Abstract
Hydrazone formation and similar reactions are highly versatile and specific, but their application to biological systems has been limited by their characteristically slow reaction kinetics at neutral pH. Catalysis of these reactions through imine formation with aromatic amines such as aniline has broadened the applicability of these reactions to biomolecular labeling. High concentrations of the catalyst are necessary, which may be incompatible with the native structure of certain proteins. In this study, we investigated the utility of 4-aminophenylalanine (4a-Phe) as a catalyst for these reactions. We find that 4a-Phe is nearly as effective as aniline in catalyzing hydrazone formation between the reactive amino acid 3-formyltyrosine (3f-Tyr) and hydrazine-containing fluorophores, both free in solution and incorporated into the protein tubulin. The catalyst 4a-Phe maintains ~70% of the catalytic efficacy of aniline and is less detrimental to the native structure of tubulin. Examination of the temperature dependence of imine formation between 3f-Tyr and 4a-Phe shows an increase in imine concentration accompanying a decrease in temperature, confirming the exothermic nature of the equilibrium reaction. Interestingly, decreasing the temperature of the 4a-Phe-catalyzed hydrazone reaction between 3f-Tyr and the fluorophore 7-hydrazinyl-3-methylcoumarin increases the overall rate of the reaction. This result indicates that the temperature dependence of the catalyst-aldehyde equilibrium is greater than the temperature dependence of the rate constant for hydrazone formation from this intermediate, and that the rate of hydrazone formation a direct function of the concentration of the intermediate imine. These results provide a platform for conducting nucleophilic catalysis under conditions that are more compatible with biomolecular targets than previously demonstrated, thereby expanding the utility of hydrazone-ligations in biological systems.
INTRODUCTION
Site-specific chemical modification of biomolecules relies on reactions of functional groups with reactivities that are orthogonal to endogenous moieties.1 Ideally, such reactions occur rapidly under conditions that retain the native structure of the target; however, in practice most labeling systems fail to meet all criteria. Condensation reactions of aldehydes and ketones with nucleophiles such as hydrazines, hydrazides and hydroxylamines possess many attractive properties for ligations in biological systems.2-4 They occur readily in aqueous solutions without major side reactions, and form covalent bonds with varying degrees of reversibility, tunable by the nature of the reactants. The usefulness of this class of reactions has been limited by its characteristic pH sensitive kinetics -- the rate of the condensation reaction is typically greatest near the pKa of the nucleophile,5 dropping off sharply at higher and lower pH.6 Because of this kinetic profile, biomolecules that require neutral pH to maintain structural and functional integrity are not amenable to direct ligation reactions with the less basic members of this group.
Our lab has developed a hydrazine-tag aldehyde-target based labeling system that employs reaction of a protein containing 3-formyl-L-tyrosine (3f-Tyr), a synthetic tyrosine derivative, with a hydrazine-containing probe.7 In this manifestation, the unnatural amino acid is appended to α-tubulin by a highly specific enzymatic reaction. Use of an aromatic hydrazine rather than a commercially available hydrazide as the nucleophile partially alleviated the problem of slow reaction at neutral pH owing to the greater basicity of the aromatic hydrazine. The aromatic hydrazine, a coumarin derivative synthesized in our lab, has an additional desirable feature, which is a red shift in its absorption and emission spectra accompanied by an increase in quantum yield upon hydrazone formation.
A wider variety of probes would increase the number of applications available for the labeled protein. However, our selection of probes has been limited precisely because our protein of interest, α,β,-tubulin, is highly temperature and pH sensitive.8-11 The commercial hydrazide-containing probes react too slowly at neutral pH and the protein is not stable at lower pH. Nucleophilic catalysis of these reactions using amines such as aniline and aniline derivatives (e.g. p-anisidine), which form a reactive imine intermediate with the target carbonyl, provides a potential solution to this problem. Aniline has been shown to be effective in aqueous buffered systems as well as with biomolecules and biomolecular conjugates.3,12-15 However, it was not known at the outset of this investigation whether aniline catalysis would be amenable for use with fragile biomolecular targets such as tubulin. Millimolar concentrations of aniline are necessary to produce an appreciable amount of imine intermediate, as the equilibrium constant for imine formation is quite small (<10 M−1 for bisaryl imines).16
In this study, we explore the use of 4-aminophenylalanine (4a-Phe), a commercially available aromatic amine derivative of phenylalanine (Phe), as a catalyst for hydrazone ligations in aqueous buffer at neutral pH. We reasoned that the more hydrophilic zwitterionic molecule would be less likely to denature a protein but would retain the catalytic efficacy of aniline. Native but not denatured tubulin will assemble into microtubules, so the effect of the catalyst is assessed as the ability of the protein to form microtubules in the presence of the additive. The catalytic efficacies of aniline and 4a-Phe are compared with the protein and using a model system containing 3f-Tyr and 7-hydrazinyl-3-methylcoumarin (coumarin hydrazine, CH).
We also explore the effect of temperature on the catalytic reaction. Since bisaryl imine formation is exothermic,17 the concentration of imine in solution can be increased by decreasing temperature. It was hoped that the increase in imine concentration would have a greater effect on the overall rate than the expected decrease in the rate of imine formation at the lower temperature. Finally, the ability of 4a-Phe to catalyze hydrazone formation with different fluorescent dyes is measured with both the model compound 3f-Tyr and 3f-Tyr-labeled tubulin. We find that 4a-Phe effectively catalyzes hydrazone formation between the unnatural amino acid and a variety of hydrazine-containing substrates, and that the labeling reaction is in fact enhanced at low temperature.
