Abstract
Erlotinib is effective for epidermal growth factor receptor (EGFR) mutant lung cancer, but CNS penetration at standard daily dosing is limited. We previously reported that intermittent “pulsatile” administration of high-dose (1500 mg) erlotinib once weekly was tolerable and achieved concentrations in cerebrospinal fluid exceeding the half maximal inhibitory concentration for EGFR mutant lung cancer cells in a patient with leptomeningeal metastases; we now expand this paradigm to a series of 9 patients. We retrospectively identified patients with EGFR mutant lung cancer treated with pulsatile erlotinib for CNS metastases (brain and/or leptomeningeal) that occurred despite conventional daily erlotinib or other EGFR tyrosine kinase inhibitors. Mutations in available lung and CNS tissue were correlated with efficacy. Erlotinib was administered as monotherapy at a median dose of 1500 mg weekly. Best CNS radiographic response was partial in 67% (6/9, including 2 with isolated leptomeningeal metastases), stable disease in 11% (1/9), and progressive disease in 22% (2/9). Median time to CNS progression was 2.7 months (range, 0.8–14.5 months) and median overall survival was 12 months (range, 2.5 months–not reached). Treatment was well tolerated. No acquired resistance mutations in EGFR were identified in the CNS metastases of 4 patients, including 1 harboring T790M outside the CNS. Pulsatile erlotinib can control CNS metastases from EGFR mutant lung cancer after failure of standard daily dosing. CNS disease may not harbor acquired resistance mutations that develop systemically. A prospective trial is planned.
Keywords: CNS metastases, EGFR, erlotinib, lung cancer, pulsatile dosing
Somatic mutations in the epidermal growth factor receptor (EGFR) tyrosine kinase domain are found in up to 25% of non-small cell lung cancers (NSCLCs).1 Nearly 90% of these mutations occur as deletions in exon 19 or as a single missense mutation at position 858 on exon 21. These mutations are associated with a high rate of response to EGFR tyrosine kinase inhibitors (TKIs), such as erlotinib (Tarceva; OSI Pharmaceuticals/Genentech) and gefitinib (Iressa; AstraZeneca).2–4 However, secondary mutations during therapy lead to acquired EGFR TKI resistance.5 For example, T790M substitution in EGFR exon 20 has been reported in approximately 50% of cases with acquired resistance to EGFR TKIs.6 In addition, MET amplification was found after TKI treatment of NSCLC in up to 20% of patients.7
Approximately one-third of patients develop CNS metastases after initial response to EGFR TKIs.8–10 However, CNS metastases do not consistently harbor acquired resistance mutations found in synchronous disease outside the CNS.11,12 Therefore, CNS metastases may retain EGFR TKI sensitivity if sufficient drug concentrations can be achieved in brain parenchyma for brain metastases or in cerebrospinal fluid (CSF) for leptomeningeal metastases. We previously demonstrated that the concentration of CSF erlotinib during standard daily dosing of 150 mg is inadequate to kill EGFR mutant NSCLC cells.12 By contrast, high-dose weekly administration of at least 2000 mg both is tolerable13 and achieves therapeutic CSF concentration.12 Moreover, such “pulsatile” kinase inhibition induces cancer cell apoptosis as effectively as chronic inhibition in other settings.14 Others also reported increased CSF penetration with high-dose gefitinib,11 as well as tolerability of pulsatile dosing with the EGFR TKI lapatinib.15 We recently reported a single case of CNS metastases (leptomeningeal) from NSCLC that responded to pulsed-dose erlotinib after failure of low-dose daily treatment.12 Here, we expand our experience to a series of 9 cases with molecular correlates of efficacy.
Methods
Using departmental databases from Memorial Sloan-Kettering Cancer Center, we retrospectively identified patients with EGFR mutant lung cancer treated with pulsatile erlotinib for CNS metastases that developed or worsened following prior therapy with an EGFR TKI at standard dosing. Patients who received at least 1 pulsatile erlotinib dose and underwent at least 1 follow-up CNS imaging study to assess response were included. Patients who did not have a documented EGFR TKI sensitizing mutation in pretreatment tissue were excluded. There was no maximum age or minimum performance status required.
