Abstract
An increase in the incidence of CNS tumors has been observed in many countries in the last decades. The reality of this trend has been much debated, as it has happened during a period when computer-assisted tomography and MRI have dramatically improved the detection of these tumors. The Gironde CNS Tumor Registry provides here the first data on CNS tumor incidence and trends in France for all histological types, including benign and malignant tumors, for the period 2000–2007. Incidence rates were calculated globally and for each histological subtype. For trends, a piecewise log-linear model was used. The overall annual incidence rate was found to be 17.6/100 000. Of this rate, 7.9/100 000 were neuroepithelial tumors and 6.0/100 000 were meningiomas. An overall increase in CNS tumor incidence was observed from 2000 to 2007, with an annual percent change (APC) of +2.33%, which was explained mainly by an increase in the incidence of meningiomas over the 8-year period (APC = +5.4%), and also more recently by an increase in neuroepithelial tumors (APC = +7.45% from 2003). The overall increase was more pronounced in women and in the elderly, with an APC peaking at +24.65% in subjects 85 and over. The increase in the incidence rates we observed may have several explanations: not only improvements in registration, diagnosis, and clinical practice, but also changes in potential risk factors.
Keywords: central nervous system neoplasms, epidemiology, incidence, population register, trends
Primary tumors of the CNS are not frequent compared with many other tumor sites. In a survey of 24 sites in France, they were the 15th and 13th most common sites of cancer in males and females, respectively, and represented about 5300 new cases and 3200 deaths per year in 2000 (about 2% of all cancers for incidence and mortality) (http://www.invs.sante.fr/publications/2003/rapport_cancer_2003/). However, they are a public health concern because of their poor prognosis and the many unsolved questions about their epidemiology. Since the mid-1980s, clinical reports and epidemiological literature have pointed to their increase in many countries,1–11 which has been more pronounced in subgroups like elderly persons (+5-fold among those ≥85 y in a 10-y period in the US)11–14 and children.15–18 Over time, several hypotheses have been proposed to explain the trends.
In the 1970s, it was debated whether the introduction of x-ray computer-assisted tomography, which improved the detection of CNS tumors, could have been responsible for all or part of the increase in incidence.3,9,19 In the 1980s, the same question was raised concerning the impact of MRI.20 During the same period, the contribution of acquired immune deficiency syndrome (AIDS) to a rise in brain lymphomas was considered,19 with the assumption that these tumors would be the most frequent in the CNS in the US by the year 2000.21 In the 1990s, a hypothesis was that the sharp increase in mobile phone use could have had an impact on incidence.22 Exploring the various hypotheses has been difficult owing to the lack of standardization among tumor registries over time in such areas as changes in coding classifications, differences in types of tumors recorded, differences in health care, variations in the proportion of tumors without histological confirmation, autopsy rates, and misclassification of secondary brain tumors as primary.
Since the recommendations of the International Agency for Research on Cancer at the end of the 1990s,23 most registries have taken into account standardized reporting definitions and have started collecting data on tumors with benign or uncertain behavior as well as malignant tumors.24,25 For these reasons, the 2000s represent the first decade where no major event created an artifactual increase in CNS tumor recording and offer the opportunity to obtain realistic data on incidence and trends for major histological subtypes. Getting high-quality and detailed data is critical for providing a good basis for etiological clues, since differences in age, sex, and other individual characteristics may be related to risk factors.
A primary CNS register was implemented in France in 1999, and the first data covering the period 1999–2001 showed rather high incidence rates (IRs) compared with other published data.26 Here we present data from this registry over an 8-year period (2000–2007).
Material and Methods
Cancer surveys in France are based on a network of 25 population-based registries, which in 2009 covered 18.5% of the population (about 11 million people). Since 1991 they have been connected in a network called FRANCIM (France Cancer Incidence et Mortalité), which is now funded by the French Institute for Health (Institut de Veille Sanitaire) and the French Institute in charge of Cancer (Institut National du Cancer). The network includes 13 “general” registries collecting data from all tumor sites, including the CNS, and 12 “specific” registries devoted to specific sites or populations. One of them specifically collects data about primary tumors of the CNS. It is based in Gironde, an administrative area in southwestern France, where about 1 400 000 inhabitants were recorded as living in 2007. The French general registries published data on gliomas in 1997,27 and some others on brain tumors in 2006,28 but the lack of standardization in definitions and collection methods among registries did not allow incidence and trends for all histological subtypes to be assessed.
The Gironde area is served by a university teaching hospital with 2 neurology departments (1 for adults and 1 pediatric), 2 neurosurgery departments, and 1 neuropathology laboratory, and by a Regional Comprehensive Cancer Center with 1 oncology and 1 radiotherapy department involved in brain tumor treatment. Twenty-two CT and 16 MRI machines are now available in the Gironde area, installed in the 1980s in the largest health centers. Patients from Gironde with suspected CNS tumors are unlikely to be referred outside the area, as the regional neurosciences center is based in Bordeaux.
From May 1999, all patients who lived in Gironde in whom any new primary tumor of the CNS was diagnosed, whether symptomatic or asymptomatic, were prospectively registered. Spinal tumors were included. We excluded pituitary tumors, tumors associated with AIDS, recurrence of tumors, and metastatic tumors. No case was obtained from autopsy or from a death certificate only. Collection of the data is exhaustive because of the collaboration of a work group comprising practitioners (neurosurgeons, neuropathologists, medical oncologists, radiotherapists, neurologists, etc) involved in the diagnosis and therapeutic management of patients. To identify eligible cases and minimize the number of cases that may have been missed, multiple overlapping sources were used: (1) clinical reports obtained through registration forms filled in by practitioners, (2) extractions from the French National Hospital Database (PMSI) for relevant discharge data, (3) neuropatholology reports, (4) requests to the French Health Insurance Organization (Affections de Longue Durée) for free health treatment for CNS tumors, and (5) death certificates.
