
Discussion
The mechanism of fruit ripening has long been studied, and ethylene has been demonstrated to be an important regulator of climacteric fruit (Barry and Giovannoni, 2007). However, the mechanism of non-climacteric fruit ripening is still unclear. Other plant hormones or effectors have also been shown to be involved in regulating fruit development and ripening (Vriezen et al., 2008; Nitsch et al., 2009). Among them, ABA has pleiotropic biological effects and correlates with multiple processes during fruit ripening (Vendrell and Buesa, 1989; Buta and Spaulding, 1994; Kojima, 1996; Kondo and Tomiyama, 1998; Alferez et al., 2005; Cantin et al., 2007). Therefore, in recent years, our laboratory has committed to studying the biological functions of ABA in the regulation of fruit development and ripening (Zhang et al., 2009a, b; Ren et al., 2010a, b; Sun et al., 2010). However, the molecular mechanism of how ABA regulates fruit ripening has still not been fully clarified. Encouragingly, the ABA signal transduction pathway has been established by identification of three core components: PYL/RCAR, PP2C, and SnRK2 (Ma et al., 2009; Park et al., 2009). This breakthrough provided new leads for investigating the issue mentioned above.
The results in Fig. 1 and in Supplementary Figs S1–S5 at JXB online showed that the SlPYL, SlPP2C, and SlSnRK2 genes identified in tomato are homologous to AtPYL, AtPP2C, and AtSnRK2 in Arabidopsis, respectively. Additionally, most of their deduced amino acid sequences are well conserved in functional residues or domains (Fig. 1; Supplementary Figs S1–S4) within each gene family. Therefore, it can be presumed that SlPYL genes encode ABA receptors in tomato. Meanwhile, it is worth noting the presence of an amino acid mutation (R146K) in the latch domain of SlPYL7 (Fig. 1A; Supplementary Fig. S1), as previously reported (Melcher et al., 2009), which may decrease the ability of SlPYL7 to respond to ABA. Compared with other members of the SlPP2C family (Fig. 1B; Supplementary Fig. S2), the deduced amino acid sequences of SlPP2C, SlPP2C3, and SlPP2C4 had significant differences in residues interacting with PYLs and ABA. However, as the SlPP2C gene was previously shown to encode an enzymatically active PP2C in tomato (Tao et al., 2004), it can be presumed that all SlPP2C genes encode PP2Cs, but only SlPP2C1, SlPP2C2, SlPP2C5, and SlPP2C6 may be involved in ABA signal transduction in tomato. Within the SlSnRK2 gene family, only SlSnRK2.3 and SlSnRK2.4 belonged to the SnRK2 subclass III (Fig. 1C; Supplementary Fig. S5B) and contained a D-rich C-terminal domain II (Fig. 1D; Supplementary Figs S3, S4) which was shown to be essential for transducing ABA signals (R Yoshida et al., 2006). Therefore, SlSnRK2.3 and SlSnRK2.4 may encode ABA signal transduction core components in tomato, while the other SlSnRK2 genes may encode SnRK2. However, whether they can transduce ABA signals will still need to be explored in further studies.
The variations in expression of many genes mentioned above were associated with the process of fruit development and ripening (Figs 2A, 3A, 4A) and ABA content (Fig. 6). Therefore, it can be presumed that the ABA signal transduction core components are involved in the regulation of fruit development and ripening at the transcriptional level. Within the SlPYL gene family (Fig. 2A), SlPYL1, SlPYL2, SlPYL3, and SlPYL6 were the major genes involved in the regulation of fruit development and ripening. Among them, SlPYL1 and SlPYL2 may have functional roles throughout the whole process of fruit development and ripening. Meanwhile, SlPYL3 and SlPYL6 may carry out regulatory functions mainly at the early development (IM and MG) stages and later ripening stages, respectively. Within the SlPP2C gene family (Fig. 3A), SlPP2C1 and SlPP2C5 were the main genes regulating both fruit development and ripening processes. Of the SlSnRK2 gene family (Fig. 4A), SlSnRk2.3 and SlSnRK2.4 may play key roles in the regulation of fruit development and ripening in all maturity stages, and SlSnRK2.3 may play a more important role during the breaker stages.
