Abstract
Sorafenib is an inhibitor of multiple kinases that has demonstrated antiproliferative and antiangiogenic activity in a number of in vitro and in vivo model systems. A phase I study was conducted to determine the maximum tolerated dose (MTD) of sorafenib in patients with recurrent malignant glioma. Sorafenib was given orally, twice a day (BID), continuously in 28-day cycles. The dose was escalated in 2 groups of patients stratified by use of enzyme-inducing antiseizure drugs (±EIASDs). Dose-limiting toxicity (DLT) was defined as any grades 3–4 nonhematological toxicity, grade 4 hematological toxicity, and febrile neutropenia. The number of evaluable patients enrolled in the +EIASD and −EIASD arms were 23 and 24, respectively. DLTs were predominantly dermatological and gastrointestinal effects, as observed in previous clinical trials of sorafenib. The MTD was 600 mg BID for patients receiving EIASDs and 800 mg BID for those who were not. The plasma pharmacokinetics of sorafenib were not significantly affected by the concurrent administration of EIASDs. The MTD of sorafenib given orally BID on a continuous basis was established as 600 mg BID in patients with malignant glioma who were concurrently receiving EIASDs and 800 mg BID in those who were not. Further evaluation is warranted of sorafenib at the recommended MTD against recurrent or progressive malignant glioma in combination with other molecularly targeted drugs or in the newly diagnosed setting concurrent with chemoradiation.
Keywords: angiogenesis, glioma, pharmacokinetics, proliferation, targeted therapy
An estimated 51, 410 new cases of primary brain cancer affected individuals in the United States in 2007.1
Current treatment relies primarily on surgery, radiation therapy, and chemotherapy. Despite recent advances in overall survival, malignant gliomas continue to generate a substantial loss of neurological quality of life for patients and a burden on caregivers.2,3 The available treatment modalities remain limited in their capacity for selectively targeting glioma cells and may result in the loss of neurological function as a side effect or toxicity.4 Current efforts and recent advances in the laboratory setting have improved our understanding of the molecular events promoting tumor cell behaviors such as proliferation, angiogenesis, invasion, and resistance, which are the direct causes of morbidity and mortality for patients. The development of treatment modalities targeted to specific molecular events, thus blocking the downstream detrimental behaviors, will hopefully translate into meaningful improvements for patients and their caregivers.
Malignant gliomas are characterized by an intensely angiogenic phenotype resulting from high levels of vascular endothelial growth factor (VEGF) expression.5 Treatment of patients with bevacizumab, a VEGF-neutralizing antibody, resulted in significant radiographic responses and improved outcome.6 A common molecular event that characterizes glioblastoma is constitutive activation of the ras-raf-MAPK (mitogen-activated protein kinase) signaling pathway.7 The downstream gene transcription effects promote tumor cell proliferation, invasion, and antiapoptotic behavior.
Sorafenib is an orally available synthetic biphenylurea compound that inhibits both the Raf/MEK/ERK and VEGF signaling pathways. It has a favorable kinase selectivity to Raf kinase and the VEGF receptors 1, 2, and 3; platelet-derived growth factor receptor-beta; fms-like tyrosine kinase-3; and c-Kit protein.8,9 A phase I clinical trial in patients with advanced solid cancers established a maximum tolerated dose (MTD) of 400 mg given twice a day (BID), with predominant adverse events of skin and gastrointestinal toxicity.10 Sorafenib has demonstrated clinical benefit resulting in regulatory approval in renal cell and hepatocellular carcinoma.11,12
The rationale for evaluating sorafenib in the treatment of malignant glioma is provided by the abundance of target kinases in an activated state promoting disease behavior detrimental to patient outcomes. This report describes the results of a phase I clinical trial undertaken to determine the toxicity profile, MTD, pharmacokinetics, and preliminary assessment of the antitumor activity of sorafenib in patients with recurrent or progressive malignant gliomas.
Patients and Methods
Patient Selection
This study was sponsored by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute and was conducted by the New Approaches to Brain Tumor Therapy Consortium (see www.nabtt.org for participating institutions). The protocol was reviewed and approved by CTEP and the institutional review board at each participating institution.