Medical data were individually reviewed to ensure that the diagnoses were eligible, to check for the diagnosis date (from April 1999), and to fill in exclusion criteria. All duplicate cases were thoroughly searched and excluded. To ensure completeness, a periodic review of archives and pathology records was performed in the relevant departments and laboratories. Diagnosis was based on clinical and radiological data with or without histological confirmation. Whenever a surgical specimen was available for neuropathological analysis, the slides were systematically reviewed by a pathologist not involved in the initial diagnosis. When biopsy or surgical resection of the tumor was not possible, an assessment was made by experienced neuroradiologists and neurosurgeons (A.H., H.L., T.T.) to ascertain the diagnosis.
The following parameters were systematically recorded: date of birth, sex, postal code of residence, date of diagnosis, topography, and tumor histological type and grade.
Tumors were classified by grouping 4-digit histology codes from the International Classification of Diseases for Oncology, third edition, into broad histology subgroups based on the recommendations from the 2000 Consensus Conference on Brain Tumor Definition for Registration.25
Overall, age- (5-y age groups) and sex-specific crude IRs were calculated and expressed per 100 000 per year. Population estimates by gender and calendar year were supplied by the Institut National de la Statistique et des Études Économiques (INSEE) (http://www.insee.fr/en/bases-de-donnees/default.asp?page=recensements.htm). The study population was divided into children (<15 y), young adults (15–24 y), middle-aged adults (25–64 y), and elderly persons (65–84 y and ≥85 y separately). Rates were presented according to the rural/urban status assigned to the place of residence at time of initial diagnosis, obtained from the INSEE urban zoning classification (http://www.insee.fr/fr/methodes/default.asp?page=zonages/unites_urbaines.htm). The annual age-standardized IRs (per 100 000) were calculated globally and separately for men and women. The age distributions of populations in Europe, in the US, and in the world were used as references.
For trends, we estimated a piecewise log-linear model with constant variance and uncorrelated errors using Joinpoint Software (version 3.4.2) available on the Surveillance, Epidemiology, and End Results (SEER) *Stat pages on the US National Cancer Institute website (http://srab.cancer.gov/joinpoint/). The method implemented in this software allows choosing the number and the locations of joinpoints and testing whether an apparent change in trend is statistically significant. The tests of significance use a Monte Carlo permutation method. We chose to use only one joinpoint because of the limited number of points in our series. The date of onset was aggregated into calendar years. We characterized trends in IRs by estimating annual percent changes (APCs). P < .05 was considered statistically significant.
Here we present data collected from January 2000 to December 2007.
Results
General Characteristics
From 2000 to 2007, a total of 1907 new primary CNS tumors were registered among the 1 407 500 inhabitants of Gironde, corresponding to an overall crude rate of 17.6/100 000, unchanged when standardizing on the French population. To enable international comparisons, standardized rates were calculated as follows: 17.5/100 000 (reference population, Europe), 15.9/100 000 (reference population, US; used by SEER and the Central Brain Tumor Registry of the United States), and 12.1/100 000 (reference population, world; used by the International Agency for Research on Cancer for the calculation of cancer in the 5 continents).
Overall, 1513 tumors (79.3%) were histologically confirmed (from 69.6% of meningeal tumors to 87.8% of neuroepithelial tumors) (Table 1). In cases with histological confirmation, 43.6% were supratentorial; 27.4% were in the brain, with no other details given; 16.5% were meningeal; 5.8% were intracranial and intraspinal; 4.8% were infratentorial; and 1.9% were ventricular. The proportion of tumors in the brain with no other details given was higher in tumors without histology (49.7%).
Table 1.
Distribution and crude incidence rates (IRs) of CNS tumors by site globally and according to histological confirmation, Gironde CNS Tumor Registry, 2000–2007
| Site | All Cases |
Cases with Histological Confirmation |
Cases without Histological Confirmation |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | IR | n | % | IR | n | % | IR | |
| Supratentorial | 785 | 41.16 | 7.25 | 660 | 43.62 | 6.09 | 125 | 31.73 | 1.15 |
| Ventricular | 30 | 1.57 | 0.28 | 29 | 1.92 | 0.27 | 1 | 0.25 | 0.01 |
| Infratentorial | 87 | 4.56 | 0.80 | 72 | 4.76 | 0.66 | 15 | 3.81 | 0.14 |
| Unspecified | 611 | 32.04 | 5.64 | 415 | 27.43 | 3.83 | 196 | 49.75 | 1.81 |
| Meningeal | 283 | 14.84 | 2.61 | 250 | 16.52 | 2.31 | 33 | 8.38 | 0.30 |
| Intracranial and intraspinal | 111 | 5.82 | 1.02 | 87 | 5.75 | 0.80 | 24 | 6.09 | 0.22 |
| Total | 1907 | 100.00 | 17.60 | 1513 | 100.00 | 13.97 | 394 | 100.00 | 3.64 |
Distribution and Incidence by Histological Subtype and Sex
The incidence of tumors by histological subtype and sex is presented in Table 2 in the form of rates that are crude and standardized for Europe, the US, and the world. The overall incidence was higher in women (18.8/100 000) than in men (16.3/100 000). In the whole population, neuroepithelial tumors were the most frequent (n = 860, 45.1%) and corresponded to a 7.9/100 000 crude IR, ahead of meningeal tumors (n = 652, 34.2%; IR = 6.0/100 000), neurinomas (n = 234, 12.3%; IR = 2.2/100 000), lymphomas (n = 63, 3.3%; IR = 0.6/100 000), and other tumors (n = 98, 5.1%; IR = 0.9/100 000). The pattern differed with gender: more than half (57.2%) of the tumors in men were neuroepithelial, but only 35.4% in women were, whereas meningeal tumors were the most frequent (45.5%) in women but represented only 20.0% of tumors in men. Histological subtypes are presented in more detail in Table 3. Glioblastoma represented 28.2% of all tumors (35.0% in men, 22.7% in women) and 62.4% of neuroepithelial tumors. Mixed gliomas, including oligoastrocytomas and anaplastic oligoastrocytomas, were ranked second among neuroepithelial tumors, ahead of anaplastic astrocytomas.