Brain and/or spine MRI scans to assess CNS radiographic response were reviewed by 2 neuro-oncologists (C.G., A.B.L.) and a neuroradiologist (A.I.H) using Response Evaluation Criteria in Solid Tumors (RECIST) 1.1.16 In patients treated previously with stereotactic radiosurgery (SRS), we evaluated SRS-naive lesion(s) to avoid the potential for mislabeling improved radionecrosis as a response. Time to progression and survival were calculated by the Kaplan–Meier method. Clinical data were updated as of May 19, 2011. Testing for EGFR sensitizing mutations was performed on all available tissue, using previously described methods.4,17 Acquired resistance specimens, when available, were tested for the EGFR exon 20 T790M mutation using a highly sensitive locked nucleic acid assay developed at our institution. MET amplification was evaluated by fluorescence in situ hybridization in acquired resistance specimens when adequate tissue was available, using previously described methods.7 This study (including molecular analyses of tissue and clinical annotation) was approved by the institutional review board of Memorial Sloan-Kettering Cancer Center.
Results
Patients
We studied 7 women and 2 men (Table 1) with a median age of 57 years at the start of pulsatile erlotinib (range, 44–76 years) and a median KPS of 80 (range, 50–90). Pulsatile erlotinib was started for newly diagnosed CNS metastases in 3 patients and for recurrent/progressive CNS disease in 6 (Table 1). Five had coexistent brain and leptomeningeal metastases, 1 isolated brain metastases, and 3 isolated leptomeningeal metastases. Six patients had additional metastases outside the CNS, while 3 had isolated CNS metastases. Pulsatile erlotinib was administered as monotherapy to all patients at a median dose of 1500 mg once per week (range, 900–1500 mg).
Table 1.
Baseline characteristics at start of pulsatile erlotinib
| Patient | Gender | Age | KPS | Therapy for CNS disease before Pulsatile Erlotinib | Type of CNS disease | Metastases Outside the CNS | Prior EGFR TKI |
|---|---|---|---|---|---|---|---|
| 1 | Woman | 44 | 70 | Resection; SRS; WBRT; docetaxel + cisplatin + daily erlotinib | Brain | Yes | Erlotinib |
| 2 | Woman | 76 | 70 | WBRT | Brain + lepto | Yes | Afatinib |
| 3 | Woman | 57 | 80 | None | Lepto | No | Erlotinib |
| 4a | Woman | 57 | 80 | None | Lepto | Yes | Erlotinib |
| 5 | Woman | 69 | 50 | Daily erlotinib + pemetrexed | Lepto | No | Erlotinib |
| 6 | Man | 49 | 60 | WBRT | Brain + lepto | Yes | Gefitinib |
| 7 | Woman | 58 | 90 | Daily erlotinib | Brain + lepto | Yes | Erlotinib |
| 8 | Woman | 49 | 80 | None | Brain + lepto | No | Erlotinib |
| 9 | Man | 60 | 90 | Pemetrexed + bevacizumab + carboplatin, then pemetrexed + bevacizumab + daily erlotinib, then daily erlotinib | Brain + lepto | Yes | Erlotinib |
Abbreviations: SRS, stereotactic radiosurgery; WBRT, whole brain radiation therapy; Brain, parenchymal brain metastases; Lepto, leptomeningeal metastases; TKI, tyrosine kinase inhibitor.
aReported previously.12
Efficacy
By formal RECIST evaluation, best CNS radiographic response was partial in 4 (44%), noncomplete response/nonprogressive disease in 2, stable disease in 1, and progressive disease in 2 (Table 2). However, we also noted significant radiographic improvement in both patients with isolated leptomeningeal disease (Fig. 1, Table 2). Including these patients, the response rate was 67% (6/9). In all patients the dose of corticosteroids was stable or decreasing at the time of best response assessment. Median time to best response was 3.3 months (range, 0.7–6.0 months) for patients without progressive disease as best response.
Table 2.
Response, time to progression, and survival following pulsatile therapy
| Patient | Best CNS response | Best response outside CNS | CNS TTP (mo) | OS (mo) | Major toxicity during Pulsatile Erlotinib (grade) | Treatment(s) after Pulsatile Erlotinib |
|---|---|---|---|---|---|---|
| 1 | SD | SD | 3.2 | 5.9 | Rash (2), CNS hemorrhage (1) | Pemetrexed, paclitaxel |
| 2 | PR | NE | 2.7 | 2.9 | None | None |
| 3 | PRa | SD | 14.5 | >25.4 | None | WBRT, daily erlotinib |
| 4 | PRa | NE | 1.8 | 15.3 | Diarrhea (1) | WBRT, cetuximab, daily erlotinib, gemcitabine, everolimus |
| 5 | PD | PD | 0.8 | 6.2 | Fatigue (1) | Daily erlotinib |
| 6 | PR | NE | 9.5 | 12.0 | CNS hemorrhage (1) | None |
| 7 | PR | SD | 7.6 | 17.5 | Rash (1) | Added bevacizumab, pemetrexed |
| 8 | PR | NE | 2.4 | >11.3 | CNS hemorrhage (1), nausea (1), hair thinning (1) | Pemetrexed |
| 9 | PD | PD | 1.2 | 3.4 | Fatigue (1) | Cetuximab, afatinib |
Abbreviations: TTP, time to progression; OS, overall survival; SD, stable disease, PR, partial response; CR, complete response; NE, not evaluable; PD, progressive disease; >, patient alive (censored for survival) at time of analysis.
