Abstract
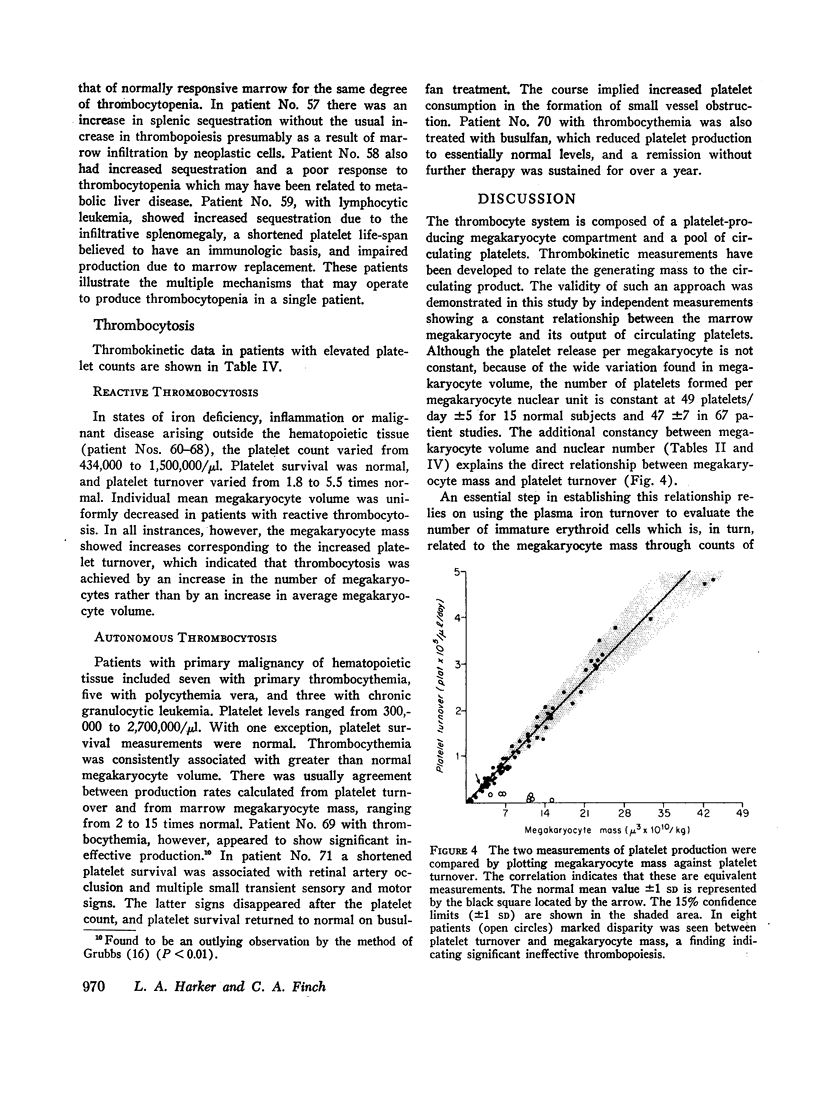
Platelet production, distribution, and destruction have been quantitated in normal man and in selected patients with platelet disorders. In most instances, total production as calculated from the megakaryocyte mass agreed with production estimated from platelet turnover. In patients with megaloblastosis, a discrepancy between these two measurements indicated the presence of ineffective thrombopoiesis.