Table 2.
Crude and standardized incidence rates (reference populations: Europe, US, world) by sex and histological type, Gironde CNS Tumor Registry, 2000–2007
| Crude Rates/100 000 | Standardized Rates/100 000 |
|||
|---|---|---|---|---|
| Europe | US | World | ||
| Men | 16.31 | 15.87 | 14.92 | 11.66 |
| Neuroepithelial tumors | 9.33 | 9.06 | 8.52 | 6.92 |
| Meningeal tumors | 3.26 | 3.16 | 2.94 | 2.04 |
| Cranial and spinal nerve tumors | 2.35 | 2.40 | 2.22 | 1.77 |
| Lymphomas | 0.56 | 0.51 | 0.48 | 0.30 |
| Other tumors | 0.81 | 0.74 | 0.76 | 0.63 |
| Women | 18.79 | 18.98 | 16.94 | 12.52 |
| Neuroepithelial tumors | 6.66 | 6.74 | 5.99 | 4.68 |
| Meningeal tumors | 8.56 | 8.60 | 7.70 | 5.33 |
| Cranial and spinal nerve tumors | 1.98 | 2.07 | 1.88 | 1.49 |
| Lymphomas | 0.60 | 0.61 | 0.51 | 0.33 |
| Other tumors | 0.99 | 0.95 | 0.87 | 0.68 |
| All | 17.60 | 17.48 | 15.95 | 12.08 |
| Neuroepithelial tumors | 7.94 | 7.86 | 7.23 | 5.81 |
| Meningeal Tumors | 6.02 | 5.98 | 5.36 | 3.67 |
| Cranial and spinal nerves tumors | 2.16 | 2.23 | 2.05 | 1.63 |
| Lymphomas | 0.58 | 0.56 | 0.50 | 0.32 |
| Other tumors | 0.90 | 0.85 | 0.82 | 0.66 |
Table 3.
Distribution of CNS tumors by histological type and sex, Gironde CNS Tumor Registry, 2000–2007
| Histology | Males |
Females |
Total |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | IR | n | % | IR | n | % | IR | |
| Neuroepithelial tumors | |||||||||
| Diffuse astrocytoma | 20 | 2.4 | 0.39 | 13 | 1.2 | 0.23 | 33 | 1.7 | 0.30 |
| Anaplastic astrocytoma | 19 | 2.2 | 0.37 | 21 | 2.0 | 0.37 | 40 | 2.1 | 0.37 |
| Glioblastoma | 296 | 35.0 | 5.71 | 241 | 22.7 | 4.27 | 537 | 28.2 | 4.96 |
| Pilocytic astrocytoma | 14 | 1.7 | 0.27 | 13 | 1.2 | 0.23 | 27 | 1.4 | 0.25 |
| Unique astrocytoma variantsa | 4 | 0.5 | 0.08 | 2 | 0.2 | 0.04 | 6 | 0.3 | 0.06 |
| Oligodendroglioma | 8 | 0.9 | 0.15 | 6 | 0.6 | 0.11 | 14 | 0.7 | 0.13 |
| Anaplastic oligodendroglioma | 2 | 0.2 | 0.04 | 7 | 0.7 | 0.12 | 9 | 0.5 | 0.08 |
| Ependymoma/anaplastic ependymoma | 18 | 2.1 | 0.35 | 13 | 1.2 | 0.23 | 31 | 1.6 | 0.29 |
| Ependymoma variants (myxopapillary ependymoma) | 6 | 0.7 | 0.12 | 2 | 0.2 | 0.04 | 8 | 0.4 | 0.07 |
| Mixed gliomab | 33 | 3.9 | 0.64 | 20 | 1.9 | 0.35 | 53 | 2.8 | 0.49 |
| Astrocytoma, NOS | 8 | 0.9 | 0.15 | 4 | 0.4 | 0.07 | 12 | 0.6 | 0.11 |
| Glioma malignant, NOS | 18 | 2.1 | 0.35 | 8 | 0.8 | 0.14 | 26 | 1.4 | 0.24 |
| Choroid plexus | 2 | 0.2 | 0.04 | 2 | 0.2 | 0.04 | 4 | 0.2 | 0.04 |
| Neuroepithelial | 3 | 0.4 | 0.06 | 2 | 0.2 | 0.04 | 5 | 0.3 | 0.05 |
| Benign and malignant neuronal/glial, neuronal and mixed | 14 | 1.7 | 0.27 | 16 | 1.5 | 0.28 | 30 | 1.6 | 0.28 |
| Pineal parenchymal | 1 | 0.1 | 0.02 | 0 | 0.0 | 0.00 | 1 | 0.1 | 0.01 |
| Embryonal/primitive/medulloblastoma | 18 | 2.1 | 0.35 | 6 | 0.6 | 0.11 | 24 | 1.3 | 0.22 |
| Tumors of cranial and spinal nerves | 122 | 14.4 | 2.35 | 112 | 10.6 | 1.98 | 234 | 12.3 | 2.16 |
| Meningeal Tumors | |||||||||
| Meningioma | 146 | 17.3 | 2.81 | 464 | 43.7 | 8.22 | 610 | 32.0 | 5.63 |
| Other mesenchymal, benign and malignant | 6 | 0.7 | 0.12 | 9 | 0.8 | 0.16 | 15 | 0.14 | 0.8 |
| Hemangioblastoma | 17 | 2.0 | 0.33 | 10 | 0.9 | 0.18 | 27 | 1.4 | 0.25 |
| Lymphomas | 29 | 3.4 | 0.56 | 34 | 3.2 | 0.60 | 63 | 3.3 | 0.58 |
| Germ cell tumors and cystsc | 6 | 0.7 | 0.12 | 4 | 0.4 | 0.07 | 10 | 0.5 | 0.09 |
| Tumors of the sellar region (craniopharyngiomas) | 10 | 1.2 | 0.19 | 12 | 1.1 | 0.21 | 22 | 1.2 | 0.20 |
| Chordoma/chondrosarcoma | 2 | 0.2 | 0.04 | 3 | 0.3 | 0.05 | 5 | 0.3 | 0.05 |
| Unclassified tumors | 24 | 2.8 | 0.46 | 37 | 3.5 | 0.66 | 61 | 3.2 | 0.56 |
| Total | 846 | 100.0 | 16.31 | 1061 | 100.0 | 18.79 | 1907 | 100.0 | 17.60 |
Abbreviations: IR, incidence rate; NOS, not otherwise specified.