Recent studies suggested that ABA may be involved in regulating the onset of fruit ripening (Zhang et al., 2009a; Sun et al., 2010). In this present work, it was also found that the ABA content peaked at the breaker stages (Fig. 6), corresponding to the peak of the highly expressed genes SlPYL1, SlPYL2, SlPP2C1, SlPP2C5, and SlSnRK2.3 (Figs 2A, 3A, 4A). Therefore, these genes may also be involved in regulating fruit ripening onset.
To explore the correlations between gene expression and ABA content, the highly expressed genes were investigated in response to exogenous ABA treatment in fruit. The results in Fig. 5 indicated that at early developmental stages, especially at the IM stage, expression of most genes positively correlated with ABA accumulation. In contrast, there was no clear correlation between gene expression and ABA content accumulation at the B and T stages. For example, SlPP2C1 and SlPP2C5, which varied with the fruit ripening process and ABA content, had almost no response to exogenous ABA treatment (Fig. 5B); while SlPP2C2 and SlPP2C6 which changed little during different maturity stages (Fig. 3A) were up-regulated by exogenous ABA (Fig. 5B). However, direct experimental evidence is still needed to clarify the exact relationship between changes in expression of ABA signal transduction core components and endogenous ABA content.
Another interesting result worth noting was that, within the SlPYL gene family, the highly expressed genes SlPYL1, SlPYL2, and SlPYL3 were on the same branch of the phylogenetic tree (Fig. 1A) and shared a high identity in their amino acid sequences (Supplementary Table S2 at JXB online). Therefore, it can be presumed that, in tomato plants which produce a typical fleshfruit, some ABA receptors may evolve to take on more tasks in regulating fruit development and ripening.
The expression patterns of genes encoding ABA signal transduction core components were also investigated in response to dehydration in tomato leaf. The results (Figs 2B, 3B, 4B) were similar to those found in Arabidopsis and Z. mays (Chak et al., 2000; Santiago et al., 2009), suggesting that members in each gene family of the ABA signal transduction core components had similar basic characteristics allowing them to respond to dehydration at the transcriptional level.
All the findings herein were based on analysis at the transcriptional level. However, protein accumulation may not always correspond with the mRNA levels (Kevany et al., 2007). Additionally, regulation of ABA signal transduction occurs through protein–protein interactions between PYLs and PP2Cs, and also between PP2Cs and SnRK2s (Fujii et al., 2009; Kline et al., 2010; Umezawa et al., 2010). To better understand the molecular mechanism involving ABA in the regulation of fruit development and ripening, it is intended to investigate in further studies the functions of SlPYLs, SlPP2Cs, and SlSnRK2s at the protein level by utilizing the yeast two-hybrid method and RNA interference (RNAi) technology combined with proteomic analysis.
Conclusion
In this study, SlPYL, SlPP2C, and SlSnRK2 genes encoding the ABA signal core components from tomato were isolated in silico and confirmed to be homologous to the Arabidopsis AtPYL, AtPP2C, and AtSnRK2 genes, respectively. The transcriptional regulation of these genes was determined by detecting the expression patterns and levels of SlPYL, SlPP2C, and SlSnRK2 genes, which varied during fruit development and ripening, as well as during dehydration in tomato leaf. Therefore, the ABA receptors and transduction pathway in tomatoes may function to regulate fruit development and response to drought stress.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Sequence alignment of the PYL family.
Figure S2. Sequence alignment of the PP2C family.
Figure S3. Sequence alignment of the SnRK2 family.
Figure S4. Sequence alignment of subclass III SnRK2s.
Figure S5. Phylogenetic trees of PP2C and SnRK families in tomato and Arabidopsis.
Figure S6. Expression of the SAND gene at different stages of maturity of tomato fruit.
Figure S7. Expression of SlNCED1 and SlCYP707A2 genes in dehydration-treated leaf and ABA-treated fruit.
Table S1. Real-time PCR primers used in this study.
Table S2. Similarity of SlPYLs based on deduced amino acids.
Table S3. Similarity of SlPP2Cs based on deduced amino acids.
Table S4. Similarity of SlSnRK2s based on deduced amino acids.