aPatient had clear partial response of isolated leptomeningeal metastases, designated by RECIST as non-CR/non-PD.
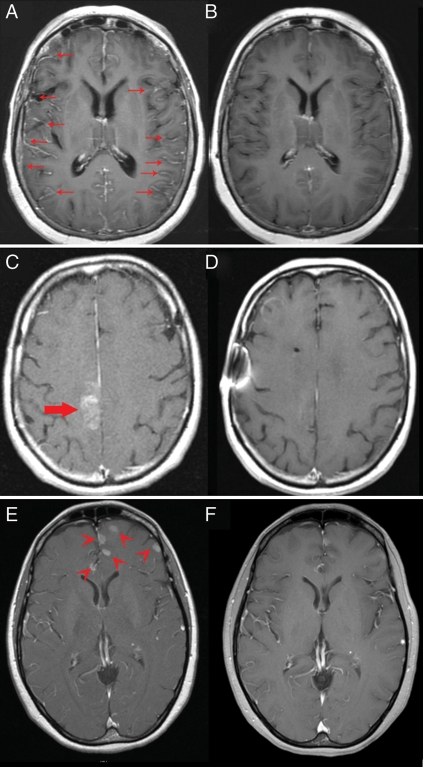
Fig. 1.
Response of CNS metastases to pulsatile erlotinib in 3 patients. Contrast (gadolinium)-enhanced axial T1 MRI sequences in patient #3 with leptomeningeal metastases (arrows) before (A) and after (B) 6 months of therapy. Patient #6 with coexistent brain (large arrow) and leptomeningeal metastases (not shown) before (C) and after (D) 5 months of therapy. Patient #8 with coexistent brain (arrow heads) and leptomeningeal metastases (not shown) before (E) and after (F) 2 months of therapy.
Median time to CNS progression was 2.7 months (range, 0.8–14.5 months) and median overall survival was 12.0 months (range, 2.9 months–not reached) after initiation of pulsatile erlotinib (Table 2). Best response of disease outside the CNS was assessable in 5 patients; 3 showed stable disease and 2 progressed.
Toxicity
Major observed toxicities included rash (grades 1–2, n = 2), fatigue (grade 1, n = 2), diarrhea (grade 1, n = 1), nausea (grade 1, n = 1), hair thinning (grade 1, n = 1), and asymptomatic intratumoral CNS hemorrhage (grade 1, n = 3, none receiving therapeutic anticoagulation) that did not affect treatment; no grade ≥3 toxicities were observed (Table 2).
EGFR Mutations and MET Amplification
Tumor specimens were submitted for EGFR genotyping, and all patients were found to have tumors harboring mutations: exon 19 deletion (n = 3), exon 19 insertion (n = 1), exon 21 L858R substitution (n = 4), and combined exon 18 G719S/exon 21 L861Q substitutions (n = 1) (Table 3). Following acquired resistance to standard dosing of EGFR TKIs, non-CNS tissue was obtained in 3 patients, all of whom harbored exon 20 T790M.
Table 3.
Molecular analyses
| Patient | Baseline EGFR mutation outside-CNS | Sensitizing mutation in CNS at time of acquired resistance | Acquired resistance mutation outside CNS | Acquired resistance mutation in CNS |
|---|---|---|---|---|
| 1 | Exon 19 deletion | Exon 19 deletion | Undetermined | No exon 20 T790M, no MET amplification |
| 2 | Exon 19 insertion | Undetermined | Undetermined | Undetermined |
| 3 | Exon 21 L858R | Exon 21 L858R | Undetermined | No exon 20 T790M |
| 4 | Exon 21 L858R | Exon 21 L858R | Exon 20 T790M | No exon 20 T790M |
| 5 | Exon 19 deletion | Undetermined | Undetermined | Undetermined |
| 6 | Exon 21 L858R | Exon 21 L858R | Undetermined | No exon 20 T790M |
| 7 | Exon 18 G719S, L861Q | Undetermined | Undetermined | Undetermined |
| 8 | Exon 19 deletion | Undetermined | Exon 20 T790M, no MET amplification | Undetermined |
| 9 | Exon 21 L858R | Undetermined | Exon 20 T790M, no MET amplification | Undetermined |
Sensitizing EGFR mutations in CNS specimens (1 brain, 3 CSF) were found in all 4 cases with available material for analysis. Three had an exon 21 L858R substitution, and 1 an exon 19 deletion. T790M was not detected in any of these 4 cases, and MET amplification was not observed in the single case tested. In the remaining cases, obtaining additional CNS samples was either impractical or not clinically indicated, precluding further analysis.
