Abstract
We describe the synthesis, properties, and application of a new fluorescent potassium chemosensor, KS2, for K+ sensing and imaging in live cells. By virtue of a strong electron-withdrawing group, 2-dicyanomethylene-3-cyano-4,5,5-trimethyl-2,5-dihydrofuran (TCF), with a triazacryptand (TAC) ligand, the new sensor can respond to K+ up to 1.6 M. This is the first highly selective intracellular sensor suitable for sensing K+ over a broad and high concentration range. Confocal fluorescence microscopy has established the utility of KS2 for live-cell K+ detection. Application of KS2 combined with other sensors will be of great benefit for investigating cellular metabolism, detecting and diagnosing diseases including cancer, and monitoring responses to therapy.
Potassium makes up about 0.4 percent of the mass in the human body and is the most abundant intracellular cation. Potassium ions (K+) play diverse roles in biological processes including muscle contraction, heartbeat, nerve transmissions, and kidney functions. Abnormal K+ fluctuations are early indicators of diseases such as alcoholism, anorexia, bulimia, heart disease, diabetes, AIDS, and cancer.1 Confocal fluorescence microscopy has proven to be an important tool in understanding metal ion biology2, and has the potential to measure changes of K+ concentrations with a high spatial and temporal fidelity. One of the earliest and best known intracellular fluorescent K+ probes is potassium-binding benzofuran isophthalate (PBFI), which uses a diaz-18-crown-6 as a ligand and a benzofuran derivative as the fluorophore. PBFI, however, suffers a poor selectivity to sodium ions (Na+).3 To alleviate this problem, He et al developed a ligand based on a triazacryptand platform, which features excellent selective responses for K+ over competing sodium ions.3 Verkman’s group developed other fluorescent K+ sensors based on this ligand.4-6 He et al and Verkman et al chemically grafted their K+ probes onto biocompatible polymers for extracellular K+ sensing. Physiological extracellular K+ concentrations are around 5 mM,7 while cells usually contain about 150 mM of intracellular K+.8 Erythrocytes and epidermal cells contain more intracellular K+ with homeostasis concentrations of 200 mM and 475 mM, respectively.8 For myocytes, the intracellular K+ concentration is even high at mole levels.8 Therefore, ideal intracellular K+ sensors should be applicable for measurement of high K+ concentrations with a relatively large dissociation constant (Kd) (for example, around 100 mM). Commercially available PBFI has a millimolar Kd (6.6 mM),9 which is not ideal for highly concentrated intracellular K+ sensing. Although the Kd values of the K+ sensors possessing the triazacryptand ligands were not reported, the Kd values could be estimated to be close to PBFI based on the published figures by He et al and Verkman et al.3-6
Herein, we report a new red fluorescent potassium ion sensor (KS2) (Scheme 1). The sensor was constructed using a strong electron-withdrawing group, 2-dicyanomethylene-3-cyano-4,5,5-trimethyl-2,5-dihydrofuran (TCF), and an electron-donating group, an aniline derivative of the triazacryptand (TAC) ligand for K+ sensing. TCF has been known as a strong acceptor and was initially used for non-linear optical materials10, 11, and later for red fluorophores for bioimaging12, 13 and pH sensing.14 Because of the strong delocalization of electrons in the push-pull fluorophore in KS2, the complex ability of the nitrogen atom of the aniline group, a component of the ligand for the potassium ion, decreased significantly. Such a decrease may result in a blunted binding ability of the whole ligand in KS2 and decreased sensitivity of the intramolecular charge transfer (ICT) to the complexation with K+ to achieve a Kd of around one-tenth mole level.
Scheme 1.
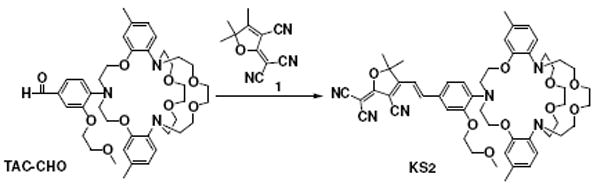
Synthesis of KS2 and its sensing mechanism
KS2 is constructed using strong electron-donating (aniline) and electron-withdrawing (TCF) groups, yielding an absorption maximum at 560 nm. The extinction coefficient (ε) at 560 nm is 3.84 × 104 M-1 cm-1 (supporting information S-Figure 1 and S-Table 1). KS2 displays weak fluorescence in its free form with a quantum yield (η) of 0.11%. Upon addition of K+, the fluorescence intensity of KS2 increases by ca. 4 and 50 fold at K+ concentrations of 140 mM and 1400 mM (Figure 1a and 1b), respectively, resulting in corresponding η of 0.52% and 5.6% (S-Table 1). Kd was determined by the Benesi-Hildebrand equation (equation 1),15
| (1) |
where F0 is the integrated fluorescence intensity of a free sensor, F is the observed integrated fluorescence intensity, Fcomplex is the emission of the ligand–metal ion complex, and [M] is the metal ion concentration. When F0/(F0 - F) is plotted against 1/[M], the binding constant is given by the ratio intercept/slope. Curve fitting of the KS2 fluorescence intensity against the reciprocal of the K+ concentration (1/[ K+ ]), this Benesi-Hildebrand plot yields a linear fit (Figure 1c), where the Kd was estimated to be 88 mM. The linear fit evidences a 1:1 complexation behavior.