References
- Alferez F, Sala JM, Sanchez-Ballesta MT, Mulas M, Lafuente MT, Zacarias L. A comparative study of the postharvest performance of an ABA-deficient mutant of oranges I. Physiological and quality aspects. Postharvest Biology and Technology. 2005;37:222–231. [Google Scholar]
- Barry CS, Giovannoni JJ. Ethylene and fruit ripening. Journal of Plant Growth Regulation. 2007;26:143–159. [Google Scholar]
- Belin C, Franco PO, Bourbousse C, Chaignepain S, Schmitter JM, Vavasseur A, Giraudat J, Barbier-Brygoo H, Thomine S. Identification of features regulating OST1 kinase activity and OST1 function in guard cells. Plant Physiology. 2006;141:1316–1327. doi: 10.1104/pp.106.079327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M, Barbier-Brygoo H, Lauriere C. Identification of nine sucrose nonfermenting 1-related protein kinases 2 activated by hyperosmotic and saline stresses in Arabidopsis thaliana. Journal of Biological Chemistry. 2004;279:41758–41766. doi: 10.1074/jbc.M405259200. [DOI] [PubMed] [Google Scholar]
- Buta JG, Spaulding DW. Changes in indole-3-acetic acid and abscisic acid levels during tomato (Lycopersicon esculentum Mill.) fruit development and ripening. Journal of Plant Growth Regulation. 1994;13:163–166. [Google Scholar]
- Cantin CM, Fidelibus MW, Crisosto CH. Application of abscisic acid (ABA) at veraison advanced red color development and maintained postharvest quality of ‘Crimson Seedless’ grapes. Postharvest Biology and Technology. 2007;46:237–241. [Google Scholar]
- Chak RKF, Thomas TL, Quatrano RS, Rock CD. The genes ABI1 and ABI2 are involved in abscisic acid- and drought-inducible expression of the Daucus carota L. Dc3 promoter in guard cells of transgenic Arabidopsis thaliana (L.) Heynh. Planta. 2000;210:875–883. doi: 10.1007/s004250050692. [DOI] [PubMed] [Google Scholar]
- Chernys JT, Zeevaart ADJ. Characterization of the 9- cis-epoxycarotenoid dioxygenase gene family and the regulation of abscisic acid biosynthesis in avocado. Plant Physiology. 2000;124:343–354. doi: 10.1104/pp.124.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exposito-Rodriguez M, Borges AA, Borges-Perez A, Perez JA. Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biology. 2008;8:131–143. doi: 10.1186/1471-2229-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, Cutler SR, Sheen J, Rodriguez PL, Zhu JK. In vitro reconstitution of an abscisic acid signaling pathway. Nature. 2009;462:660–664. doi: 10.1038/nature08599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Nakashima K, Yoshida T, et al. Three SnRK2 protein kinases are the main positive regulators of abscisic acid signaling in response to water stress in Arabidopsis. Plant and Cell Physiology. 2009;50:2123–2132. doi: 10.1093/pcp/pcp147. [DOI] [PubMed] [Google Scholar]
- Furihata T, Maruyama K, Fujita Y, Umezawa T, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K. Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proceedings of the National Academy of Sciences, USA. 2006;103:1988–1993. doi: 10.1073/pnas.0505667103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galpaz N, Wang Q, Menda N, Zamir D, Hirschberg J. Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content. The Plant Journal. 2008;53:717–730. doi: 10.1111/j.1365-313X.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garcia MP, Rodriguez D, Nicolas C, Rodriguez PL, Nicolas G, Lorenzo O. Negative regulation of abscisic acid signaling by the Fagus sylvatica FsPP2C1 plays a role in seed dormancy regulation and promotion of seed germination. Plant Physiology. 2003;133:135–144. doi: 10.1104/pp.103.025569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabak EM, Chan CWM, Gribskov M, et al. The Arabidopsis CDPK–SnRK superfamily of protein kinases. Plant Physiology. 2003;132:666–680. doi: 10.1104/pp.102.011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huai J, Wang M, He J, Zheng J, Dong Z, Lv H, Zhao J, Wang G. Cloning and characterization of the SnRK2 gene family from. Zea mays. Plant Cell Reports. 2008;27:1861–1868. doi: 10.1007/s00299-008-0608-8. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Joyce DC, Macnish AJ. Effect of abscisic acid on banana fruit ripening in relation to the role of ethylene. Journal of Plant Growth Regulation. 2000;19:106–111. doi: 10.1007/s003440000011. [DOI] [PubMed] [Google Scholar]