Criteria required for entry into the study included: age ≥18 years; histologically proven malignant glioma (glioblastoma multiforme, anaplastic astrocytoma, or anaplastic oligodendroglioma); progressive or recurrent tumor after radiation therapy with or without chemotherapy; measurable tumor by MRI or CT imaging; complete recovery from toxicity of previous therapies; 3 months from completion of radiation therapy to study entry; ≤2 prior chemotherapy regimens; KPS ≥60%; and Mini Mental State Exam score ≥15. Eligibility also required demonstrating acceptable hematological parameters (absolute neutrophil count ≥1500/μL; platelet count ≥100 000/μL; normal coagulation tests), renal function (serum creatinine ≤1.7 mg/dL), and hepatic function (total bilirubin ≤1.5 mg/dL; serum levels of aspartate aminotransferase and alanine aminotransferase ≤4 times the upper limit of normal). Conditions resulting in exclusion from the study included: a serious concurrent infection, illness, or medical condition; females who were pregnant or nursing; any concurrent cancer therapy except for corticosteroids; a concurrent malignancy or a prior neoplasm that had been curatively treated with a disease-free interval <5 years; evidence of bleeding diathesis; or requirement for therapeutic anticoagulation. Agreement to practice adequate birth control methods was required for fertile patients. Each patient was required to sign a written informed consent document, satisfying all federal and institutional policies and regulations, as a condition of registering for participation in the study. All evaluations required to document the eligibility criteria were performed within 14 days of initiating therapy.
Treatment Plan
Patients were stratified into 1 of 2 arms based upon preexisting use of antiseizure drugs. Patients taking antiseizure drugs that are known inducers of hepatic drug metabolizing enzymes were assigned to the +EIASD group. Patients assigned to the −EIASD group were either not being treated with an antiseizure drug or were taking a medication that did not significantly induce hepatic enzymes. The dose of sorafenib was escalated independently in these 2 groups. An appropriate corticosteroid dose was determined for each patient before beginning the first cycle of therapy and not changed until after the first tumor imaging scan was obtained. Patients were permitted to receive other medications for adequate supportive care, especially those who developed hand/foot syndrome. The routine use of antiemetics was not required.
Sorafenib (Bayer Pharmaceuticals) was supplied as an immediate-release film-coated tablet containing 200 mg of the free base of the drug as a tosylate salt. Sorafenib was administered orally on a continuous BID schedule. The dose was taken with at least 1 cup of water without regard to meals. The starting dose was 200 mg BID and the dose was escalated at a constant increment of 200 mg BID. The MTD was based upon dose-limiting toxicity (DLT) that occurred during the initial 28-day cycle of therapy. Groups of 3 patients were initially evaluated at each dose level. Dose escalation proceeded in the absence of a DLT in any patient evaluated at a given dose level. An additional 3 patients were entered into a dose level if a DLT occurred in 1 of 3 patients or into the preceding dose level if 2 of 3 patients had a DLT. Dose escalation continued if 1 of 6 patients experienced a DLT. The MTD was defined if 2 of 6 patients had a DLT, unless the toxicity was grade ≥4 in both patients, in which case the previous dose was the MTD. The preceding dose was also defined as the MTD if a DLT occurred in >2 of 6 patients.
Additional cycles of therapy with the same dose of sorafenib were repeated every 28 days in patients who did not experience a DLT and continued to satisfy all eligibility requirements. Dosing was stopped in the event of a DLT. Retreatment could be delayed for a maximum of 21 days to permit complete recovery from toxicities attributed to the previous dose or a severe adverse event unrelated to the study drug. A reduction in the dose to the preceding level was required for continued treatment in the event of a DLT that resolved to grade ≤1 or baseline within this time frame. Patients experiencing a DLT while receiving the 200-mg starting dose were removed from the study. Therapy was also permanently discontinued upon evidence of disease progression, treatment delay >21 days, or patient request for any reason.