Figure 1.
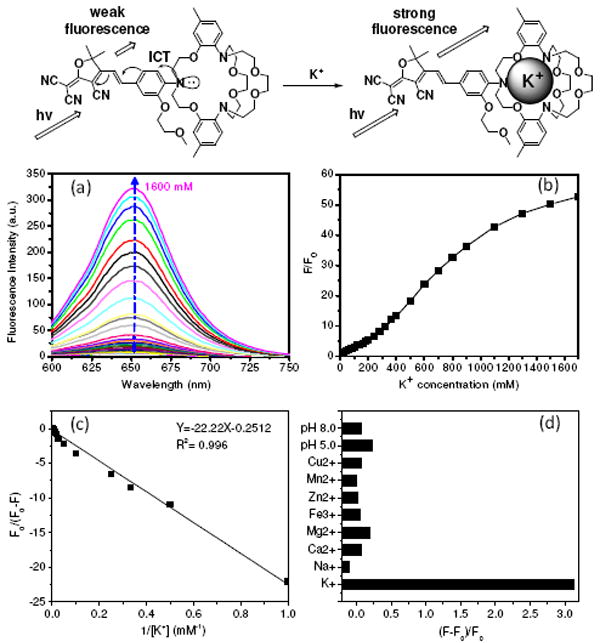
(a) Fluorescence change of KS2 in HEPES buffer (pH 7.2) containing different KCl concentrations, excited at 561 nm. (b) Changes of fluorescence intensities at 650 nm at different K+ concentrations. F is the fluorescence intensity at various conditions. F0 is the emission intensity before the titration (with 0 mM K+). (c) Benesi-Hildebrand plot of KS2. (d) Fluorescence intensity change of KS2 in the presence of various biological cations at their physiological concentrations [K+ (150 mM), Na+ (15 mM), Ca2+ (2.0 mM), Mg2+ (2.0 mM), Zn2+ (2.0 mM), Fe3+ (50 μM), Mn2+ (50 μM), and Cu2+ (50 μM)] and different pH values [pH (5.0) and pH (8.0)].
The KS2 was tested against physiological concentration levels of the following metal ions: K+, Na+, Ca2+, Mg2+, Fe3+, Zn2+, Mn2+, and Cu2+. The results revealed that the potassium ion had the greatest influence on the sensor’s emissions (Figure 1d). Furthermore, this trend was also present when the concentrations of Na+, Ca2+, and Mg2+ were increased to 140 mM (S-Figure 2). These results showed the high selectivity of KS2 to K+. KS2 was not sensitive to pH in the range of 5 to 8. The high selectivity to K+ with a large Kd suggested that KS2 be useful for intracellular K+ sensing in a wide range of biological condition.
Cytotoxicity of KS2 to human glioblastoma U87MG cells was studied using Trypan blue staining. After internalizing the sensor with cells for 2 hours, more than 97% of the cells were viable, showing the non-cytotoxicity of KS2 to U87MG cell line under our experimental conditions. Confocal images of U87MG cells internalized with KS2 showed that KS2 could be taken up by cells within 10 minutes. Red fluorescence was observed in the cytoplasm area, which was further confirmed by a minimum colocalization with blue emission from the nucleic staining probe (Hoechst 33342) (Figure 2). Furthermore, the sensor did not have a specific colocalization in mitochondria or lysosomes, showing that KS2 has a non-specific distribution and will therefore monitor potassium ions in the cytoplasm and in any of these compartments (S-Figure 4 and S-Figure 5).
Figure 2.