Discussion
We report 9 patients with EGFR mutant NSCLC with CNS (brain and/or leptomeningeal) metastases treated with pulsatile erlotinib weekly as a single agent. All developed CNS disease as part of progression following initiation of standard daily dosing of EGFR TKIs. We used RECIST for evaluating radiographic changes during therapy because, to our knowledge, no other response criteria are widely accepted for evaluation of brain metastases from solid tumors such as NSCLC. For example, the traditional Macdonald criteria18 and the newly published criteria of the Response Assessment in Neuro-Oncology working group19 are intended for evaluation of primary brain tumors (especially glioblastoma) rather than brain metastases.
By formal RECIST evaluation, response of CNS disease was observed in 44% (4/9) of patients. However, 2 of 3 patients with isolated leptomeningeal metastases (without coexistent parenchymal brain metastases) achieved clear partial radiographic responses (Fig. 1). RECIST defines leptomeningeal metastases as “nontarget” lesions, and clear but incomplete responses, such as those we observed, are designated as “noncomplete response/nonprogressive disease” rather than either partial response or stable disease. Therefore, if the 2 patients with clearly improved leptomeningeal disease (Fig. 1) were designated as partial responders, then the response rate would increase to 67% (6/9) (Table 2). Increased corticosteroids did not account for responses.20
Although the median time to progression was only 2.7 months, the median overall survival was 12.0 months. In context, median survival after whole brain radiotherapy for brain metastases is 4.9 months.21 However, the natural history of EGFR mutant disease is often more favorable than for EGFR wild-type disease. For example, Eichler et al. reported median survival of 14.5 months from diagnosis of brain metastases from EGFR mutant lung cancer.10 In addition, some of the responses we observed were not durable.
Moreover, all patients in our series had worsening CNS metastases during or following treatment with an EGFR TKI at standard dosing, which was not addressed in the Eichler series.10 Heon et al. reported median survival of approximately 5 months among patients with EGFR mutant NSCLC following the development of new or worsening CNS metastases after conventional EGFR TKI therapy.8 In addition, all but 1 (89%, 8/9) of our patients had leptomeningeal metastases, which is generally considered more refractory to treatment than isolated parenchymal brain metastases, and this issue was not analyzed in detail in the Eichler10 or Heon8 series.
We identified an EGFR TKI sensitizing mutation in all tested CNS tissue and acquired resistance mutations in none. Three patients had T790M in disease outside the CNS (cases 4, 8, and 9; Table 3), including one without T790M in the CNS (case #4). Therefore, we did not address whether pulsatile therapy could overcome molecular resistance mechanisms in the CNS. However, the available data suggest it cannot. For example, no patient had a response outside the CNS, although only 1 (case #9) had both documented T790M and was re-evaluated systemically following pulsatile therapy. Best response in this case was progressive disease (Table 2).
There are several limitations to our study, including the small size, the retrospective design, the difficulty of determining response of isolated leptomeningeal disease, and limited availability of tissue for molecular analysis in some cases.
However, our results suggest that pulsatile erlotinob at approximately 1500 mg per week is safe and has activity in patients with CNS disease from EGFR mutant NSCLC even when systemic resistance has developed and been confirmed. Poor penetration of erlotinib when administered at standard low doses daily may explain in part the failure to achieve control of CNS metastases, rather than acquired resistance mutations such as T790M.8,11,12,22 A prospective trial is planned.
Conflict of interest statement. Consultant/Advisory Board: MolecularMD (W.P.), AstraZeneca (W.P.), Boehringer-Ingelheim (M.G.K.), Pfizer (M.G.K.), Roche/Genentech/OSI (V.A.M., A.B.L.), Eisai (A.B.L.), Enzon (A.B.L.), Merck/Schering Plough (J.L.C., A.B.L.), Bristol Myers-Squibb (W.P., A.B.L.), Symphony Evolution (W.P.); Campus Bio (A.B.L.) Cephalon (A.B.L.), ImClone (A.B.L.), GSK (A.B.L.); Rights to EGFR T790M testing were licensed on behalf of W.P. to MolecularMD by Memorial Sloan-Kettering Cancer Center. All other authors: None declared.