EXPERIMENTAL PROCEDURES
Reagents
Texas red hydrazide (TxRed, 90% single isomer) and 7-diethylaminocoumarin-3-carboxylic acid, hydrazide (DCCH) were purchased from Molecular Probes (Eugene, OR). CH and 3f-Tyr were synthesized as described previously.7 The probe naphthalene-2-ylhydrazine (naphthalene hydrazine, NH) was synthesized as detailed in Supporting Information. Stock solutions of TxRed, DCCH, and NH were made in DMSO and stored at −20 °C. Stocks of CH were made fresh in PME buffer (100 mM PIPES, 1 mM MgSO4, and 2 mM EGTA at pH 6.90) and discarded after 24 hours. A stock solution of 3f-Tyr was made in PME and stored at 4 °C. Concentrations of TxRed, DCCH, and CH were determined spectrophotometrically using extinction coefficients of 109,000 M−1cm−1 at 588 nm (TxRed, DMSO), 46,000 M−1cm−1 at 420 nm (DCCH, DMSO), and 19,000 M−1cm−1 at 346 nm (CH, PME).7 The concentration of NH was determined by mass. The source of 4-amino-DL-phenylalanine hydrate (4a-Phe) was Sigma-Aldrich (>97% pure). All other chemicals were purchased from Sigma-Aldrich and were reagent grade or better. All experiments were performed in PME buffer unless otherwise noted. The reactions presented in this paper were conducted in PME at pH 6.9 for compatibility with tubulin. Many of these experiments were also performed in 0.1 M phosphate buffer at pH 7.0, and no difference in the results obtained with the two buffer systems was observed.
Spectral Characteristics and Kinetics of 3f-Tyr Imine Formation
Imine formation was monitored by absorption difference spectroscopy. Dual chambered cuvettes were loaded with identical volumes of 800 μM 3f-Tyr and 20 mM of either aniline, Phe, or 4a-Phe. The cuvette was then placed in an HP 8453 UV-Vis spectrophotometer equipped with a multi-cell thermostated cuvette holder and equilibrated to 25 °C. After blanking, the solutions mixed rapidly by inversion, halving the concentration and doubling the path-length of each solution. Absorption difference spectra were collected at 30 s intervals. Steady state was reached in less than one hour, so the difference spectra of the equilibrated imine solution was also monitored at steady state. There was no change in the difference spectrum after steady state was achieved. The absorbance maximum at steady state (aniline: 440 nm, Phe: 405 nm, 4a-Phe:415 nm) was then plotted as a function of time according to the following equation for a reversible pseudo first-order reaction approaching equilibrium:
where ΔAeq is the absorption difference at equilibrium, ΔAt is the absorption difference at time t, and kobs is the observed rate constant for approach to equilibrium, which is the sum of the forward and reverse rate constants. Values for kobs were obtained from a linear fit done in SigmaPlot 10.0 (Systat Software Inc., San Jose, CA).
Relative Initial Rates of Formation
For measurement of 3f-Tyr-4a-Phe imine formation kinetics, 800 M 3f-Tyr and 20 mM 4a-Phe were loaded into either side of a dual chamber cuvette as described above. The reaction vessels were equilibrated at 0 °C, 25 °C, or 37 °C before mixing and maintained at those temperatures throughout the course of the reaction. Imine formation was monitored by the change in the absorption difference spectrum at 415 nm as a function of time. Reactions were compared based on initial rates, which were calculated for each reaction as the slope of the linear portion of the absorption vs. time plot. The initial rate of each reaction was compared to its initial rate at 25° C to yield a unitless relative initial rate.
Kinetics of Hydrazone Formation
Apparent pseudo-first order rate constants in the presence and absence the catalysts were determined by absorption difference spectroscopy in a CH and 3f-Tyr test system. Identical volumes of 800 μM 3f-Tyr and 80 μM CH were loaded into separate chambers of a dual chamber cuvette with or without 10 mM of aniline, Phe, or 4a-Phe in both chambers. (Note that including the catalyst in both chambers allows the concentration of catalyst to be constant throughout the experiment.) The solutions were allowed to equilibrate before the 3f-Tyr and hydrazine were mixed. The spectrophotometer was blanked, the solutions mixed, and reactions monitored as above at 400 nm. The resulting kinetic trace was then fit as a single pseudo first-order reaction according to the equation:
where ΔA(t) is the absorption difference at time t, ΔAfinal is the absorption difference at completion, and k’ is the apparent pseudo first-order rate constant. These data are treated as an irreversible reaction because the hydrazones are not observed to dissociate under harsher reaction conditions (vide infra). Fitting was accomplished using SigmaPlot 10.0. For the reaction of TxRed, NH, and DCCH with 3f-Tyr, only 4a-Phe was used as a catalyst, the wavelengths monitored were 610 nm (TxRed), 370nm (NH), and 460 nm (DCCH); 4% DMSO was included in both chambers to solublize the probes and their hydrazones. The concentration of fluorophore was 40 μM after mixing in experiments with CH, TxRed, and NH. The concentration of DCCH was reduced to produce a final concentration of 20 μM due to low solubility of the hydrazone product from this reaction. Corroborating fluorescence experiments were conducted as previously described.7
Temperature dependent rate constants were determined in the same manner, except the reactions were equilibrated at 0 °C or 37 °C in the thermostated multi-cell holder prior to mixing and held at that temperature throughout the reaction and CH was the only probe used.