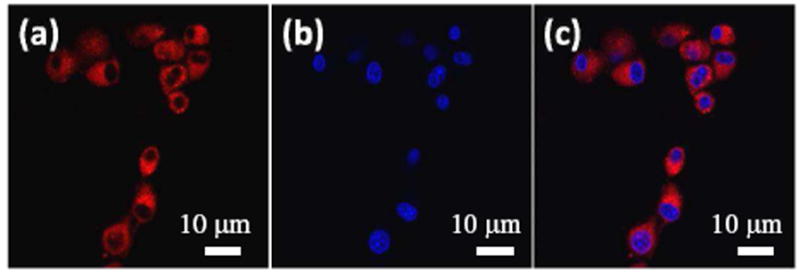
Confocal fluorescence images of KS2 (4 μM) in U87MG cells co-stained with Hoechst 33342 (10 μM). (a) Red emission from KS2 excited at 561 nm; (b) Blue emission from Hoechst 33342 excited at 405 nm; (c) Overlay of (a) and (b).
To monitor the stimuli-response of the intracellular K+ level, cells internalized with 4 μM of KS2 for 10 minutes were treated with a mixture of nigericin, bumetanide, and ouabain (Figure 3) at 37°C. The decrease of the fluorescence intensities indicated the efflux of the intracellular K+ from cells (Figure 3a, 3c, and 3e). The combination of the ionophore nigericin with influx inhibitors of bumetanide (inhibitor of Na+, K+, 2Cl--cotransport) and ouabain (inhibitor of Na+, K+, ATPase pump) is an effective K+-efflux stimulator. 16 This mixture effectively induced K+ efflux as shown in fluorescence images (Figure 3). Meanwhile, cell shrinkage with a preservation of an intact plasma membrane was observed after 60 mins of stimulation. It has been known that depletion of intracellular K+ will result in cell shrinkage, activated caspases, and DNA fragmentation, all of which are features of apoptosis.16, 17 Therefore, the phenomenon observed in Figure 3 indicated that U87MG cells underwent the apoptotic change through the K+ efflux induced by nigericin, ouabain, and bumetanide. A control experiment without the drug stimulation did not show obvious fluorescence intensity changes nor morphological shrinkages (S-Figure 8).
Figure 3.
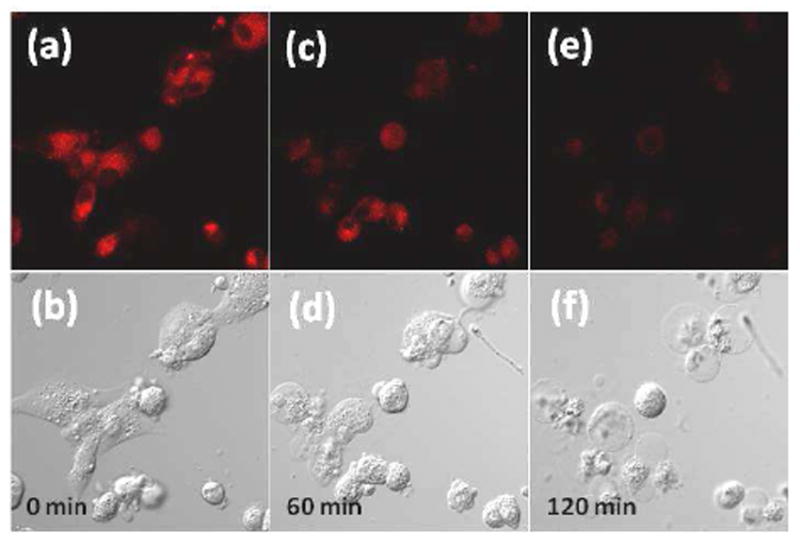
Time-dependent intracellular K+ in U87MG cells after treatment with a mixture of nigericin, bumetanide, and ouabain for 0 min (a and b), 60 minutes (c and d), and 120 minutes (e and f). Top: fluorescence images; Bottom: bright field images. Concentrations of KS2, nigericin, bumethanide, and ouabain for this study were 4 μM, 5 μM, 10 μM, and 10 μM, respectively. More detailed time dependence was given in S-Figure 7 of the supporting information.