aUnique astrocytoma variants correspond to subependymoma (n = 3) and subependymal giant cell astrocytoma (n = 3).
bMixed gliomas correspond to anaplastic oligoastrocytoma (n = 23) and oligoastrocytoma (n = 30).
cGerm cell tumors and cysts correspond to germinoma (n = 4), mature teratoma (n = 1), dermoïd cyst (n = 3), and choriocarcinoma (n = 1).
Incidence by Age and Sex
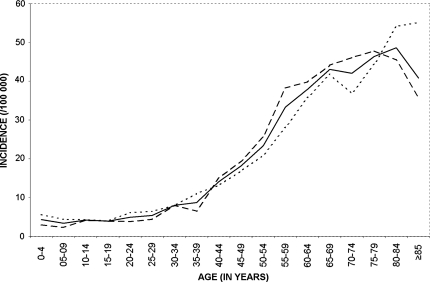
IRs are presented by age and sex in Figure 1. In children, IRs were 4.3/100 000 in the very first years of life and between 3.4 and 4.2 from 5 to 19 years of age. Between 20 and 39 years of age, the incidence slightly increased from 4.9 to 8.7. The incidence exceeded 10/100 000 after 40 years, 20/100 000 after 50, 30/100 000 after 55, and finally culminated over 40/100 000 after 65. The incidence was higher in men than in women before 40 years of age, then the opposite pattern was observed until 80 years. After this age, a decrease was observed in women, but not in men, which drove the overall tendency of CNS tumors downward for subjects over 85.
Fig. 1.
Annual incidence of CNS tumor by age and sex, Gironde CNS Tumor Registry, 2000–2007.  Men
Men  Women
Women  Total.
Total.
Histological Types by Age and Sex
The distribution of histological types varied considerably within age groups and between the sexes (Fig. 2). In children younger than 15 years, more than 80% of all tumors were neuroepithelial, other types being very rare (≤2 tumors registered every year). In adolescents and young adults (15−24 y), neuroepithelial tumors remained predominant (60.6%), with a higher frequency in men than in women (64.9% vs 55.2%), but neurinomas and meningiomas represented 13.6% and 9.1%, respectively, both with higher frequency in women than in men. In this age group, cranopharyngiomas (in men and women) and germ cell tumors (in men) also exceeded 5% of the tumors. In adults between 25 and 64, the proportion of neuroepithelial tumors was 40.8% (53.1% in men, 30.5% in women), and the proportion of meningiomas was 36.6% (20.3% in men, 50.5% in women). In men the proportion of neurinomas (20.9%) was very similar to that of meningiomas, whereas it was only 13.8% in women. All other types represented less than 5% of the cases. In elderly persons 65−84 years, the distribution was quite similar, but a lower proportion of neurinomas was observed in women (7.6%) and in men (7.2%) than in the previous age group. On the other hand, the proportion of lymphoma was higher (5.8% in men, 4.4% in women). In elderly persons 85 years and older, the proportion of unclassified tumors reached 10%. In this age group, meningiomas were the most frequent type (46.7%), corresponding to 51.7% of the tumors in women and 37.5% in men. Neuroepithelial tumors (34.4%) and lymphomas (6.7%) were the only other types exceeding 5% of the tumors. Incidence by age, sex, and histological subtypes are presented in Table 4.
Fig. 2.
Distribution of main histological types by sex and age, Gironde CNS Tumor Registry, 2000–2007.  Neuroepithelial tumors,
Neuroepithelial tumors,  meningeal tumors,
meningeal tumors,  cranial and spinal nerve tumors,
cranial and spinal nerve tumors,  lymphomas,
lymphomas,  others (local extension from regional tumors, craniopharyngiomas, germ cell tumors and cysts, unclassified tumors.
others (local extension from regional tumors, craniopharyngiomas, germ cell tumors and cysts, unclassified tumors.
Table 4.