Temperature Dependent Imine Formation
A mixture of 400 μM 3f-Tyr and 10 mM 4a-Phe in a 1 cm path length cuvette was placed in the thermostated multi-cell holder, equilibrated to 25 °C, and allowed to react until equilibrium was achieved. The sample was then blanked, the temperature decreased to 0 °C, allowed to react until equilibrium, and a difference spectrum taken. The temperature was raised back to 25 °C, raised up to 37 °C, and finally cooled back to 25 °C, with a spectrum taken after the reactions reached equilibrium at every temperature. The criterion for equilibrium was no change in the difference spectrum for 2 minutes.
Tubulin Purification
Tubulin was isolated from bovine brains by two cycles of temperature-dependent polymerization and depolymerization followed by phosphocellulose ion-exchange chromatography.18 The protein containing fractions were then pooled, drop frozen, and stored in liquid nitrogen until use. Prior to use, frozen aliquots were gently thawed and desalted into PME buffer by the method of Penefsky.19 The concentration was determined spectrophotometrically using an extinction coefficient of 114,000 M−1cm−1 at 280 nm, which was calculated from sequence data as previously described with modification to account for the two bound guanosine nucleotides per tubulin dimer.20 A detailed procedure can be found in Supporting Information.
Effect of Catalyst on Microtubule Formation
Tubulin (10 μM) was incubated with varying concentrations of the catalyst for 4 hrs at room temperature in PME. The samples were then equilibrated to 37 °C in 1 cm path length cuvettes in the UV-Vis spectrophotometer in a thermostated multi-cell holder and baselines taken. Polymerization was initiated by addition of 10% v/v DMSO and 1 mM GTP. Polymerization activity was monitored as apparent absorption increase at 400 nm, taking the plateau value to be polymerization extent. The plateau values were graphed on a semi-log plot versus amine concentration, and the IC50 value extracted from a non-linear sigmoidal fit done in SigmaPlot 10.0.21
Preparation of 3f-Tyr Hybridized Tubulin
Tubulin was hybridized with 3f-Tyr using recombinant human tubulin-tyrosine ligase fused to glutathione transferase (GST-TTL) as previously described with minor modification.7 Purified tubulin (40 - 80 μM) in PME buffer was treated with carboxypeptidase A (Sigma) for 30 min at 37 °C to remove the C-terminal tyrosine from α-tubulin. The reaction was stopped by addition of 20 mM DTT. Tubulin was equilibrated into TTL buffer (25mM MES, 150 mM KCl, 27 μM MgCl2, 2.5 mM ATP, 1 mM DTT, and 1.5% v/v glycerol at pH 6.8) by rapid gel filtration using Sephadex G-50. The protein was incubated with 1 mM 3f-Tyr and 0.45 mg mL−1 GST-TTL for 30 min at 37 °C. Excess ligand was removed by rapid gel filtration in Sephadex G-50 into PME buffer, and the concentration of tubulin determined spectrophotometrically as above, subtracting the absorbance due to the 0.45 mg mL−1 GST-TTL (ε280 nm= 100,000 M−1cm−1, MW = 71078). The extinction coefficient was calculated using the same method used for tubulin, and is detailed in Supporting Information.
Fluorescent Labeling of α-Tubulin
Tubulin hybridized with 3f-Tyr (40 μM) was incubated with 400 μM hydrazine-containing probe (CH, NH, DCCH, or TxRed) in the presence or absence of 10 mM 4a-Phe, along with an unmodified tubulin control. In the NH, DCCH, and TxRed reactions, 4% DMSO was also included. Reactions were allowed to progress for 15 minutes and 60 minutes at either RT or 0 °C, and then immediately subjected to reducing SDS-PAGE without boiling. The gels were imaged under long-wavelength UV light. The same gels were then stained with Coomassie Brilliant Blue and imaged again under white light.
RESULTS & DISCUSSION
The application of hydrazone ligations to biological systems has been limited by the slow kinetics of such reactions at neutral pH.23 Rate enhancement of similar reactions by nucleophilic catalysis was first detailed by Jencks, who noted that certain amines were more effective catalysts of semicarbazone formation than predicted by a general acid catalysis mechanism and who then detailed the nucleophilic catalysis mechanism.22,24 The recent application of this principle to biological systems by Dawson and coworkers has led to a plethora of new hydrazone and oxime ligations.3,12-15,25-27 The work presented here represents a continued expansion of nucleophilic catalysis in biological systems through the application of a catalyst that is more biocompatible than aniline.
Reactive Imine Formation
The catalyst 4a-Phe possesses two potential nucleophiles for imine formation: the aromatic amine of the side chain and the aliphatic α-amine. Therefore the unmodified amino acid Phe was evaluated in parallel with aniline and 4a-Phe. Kinetics of imine formation between the three catalysts (aniline, Phe, and 4a-Phe) and the model compound 3f-Tyr were followed by absorption difference spectroscopy in aqueous buffer at neutral pH. All three catalysts formed imines with 3f-Tyr with distinct absorption difference characteristics (Figure 1, Table 1). The aniline imine peak (aromatic amine) in the absorption difference spectrum is significantly red shifted from that of the Phe imine peak (α-amine) at equilibrium, presumably because of extended conjugation in the bisaryl product. The shape of the absorption difference spectrum of the Phe – 3f-Tyr reaction is consistent throughout the reaction, indicating that a single product is formed. In the aniline – 3f-Tyr reaction, the peak shape changes slightly during the reaction. The absorption maximum shifts slightly to the blue over the course of the reaction, which may be attributed to a small amount of imine formation between the α-amine and the aldehyde side chain of 3f-Tyr. The maximum of absorption difference spectrum of the 4a-Phe reaction shifts substantially during the course of the reaction, from the 445 nm (aromatic amino imine) region towards the 405 nm (α-amino imine) region. Taken together, these results indicate that imines with the o-hydroxybenzaldehyde moiety of 3f-Tyr form at both amino groups in 4a-Phe, and that the aromatic amino imine forms more quickly.