- Kevany BM, Tieman DM, Taylor MG, Cin VD, Klee HJ. Ethylene receptor degradation controls the timing of ripening in tomato fruit. The Plant Journal. 2007;51:458–467. doi: 10.1111/j.1365-313X.2007.03170.x. [DOI] [PubMed] [Google Scholar]
- Kline KG, Sussman MR, Jones AM. Abscisic acid receptors. Plant Physiology. 2010;154:479–482. doi: 10.1104/pp.110.160846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Murata M, Minami H, Yamamoto S, Kagaya Y, Hobo T, Yamamoto A, Hattori T. Abscisic acid-activated SNRK2 protein kinases function in the gene-regulation pathway of ABA signal transduction by phosphorylating ABA response element-binding factors. The Plant Journal. 2005;44:939–949. doi: 10.1111/j.1365-313X.2005.02583.x. [DOI] [PubMed] [Google Scholar]
- Kojima K. Distribution and change of endogenous IAA and ABA in asparagus spear and orange fruit. Chemical Regulation of Plants, Japan. 1996;31:68–71. [Google Scholar]
- Kondo S, Tomiyama A. Changes of free and conjugated ABA in the fruit of Satohnishiki sweet cherry and the ABA metabolism after application of (s)-(+)-ABA. Journal of Horticultural Science and Biotechnology. 1998;73:467–472. [Google Scholar]
- Krochko JE, Abrams GD, Loewen MK, Abrams SR, Cutler AJ. (+)-Abscisic acid 8'-hydroxylase is a cytochrome P450 monooxygenase. Plant Physiology. 1998;118:849–860. doi: 10.1104/pp.118.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn JM, Boisson-Dernier A, Dizon MB, Maktabi MH, Schroeder JI. The protein phosphatase AtPP2CA negatively regulates abscisic acid signal transduction in Arabidopsis, and effects of abh1 on AtPP2CA mRNA. Plant Physiology. 2006;140:127–139. doi: 10.1104/pp.105.070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J, Bouvier-Durand M, Morris PC, Guerrier D, Chefdor F, Giraudat J. Arabidopsis ABA response gene ABI1: features of a calcium-modulated protein phosphatase. Science. 1994;264:1448–1452. doi: 10.1126/science.7910981. [DOI] [PubMed] [Google Scholar]
- Leung J, Merlot S, Giraudat J. The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. The Plant Cell. 1997;9:759–771. doi: 10.1105/tpc.9.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FH, Fu FL, Sha LN, He L, Li WC. Differential expression of serine/threonine protein phosphatase type-2C under drought stress in maize. Plant Molecular Biology Reporter. 2009;27:29–37. [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–1068. doi: 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- Melcher K, Ng LM, Zhou XE, et al. A gate–latch–lock mechanism for hormone signalling by abscisic acid receptors. Nature. 2009;462:602–608. doi: 10.1038/nature08613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot S, Gosti F, Guerrier D, Vavasseur A, Giraudat J. The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. The Plant Journal. 2001;25:295–303. doi: 10.1046/j.1365-313x.2001.00965.x. [DOI] [PubMed] [Google Scholar]
- Meyer K, Leube MP, Grill E. A protein phosphatase 2C involved in ABA signal transduction in. Arabidopsis thaliana. Science. 1994;264:1452–1455. doi: 10.1126/science.8197457. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Koga R, Bohnert HJ, Fukuhara T. Tissue- and environmental response-specific expression of 10 PP2C transcripts in Mesembryanthemum crystallinum. Molecular Genetics and Genomics. 1999;261:307–316. doi: 10.1007/s004380050971. [DOI] [PubMed] [Google Scholar]