Distribution and standardized incidence rates (reference populations: Europe, US, world) by age, sex, and histological type, Gironde CNS Tumor Registry, 2000–2007
| 0–14 y |
15–24 y |
25–64 y |
65–84 y |
85 y and over |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | Standardized Rates/100 000 |
% | Standardized Rates/100 000 |
% | Standardized Rates/100 000 |
% | Standardized Rates/100 000 |
% | Standardized Rates/100 000 |
||||||||||||
| Europe | US | World | Europe | US | World | Europe | US | World | Europe | US | World | Europe | US | World | |||||||
| Neuroepithelial tumors | Men | 50.7 | 3.90 | 3.94 | 3.94 | 36.4 | 3.29 | 3.27 | 3.24 | 24.4 | 8.53 | 8.48 | 7.81 | 24.3 | 25.65 | 25.63 | 25.56 | 15.6 | 22.88 | 23.41 | 23.90 |
| Women | 30.7 | 2.58 | 2.50 | 2.49 | 24.2 | 2.11 | 2.13 | 2.13 | 16.5 | 5.72 | 5.52 | 4.94 | 22.9 | 17.33 | 17.29 | 17.12 | 18.9 | 10.52 | 10.48 | 10.43 | |
| All | 81.3 | 3.26 | 3.23 | 3.24 | 60.6 | 2.71 | 2.72 | 2.70 | 40.8 | 7.10 | 6.99 | 6.39 | 47.2 | 20.65 | 20.91 | 20.90 | 34.4 | 13.77 | 14.46 | 14.77 | |
| Meningeal tumors | Men | 4.0 | 0.33 | 0.31 | 0.31 | 3.0 | 0.27 | 0.27 | 0.26 | 9.3 | 3.35 | 3.23 | 2.84 | 8.7 | 8.94 | 9.31 | 8.84 | 13.3 | 21.76 | 22.54 | 23.52 |
| Women | 1.3 | 0.11 | 0.11 | 0.10 | 6.1 | 0.54 | 0.54 | 0.54 | 27.3 | 9.22 | 9.15 | 7.84 | 25.9 | 20.05 | 19.86 | 20.05 | 33.3 | 18.11 | 19.23 | 19.53 | |
| All | 5.3 | 0.22 | 0.21 | 0.21 | 9.1 | 0.40 | 0.40 | 0.40 | 36.6 | 6.34 | 6.22 | 5.32 | 34.6 | 15.61 | 15.29 | 15.03 | 46.7 | 19.07 | 20.25 | 20.81 | |
| Cranial and spinal nerve tumors | Men | 2.7 | 0.18 | 0.20 | 0.20 | 6.1 | 0.55 | 0.55 | 0.53 | 9.6 | 3.40 | 3.35 | 3.08 | 2.9 | 3.35 | 3.11 | 3.41 | 1.1 | 1.98 | 2.02 | 2.29 |
| Women | 1.3 | 0.11 | 0.11 | 0.11 | 7.6 | 0.67 | 0.66 | 0.66 | 7.5 | 2.49 | 2.51 | 2.28 | 4.5 | 3.85 | 3.65 | 4.03 | 1.1 | 0.79 | 0.64 | 0.69 | |
| All | 4.0 | 0.15 | 0.15 | 0.15 | 13.6 | 0.61 | 0.60 | 0.60 | 17.1 | 2.94 | 2.93 | 2.68 | 7.4 | 3.65 | 3.42 | 3.75 | 2.2 | 1.10 | 1.07 | 1.21 | |
| Lymphomas | Men | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.00 | 0.00 | 0.00 | 1.1 | 0.39 | 0.39 | 0.34 | 2.3 | 2.32 | 2.46 | 2.26 | 2.2 | 3.55 | 4.02 | 4.12 |
| Women | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.00 | 0.00 | 0.00 | 1.2 | 0.42 | 0.40 | 0.35 | 2.6 | 2.05 | 1.97 | 2.06 | 4.4 | 2.71 | 2.89 | 2.99 | |
| All | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.00 | 0.00 | 0.00 | 2.3 | 0.40 | 0.39 | 0.34 | 4.9 | 2.16 | 2.18 | 2.15 | 6.7 | 2.93 | 3.24 | 3.35 | |
| Other tumors | Men | 4.0 | 0.31 | 0.31 | 0.31 | 10.6 | 0.96 | 0.96 | 0.95 | 1.5 | 0.53 | 0.55 | 0.54 | 2.0 | 1.80 | 2.16 | 1.70 | 3.3 | 4.06 | 5.38 | 4.52 |
| Women | 5.3 | 0.41 | 0.44 | 0.43 | 6.1 | 0.53 | 0.53 | 0.52 | 1.6 | 0.58 | 0.55 | 0.54 | 3.8 | 2.65 | 2.84 | 2.50 | 6.7 | 3.84 | 3.85 | 3.88 | |
| All | 9.3 | 0.36 | 0.37 | 0.37 | 16.7 | 0.75 | 0.75 | 0.74 | 3.1 | 0.56 | 0.55 | 0.54 | 5.8 | 2.31 | 2.54 | 2.14 | 10.0 | 3.90 | 4.32 | 4.09 | |
Trends over the 8-year Period
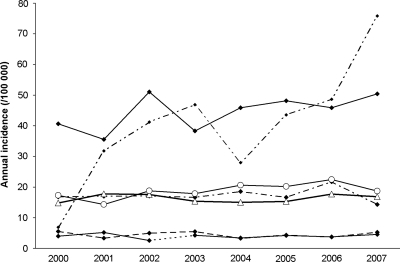
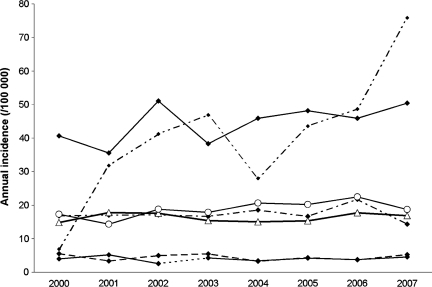
When considering all tumors together, the APC over the period 2000–2007 was +2.33% and was statistically significant (P = .04) (Table 5). The increase in the annual rates standardized on the French population was lower in men (+0.65%) and was not statistically significant, while it reached +3.88% in women and was nearly significant (P = .06). The IRs in the age groups younger than 65 years were quite stable over the period (Fig. 3). A nonsignificant increase was observed in the 65- to 84-year-old group (+3.30%), while a sharp increase in annual rates was observed in the group 85 years and older (+24.64%, P = .03). The joinpoint analysis identified 2 trends for neuroepithelial tumor IRs: in the early years of the study (2000–2003), a 7.46% annual decrease was found (P = .12), while a nearly significant increase was observed thereafter (+7.45% for the period 2003–2007) (P = .05). A clearer tendency was observed for meningiomas, with an APC of +5.40% for the whole period, which was statistically significant (P = .02). This trend included meningiomas both with (+6.3%) and without (+3.9%) histological confirmation. There were only 52 asymptomatic patients in the 8-year period, but these presented a sharper increase (+23.8%) than did symptomatic patients (+3.6%). No tendency was observed for cranial and spinal nerve tumors, and unstable results were obtained for other histological types owing to the limited numbers over the 8-year period. The changes in the rates over the period did not appear to be related to the place of residence at the time of diagnosis, as the APC was similar in urban (+2.13%) and rural (+3.07%) settings.