KS2 can also be used to monitor K+ influx through stimuli. Cells internalized with 4 μM of KS2 for 10 minutes were treated with 20 mM KCl in medium at 37°C for 1 hour. This just induced a slight fluorescence intensity increase (data not shown). While obvious fluorescence increase was observed after cells were treated with 5 μM isoproterenol and the 20 mM KCl in medium for 40 minutes at 37°C (S-Figure 9). Isoproterenol was reported to be a potassium ion influx stimulator by stimulating cyclic adenosine monophosphate (cAMP) generation, which associates with physiology change in K+ transport.18 The average fluorescence intensity of cells after the treatment was 33% greater than that before the treatment; which was quantified using ImageJ software – a public domain developed at the National Institute of Health.19
The sensor was further demonstrated for in-situ observation of dynamic potassium ion influx and then efflux through the changes of fluorescence (Figure 4) stimulated by an ionophore nigericin. Cells internalized with 4 μM of KS2 at 37°C for 10 minutes were washed with 20 mM KCl-containing fresh medium. Nigericin (20 μM at its final concentration) was added in 20 mM KCl-containing medium to help K+ to across cell membrane. Enhanced fluorescence was immediately observed after the addition of nigericin, indicating potassium ion influx occurred. The potassium influx peaked after 3 minutes with an average of fluorescence increase of 370%. After 3 minutes, potassium efflux was observed by the decrease of the fluorescence. The efflux stabilized after 15 minutes, and the fluorescence intensity after the stabilization was about 25% below that of before the stimulation by nigericin. The ionophore nigericin was used to stimulate K+ influx for yeast Saccharomyces cerevisiae.20 It was also reported to be a K+ efflux stimulator for human colorectal adenocarcinoma HT-29 cells.4 The observation of the K+ influx and then efflux of U87MG cells using KS2, demonstrating its further utility in measuring the kinetic of K+ transport.
Figure 4.
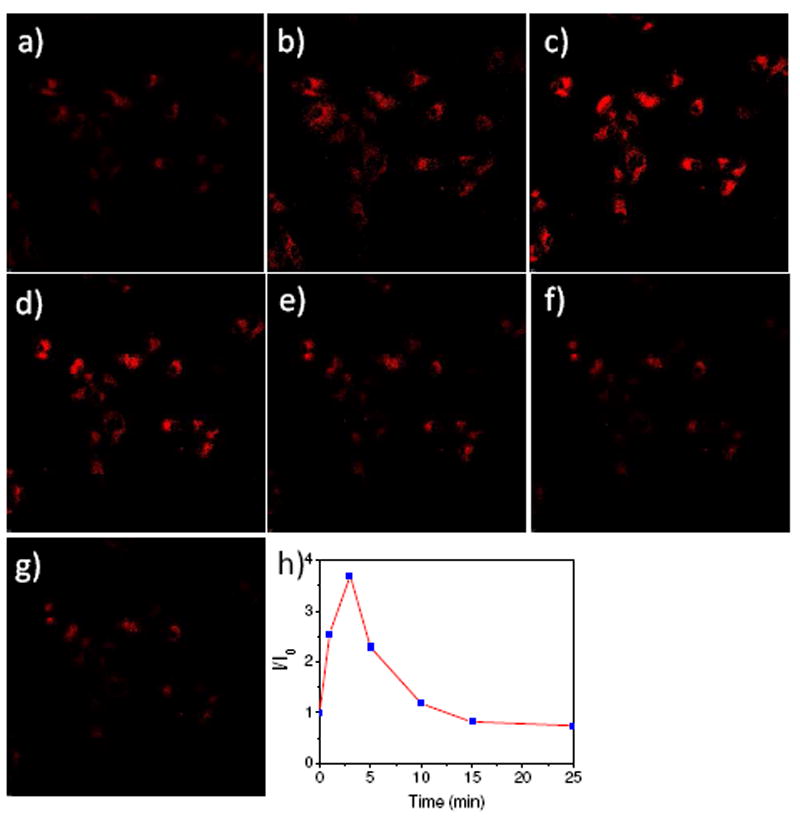
Time-dependent fluorescence of U87MG cells stimulated by nigericin observed under confocal fluorescence microscope. a is time of 0 before the addition of nigericin; b, c, d, e, f, and g are time of 1, 3, 5, 10, 15 and 25 minutes, respectively, after the addition of nigericin (20 μM, final concentration) into the 20 mM KCl-containing medium; h gives the average fluorescence intensity ratios measured by ImageJ. I0 is the average fluorescence intensity from figure a; I is the average fluorescence intensity at different times. The enlarged figure was given in S-Figure 10 of the supporting information.
In summary, we described the synthesis, properties, and cellular application of KS2 - a new fluorescent chemosensor for K+ sensing and imaging. By constructing a strong electron withdrawing group TCF with the triazacryptand ligand, the new sensor can respond to K+ in a broad range up to 1600 mM. This is the first highly selective intracellular K+ sensor that is capable of being used in such a broad range for highly concentrated K+ sensing. Moreover, confocal microscopy experiments established that KS2 can be used for detecting K+ levels within living cells. Application of KS2 combined with other sensors for cellular metabolism investigation, disease/cancer detection and diagnosis is underway.