Figure 1.
Time-dependent absorption difference spectra for aniline (A), Phe (B), or 4a-Phe (C) reacting with 3f-Tyr at 25 °C in PME buffer, pH 6.9. The concentration of 3f-Tyr was 400 μM and the concentrations of aniline, Phe or 4a-Phe were 10 mM. Arrows indicate the wavelength at the maximum of the absorption difference spectrum at each time point. The peaks at t=0 are due to the reaction that occurred during the seconds it took to mix the samples.
Table 1.
Observed Rate to Equilibrium for Imine Formation with 3f-Tyr
| Amine | kobs × 10−3 (s−1)a | λmax (nm)b |
|---|---|---|
| Aniline | 5.3 ± 0.2 | 445-440 |
| 4a-Phe | 3.7 ± 0.1 | 443-415 |
| Phe | 1.4 ± 0.1 | 405 |
Rate constant for equilibration of 400 μM 3f-Tyr and 10 mM of each substance at 25 °C in PME at pH 6.9, determined as described under Experimental Procedures.
Range of maximum wavelength of the absorption difference spectrum over the course of the reaction and monitored by absorption difference spectroscopy.
The relative rates at which each reaction approaches equilibrium were assessed by analyzing the data of absorbance change as a function of time as described under Experimental Procedures. The observed rate constant to equilibrium for each system is reported in Table 1. The wavelength of the absorption maximum at equilibrium was used in the calculation, since the goal was to assess equilibration rate of the entire system, which includes both α- and aromatic amines. Overall, it is observed that aniline approaches its equilibrium the fastest, Phe the slowest, and 4a-Phe at an intermediate rate.
Catalytic Efficacy and Protein Stability
The search for a more biocompatible catalyst was spurred by our interest in site-specific fluorescent labeling of the protein tubulin. When tubulin was exposed to 100 mM aniline, the concentration used in most studies currently aimed at adapting the catalysis to biomolecules, it was completely denatured and inactivated in less that 1 hour (data not shown). We therefore investigated the effects of aniline, 4a-Phe and Phe on the native structure of tubulin through polymerization activity assays. Native tubulin can be induced to assemble into microtubules, and denaturants inhibit this activity. Aniline is about 3-fold more damaging to tubulin’s ability to polymerize than 4a-Phe; interestingly, unmodified Phe did not appear to affect the assembly process at equivalent concentrations (Table 2). The results suggest the aminopropanoic acid moiety of Phe is compatible with the protein, and inclusion of this moiety in 4a-Phe protects the protein from the detrimental effects of aniline. Based on these results, the concentration of catalyst for subsequent experiments was fixed at 10 mM.
Table 2.
Effect of Catalyst on the Native State of Tubulin and the Relative Efficacy of the Catalyst
| Catalyst | IC50 (mM)a | k (10−4)(s−1)b |
|---|---|---|
| Uncatalyzed | - | 3.5 ± 0.2 |
| Aniline | 28 ± 3 | 66 ± 0.8 |
| 4a-Phe | 88 ± 13 | 45 ± 0.4 |
| Phe | >100 | 5.5 ± 0.08 |
IC50 for tubulin polymerization activity. Tubulin (10 μM) was incubated with varying concentrations of the catalyst in PME (pH 6.9) for 4 hours at 25 °C and then polymerized at 37 °C
Pseudo first-order rate constant for the reaction of 40 μM CH with 400 μM 3f-Tyr in the presence of 10 mM catalyst at 25 °C in PME (pH 6.9). Note the increased IC50 for 4a-Phe relative to aniline while still maintaining the majority of the rate enhancement for the reaction, indicating its utility as a biocompatible catalyst.
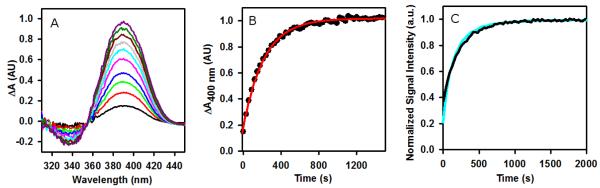
The relative catalytic efficacies of the molecules were evaluated by measuring the reaction rate of hydrazone formation between 3f-Tyr and CH as a function of catalyst. The reactants were selected because of their relevance to our tubulin labeling application, appropriate water solubilities of both the components and their resulting hydrazones, and reasonable uncatalyzed reaction kinetics for comparison.7 Reactions were monitored by absorption difference spectroscopy as detailed in Experimental Procedures. A representative experiment is shown in Figure 2. An isosbestic point in the spectra is observed, which indicates that a single transformation is measured by the technique (i.e, hydrazine to hydrazone). Although the imine between the catalyst and 3f-Tyr absorbs in this region of the spectrum, its absorbance does not contribute to the observed difference spectrum. The 3f-Tyr/4aPhe solution was allowed to equilibrate before the hydrazone reaction was initiated; thus, absorption due to the initial imine is eliminated by blanking the sample. The large excess of 3f-Tyr relative to CH ensures that the fractional change in imine concentration over the course of the reaction is small.
Figure 2.
A. Absorption difference spectra for the reaction of CH (40 μM) with 3f-Tyr (400 μM) at 25 °C in PME buffer, pH 6.9 in the presence of 4a-Phe (10 mM). B. Absorption difference of the reaction shown in panel A at 400 nm as a function of time. Solid line: data fit as a single pseudo-first order reaction. C. Kinetics of hydrazone formation catalyzed by 10 mM 4a-Phe monitored by absorption difference spectroscopy (black) and fluorescence spectroscopy (cyan). The signal data were normalized to arbitrary units to more clearly show the overlap of the kinetic traces.