Toxicity Assessments
A complete blood count with differentials and a platelet count were determined on a weekly basis, and a limited set of serum chemistry tests were performed for electrolytes, creatinine, blood urea nitrogen, and glucose. Hematological tests were performed twice a week upon first evidence of hematological toxicity until recovery of an absolute neutrophil count ≥1500/µL and a platelet count ≥100 000/µL. The entire series of evaluations performed to assess patient eligibility, with the exception of the electrocardiogram, chest x-ray, and pregnancy test, were repeated within 5 days of beginning every odd-numbered cycle of therapy and within 7 days of stopping further treatment. In addition, the medical history, physical and neurological examinations, and performance status were repeated before starting every even-numbered cycle of therapy. Toxicities were characterized and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events V.3.0 (http://www.ctep.cancer.gov). DLT was defined as any of the following adverse events: absolute neutrophil count ≤500/µL; platelet count ≤25 000/µL; febrile neutropenia; any nonhematological toxicities of grade >3; or treatment delay >14 days because of treatment-related toxicity.
Evaluation of Response
Tumor size was measured in 2 dimensions, preferably by MRI, with CT used only for those patients who were unable to undergo MRI for physical or medical reasons. A baseline image of the tumor was obtained within 14 days of beginning treatment. This scan was obtained within 48 h after surgery for patients undergoing a tumor resection. Imaging studies were repeated every 8 weeks within 5 days of beginning every odd-numbered cycle of therapy until relapse. Response to therapy was defined as previously described with a minimum time interval of 4 weeks for the radiographic and neurological improvements.13 The first indication of an objective response was confirmed by repeating the tumor measurement after completing the next treatment cycle. All patients deemed to have a radiological response at the treating institutions were centrally reviewed to confirm response. All patients were followed for survival, which was measured from the time of entry into the study.
Pharmacokinetics
Patients received only 1 dose of sorafenib on the first day of cycle 1 to facilitate definition of the plasma concentration-time profile. Blood specimens (7 mL) were drawn from a peripheral arm vein into tubes containing freeze-dried sodium heparin before dosing and at the following times after taking the first dose of sorafenib: 0.5, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, and 24.0 h. Twice daily dosing commenced on day 2 after collecting the 24-h sample. Samples were also acquired on day 15 according to this same schedule, except for the 24-h sample. Sample tubes were mixed by inversion and placed in ice until centrifuged (1300 g, 10 min, 4°C) within 15 min. The plasma was stored at −70°C until assayed.
The concentration of sorafenib in plasma was determined by reversed-phase high performance liquid chromatography with tandem mass spectrometry detection. This assay was adapted from the analytical method used for pharmacokinetic drug level monitoring in the initial phase I clinical trials of sorafenib,10 which has not been published. Plasma (100 µL) was mixed vigorously with 300 µL of acetic acid (1%, v/v) in acetonitrile containing the internal standard (carbanilide, 8 µg/mL) and centrifuged (10 000 g, 5 min). After diluting the supernatant with an equivalent volume of 10-mM ammonium formate buffer, pH 3.0, the sample solution (10 µL) was injected onto a Luna phenyl-hexyl high-performance liquid chromatography column (5 µm, 15 × 4.6 mm), preceded by a 0.5-µm inline filter guard column (4.0 × 3.0 mm) of the same stationary phase (Phenomenex). The column was eluted with a mobile phase composed of acetonitrile/10-mM ammonium formate buffer, pH 3.0 (60:40, v/v) at 1.0 mL/min.
An Agilent 1100 series XCT ion trap mass spectrometer with an atmospheric pressure ionization-electrospray interface was used for detection. Operating parameters of the mass spectrometer were individually optimized to maximize the signal for the most abundant product ions resulting from the isolation and fragmentation of the [M+H]+ ions for sorafenib (m/z 465) and the internal standard (m/z 213). Nitrogen was used as the nebulizing gas (50 psi) and drying gas (10 L/min, 350°C). Positive ion tandem mass spectrometry was performed in multiple reaction monitoring mode using helium as the collision gas. The internal standard was monitored with an m/z 50–300 scan range, an m/z 50 fragmentation cutoff, and a 1.0-V fragmentation amplitude from 1.6 to 4.0 min. Sorafenib was monitored with an m/z 100–600 scan range, an m/z 155 fragmentation cutoff, and a 1.2-V fragmentation amplitude from 4.0 to 6.0 min. The extracted product ion chromatograms for the internal standard (m/z 94) and sorafenib (m/z 252, 270, and 425) were integrated to provide peak areas.