Table 5.
Annual percent change (APC) in standardized rates according to sex, age, histology, and urban/rural place of residency, Gironde CNS Tumor Registry, 2000–2007
| n | APC | 95% CI, APC | Tendency P-value | |
|---|---|---|---|---|
| All CNS tumors | 1907 | +2.33 | [+0.20; +4.52] | .04 |
| Sex | ||||
| Men | 846 | +0.65 | [−2.69; +4.09] | .66 |
| Women | 1061 | +3.88 | [−0.22; +8.14] | .06 |
| Age | ||||
| 0–14 y | 75 | +0.68 | [−7.58; +9.67] | .85 |
| 15–24 y | 66 | −1.06 | [−9.72; +8.44] | .79 |
| 25–64 y | 989 | +0.14 | [−4.50; +5.02] | .94 |
| 65–84 y | 687 | +3.30 | [−1.06; +7.84] | .12 |
| 85 y and over | 90 | +24.65 | [+3.03; +50.80] | .03 |
| Histology | ||||
| Neuroepithelial tumors | 860 | +1.14 | [−2.95; +15.41] | .53 |
| Meningeal tumors | 652 | +5.40 | [+1.15; +9.83] | .02 |
| Cranial and spinal nerve tumors | 234 | −0.88 | [−4.86; + 3.28] | .62 |
| Other tumors* | 161 | +2.38 | [−9.08; +15.29] | .65 |
| Place of residence at time of diagnosis | ||||
| Urban | 1553 | +2.13 | [−0.29; +4.60] | .075 |
| Rural | 353 | +3.07 | [−2.36;+ 8.81] | .221 |
Abbreviation: CI, confidence interval. *Lymphomas (n = 63), germ-cell tumors (n = 10), tumors of the sellar region (n = 22), local extensions from regional tumors (n = 5), unclassified tumors (n = 61).
Fig. 3.
Trends in crude incidence by age group and by sex, Gironde CNS Tumor Registry, 2000–2007.  0–14 y
0–14 y  25–64 y
25–64 y  15–24
15–24  65–84 y
65–84 y  ≥ 85 y.
≥ 85 y.  Women
Women  Men.
Men.
Discussion
The Gironde CNS Tumor Registry provides here the first data on CNS tumor incidence and trends in France for all histological types, including benign and malignant tumors. The overall annual IR was found to be 17.6/100 000 for the 2000–2007 period (15.9 and 12.1/100 000 when standardized on the US and the world population, respectively), which is higher than most previous published data. Over comparable periods, the California Cancer Registry found a 14.3/100 000 IR, standardized on US population,29 and the National Cancer Registration System in England found a 9.2/100 000 incidence, standardized on world population.30 When excluding pituitary tumors, which were not considered here, this incidence was even lower, from 1 to 2/100 000.
The most comparable data are from the Austrian Brain Tumor Registry, which found an 18.1/100 000 overall IR (standardized on US population), corresponding to a 16.3/100 000 rate when excluding tumors of the sellar region.31 Concerning histological types, they found IRs of 7.3/100 000 for neuroepithelial tumors and of 5.3/100 000 for meningeal tumors, which are comparable to the rates we found in the Gironde (7.2/100 000 and 5.4/100 000, respectively).
The global characteristics of CNS tumors included in the Gironde Tumor Registry were consistent with the findings of most other studies: overall incidence increased dramatically with age, and neuroepithelial tumors in men and meningeal tumors in women were the predominant histological categories.
There was an overall increase in CNS tumor incidence from 2000 to 2007, with an APC of +2.33%, which was explained mainly by an increase in the incidence of meningiomas over the 8-year period (APC = +5.4%), and also more recently by an increase in neuroepithelial tumors (APC = +7.45% from 2003). The overall increase was more pronounced in women and in elderly persons, with an APC peaking at +24.65% in subjects 85 years and older. Even if some authors did not detect any clear change in any subgroup in the past decade,1 numerous reports have found an overall increase in brain tumors with time, with a considerably greater rise in older age. However, most of those studies concerned periods when diagnostic tools, although improved, were not performing as well as nowadays. Christensen found a 3.9% increase in meningioma from 1943 to 1997 in Denmark.2 Klaeboe also observed an overall increasing incidence of intracranial meningioma in Scandinavian countries for the period 1968–1997, which was more pronounced in women.32 In elderly persons, the likelihood of other cancers (breast, lung) commonly associated with brain metastases is greatest, so misclassification of some secondary tumors could be suspected, especially in the absence of histological data. However, the percentage of tumors without histology was not found increased in this age group in our registry, so this hypothesis is not likely to explain the whole increase.