- Muller AH, Hansson M. The barley magnesium chelatase 150-kD subunit is not an abscisic acid receptor. Plant Physiology. 2009;150:157–166. doi: 10.1104/pp.109.135277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N, Hitomi K, Arvai AS, Rambo RP, Hitomi C, Cutler SR, Schroeder JI, Getzoff ED. Structural mechanism of abscisic acid binding and signaling by dimeric PYR1. Science. 2009;326:1373–1379. doi: 10.1126/science.1181829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N, Yoshida T, Kitahata N, Asami T, Shinozaki K, Hirayama T. ABA-Hypersensitive Germination1 encodes a protein phosphatase 2C, an essential component of abscisic acid signaling in Arabidopsis seed. The Plant Journal. 2007;50:935–949. doi: 10.1111/j.1365-313X.2007.03107.x. [DOI] [PubMed] [Google Scholar]
- Nitsch LMC, Oplaat C, Feron R, Ma Q, Wolters-Arts M, Hedden P, Mariani C, Vriezen WH. Abscisic acid levels in tomato ovaries are regulated by LeNCED1 and. SlCYP707A1. Planta. 2009;229:1335–1346. doi: 10.1007/s00425-009-0913-7. [DOI] [PubMed] [Google Scholar]
- Pandey S, Nelson DC, Assmann SM. Two novel GPCR-type G proteins are abscisic acid receptors in. Arabidopsis. Cell. 2009;136:136–148. doi: 10.1016/j.cell.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Park SY, Fung P, Nishimura N, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Sun L, Wang C, Zhao S, Leng P. Expression analysis of the cDNA for magnesium chelatase H subunit (CHLH) during sweet cherry fruit ripening and under stress conditions. Plant Growth Regulation. 2010 b;63:301–307. [Google Scholar]
- Ren J, Sun L, Wu J, Zhao S, Wang C, Wang Y, Ji K, Leng P. Cloning and expression analysis of cDNAs for ABA 8'-hydroxylase during sweet cherry fruit maturation and under stress conditions. Journal of Plant Physiology. 2010 a;167:1486–1493. doi: 10.1016/j.jplph.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Rodrigo MJ, Alquezar B, Zacarias L. Cloning and characterization of two 9-cis-epoxycarotenoid dioxygenase genes, differentially regulated during fruit maturation and under stress conditions, from orange (Citrus sinensis L. Osbeck) Journal of Experimental Botany. 2006;57:633–643. doi: 10.1093/jxb/erj048. [DOI] [PubMed] [Google Scholar]
- Rodriguez PL, Benning G, Grill E. ABI2,a second protein phosphatase 2C involved in abscisic acid signal transduction in Arabidopsis. FEBS Letters. 1998;421:185–190. doi: 10.1016/s0014-5793(97)01558-5. [DOI] [PubMed] [Google Scholar]
- Saez A, Apostolova N, Gonzalez-Guzman M, Gonzalez-Garcia MP, Nicolas C, Lorenzo O, Rodriguez PL. Gain-of-function and loss-of-function phenotypes of the protein phosphatase 2C HAB1 reveal its role as a negative regulator of abscisic acid signalling. The Plant Journal. 2004;37:354–369. doi: 10.1046/j.1365-313x.2003.01966.x. [DOI] [PubMed] [Google Scholar]
- Santiago J, Dupeux F, Betz K, Antoni R, Gonzalez-Guzman M, Rodriguez L, Marquez JA, Rodriguez PL. Plant Science. 2010. Structural insights into PYR/PYL/RCAR ABA receptors and PP2Cs. (in press) [DOI] [PubMed] [Google Scholar]
- Santiago J, Rodrigues A, Saez A, Rubio S, Antoni R, Dupeux F, Park SY, Márquez JA, Cutler SR, Rodriguez PL. Modulation of drought resistance by the abscisic acid receptor PYL5 through inhibition of clade A PP2Cs. The Plant Journal. 2009;60:575–588. doi: 10.1111/j.1365-313X.2009.03981.x. [DOI] [PubMed] [Google Scholar]
- Shen YY, Wang XF, Wu FQ, et al. The Mg-chelatase H subunit is an abscisic acid receptor. Nature. 2006;443:823–826. doi: 10.1038/nature05176. [DOI] [PubMed] [Google Scholar]
- Shin R, Alvarez S, Burch AY, Jez JM, Schachtman DP. Phosphoproteomic identification of targets of the Arabidopsis sucrose nonfermenting-like kinase SnRK2.8 reveals a connection to metabolic processes. Proceedings of the National Academy of Sciences, USA. 2007;104:6460–6465. doi: 10.1073/pnas.0610208104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SH, Tan BC, Gage DA, Zeevaart JA, McCarty DR. Specific oxidative cleavage of carotenoids by VP14 of maize. Science. 1997;276:1872–1874. doi: 10.1126/science.276.5320.1872. [DOI] [PubMed] [Google Scholar]