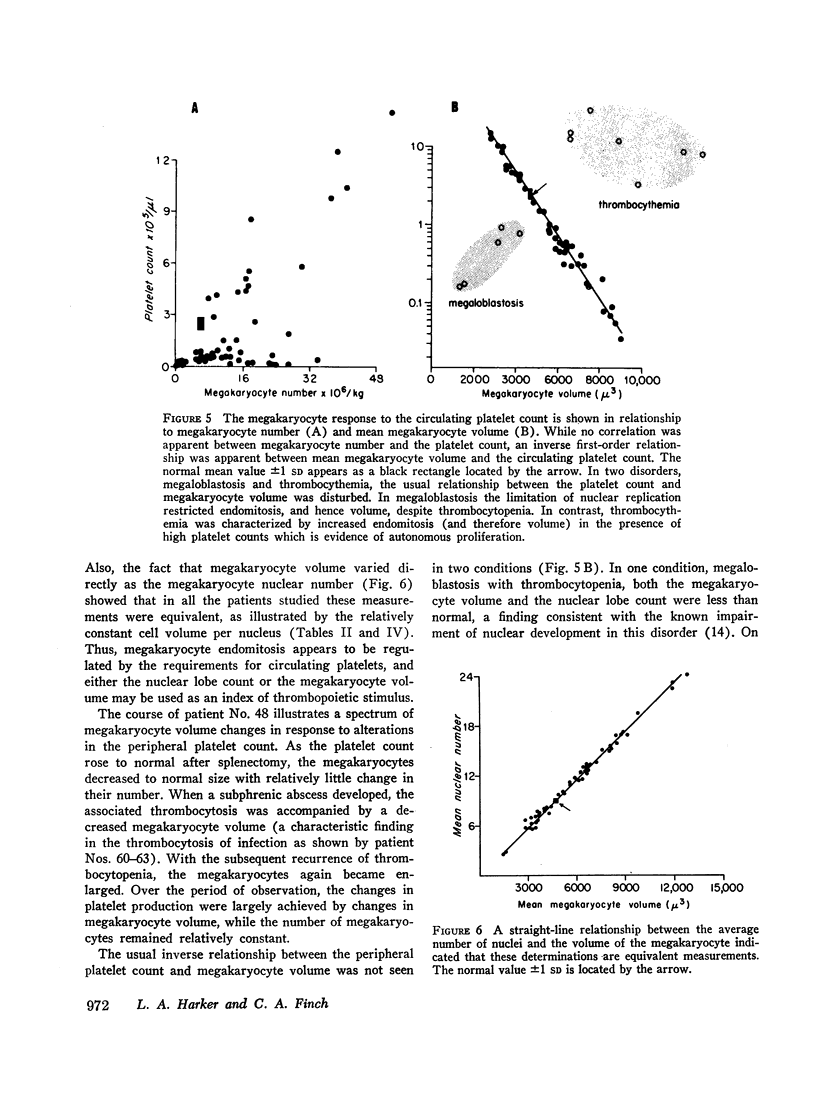
Thrombopoiesis was regulated by (a) alterations in megakaryocyte number, and (b) changes in megakaryocyte volume (produced by changes in endomitosis). The volume-endomitosis changes were closely related to the peripheral platelet count and were a useful indicator of thrombopoietic stimulus.
Thrombocytopenic disorders have been classified on the basis of the disturbed physiology into disorders of (a) production (hypoproliferative or ineffective), (b) distribution (splenic pooling), or (c) destruction (immune or consumptive). Less than a twofold increase in platelet production in the presence of significant thrombocytopenia was taken to represent impaired proliferation.
Thrombocytosis was classified as reactive or autonomous. Reactive thrombocytosis was consistently associated with a mean megakaryocyte volume and endomitosis less than normal but appropriate for the elevated circulating platelet count. In contrast, the average megakaryocyte volume and nuclear number were always greater than normal in thrombocythemia findings indicating autonomy.