The increase in the IRs we observed may have several explanations: there may have been not only improvements in registration, diagnosis, and clinical practice, but also changes in potential risk factors. Interestingly, our results were obtained during a period when no major changes in the registration procedure or in access to imaging technologies occurred. However, we cannot completely rule out that clinical practice may have evolved and that physicians increased their willingness to pursue a diagnosis for older patients, although this is not likely to explain the difference in increase between histological subtypes and gender. Moreover, a number of statistical hypotheses have been tested, particularly with respect to changes in APC, and P-values have not been adjusted for multiple comparisons. Then confirmation from other locales would be advisable.
The more pronounced increase in incidence for women than for men suggests the possible role of gender-related factors. Increased use of hormones in women since the 1960s with the introduction of oral contraception, hormone replacement therapy, and sterility treatment is a potential explanation. Another is that exposure to electromagnetic fields and to some chemicals has also increased in recent years and constitutes a hypothesis for changes over time, even if not obviously gender related.
The debate that has lasted for more than 2 decades about the reasons that CNS tumors increased in most countries and especially in elderly persons does not seem completely closed. At the end of the 1990s, data from the US, the Scandinavian countries, and recently from England33 tended to show a stabilization or a slight decrease in rates, which pleads for the hypothesis of an artifactual increase in the 1980s and 1990s likely related to progress in imaging and a better specificity in diagnosis. However, the more recent trends were calculated until 1999 in the Central Brain Tumor Registry of the United States4 and until 2003 in the Scandinavian countries and England,1,33 which were before the increase appeared for gliomas in our study. Thus there will be interest in coming years to compare updated trends from other countries, with specific focus on subjects over 85 and on nonmalignant tumors, in order to interpret the results we have found here in France.
Conflict of interest statement. None declared.
Funding
This work was supported by the Institut de Veille Sanitaire and the Institut National du Cancer.
Acknowledgments
The Gironde CNS Tumor Registry is funded by the Institut de Veille Sanitaire and the Institut National du Cancer. We thank all the clinicians of the Bordeaux University Teaching Hospital who contributed to data collection.
References
- 1.Deltour I, Johansen C, Auvinen A, Feychting M, Klaeboe L, Schüz J. Time trends in brain tumour incidence rates in Denmark, Finland, Norway, and Sweden, 1974–2003. J Natl Cancer Inst. 2009;101:1721–1724. doi: 10.1093/jnci/djp415. [DOI] [PubMed] [Google Scholar]
- 2.Christensen HC, Kosteljanetz M, Johansen C. Incidences of gliomas and meningiomas in Denmark, 1943 to 1997. Neurosurgery. 2003;52:1327–1333. doi: 10.1227/01.neu.0000064802.46759.53. [DOI] [PubMed] [Google Scholar]
- 3.Helseth A. The incidence of primary central nervous system neoplasms before and after computerized tomography availability. J Neurosurg. 1995;83:999–1003. doi: 10.3171/jns.1995.83.6.0999. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman S, Propp JM, McCarthy BJ. Temporal trends in incidence of primary brain tumors in the United States, 1985–1999. Neuro-Oncology. 2006;8:27–37. doi: 10.1215/S1522851705000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hess KR, Broglio KR, Bondy ML. Adult glioma incidence trends in the United States, 1977–2000. Cancer. 2004;101:2293–2299. doi: 10.1002/cncr.20621. [DOI] [PubMed] [Google Scholar]
- 6.Kuratsu J, Ushio Y. Epidemiological study of primary intracranial tumours in elderly people. J Neurol Neurosurg Psychiatr. 1997;63:116–118. doi: 10.1136/jnnp.63.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lönn S, Klaeboe L, Hall P, et al. Incidence trends of adult primary intracerebral tumors in four Nordic countries. Int J Cancer. 2004;108:450–455. doi: 10.1002/ijc.11578. [DOI] [PubMed] [Google Scholar]
- 8.Johannesen TB, Angell-Andersen E, Tretli S, Langmark F, Lote K. Trends in incidence of brain and central nervous system tumors in Norway, 1970–1999. Neuroepidemiology. 2004;23:101–109. doi: 10.1159/000075952. [DOI] [PubMed] [Google Scholar]
- 9.Polednak AP. Interpretation of secular increases in incidence rates for primary brain cancer in Connecticut adults, 1965–1988. Neuroepidemiology. 1996;15:51–56. doi: 10.1159/000109889. [DOI] [PubMed] [Google Scholar]
- 10.Pirouzmand F, Sadanand V. The incidence trends of primary brain tumors in Saskatchewan from 1970 to 2001. Can J Neurol Sci. 2007;34:181–186. doi: 10.1017/s0317167100006016. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Tovar M, de Pedro-Cuesta J, Pollán-Santamaría M, López-Abente G. Time-trend analysis of mortality from malignant tumors of the nervous system in Spain, 1952–1986. J Neurol Sci. 1995;131:15–20. doi: 10.1016/0022-510x(95)00013-r. [DOI] [PubMed] [Google Scholar]