Funding
Funding was provided by the Geoffrey Beene Cancer Research Center of Memorial Sloan-Kettering Cancer Center, The Brain Tumor Center of Memorial Sloan-Kettering Cancer Center, Joan's Legacy, the Doris Duke Charitable Foundation, and the National Institutes of Health (R01CA121210 to W.P.).
Acknowledgments
We thank Lisa M. DeAngelis, MD, and Ingo K. Mellinghoff, MD, for critical review of the manuscript, and Judith A. Lampron for invaluable editorial assistance. This work was presented as a poster at the 2010 Society for Neuro-Oncology annual meeting.
References
- 1.Pao W, Miller VA. Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non-small-cell lung cancer: current knowledge and future directions. J Clin Oncol. 2005;23:2556–2568. doi: 10.1200/JCO.2005.07.799. doi:10.1200/JCO.2005.07.799. [DOI] [PubMed] [Google Scholar]
- 2.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. doi:10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 3.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. doi:10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 4.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. doi:10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. doi:10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 6.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. doi:10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. doi:10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2010;16:5873–5882. doi: 10.1158/1078-0432.CCR-10-1588. doi:10.1158/1078-0432.CCR-10-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omuro AM, Kris MG, Miller VA, et al. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer. 2005;103:2344–2348. doi: 10.1002/cncr.21033. doi:10.1002/cncr.21033. [DOI] [PubMed] [Google Scholar]
- 10.Eichler AF, Kahle KT, Wang DL, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol. 2010;12:1193–1199. doi: 10.1093/neuonc/noq076. doi:10.1093/neuonc/noq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackman DM, Holmes AJ, Lindeman N, et al. Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol. 2006;24:4517–4520. doi: 10.1200/JCO.2006.06.6126. doi:10.1200/JCO.2006.06.6126. [DOI] [PubMed] [Google Scholar]
- 12.Clarke JL, Pao W, Wu N, Miller VA, Lassman AB. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol. 2010;99:283–286. doi: 10.1007/s11060-010-0128-6. doi:10.1007/s11060-010-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milton DT, Azzoli CG, Heelan RT, et al. A phase I/II study of weekly high-dose erlotinib in previously treated patients with nonsmall cell lung cancer. Cancer. 2006;107:1034–1041. doi: 10.1002/cncr.22088. doi:10.1002/cncr.22088. [DOI] [PubMed] [Google Scholar]
- 14.Shah NP, Kasap C, Weier C, et al. Transient potent BCR-ABL inhibition is sufficient to commit chronic myeloid leukemia cells irreversibly to apoptosis. Cancer Cell. 2008;14:485–493. doi: 10.1016/j.ccr.2008.11.001. doi:10.1016/j.ccr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Chien AJ, Illi JA, Ko AH, et al. A phase I study of a 2-day lapatinib chemosensitization pulse preceding nanoparticle albumin-bound Paclitaxel for advanced solid malignancies. Clin Cancer Res. 2009;15:5569–5575. doi: 10.1158/1078-0432.CCR-09-0522. doi:10.1158/1078-0432.CCR-09-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. doi:10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Pan Q, Pao W, Ladanyi M. Rapid polymerase chain reaction-based detection of epidermal growth factor receptor gene mutations in lung adenocarcinomas. J Mol Diagn. 2005;7:396–403. doi: 10.1016/S1525-1578(10)60569-7. doi:10.1016/S1525-1578(10)60569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 19.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. doi:10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 20.Watling CJ, Lee DH, Macdonald DR, Cairncross JG. Corticosteroid-induced magnetic resonance imaging changes in patients with recurrent malignant glioma. J Clin Oncol. 1994;12:1886–1889. doi: 10.1200/JCO.1994.12.9.1886. [DOI] [PubMed] [Google Scholar]
- 21.Mehta MP, Rodrigus P, Terhaard CH, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21:2529–2536. doi: 10.1200/JCO.2003.12.122. doi:10.1200/JCO.2003.12.122. [DOI] [PubMed] [Google Scholar]
- 22.Balak MN, Gong Y, Riely GJ, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12:6494–6501. doi: 10.1158/1078-0432.CCR-06-1570. doi:10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]