Full text
PDF
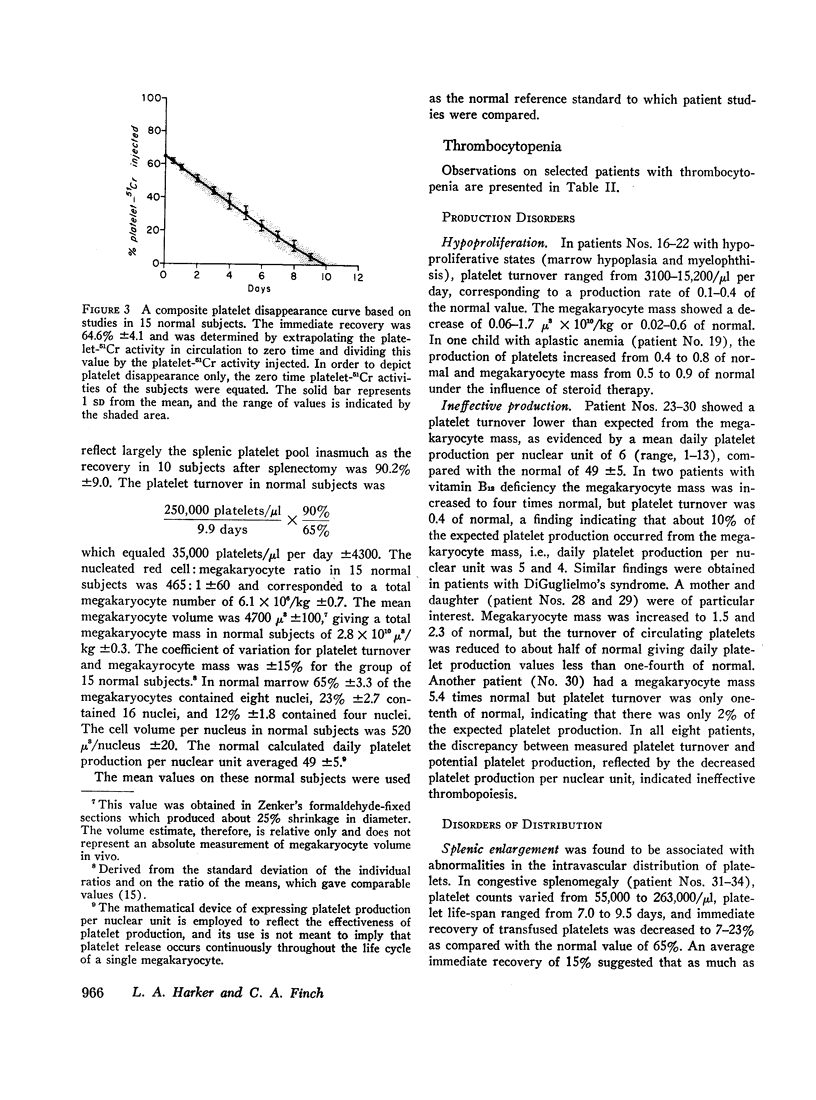
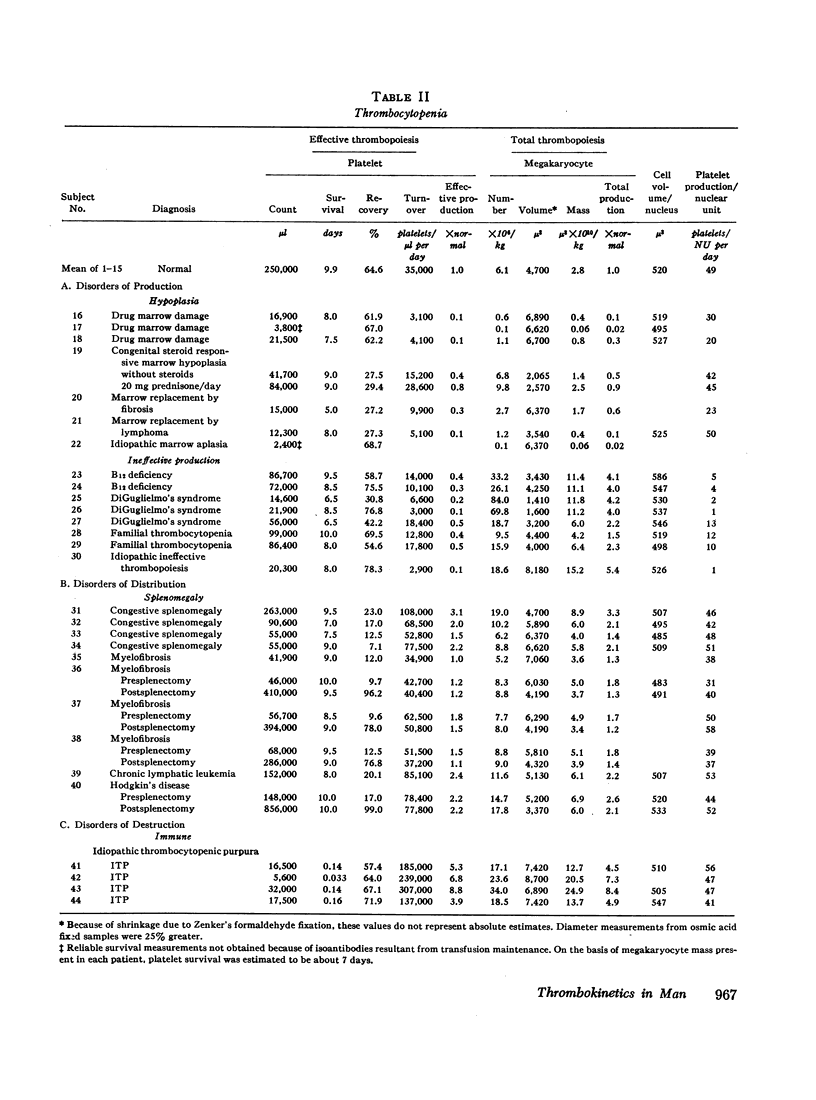
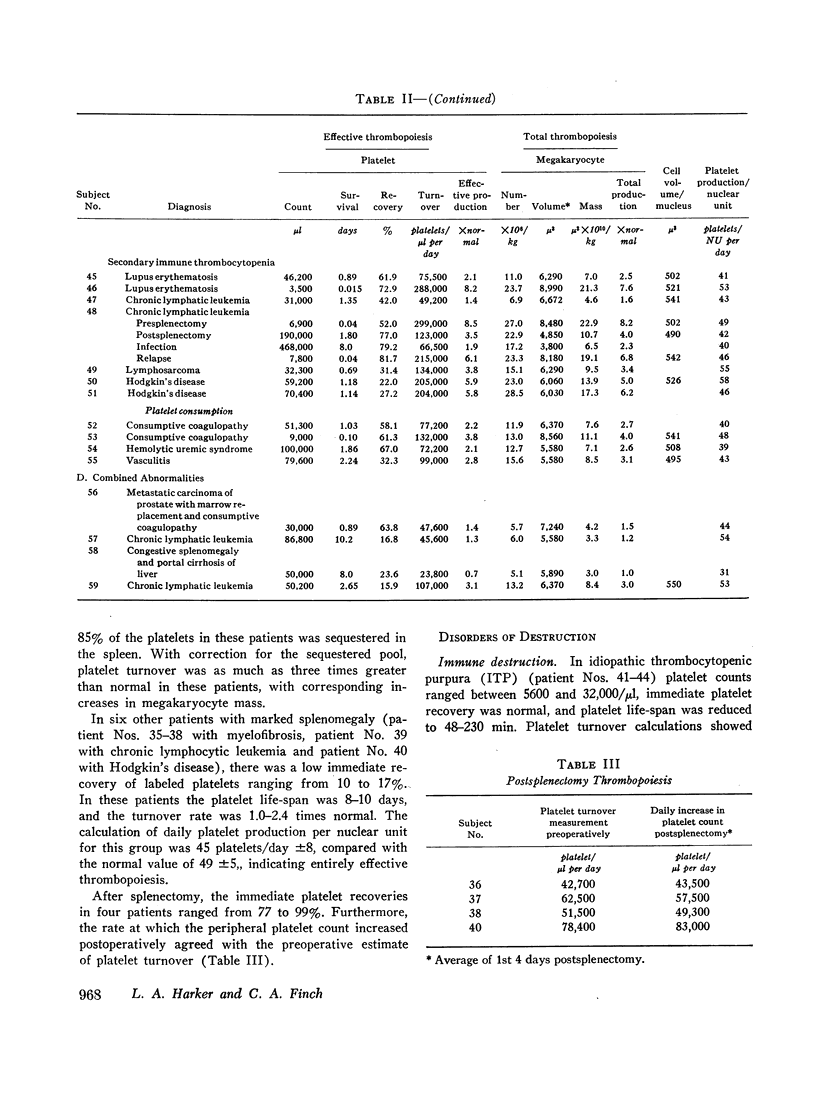
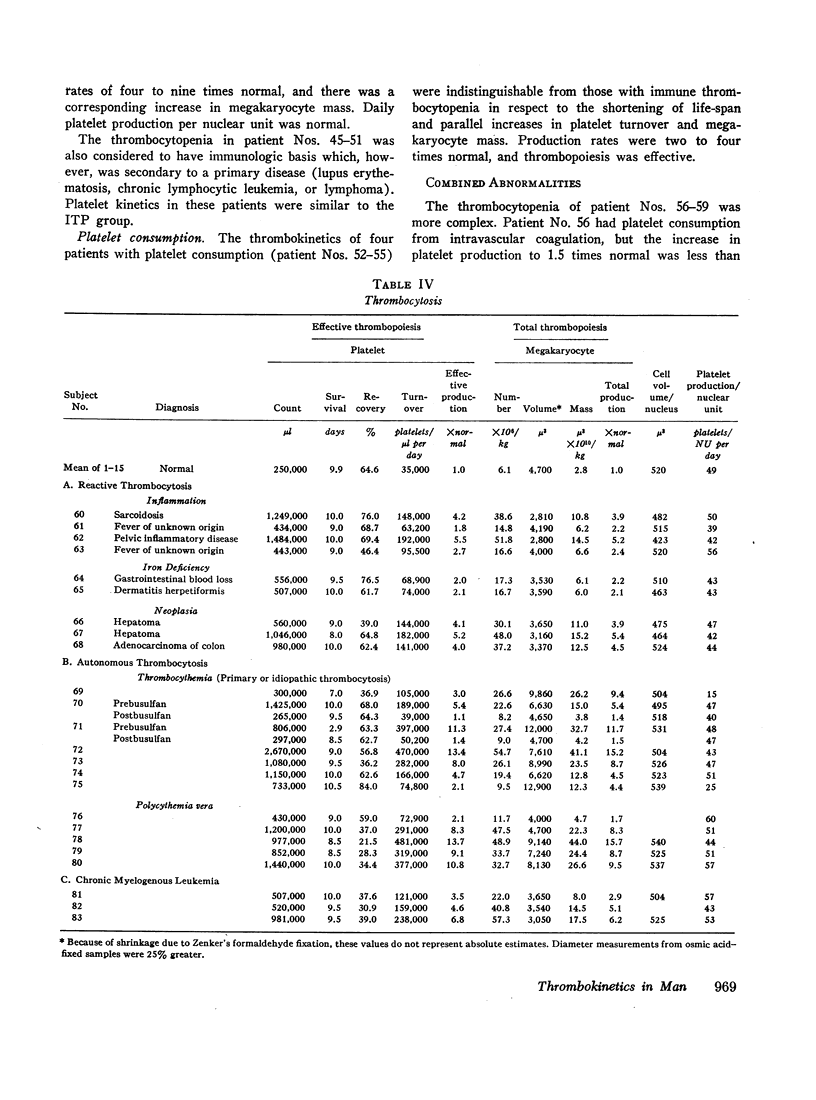



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abildgaard C. F., Simone J. V. Thrombopoiesis. Semin Hematol. 1967 Oct;4(4):424–452. [PubMed] [Google Scholar]
- Adamson J. W., Finch C. A. Mechanisms of erythroid marrow activation. Trans Assoc Am Physicians. 1966;79:419–425. [PubMed] [Google Scholar]
- Aster R. H. Effect of anticoagulant and ABO incompatibility on recovery of transfused human platelets. Blood. 1965 Dec;26(6):732–743. [PubMed] [Google Scholar]
- Aster R. H. Pooling of platelets in the spleen: role in the pathogenesis of "hypersplenic" thrombocytopenia. J Clin Invest. 1966 May;45(5):645–657. doi: 10.1172/JCI105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aster R. H. Studies of the mechanism of "hypersplenic" thrombocytopenia in rats. J Lab Clin Med. 1967 Nov;70(5):736–751. [PubMed] [Google Scholar]