- 12.Kuratsu J, Takeshima H, Ushio Y. Trends in the incidence of primary intracranial tumors in Kumamoto, Japan. Int J Clin Oncol. 2001;6:183–191. doi: 10.1007/pl00023928. [DOI] [PubMed] [Google Scholar]
- 13.Grieg NH, Ries LG, Yancik R, Rapoport SI. Increasing annual incidence of primary malignant brain tumors in the elderly. J Natl Cancer Inst. 1990;82:1621–1624. doi: 10.1093/jnci/82.20.1621. [DOI] [PubMed] [Google Scholar]
- 14.Lowry JK, Snyder JJ, Lowry PW. Brain tumors in the elderly: recent trends in a Minnesota cohort study. Arch Neurol. 1998;55:922–928. doi: 10.1001/archneur.55.7.922. [DOI] [PubMed] [Google Scholar]
- 15.Smith MA, Freidlin B, Ries LA, Simon R. Increased incidence rates but no space-time clustering of childhood astrocytoma in Sweden, 1973–1992: a population-based study of pediatric brain tumors. Cancer. 2000;88:1492–1493. doi: 10.1002/(sici)1097-0142(20000315)88:6<1492::aid-cncr30>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Cho K, Wang K, Kim S, Shin S, Chi JG, Cho B. Pediatric brain tumors: statistics of SNUH, Korea (1959–2000) Childs Nerv Syst. 2002;18:30–37. doi: 10.1007/s00381-001-0547-y. [DOI] [PubMed] [Google Scholar]
- 17.Keene DL, Hsu E, Ventureyra E. Brain tumors in childhood and adolescence. Pediatr Neurol. 1999;20:198–203. doi: 10.1016/s0887-8994(98)00139-8. [DOI] [PubMed] [Google Scholar]
- 18.McKinney PA. Brain tumours: incidence, survival, and aetiology. J Neurol Neurosurg Psychiatr. 2004;75:12–17. doi: 10.1136/jnnp.2004.040741. (suppl 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang SL, Kuo TH, Lieu AS, et al. The change of relative incidences of intracranial tumors after the use of computed tomography in Taiwan. Kaohsiung J Med Sci. 2000;16:345–350. [PubMed] [Google Scholar]
- 20.Jukich PJ, McCarthy BJ, Surawicz TS, Freels S, Davis FG. Trends in incidence of primary brain tumors in the United States, 1985–1994. Neuro-Oncology. 2001;3:141–151. doi: 10.1093/neuonc/3.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corn BW, Marcus SM, Topham A, Hauck W, Curran WJ. Will primary central nervous system lymphoma be the most frequent brain tumor diagnosed in the year 2000? Cancer. 1997;79:2409–2413. [PubMed] [Google Scholar]
- 22.Kundi M. The controversy about a possible relationship between mobile phone use and cancer. Environ Health Perspect. 2009;117:316–324. doi: 10.1289/ehp.11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parkin DM, Shanmugaratnam K, Sobin L, Ferlay J, Whelan SL. Histological Groups for Comparative Studies. Lyon: IARC Press; 1998. [Google Scholar]
- 24.McCarthy BJ, Kruchko C. Consensus conference on cancer registration of brain and central nervous system tumors. Neuro-Oncology. 2005;7:196–201. doi: 10.1215/S115285170400050X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarthy BJ, Surawicz T, Bruner JM, Kruchko C, Davis F. Consensus Conference on Brain Tumor Definition for Registration. Neuro-Oncology. 2002;4:134–145. doi: 10.1215/15228517-4-2-134. November 10, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elia-Pasquet S, Provost D, Jaffré A, et al. Incidence of central nervous system tumors in Gironde, France. Neuroepidemiology. 2004;23:110–117. doi: 10.1159/000075953. [DOI] [PubMed] [Google Scholar]
- 27.Fleury A, Menegoz F, Grosclaude P, et al. Descriptive epidemiology of cerebral gliomas in France. Cancer. 1997;79:1195–1202. doi: 10.1002/(sici)1097-0142(19970315)79:6<1195::aid-cncr19>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 28.Ménégoz F, Martin E, Danzon A, et al. Incidence and mortality of central nervous system tumors in France: trends over the period 1978–2000 and influence of registration practices on results. Rev Epidemiol Sante Publique. 2006;54:399–406. doi: 10.1016/s0398-7620(06)76738-4. [DOI] [PubMed] [Google Scholar]
- 29.Brown M, Schrot R, Bauer K, Letendre D. Incidence of first primary central nervous system tumors in California, 2001–2005. J Neurooncol. 2009;94:249–261. doi: 10.1007/s11060-009-9864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arora RS, Alston RD, Eden TOB, Estlin EJ, Moran A, Birch JM. Age-incidence patterns of primary CNS tumors in children, adolescents, and adults in England. Neuro-Oncology. 2009;11:403–413. doi: 10.1215/15228517-2008-097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wöhrer A, Waldhör T, Heinzl H, et al. The Austrian Brain Tumour Registry: a cooperative way to establish a population-based brain tumour registry. J Neurooncol. 2009;95:401–411. doi: 10.1007/s11060-009-9938-9. [DOI] [PubMed] [Google Scholar]
- 32.Klaeboe L, Lonn S, Scheie D, et al. Incidence of intracranial meningiomas in Denmark, Finland, Norway and Sweden, 1968–1997. Int J Cancer. 2005;117:996–1001. doi: 10.1002/ijc.21255. [DOI] [PubMed] [Google Scholar]
- 33.Arora RS, Alston RD, Eden TOB, et al. Are reported increases in incidence of primary CNS tumours real? An analysis of longitudinal trends in England, 1979–2003. Eur J Cancer. 2010;46:1607–1616. doi: 10.1016/j.ejca.2010.02.007. [DOI] [PubMed] [Google Scholar]