Study samples were independently assayed in duplicate, on separate days, together with a series of 8 calibration standards of sorafenib in human donor plasma at concentrations ranging from 25 to 1000 ng/mL, drug-free plasma assayed with and without addition of the internal standard, and 4 quality control samples. The relationship between the drug/internal standard peak area ratio and known concentration of sorafenib in each calibration standard was analyzed by weighted linear regression. The slope and y-intercept of the best-fit line were used to calculate the drug concentration in study samples. Specimens with concentrations exceeding the upper range of the standard curve were reassayed upon dilution with drug-free human plasma. Sorafenib was determined with an interday accuracy of 113.3% and a precision of 17.5% at the lowest concentration included in the calibration curves (25 ng/mL). Interday accuracy of the assay for measuring quality control samples of sorafenib in human plasma at concentrations of 60, 300, and 900 ng/mL ranged from 95.3% to 103.6% of the known concentrations with a precision of 7.1–10.3%. A fourth quality control sample with an added drug concentration of 10 000 ng/mL that was assayed after dilution 20 times with blank plasma had an accuracy of 102.8% and a precision of 11.8%.
Actual sample times were calculated relative to the time that the preceding dose of sorafenib was taken. Sorafenib plasma concentration-time curves were analyzed by standard noncompartmental methods using WinNonlin Professional 5.0 software (Pharsight).14 Area under the curve (AUC) for plasma concentration-time to the last data point, either 12 h (AUC0–12) or 24 h (AUC0–24), was estimated using the log-linear trapezoidal algorithm. Minimum concentrations and maximum concentrations (Cmax) of the drug in plasma were based upon the observed values in the 2 sets of samples obtained from each patient. Geometric means of the pharmacokinetic variables were calculated for the groups of patients evaluated at each dose level in both treatment arms.15,16 The jackknife technique was used to estimate the SDs of the geometric means.17 The percent difference between mean values of the pharmacokinetic variables in the +EIASD and −EIASD treatment arms was calculated at each dose level. The general linear model was used to statistically compare the pharmacokinetic variables, after logarithmic transformation, between the 2 treatment arms using SYSTAT version 9.0 software (SPSS). Dose was defined as a continuous predictor and EIASD use as a categorical predictor with assigned values of 1 for the +EIASD arm and −1 for the −EIASD arm. Significant difference was considered as P < .05.
Statistical Considerations
Patient characteristics and toxicities were summarized using appropriate descriptive statistics. Confidence intervals were calculated using standard methods. These analyses were performed using SAS version 9.1.3 (SAS Institute).
Results
Patient Characteristics
Twenty-three patients were enrolled into the +EIASD arm and 24 into the −EIASD arm. All patients had had prior surgery and had completed radiation therapy. The mean age was 52 years (range, 18–79 years). The median KPS was 90 (range, 60–100), and the prior average number of chemotherapy regimens was 1 (range, 1–2). There were 29 patients with glioblastoma multiforme, 8 with anaplastic astrocytoma, 6 with anaplastic oligodendroglioma, and 4 with other gliomas (mixed glioma, malignant glioma, infiltrating glioma, and oligodendroglioma well differentiated).
Toxicity
For patients in the +EIASD arm, there were no DLTs in dose level 1 (200 mg) and 1 DLT (grade 3 hand/foot syndrome) among the first 3 patients evaluated in dose level 2 (400 mg BID). This prompted expansion of the cohort to 6 patients, and no additional DLTs were observed. None of the patients in dose level 3 (600 mg BID) experienced a DLT. One of the first 3 patients evaluated in dose level 4 (800 mg BID) had a DLT (grade 3 hand/foot syndrome), and 2 of the 3 additional patients entered into this dose level also had DLTs (grade 3 joint pain, grade 3 hypophosphatemia). The MTD for the +EIASD arm was therefore established as 600 mg BID.
For patients in the −EIASD arm, there were no DLTs noted in dose level 1. For dose level 2, a single grade 3 pruritus/itching was observed in the first 3 patients, and the cohort was expanded to 6. No additional DLTs were described in this cohort. There were no DLTs noted for dose level 3. At dose level 4, a grade 3 hand/foot syndrome occurred in the first 3 patients, and the cohort was expanded to 6. A total of 5 patients were accrued to this expanded cohort, as 2 had to be replaced and were not evaluable for toxicity. In the expanded cohort, there were no additional DLTs. The arm continued escalation to dose level 5 (1000 mg BID). There were 3/3 DLTs in the first 3 patients, with grade 4 fatigue and grade 3 nausea, and 2 patients had grade 3 hand/foot syndrome. The MTD was established as 800 mg BID for this arm.