To further demonstrate that the absorption difference spectra are proportional to hydrazone formation, the reaction kinetics was monitored by fluorescence under the same experimental conditions. We have shown previously that the reaction between CH and 3f-Tyr produces a hydrazone with distinct absorption and emission properties, and that the fluorescence of the hydrazone can be observed without interference by unreacted hydrazine.7 Figure 2C shows the kinetic trace of the fluorescence emission increase overlaid with absorption difference spectra data. The concurrence of the two data sets points to the fluorescence and absorbance signals corresponding to the same process, hydrazone formation. Calculated pseudo-first order rate constants for each signal are within experimental error of one another. In harmony with previous studies, all three catalysts increased the observed pseudo first-order rate constant for the formation of the 3f-Tyr-CH hydrazone (Table 2).22 Aniline catalyzed the reaction most effectively (~19 fold), Phe the least effectively (~2 fold), with 4a-Phe at an intermediate effectiveness (~13 fold). These data support the notion that the aromatic imine is the more reactive intermediate, and when both aromatic and α-amino groups are present they may compete with each other, the net result of which is a decrease in the overall reaction rate (Scheme 1). However, this is counterbalanced by the increased concentrations of 4a-Phe that can be used because of its compatibility with the protein.
Scheme 1.
Proposed reaction pathway for 4a-Phe catalyzed hydrazine-ligations with o-hydroxyl aromatic aldehydes attached to biomolecules.
Alternative Tags
We also investigated the generalizability of 4a-Phe catalysis to varying classes of hydrazine-containing molecules using the model reaction. This was accomplished by calculating apparent pseudo first-order rate constants for reactions between 3f-Tyr and NH, DCCH, and TxRed (Table 3). The catalysis was effective for all three molecules, yielding rate enhancements of 9-, 130-, and 3-fold, respectively. Note that the rate of hydrazone formation with the more reactive aromatic hydrazines (CH and NH) is less affected by the catalyst than that of the less reactive hydrazide (DCCH). It is unclear why catalysis was less effective in the TxRed reaction. It may be that the increased sterics of the imine intermediate counteracts the greater reactivity of the iminium ion compared to the aromatic aldehyde.
Table 3.
Apparent Pseudo First-Order Rate Constants for Hydrazone Formation Between 3f-Tyr and Hydrazine-Containing Probes Catalyzed by 4a-Phe
| Molecular Tag | kuncat (10−4)(s−1)a | kcat(10−4) (s−1)b |
|---|---|---|
| NH | 5.2 ± 0.3 | 45.2 ± 0.4 |
| DCCH | 0.11 ± 0.01 | 14.2 ± 0.04 |
| TxRed | 0.60 ± 0.12 | 1.8 ± 0.1 |
Apparent pseudo first-order rate constants for the reaction of hydrazines with 3f-Tyr in the absence and presenceb of 4a-Phe
Apparent pseudo first-order rate constants for the reaction of hydrazines with 3f-Tyr in the presence of 4a-Phe.
Experiments were performed with 400 μM 3f-Tyr with 40 μM molecular tag (20 μM in the case of DCCH) in the presence or absence of 10 mM 4a-Phe at 25 °C in PME (pH 6.9).
Temperature Dependence of 4a-Phe and 3f-Tyr Reactions
A solution variable that frequently affects the stability of biological molecules is temperature. Proteins and nucleic acid polymers tend to be more stable at lower temperatures; the native conformation of tubulin is particularly sensitive to variations in temperature.8,9 According to equilibrium thermodynamic parameters measured for related o-hydroxyl aldehyde-amine systems, imine formation is exothermic.17 Therefore, a decrease in temperature would be expected to increase the concentration of imine in solution, which may then increase the overall efficacy of the catalyst.
Absorption difference spectroscopy was used to assess the effect of temperature on the relative equilibrium concentration of imine in a solution of the model compounds 3f-Tyr and 4a-Phe (Figure 3). The solution of the two components was equilibrated to room temperature (25 °C), and the spectrophotometer was blanked. The temperature was decreased to 0 °C, and absorption spectra were collected until no change in the spectrum was observed. A positive band was observed in the absorption difference spectrum that peaks near 440 nm, which is indicative of an increase in the concentration of the aromatic amino imine. This process was fully reversed when the temperature of the solution was restored to 25 °C. Subsequent warming the solution to 37 °C caused a decrease in absorbance in the 440 nm region, which is consistent with dissociation of aromatic amino imine. This process was also fully reversed when cooled to 25 °C. Therefore, the imine concentration in the solution can be reversibly increased or decreased by lowering or raising the solution temperature.
Figure 3.

Effect of temperature on the absorption difference spectrum of 4a-Phe plus 3f-Tyr. A solution of 10 mM 4a-Phe and 400 μM 3f-Tyr in PME buffer (pH 6.9) was mixed at 25 °C and equilibrated until no change in the absorption spectrum was observed. The instrument was then referenced to this solution (curve 1). Without removing the cuvette from the instrument, the temperature was decreased to 0 °C and the spectrum was monitored until no further change in the absorption spectrum was observed, at which point a spectrum was taken (curve 2). The process of temperature change and equilibration was repeated on the same sample, which was not removed from the instrument. Curve 3: after equilibration of 0 °C solution to 25 °C. Curve 4: after equilibration of 25 °C solution to 37 °C. Curve 5: after equilibration of 37 °C solution to 25 °C. The fully reversible increase in the 440 nm region at 0 °C and decrease at 37 °C indicates aromatic amino imine concentration increases at 0 °C and decreases at 37 °C relative to the concentration at room temperature (25 °C).