- BRECHER G., SCHNEIDERMAN M., CRONKITE E. P. The reproducibility and constancy of the platelet count. Am J Clin Pathol. 1953 Jan;23(1):15–26. doi: 10.1093/ajcp/23.1.15. [DOI] [PubMed] [Google Scholar]
- Bull B. S., Schneiderman M. A., Brecher G. Platelet counts with the Coulter counter. Am J Clin Pathol. 1965 Dec;44(6):678–688. doi: 10.1093/ajcp/44.6.678. [DOI] [PubMed] [Google Scholar]
- COHEN P., GARDNER F. H., BARNETT G. O. Reclassification of the thrombocytopenias by the Cr51-labeling method for measuring platelet life span. N Engl J Med. 1961 Jun 22;264:1294–contd. doi: 10.1056/NEJM196106222642506. [DOI] [PubMed] [Google Scholar]
- COLEMAN D. H., DONOHUE D. M., FINCH C. A., MOTULSKY A. G., REIFF R. H. Erythrokinetics in pernicious anemia. Blood. 1956 Sep;11(9):807–820. [PubMed] [Google Scholar]
- DONOHUE D. M., GABRIO B. W., FINCH C. A. Quantitative measurement of hematopoietic cells of the marrow. J Clin Invest. 1958 Nov;37(11):1564–1570. doi: 10.1172/JCI103749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gabriele G., Penington D. G. Regulation of platelet production: "hypersplenism" in the experimental animal. Br J Haematol. 1967 May;13(3):384–393. doi: 10.1111/j.1365-2141.1967.tb08753.x. [DOI] [PubMed] [Google Scholar]
- FRANZEN S., STRENGER G., ZAJICEK J. Microplanimetric studies on megakaryocytes in chronic granulocytic leukaemia and polycythaemia vera. Acta Haematol. 1961;26:182–193. doi: 10.1159/000206652. [DOI] [PubMed] [Google Scholar]
- Feldman J. D., Mardiney M. R., Unanue E. R., Cutting H. The vascular pathology of thrombotic thrombocytopenic purpura. An immunohistochemical and ultrastructural study. Lab Invest. 1966 Jun;15(6):927–946. [PubMed] [Google Scholar]
- Harker L. A. Kinetics of thrombopoiesis. J Clin Invest. 1968 Mar;47(3):458–465. doi: 10.1172/JCI105742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker L. A. Magakaryocyte quantitation. J Clin Invest. 1968 Mar;47(3):452–457. doi: 10.1172/JCI105741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman R. S., Henderson P. A. Control of marrow production by the level of iron supply. J Clin Invest. 1969 Mar;48(3):454–460. doi: 10.1172/JCI106002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosain F., Marsaglia G., Finch C. A. Blood ferrokinetics in normal man. J Clin Invest. 1967 Jan;46(1):1–9. doi: 10.1172/JCI105501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny R., Rozenberg M. C., Firkin B. G. The splenic platelet pool. Blood. 1966 Jan;27(1):1–16. [PubMed] [Google Scholar]
- SCHULMAN I., PIERCE M., LUKENS A., CURRIMBHOY Z. Studies on thrombopoiesis. I. A factor in normal human plasma required for platelet production; chronic thrombocytopenia due to its deficiency. Blood. 1960 Jul;16:943–957. [PubMed] [Google Scholar]
- SHULMAN N. R., MARDER V. J., WEINRACH R. S. COMPARISON OF IMMUNOLOGIC AND IDIOPATHIC THROMBOCYTOPENIA. Trans Assoc Am Physicians. 1964;77:65–78. [PubMed] [Google Scholar]