Overall, dermatological toxicity affecting 17% of the patients enrolled was the most common treatment-related adverse event observed in this study. The remainder of adverse events designated as being at least possibly related to treatment with sorafenib included fatigue, hyperglycemia, hypertension, hypophosphatemia, nausea, back pain, and joint pain. These affected individually 2% of the enrolled subjects.
Pharmacokinetics
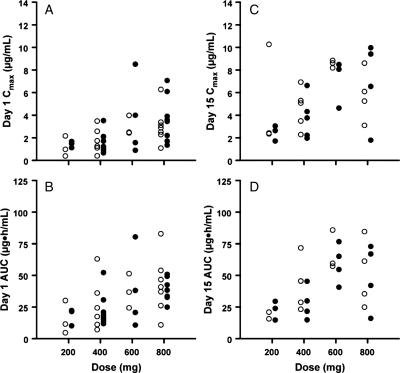
Pharmacokinetic data were available for 22 patients in the −EIASD arm and 23 patients in the +EIASD arm. Figure 1 shows the Cmax and AUC for the first dose of sorafenib and after achieving steady state for the BID dosing schedule in individual patients in both treatment groups for doses ranging from 200 to 1000 mg BID. The distribution and range of individual values for each of these variables at each dose level were very similar for patients in both treatment arms. Mean values of the sorafenib pharmacokinetic variables at each dose level and the percent difference between the two groups of patients are presented in Table 1. There were no differences in any of these parameters between the −EIASD and +EIASD treatment arms that approached statistical significance. These findings suggest that the concurrent administration of EIASDs had no discernible effect on the plasma pharmacokinetics of sorafenib in this patient population.
Fig. 1.
Plot depicting (A) the maximum drug concentration in plasma on day 1, (B) the area under the plasma concentration-time curve from 0 to 24 h for the first dose of sorafenib, (C) the maximum drug concentration in plasma for the morning dose on day 15, and (D) the area under the plasma concentration-time curve from 0 to 12 h for the morning dose given on day 15. Data points are values for patients in the −EIASD (open circles) and +EIASD (closed circles) treatment arms.
Table 1.
Mean pharmacokinetic parameters for sorafenib
| Dosea (mg) | AUC (µg h/mL)b |
Cmax (µg/mL) |
Cmin (µg/mL) | |||
|---|---|---|---|---|---|---|
| EIASD | Day 1 | Day 15 | Day 1 | Day 15 | Day 15 | |
| 200 | − | 11.8 ± 11.4 (3)c | 18.2 ± 3.7 (2) | 0.94 ± 0.85 (3) | 3.88 ± 2.96 (3) | 1.26 ± 0.80 (2) |
| + | 17.0 ± 8.0 (3) | 21.9 ± 8.0 (3) | 1.41 ± 0.30 (3) | 2.40 ± 0.74 (3) | 1.57 ± 0.99 (3) | |
| ▵% | 44.1 | 20.3 | 50.0 | −38.1 | 24.6 | |
| 400 | − | 20.7 ± 16.3 (6) | 39.9 ± 17.5 (5) | 1.44 ± 1.14 (6) | 4.31 ± 1.91 (5) | 3.91 ± 1.66 (5) |
| + | 20.1 ± 9.4 (8) | 24.9 ± 10.3 (5) | 1.47 ± 0.79 (8) | 3.42 ± 1.70 (5) | 1.68 ± 0.98 (5) | |
| ▵% | −2.9 | −37.6 | 2.1 | −20.6 | −57.0 | |
| 600 | − | 35.9 ± 13.6 (3) | 66.4 ± 14.4 (3) | 2.90 ± 0.77 (3) | 8.55 ± 0.34 (3) | 4.37 ± 1.91 (3) |
| + | 28.7 ± 25.1 (4) | 57.7 ± 15.9 (4) | 2.63 ± 2.64 (4) | 7.20 ± 2.24 (4) | 4.15 ± 1.93 (4) | |
| ▵% | −20.1 | −13.1 | −9.3 | −15.8 | −5.0 | |
| 800 | − | 36.8 ± 24.7 (7) | 46.3 ± 25.6 (4) | 2.75 ± 1.46 (7) | 5.41 ± 2.36 (4) | 3.59 ± 2.65 (4) |
| + | 36.0 ± 10.0 (8) | 42.6 ± 32.4 (4) | 3.19 ± 1.87 (8) | 5.75 ± 5.24 (4) | 2.94 ± 2.65 (4) | |
| ▵% | −2.2 | −8.0 | 16.0 | 6.3 | −18.1 | |
| 1000 | − | 43.6 ± 29.7 (3) | 81.1 (1) | 2.76 ± 2.37 (3) | 9.81 (1) | 9.81 (1) |
| P-valued | .59 | .99 | .19 | .39 | .35 | |
aA single dose was given on day 1, and continuous BID dosing began on day 2.