The temperature dependence of the kinetics of imine formation was assessed by measuring initial rates at the three temperatures. Table 4 compares the initial rates as a ratio of the initial rate of imine formation at 25 °C. At 0 °C, the initial rate of imine formation is about half of its value at room temperature, and at 37 °C, the initial rate is about 1 ½ times faster.
Table 4.
Relative Initial Rate of 4a-Phe/3f-Tyr Imine Formation at Various Temperatures
| Temperature | ki, app/ki, app, RT |
|---|---|
| 0 °C | 0.4 ± 0.1 |
| 25 °C | 1.0 ± 0.1 |
| 37 °C | 1.6 ± 0.1 |
Initial rates of formation imine at varying temperatures were determined as described under Experimental Procedures. A 400 μM solution of 3f-Tyr was reacted with 10 mM 4a-Phe at 0, 25, and 37 °C. Data are presented as the initial rate at 0 °C, 25 °C or 37 °C divided by the initial rate at room temperature (25 °C).
The net effect of temperature on the catalytic ability of 4a-Phe was assessed empirically using a 3f-Tyr and CH test system. The rate of hydrazone formation was measured at the three selected temperatures in the absence or presence of 4a-Phe catalyst (Table 5). At 25 °C, 4a-Phe increased the observed rate constant by ~13-fold. At 0 °C, 4a-Phe caused a 28-fold increase in the apparent rate constant for hydrazone formation, more than double the rate enhancement observed at 25 °C (13-fold). Although there was a slight increase in observed rate constant when the temperature was increased to 37 °C (~10 %), there was virtually no change in rate enhancement from 25 °C, which remained steady at 13-fold. The effect of temperature on the catalyzed reaction indicates that the effectiveness of 4a-Phe catalysis is governed by a mixture of thermodynamic and kinetic factors. We hypothesize that the thermodynamic favorability for imine formation at 0 °C is responsible for a marked increase in catalytic effectiveness by increasing intermediate imine concentration, which Cordes and Jencks determined to be a controlling factor governing the rate of imine-catalyzed semicarbazone formation.22 It appears that kinetic favorability at 37 °C overcomes the thermodynamic disfavorability, possibly by regenerating the consumed imine intermediate more quickly. Although it is unclear how generalizable this effect is to molecules other than the o-hydroxyl aromatic aldehyde used in our system, it may be predicted by the energetic nature of the formation of the intermediate imine -- if imine formation is sufficiently exothermic, decreasing the temperature will thermodynamically favor imine formation, thereby increasing imine concentration. If the rate constant of the subsequent transimination reaction has a smaller temperature dependence than the equilibrium constant for imine formation, as appears to be the case for the probes studied here (Figure 4, Table 5), then lowering the temperature of the reaction to increase its overall rate may be a general feature of these catalyzed reaction.
Table 5.
Effect of Temperature and 4a-Phe Catalyst on the Rate of CH/3f-Tyr Hydrazone Formation
| Temperature | kuncatm (10−4)(s−1)a | kcat(10−4) (s−1)b |
|---|---|---|
| 0 °C | 3.32 ± 0.04 | 94.4 ± 2.8 |
| 25 °C | 3.52 ± 0.20 | 44.8 ± 0.4 |
| 37 °C | 3.92 ± 0.08 | 51.6 ± 0.4 |
Apparent pseudo first-order rate constants for the reaction of 400 μM 3f-Tyr with 40 μM CH in the absence of 10 mM 4a-Phe at 0, 25, and 37 °C in PME (pH 6.9).
Apparent pseudo first-order rate constants for the reaction of 400 μM 3f-Tyr with 40 μM CH in the presence of 10 mM 4a-Phe at 0, 25, and 37 °C in PME (pH 6.9).
Figure 4.

SDS-PAGE of α-tubulin labeled with hydrazine-containing fluorophores, visualized under long wavelength UV light (top) and stained with Coomassie blue (bottom). Samples of tubulin (40 μM) in PME buffer were incubated with fluorophore (400 μM) for the specified time and temperature in the presence or absence of 10 mM 4a-Phe and immediately run on a gel. Each panel is from left to right 1) Unmodified α-tubulin (control), 2) 3f-Tyr-α-tubulin, 3) 3f-Tyr-α-tubulin + 4a-Phe. Note the increased fluorescence signal in most of the bands for the catalyzed reactions compared to the corresponding uncatalyzed reactions.
Application in Protein Labeling
To assess the applicability of 4a-Phe catalysis to protein labeling, we hybridized 3f-Tyr to α-tubulin as described previously5 and allowed it to react with hydrazine-containing molecular tags. Identical reactions were performed in the presence and absence of catalyst at both 25 °C and at 0 °C. The protein was then subjected to SDS-PAGE (Figure 4) and visualized under long-wavelength UV light. It is clear that the catalysis effectively enhanced the labeling of the protein in most cases at both room temperature and at 0 °C. The difference in the fluorescence intensities of the products in the catalyzed and uncatalyzed reactions are particularly evident at low temperature and short time points, consistent with the results in the model system. It should be noted that, although in this study the amino acid derivative 3f-Tyr is enzymatically appended to tubulin, the amino acid derivative suitable for incorporation into other proteins using unnatural amino acid mutagenesis (unpublished results) or via synthetic peptides. Therefore, the 4a-Phe-catalyzed hydrazone ligation reaction could be broadly applicable to protein labeling.