Supplementary Material
Acknowledgments
Financial support was provided by the Microscale Life Sciences Center, a NIH Center of Excellence in Genomic Sciences at Arizona State University: Grant 5P50 HG002360, Dr. Deirdre Meldrum, PI, Director.
Footnotes
SUPPORTING INFORMATION AVAILABLE. Synthesis, characterization, and experimental details (pdf). This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Kurachi Y, Jan LY, Lazdunski M. Potassium ion channels: molecular structure, function, and diseases. Academic Press; 1999. [Google Scholar]
- 2.Taki M, Wolford JL, O’Halloran TV. J Am Chem Soc. 2004;126:712–713. doi: 10.1021/ja039073j. [DOI] [PubMed] [Google Scholar]
- 3.He H, Mortellaro MA, Leiner MJP, Fraatz RJ, Tusa JK. J Am Chem Soc. 2003;125:1468–1469. doi: 10.1021/ja0284761. [DOI] [PubMed] [Google Scholar]
- 4.Namkung W, Padmawar P, Mills AD, Verkman AS. J Am Chem Soc. 2008;130:7794–7795. doi: 10.1021/ja8014499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Namkung W, Song Y, Mills AD, Padmawar P, Finkbeiner WE, Verkman AS. J Biol Chem. 2009;284:15916–15926. doi: 10.1074/jbc.M808021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padmawar P, Yao X, Bloch O, Manley GT, Verkman AS. Nat Meth. 2005;2:825–827. doi: 10.1038/nmeth801. [DOI] [PubMed] [Google Scholar]
- 7.Burnell JM, Scribner BH, Uyeno BT, Villamil MF. J Clin Invest. 1956;35:935–939. doi: 10.1172/JCI103352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sachs JR, Welt LG. J Clin Invest. 1967;46:65–76. doi: 10.1172/JCI105512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kowalczyk A, Boens N, Meuwis K, Ameloot M. Anal Biochem. 1997;245:28–37. doi: 10.1006/abio.1996.9932. [DOI] [PubMed] [Google Scholar]
- 10.Gopalan P, Katz HE, McGee DJ, Erben C, Zielinski T, Bousquet D, Muller D, Grazul J, Olsson Y. J Am Chem Soc. 2004;126:1741–1747. doi: 10.1021/ja039768k. [DOI] [PubMed] [Google Scholar]
- 11.Liao Y, Bhattacharjee S, Firestone KA, Eichinger BE, Paranji R, Anderson CA, Robinson BH, Reid PJ, Dalton LR. J Am Chem Soc. 2006;128:6847–6853. doi: 10.1021/ja057903i. [DOI] [PubMed] [Google Scholar]
- 12.Lord SJ, Conley NR, Lee H-luD, Samuel R, Liu N, Twieg RJ, Moerner WE. J Am Chem Soc. 2008;130:9204–9205. doi: 10.1021/ja802883k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouffard J, Kim Y, Swager TM, Weissleder R, Hilderbrand SA. Org Lett. 2008;10:37–40. doi: 10.1021/ol702539v. [DOI] [PubMed] [Google Scholar]
- 14.Jin Y, Tian Y, Zhang W, Jang S-H, Jen AK-Y, Meldrum DR. Anal Bioanal Chem. 2010;398:1375–1384. doi: 10.1007/s00216-010-4060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benesi HA, Hildebrand JH. J Am Chem Soc. 1949;71:2703–2707. [Google Scholar]
- 16.Andersson B, Janson V, Behnam-Motlagh P, Henriksson R, Grankvist K. Toxicol in Vitro. 2006;20:986–994. doi: 10.1016/j.tiv.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Hughes FM, Jr, Bortner CD, Purdy GD, Cidlowski JA. J Bio Chem. 1997;272:30567–30576. doi: 10.1074/jbc.272.48.30567. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa H, Bilezikian JP, Loeb J Biochim Biophys Acta. 1980;598:345–356. doi: 10.1016/0005-2736(80)90012-7. [DOI] [PubMed] [Google Scholar]
- 19.Collins TJ. BioTechniques. 2007;43(1 Suppl):25–30. doi: 10.2144/000112517. [DOI] [PubMed] [Google Scholar]
- 20.Eilam Y. J Gen Microbiol. 1982;128:2611–2614. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