bAUC0–24 for day 1 and AUC0–12 for day 15.
cValues are the geometric mean ± SD; number of patients in parentheses.
dComparison of log-transformed data for doses ranging from 200 to 800 mg between the −EIASD and +EIASD treatment groups using the general linear model.
Abbreviations: EIASD, enzyme inducing antiseizure drug; AUC, area under the plasma concentration-time curve; Cmax, maximum observed drug concentration in plasma; Cmin, minimum observed drug concentration in plasma; ▵%, percent difference between mean values in the +EIASD and −EIASD treatment groups.
Discussion
This study extends the evaluation of sorafenib into the population of malignant glioma patients and extensively evaluates pharmacokinetics at daily doses up to 2000 mg. The primary objective of the study, to define the MTD and associated toxicities, was achieved. The MTD was similar for patients in both arms of the study, consistent with the finding that the pharmacokinetics of sorafenib were not altered by the concurrent administration of EIASDs. The toxicities of sorafenib in this patient population were consistent with those described in phase I studies of systemic solid cancers.18 We observed skin reactions as the primary dose-limiting events with no hematological events of significance. Compared with previous studies, we observed less gastrointestinal toxicity, with 1 DLT attributed to diarrhea. Other gastrointestinal-related adverse events included distention, elevated hepatic enzymes, elevated lipase, abdomen pain, and perforation of the colon. These were classified as unrelated or unlikely and affected 16% of the patients in the study. Fatigue, which affected a significant percentage of patients in previous studies, was noted in a total of 4% of patients. Although the defined MTD differed for the 2 arms, this difference was due to a grade 3 hypophosphatemia in the +EIASD arm. Hypophosphatemia is a recognized side effect of sorafenib, and use as a DLT has varied in early-phase sorafenib trials. Thus, the MTD of the 2 arms may actually differ very little.
An unexpected observation of this study was the ability to escalate beyond the previously established MTD for sorafenib.10,19 The ability of malignant glioma patients to tolerate higher doses of sorafenib than patients with extraneural solid malignancies was evident in both arms of the study, suggesting that this observation was not associated with the concomitant use of EIASDs. The rationale for establishing a higher MTD in this clinical trial could relate to a more proactive approach to adverse event management, since the primary toxicities had been described and methods to minimize them were available to investigators participating in this study. Another explanation may reside in other medications commonly used by malignant glioma patients, such as corticosteriods, having a beneficial impact on sorafenib toxicities.