Hydrazone-containing conjugates have been criticized for biomolecular labeling because the reaction is an equilibrium process. It is feared that the reaction may not go to completion and that the reversibility renders the product too unstable. Equilibrium constants that have been measured for aromatic aldehyde-semicarbazide reactions in aqueous solution for aromatic semicarbazone formation in aqueous solution, which are analogous to the reactions presented here, are on the order of 105 – 106 M−1.28 Kalia and Raines argue that oximes are preferred over hydrazone linkages because of their superior resistance to hydrolysis. Their quantitative kinetic analyses show that the hydrolytic stability of hydrazone linkages is significantly affected by the structure of the hydrazone and pH at which the hydrolysis reaction is carried out. First-order rate constants of hydrazone hydrolysis decrease by 2-3 orders of magnitude from pD 5 to pD 9 in D2O. These experiments illustrate what is generally known: hydrazone stability is dependent on structures of both reactants and pH of the medium.29 The most pertinent question is whether a particular hydrazone is sufficiently stable for the biological application.
We have empirical observations to support our assertion that some hydrazones are well suited for protein labeling. Tubulin labeled with this system does not observably lose the fluorophore in neutral pH buffer under conditions that retain the native structure of the protein. The hydrazone bond remains intact during SDS-PAGE (Figure 4). Moreover, SDS-PAGE gels of tubulin labeled with CH or TxRed do not noticeably lose fluorescence even after 2 weeks of storage in standard destaining solution (containing methanol, acetic acid and water). The results suggest that the tubulin-fluorophore conjugates are stable under a variety of experimental conditions. Although the hydrazones are thermodynamically reversible, we believe that the 3f-Tyr-linked hydrazones remain intact because the products are kinetically trapped. Such behavior has been observed in hydrazone-containing dynamic covalent chemistry libraries.30
CONCLUSION
We conclude that 4a-Phe is a biocompatible catalyst for hydrazone-ligations in aqueous buffer at neutral pH with catalytic efficacy about 70% of aniline but superior compatibility with proteins. We have applied the catalysis to a site-specific bioorthogonal labeling method for conjugating molecular labels to the C-terminus of α-tubulin under conditions that preserves the activity of the fragile protein. Importantly, we demonstrate that the reactions can be effectively conducted at 0 °C, successfully increasing the extent of labeling under conditions and time-periods that are suitable for routine use with biomolecules.
Supplementary Material
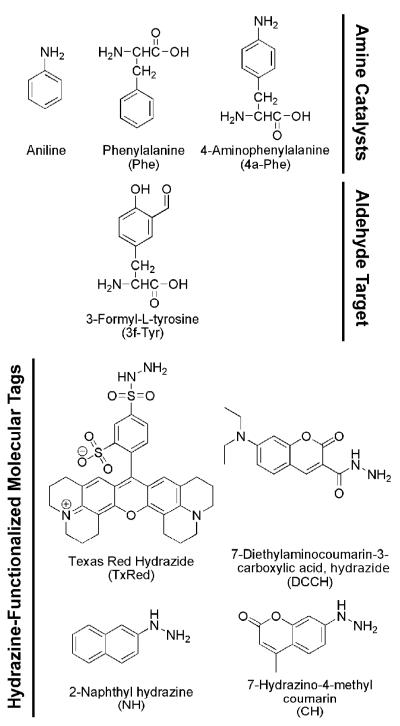
Chart 1.
Structures of the catalysts, targets, and probes.
ACKNOWLEDGEMENTS
We thank David Tuttle for his excellent scientific photography, Dr. Rebecca Kissling for her expertise and assistance in organic synthesis, and P & N packing for bovine brains for tubulin isolation. This work was supported by NIH Grant R15GM093941.
Footnotes
Supporting Information Available: Synthesis and spectral characterization of NH and its corresponding hydrazones is available. The full sequence of the GST-TTL fusion protein and calculations of the extinction coefficients for tubulin and GTS-TTL are also presented. This information is available free of charge at http://pubs.acs.org.