- Sun L, Zhang M, Ren J, Qi J, Zhang G, Leng P. Reciprocity between abscisic acid and ethylene at the onset of berry ripening and after harvest. BMC Plant Biology. 2010;10:257–268. doi: 10.1186/1471-2229-10-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Rao PK, Bhattacharjee S, Gelvin SB. Expression of plant protein phosphatase 2C interferes with nuclear import of the Agrobacterium T-complex protein VirD2. Proceedings of the National Academy of Sciences, USA. 2004;101:5164–5169. doi: 10.1073/pnas.0300084101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T, Nakashima K, Miyakawa T, Kuromori T, Tanokura M, Shinozaki K, Yamaguchi-Shinozaki K. Molecular basis of the core regulatory network in ABA responses: sensing, signaling and transport. Plant and Cell Physiology. 2010;51:1821–1839. doi: 10.1093/pcp/pcq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi-Shinozaki K, Ishihama Y, Hirayama T, Shinozaki K. Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proceedings of the National Academy of Sciences, USA. 2009;106:17588–17593. doi: 10.1073/pnas.0907095106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrell M, Buesa C. Relationship between abscisic acid content and ripening of apples. Acta Horticulturae. 1989;258:389–396. [Google Scholar]
- Vriezen WH, Feron R, Maretto F, Keijman J, Mariani C. Changes in tomato ovary transcriptome demonstrate complex hormonal regulation of fruit set. New Phytologist. 2008;177:60–76. doi: 10.1111/j.1469-8137.2007.02254.x. [DOI] [PubMed] [Google Scholar]
- Wan CY, Wilkins TA. A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.) Analytical Biochemistry. 1994;223:7–12. doi: 10.1006/abio.1994.1538. [DOI] [PubMed] [Google Scholar]
- Wu FQ, Xin Q, Cao Z, et al. The magnesium-chelatase H subunit binds abscisic acid and functions in abscisic acid signaling: new evidence in Arabidopsis. Plant Physiology. 2009;150:1940–1954. doi: 10.1104/pp.109.140731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Hobo T, Ichimura K, Mizoguchi T, Takahashi F, Aronso J, Ecker JR, Shinozaki K. ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant and Cell Physiology. 2002;43:1473–1483. doi: 10.1093/pcp/pcf188. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Umezawa T, Mizoguchi T, Takahashi S, Takahashi F, Shinozaki K. The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. Journal of Biological Chemistry. 2006;281:5310–5318. doi: 10.1074/jbc.M509820200. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Nishimura N, Kitahata N, Kuromori T, Ito T, Asami T, Shinozaki K, Hirayama T. ABA-Hypersensitive Germination3 encodes a protein phosphatase 2C (AtPP2CA) that strongly regulates abscisic acid signaling during germination among Arabidopsis protein phosphatase 2Cs. Plant Physiology. 2006;140:115–126. doi: 10.1104/pp.105.070128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa T, Tomikubo Y, Yamauchi T, Inoue A, Iwaya-Inoue M. Environmental stresses activate a tomato SNF1-related protein kinase 2 homolog, SlSnRK2C. Plant Biotechnology. 2007;24:401–408. [Google Scholar]
- Zhang M, Leng P, Zhang G, Li X. Cloning and functional analysis of 9-cis-epoxycarotenoid dioxygenase (NCED) genes encoding a key enzyme during abscisic acid biosynthesis from peach and grape fruits. Journal of Plant Physiology. 2009 b;166:1241–1252. doi: 10.1016/j.jplph.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Zhang M, Yuan B, Leng P. The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. Journal of Experimental Botany. 2009 a;60:1579–1588. doi: 10.1093/jxb/erp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.