The concurrent use of EIASDs can cause major alterations in the pharmacokinetics of many anticancer agents.20 As a result, phase I studies such as the one described in this report are needed to establish that adequate systemic exposure to novel agents is achieved before the undertaking of clinical trials to determine their effectiveness in patients with primary brain tumors. Sorafenib is eliminated by multiple mechanisms, including biliary excretion of unchanged drug, oxidative hepatic metabolism mediated predominantly by cytochrome P4503A4 (CYP3A4), and glucuronidation catalyzed by UGT1A9.21 To the best of our knowledge, this is the first report of a study designed to examine the effect of concurrently administered drugs that induce CYP3A4 activity on the clinical pharmacokinetics of sorafenib. In contrast to the expectation that inducers of CYP3A4 activity would decrease systemic exposure to sorafenib,22 it was found that the plasma pharmacokinetics of sorafenib were not significantly affected by the concomitant administration of EIASDs. Similarly, the concurrent administration of ketoconazole, a potent in vivo inhibitor of CYA3A4 activity, did not have a clinically significant effect on the pharmacokinetics of sorafenib.22 Evidently, CYP3A4 metabolism represents a relatively minor elimination pathway for sorafenib, changes in the capacity of which can be readily compensated for by other processes, including direct biliary excretion and glucuronidation, which together account for eliminating at least 80% of the administered drug.21
The pharmacokinetic behavior of sorafenib in brain cancer patients was in excellent agreement with results from prior phase I clinical trials of single-agent sorafenib.10,23–27 The mean pharmacokinetic parameters of sorafenib determined in the present study were within the range of previously reported mean values for these same doses in adult cancer patients with extraneural solid malignancies. In addition, as previously reported, we found that the mean Cmax and AUC increased in a less than proportionate manner with respect to escalations in the dose, and interpatient variability was very high.
The utility of targeted agents such as sorafenib in the treatment of malignant glioma is an area of intense clinical investigation. Compounds such as sorafenib demonstrate a sound preclinical rationale for use. Sorafenib, with multiple potential beneficial actions as both an antiproliferative agent targeting the ras-raf-MAPK signaling pathway and a VEGF-receptor inhibitor, appears particularly promising in glioma given the robust activity of both pathways in the disease. The current study supports a higher tolerated dose level than previously considered and reaffirms a toxicity profile that is expected and not unique to glioma. In the setting of heavily pretreated patients, sorafenib should be considered for further evaluation given the ease of administration, tolerable side effect profile, and favorable pharmacokinetics at the MTD established by this study.
Conflict of interest statement. None declared.
Funding
This study was supported by grants U01-CA62475 and U01-CA105689 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland. Preliminary results of this study were presented at the 2004 annual meeting of the Society of Neuro-Oncology.
References
- 1.Central Brain Tumor Registry of the United States. Statistical report: primary brain tumors in the United States, 2000–2004; 2008. http://www.cbtrus.org/reports//2007-2008/2007report.pdf. (accessed August 18, 2011).
- 2.Brown PD, Jensen AW, Felten SJ, et al. Detrimental effects of tumor progression on cognitive function of patients with high-grade glioma. J Clin Oncol. 2006;24:5427–5433. doi: 10.1200/JCO.2006.08.5605. doi:10.1200/JCO.2006.08.5605. [DOI] [PubMed] [Google Scholar]
- 3.Kvale EA, Murthy R, Taylor R, Lee JY, Nabors LB. Distress and quality of life in primary high-grade brain tumor patients. Support Care Cancer. 2009;17:793–799. doi: 10.1007/s00520-008-0551-9. doi:10.1007/s00520-008-0551-9. [DOI] [PubMed] [Google Scholar]
- 4.Laack NN, Brown PD, Ivnik RJ, et al. Cognitive function after radiotherapy for supratentorial low-grade glioma: A North Central Cancer Treatment Group prospective study. Int J Radiat Oncol Biol Phys. 2005;63:1175–1183. doi: 10.1016/j.ijrobp.2005.04.016. doi:10.1016/j.ijrobp.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. doi:10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 6.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. doi:10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 7.Lama G, Mangiola A, Anile C, et al. Activated ERK1/2 expression in glioblastoma multiforme and in peritumor tissue. Int J Oncol. 2007;30:1333–1342. [PubMed] [Google Scholar]
- 8.Wilhelm S, Chien DS. BAY 43–9006: preclinical data. Curr Pharm Des. 2002;8:2255–2257. doi: 10.2174/1381612023393026. doi:10.2174/1381612023393026. [DOI] [PubMed] [Google Scholar]
- 9.Wilhelm SM, Carter C, Tang L, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. doi:10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 10.Strumberg D, Richly H, Hilger RA, et al. Phase I clinical and pharmacokinetic study of the novel raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–972. doi: 10.1200/JCO.2005.06.124. doi:10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 11.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. doi:10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. doi:10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert MR, Supko JG, Batchelor T, Lesser G, Fisher JD, Piantadosi S, et al. Phase I clinical trial and pharmacokinetic study of irinotecan in adult patients with recurrent malignant glioma. Clin Cancer Res. 2003;9:2940–9. [PubMed] [Google Scholar]
- 14.Gabrielsson JWD. Pharmacokinetic/Pharmacodynamic Data Analysis: Concepts and Applications. 1994.