REFERENCES
- (1).Sletten EM, Bertozzi CR. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chem. Int. Ed. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).King TP, Zhao SW, Lam T. Preparation of protein conjugates via intermolecular hydrazone linkage. Biochemistry. 1986;25:5774–5779. doi: 10.1021/bi00367a064. [DOI] [PubMed] [Google Scholar]
- (3).Dirksen A, Dawson PE. Rapid Oxime and Hydrazone Ligations with Aromatic Aldehydes for Biomolecular Labeling. Bioconjugate Chem. 2008;19:2543–2548. doi: 10.1021/bc800310p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Kalia J, Raines RT. Hydrolytic Stability of Hydrazones and Oximes. Angew. Chem. Int. Ed. 2008;47:7523–7526. doi: 10.1002/anie.200802651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Reeves RL. Condensations leading to double bonds. In: Patai S, editor. The Chemistry of the Carbonyl Group. Interscience Publishers; New York, NY: 1966. pp. 567–619. [Google Scholar]
- (6).Jencks WP. Studies on the Mechanism of Oxime and Semicarbazone Formation. J. Am. Chem. Soc. 1959;81:475–481. [Google Scholar]
- (7).Banerjee A, Panosian TD, Mukherjee K, Ravindra R, Gal S, Sackett DL, Bane S. Site-Specific Orthogonal Labeling of the Carboxy Terminus of α-Tubulin. ACS Chem. Biol. 2010;5:777–785. doi: 10.1021/cb100060v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Das A, Sinha S, Acharya BR, Paul P, Bhattacharyya B, Chakrabarti G. Deuterium oxide stabilizes conformation of tubulin: a biophysical and biochemical study. BMB Rep. 2008;41:62–67. doi: 10.5483/bmbrep.2008.41.1.062. [DOI] [PubMed] [Google Scholar]
- (9).Chakrabarti G, Kim S, Gupta ML, Barton JS, Himes RH. Stabilization of tubulin by deuterium oxide. Biochemistry. 1999;38:3067–3072. doi: 10.1021/bi982461r. [DOI] [PubMed] [Google Scholar]
- (10).Burton PR, Himes RH. Electron microscope studies of pH effects on assembly of tubulin free of associated proteins. Delineation of substructure by tannic acid staining. J. Cell Biol. 1978;77:120–133. doi: 10.1083/jcb.77.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Croom HB, Correia JJ, Williams RC. The effects of elevated pH and high salt concentrations on tubulin. Arch. Biochem. Biophys. 1986;249:397–406. doi: 10.1016/0003-9861(86)90016-0. [DOI] [PubMed] [Google Scholar]
- (12).Dirksen A, Dirksen S, Hackeng TM, Dawson PE. Nucleophilic Catalysis of Hydrazone Formation and Transimination:◻Implications for Dynamic Covalent Chemistry. J. Am. Chem. Soc. 2006;128:15602–15603. doi: 10.1021/ja067189k. [DOI] [PubMed] [Google Scholar]
- (13).Dirksen A, Hackeng TM, Dawson PE. Nucleophilic catalysis of oxime ligation. Angew. Chem. Int. Ed. Engl. 2006;45:7581–7584. doi: 10.1002/anie.200602877. [DOI] [PubMed] [Google Scholar]
- (14).Dirksen A, Yegneswaran S, Dawson PE. Bisaryl hydrazones as exchangeable biocompatible linkers. Angew. Chem. Int. Ed. 2010;49:2023–2027. doi: 10.1002/anie.200906756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Bhat VT, Caniard AM, Luksch T, Brenk R, Campopiano DJ, Greaney MF. Nucleophilic catalysis of acylhydrazone equilibration for protein-directed dynamic covalent chemistry. Nat. Chem. 2010;2:490–497. doi: 10.1038/nchem.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Godoy-Alcántar C, Yatsimirsky AK, Lehn J. Structure-stability correlations for imine formation in aqueous solution. J. Phys. Org. Chem. 2005;18:979–985. [Google Scholar]
- (17).Saggiomo V, Lüning U. On the formation of imines in water--a comparison. Tetrahedron Lett. 2009;50:4663–4665. [Google Scholar]
- (18).Williams RC, Lee JC. Preparation of tubulin from brain. Meth. Enzymol. 1982;85(Pt B):376–385. doi: 10.1016/0076-6879(82)85038-6. [DOI] [PubMed] [Google Scholar]
- (19).Penefsky HS. Preparation of nucleotide-depleted F1 and binding of adenine nucleotides and analogs to the depleted enzyme. Meth. Enzymol. 1979;55:377–380. doi: 10.1016/0076-6879(79)55048-4. [DOI] [PubMed] [Google Scholar]
- (20).Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4:2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Sharma S, Poliks B, Chiauzzi C, Ravindra R, Blanden AR, Bane S. Characterization of the colchicine binding site on avian tubulin isotype betaVI. Biochemistry. 2010;49:2932–2942. doi: 10.1021/bi100159p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Cordes EH, Jencks WP. Nucleophilic Catalysis of Semicarbazone Formation by Anilines. J. Am. Chem. Soc. 1962;84:826–831. [Google Scholar]
- (23).Prescher JA, Bertozzi CR. Chemistry in living systems. Nat. Chem. Biol. 2005;1:13–21. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- (24).Cordes EH, Jencks WP. Semicarbazone Formation from Pyridoxal, Pyridoxal Phosphate, and Their Schiff Bases. Biochemistry. 1962;1:773–778. doi: 10.1021/bi00911a007. [DOI] [PubMed] [Google Scholar]
- (25).Yi L, Sun H, Wu Y, Triola G, Waldmann H, Goody RS. A highly efficient strategy for modification of proteins at the C terminus. Angew. Chem. Int. Ed. 2010;49:9417–9421. doi: 10.1002/anie.201003834. [DOI] [PubMed] [Google Scholar]
- (26).Byeon J, Limpoco FT, Bailey RC. Efficient bioconjugation of protein capture agents to biosensor surfaces using aniline-catalyzed hydrazone ligation. Langmuir. 2010;26:15430–15435. doi: 10.1021/la1021824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Thygesen MB, Munch H, Sauer J, Cló E, Jørgensen MR, Hindsgaul O, Jensen KJ. Nucleophilic catalysis of carbohydrate oxime formation by anilines. J. Org. Chem. 2010;75:1752–1755. doi: 10.1021/jo902425v. [DOI] [PubMed] [Google Scholar]
- (28).Volfenden RW, Jencks WP. The Effect of o-Substituents on Benzaldehyde Semicarbazone Formation. J. Am. Chem. Soc. 1961;83:2763–2768. [Google Scholar]
- (29).Smith PAS. Derivatives of Hydrazine and Other Hydronitrogens Having N-N Bonds. Benjamin-Cummings Publishing Co.,Subs. of Addison Wesley Longman; US: 1983. [Google Scholar]
- (30).Beeren SR, Pittelkow M, Sanders JKM. From static to dynamic: escaping kinetic traps in hydrazone-based dynamic combinatorial libraries. Chem. Commun. 2011;47:7359–7361. doi: 10.1039/c1cc12268a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.