- 15.Lacey LF, Keene ON, Pritchard JF, Bye A. Common noncompartmental pharmacokinetic variables: Are they normally or log-normally distributed. J Biopharm Stat. 1997;7:171–178. doi: 10.1080/10543409708835177. doi:10.1080/10543409708835177. [DOI] [PubMed] [Google Scholar]
- 16.Mizuta E, Tsubotani A. Preparation of mean drug concentration—time curves in plasma. A study on the frequency distribution of pharmacokinetic parameters. Chem Pharm Bull. (Tokyo). 1985;33:1620–1632. doi: 10.1248/cpb.33.1620. [DOI] [PubMed] [Google Scholar]
- 17.Miller R. The jackknife—a review. Biometrika. 1974;61:1. [Google Scholar]
- 18.Strumberg D, Awada A, Hirte H, et al. Pooled safety analysis of BAY 43-9006 (sorafenib) monotherapy in patients with advanced solid tumours: Is rash associated with treatment outcome. Eur J Cancer. 2006;42:548–556. doi: 10.1016/j.ejca.2005.11.014. doi:10.1016/j.ejca.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Strumberg D, Voliotis D, Moeller JG, et al. Results of phase I pharmacokinetic and pharmacodynamic studies of the raf kinase inhibitor BAY 43-9006 in patients with solid tumors. Int J Clin Pharmacol Ther. 2002;40:580–581. doi: 10.5414/cpp40580. [DOI] [PubMed] [Google Scholar]
- 20.Vecht CJ, Wagner GL, Wilms EB. Interactions between antiepileptic and chemotherapeutic drugs. Lancet Neurol. 2003;2:404–409. doi: 10.1016/s1474-4422(03)00435-6. doi:10.1016/S1474-4422(03)00435-6. [DOI] [PubMed] [Google Scholar]
- 21.Kane RC, Farrell AT, Saber H, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res. 2006;12:7271–7278. doi: 10.1158/1078-0432.CCR-06-1249. doi:10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- 22.Lathia C, Lettieri J, Cihon F, Gallentine M, Radtke M, Sundaresan P. Lack of effect of ketoconazole-mediated CYP3A inhibition on sorafenib clinical pharmacokinetics. Cancer Chemother Pharmacol. 2006;57:685–692. doi: 10.1007/s00280-005-0068-6. doi:10.1007/s00280-005-0068-6. [DOI] [PubMed] [Google Scholar]
- 23.Strumberg D, Clark JW, Awada A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: A review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12:426–437. doi: 10.1634/theoncologist.12-4-426. doi:10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 24.Awada A, Hendlisz A, Gil T, et al. Phase I safety and pharmacokinetics of BAY 43-9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer. 2005;92:1855–1861. doi: 10.1038/sj.bjc.6602584. doi:10.1038/sj.bjc.6602584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark JW, Eder JP, Ryan D, Lathia C, Lenz HJ. Safety and pharmacokinetics of the dual action raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res. 2005;11:5472–5480. doi: 10.1158/1078-0432.CCR-04-2658. doi:10.1158/1078-0432.CCR-04-2658. [DOI] [PubMed] [Google Scholar]
- 26.Moore M, Hirte HW, Siu L, et al. Phase I study to determine the safety and pharmacokinetics of the novel raf kinase and VEGFR inhibitor BAY 43-9006, administered for 28 days on/7 days off in patients with advanced, refractory solid tumors. Ann Oncol. 2005;16:1688–1694. doi: 10.1093/annonc/mdi310. doi:10.1093/annonc/mdi310. [DOI] [PubMed] [Google Scholar]
- 27.Duran I, Hotte SJ, Hirte H, et al. Phase I targeted combination trial of sorafenib and erlotinib in patients with advanced solid tumors. Clin. Cancer Res. 2007;13:4849–4857. doi: 10.1158/1078-0432.CCR-07-0382. doi:10.1158/1078-0432.CCR-07-0382. [DOI] [PubMed] [Google Scholar]